Abstract
Late-onset/low vulnerability alcoholics (LOAs) appear to drink less when treated with an SSRI than placebo, whereas early-onset/high vulnerability alcoholics (EOAs) show the opposite effect. We conducted a 12-week, parallel-groups, placebo-controlled trial of the efficacy of sertraline in alcohol dependence (AD). We compared the effects in LOAs vs. EOAs and examined the moderating effects of a functional polymorphism in the serotonin transporter gene. Patients (N=134; 80.6% male; 34.3% EOAs) with DSM-IV AD received up to 200 mg of sertraline (N=63) or placebo (N=71) daily. We used urn randomization and patients were genotyped for the tri-allelic 5-HTTLPR polymorphism. Planned analyses included main and interaction effects of Medication Group, Age of Onset (≤25 years vs. >25 years), and Genotype (L′/L′ vs. S’carriers) on drinking outcomes. Results showed that the moderating effect of Age of Onset on the response to sertraline was conditional on Genotype. There were no main or interaction effects among S′ allele carriers. However, in L′ homozygotes, the effects of Medication Group varied by Age of Onset (P=0.002). At the end of treatment, LOAs reported fewer drinking and heavy drinking days when treated with sertraline (P=0.011), while EOAs had fewer drinking and heavy drinking days when treated with placebo (P<0.001). The small cell sizes and high rate of attrition, particularly for L′ homozygotes, render these findings preliminary and their replication in larger samples necessary. Because AD is common, particularly in medical settings, and SSRIs are widely prescribed by practitioners, these findings have potential public health significance and warrant further evaluation.
Keywords: SSRI, Age of Onset, Alcohol Dependence, 5-HTTLPR, Pharmacogenetics, Treatment Response
INTRODUCTION
Although increasing serotonin (5-HT) consistently reduces drinking in preclinical models,1 serotonergic agonists have yielded limited and inconsistent effects on drinking in humans.2–5 Efforts to subtype alcoholics may help to reduce this inconsistency. For example, in patients with an earlier onset of alcoholism and high levels of both pre-morbid and alcohol-related problems [i.e., Type B alcoholics6], fluoxetine resulted in poorer alcohol-related treatment outcomes than placebo.7 Alcoholism subtype also moderated the effects of sertraline treatment,8 with a significantly greater likelihood of abstinence in Type A (later-onset/lower vulnerability) alcoholics treated with sertraline than placebo. In a multi-center, placebo-controlled trial, early-onset/higher vulnerability (Type II) alcoholics had a higher rate of relapse when treated with fluvoxamine than placebo.9 The converse findings have been obtained with ondansetron, a 5-HT3 antagonist, the efficacy of which is greater among early-onset alcoholics (EOAs).10,11 In summary, alcoholism subtypes may moderate the response to treatment with medications that affect serotonergic tone, which differs as a function of age of alcoholism onset. Differences in measures of central serotonergic activity may help to explain these treatment-related effects.12–15
The 5-HT transporter protein (5-HTT) is a key regulator of 5-HT tone. Variation in SLC6A4, which encodes the 5-HTT, could moderate the response to selective serotonin retuptak inhibitors (SSRIs).16 A 44-bp repeat insertion polymorphism in the 5-HTT linked promoter region (5-HTTLPR) of SLC6A4 results in long (L) and short (S) alleles.17 These alleles differentially modulate transcriptional activity of the 5-HTT promoter, yielding differences in mRNA, protein density, and 5-HT uptake activity in human lymphoblastoid cells, platelets, and brain.18–21 An A→G single nucleotide polymorphism (SNP; rs25531) in the L-specific repeat of the gene,22 also affects function, such that the LG allele is similar to the lower activity S allele.
In a meta-analysis of SSRI treatment trials of depression, L-allele homozygotes had a significantly better treatment response.16 Further, during rapid tryptophan depletion in depressed alcoholics who responded to treatment with serotonergic antidepressants, L-allele homozygotes reported greater depression than S-allele carriers.23 This replicated findings from an earlier report in depressed non-alcoholics.24
In summary, there is substantial evidence that a dichotomous typology of alcoholism, a key dimension of which is age of alcoholism onset,25 moderates the response to serotonergic medications. Variation in the gene encoding the 5-HTT may also moderate the treatment response to SSRIs. To examine these potential moderators, we conducted a parallel-groups, randomized, prospective placebo-controlled study of as a function of Age of Onset of alcohol dependence (AD) and 5-HTTLPR Genotype.
MATERIALS AND METHODS
The study was conducted between February 2004 and June 2009 at the University of Connecticut Health Center (UCHC). Recruitment was conducted primarily through advertisements, with some referrals by area clinicians. The UCHC institutional review board approved the consent form and study protocol and the study was carried out in accordance with the Declaration of Helsinki. Study participants gave written informed consent and were paid to complete daily reports and post-treatment research assessments, but not for study visits.
Patients
All patients underwent physical examination and routine laboratory testing during screening. Inclusion criteria included age 18–65 years old, a past month Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) diagnosis of AD, self-reported abstinence from alcohol for at least 3 days prior to baseline (medical detoxification, though permitted, was uncommon), ability to read English, absence of gross cognitive impairment, and an expressed intention to stop drinking. Women of childbearing potential were non-lactating, practicing reliable birth control, and had a negative serum pregnancy test. Exclusion criteria included a current DSM-IV diagnosis of dependence on any substance other than alcohol, nicotine, or caffeine; use of psychoactive drugs, disulfiram, acamprosate, or naltrexone; a current (but not past) DSM-IV major depressive disorder (MDD); evidence of psychosis; or significant untreated medical illness.
Assessments
The Structured Clinical Interview for DSM-IV26 was used at screening to assess psychiatric diagnoses and the age of onset of AD.
The Timeline Follow-Back (TLFB) method27 was used at baseline to estimate alcohol consumption during the 90-day pretreatment period and at biweekly intervals during treatment to augment daily drinking data (see below).
The Beck Depression Inventory (BDI),28 a 21-item self-report instrument, was administered at each visit to measure depressive symptom severity and suicidal risk.
Interactive voice response technology (IVR), which uses the telephone to administer survey questions,29 enabled patients to report daily on their recent experiences by pressing the keys on the keypad, with responses entered automatically in a database. The number of standard drinks of beer, wine, and liquor consumed each day and the previous evening were summed to create a total number of standard drinks per day. Patients also reported on study medication consumed, which was combined with weekly tablet counts to assess medication adherence.30
The Short Index of Problems (SIP), a 15-item, single-factor measure of alcohol-related problems,31,32 was administered at baseline and the end of treatment.
The concentration of gamma-glutamyl-transpeptidase (GGTP), a liver enzyme that reflects recent drinking, was measured at baseline and at 6 and 12 weeks of treatment.
Adverse events were measured at every visit using a self-report questionnaire.
Study Treatments
Patients were randomly assigned 1:1 to sertraline or placebo groups using a computerized urn randomization33 procedure to balance the treatment groups on Age of Onset, current age, sex, educational level, past diagnosis of MDD, and the duration of abstinence prior to screening. A research pharmacist randomized all patients and dispensed the medications to treatment staff. Sertraline (or matching placebo) was provided to patients under double-blind conditions, initially at a dosage of 50 mg/day and gradually increased to a maximum dosage of 200 mg/day based on tolerability. After 12 weeks of treatment, the study medication was tapered over 2 weeks and discontinued.
In addition to study medication, all patients received 9 structured, manual-guided, individual coping skills training sessions, modeled after interventions by McCrady et al.34 and Monti et al.35 The program included self-monitoring and functional analysis of drinking behavior and urges to drink, training and rehearsal of coping behaviors, training in cognitive coping strategies, and instruction in problem-solving and decision-making skills.
Genotyping Procedure
We used a two-stage TaqMan™ 5′ nuclease allelic discrimination assay modified from the procedure employed by Hu et al.21, 36 to differentiate S and L alleles.37,38 LA vs. LG allele-specific probes (6FAM-CCCCCCTGCACCCCCAGCATCCC-MGB and VIC-CCCCTGCACCCCCGGCATCCCC-MGB, respectively) were used to characterize the A→G SNP in the L allele. Concordant findings were obtained for S and L alleles in 24 samples (20 of which were verified by gel analysis) and for A and G alleles in 26 samples (16 of which were verified by sequencing).
Data preparation
Using IVR data supplemented by TLFB data, patients reported their drinking and medication use for 9,004 days [80% of total days, with a mean of 67.2 days (SD = 26.3; range = 2–84)]. The number of drinking days (DDs) and heavy drinking days (HDDs: ≥4 standard drinks in a day for women and ≥5 in a day for men) per week were the primary outcomes. Missing values were coded as HDDs, a conservative approach that included all patients in both analyses and yielded 12 weekly values for the drinking outcomes. Analyses of drinking behavior were repeated without imputation. We carried the last observation forward to impute missing data in the analysis of the secondary outcome measures (i.e., SIP score and GGTP concentration).
Data analysis
We used two-level hierarchical linear models (HLMs) to examine the main and interaction effects of Medication Group (sertraline = 0, placebo = 1) and Age of Onset (0 = LOA, 1 = EOA) to predict the number of DDs and HDDs per week during the 12-week treatment period. We tested interactions by multiplying together the predictors of interest and including them in the level-2 portion of the model.
Next, we augmented the models to test whether the effect of treatment varied over time (coded -11 to 0) and by 5-HTTLPR Genotype [L′/L′ (coded 0) vs. S′-carriers (coded 1)]. The LG and S alleles were grouped together as S′ (the lower expression allele) and the LA allele was designated as L′ (the higher expression allele). Week in treatment was included as a predictor in the level-1 portion of the model; level-1 slopes were modeled as a function of Medication Group, Age of Onset, and 5-HTTLPR Genotype contrasts and their multiplicative composites.
Both models included sex (0 = male, 1 = female), age (grand-mean centered), lifetime MDD (0 = no, 1 = yes), SIP total score (grand-mean centered), and drinking levels (number of DDs or of HDDs per the outcome of interest; grand-mean centered) during the 90-day pretreatment period as covariates. Intercepts and slopes were treated as random effects. We report the fixed effects with robust standard errors.
Secondary outcomes were analyzed using analysis of covariance, with the respective pretreatment measure as a covariate and Medication Group, Age of Onset, 5-HTTLPR Genotype, and their two-way and three-way interactions as factors.
RESULTS
Of 143 respondents who were screened, 134 (94%) were randomly assigned to treatment, including 63 patients who received sertraline (N = 21 EOAs and N = 42 LOAs) and 71 patients who received placebo (N = 25 EOAs and N = 46 LOAs). The on-line Supplemental Figure A shows the patient flow from screening through the end of treatment, as a function of Age of Onset and Medication Group.
Descriptive statistics
The sample was middle-aged (mean = 47.5 yr; SD = 9.8), mostly male (81%) and European American (92%), with a mean of 14.5 yrs of school (SD = 2.3).
Patients had moderate to severe alcohol dependence, with a mean of 5.8 (SD = 1.1) of 7 possible criteria for current DSM-IV AD. They had a mean SIP score of 21.3 (SD = 8.6) and 64.9% had had prior alcohol treatment. During the pretreatment period, patients drank on 67.9% of days (SD = 26.3%) and drank heavily on 56.5% of days (SD = 29.5%), with a mean of 6.4 (SD = 4.4) standard drinks per day and 9.8 (SD = 5.9) standard drinks per drinking day.
Thirty-five patients (26.1%) had a drug use disorder: cannabis (n = 23, 17.2%), cocaine (n = 26, 19.4%). Thirty-eight patients (28.4%) had a past psychiatric disorder: MDD (n = 28, 20.9%), social phobia (n = 7, 5.2%), panic disorder (n = 4, 3.0%). Four patients had a current psychiatric disorder: specific phobia (n = 1), dysthymic disorder (n = 3). The mean BDI score was 6.3 (SD = 5.9). There were no main effects of Medication Group or interaction effects (with Age of Onset) on any of the demographic or clinical indicators examined.
Treatment Completion and Research Adherence
Overall, 61.9% of patients completed the 12-week treatment. Although this did not differ significantly among the 4 groups (χ2 = 6.45, 3 df, P = 0.092) or by Medication Group (sertraline = 67.6%; placebo = 55.6%; χ2 = 1.58, 1 df, P = 0.21), when the 8 groups were compared (Supplemental Table A), there was a significant difference in completion rate (χ2 = 15.43, 7 df, P = 0.031). The completion rate was lowest in the L′L′ EOAs who were treated with sertraline (14.3%) and highest in the L′L′ EOAs who received placebo and the L′L′ LOAs who received sertraline (83.3% in both groups).
Adherence to the medication regimen during treatment was 93.8%. Although the mean maximal dosage of sertraline was significantly lower [3.4 tablets (SD = 1.0) or 169.0 mg (SD = 48.7)] than for placebo [3.7 tablets (SD = 0.8)] (F(1,130) = 4.26, P = 0.041), it did not differ by Age of Onset (P = 0.32) or Age of Onset × Medication Group (P = 0.90).
Genotypes
The distribution of S′-allele carriers and L′ homozygotes, is shown as a function of Age of Onset and Medication Group in Supplemental Table A. The genotype distribution did not differ significantly from chance (χ2 = 1.21, 3 df, P = 0.75) or Hardy Weinberg Equilibrium (χ2 = 2.36, 1 df, P = 0.12).
Drinking Behavior
Tables 2 and 3 show the results of the regression models predicting the mean number of DDs and HDDs per week, respectively. We entered the predictors into the models in two blocks. First, we entered the covariates, the dummy codes for 5-HTTLPR Genotype, Medication Group, Age of Onset of AD, and interaction to test whether Age of Onset moderated the effect of Medication Group on average drinking levels across treatment, controlling for pre-intervention drinking levels. As shown, the interaction was not significant.
Table 2.
Multilevel Regression Results for the Number of Drinking Days by Medication Group, Age of Alcohol Dependence Onset, and Genotype Group
| Predictor Variables | b | SE | t | p |
|---|---|---|---|---|
| Block 1 | ||||
| Sex | 1.027 | 0.470 | 2.185 | 0.031 |
| Age | −0.068 | 0.021 | −3.244 | 0.002 |
| Short Index of Problems Score | 0.004 | 0.024 | 0.183 | 0.855 |
| Lifetime Major Depression Diagnosis | 0.999 | 0.429 | 2.328 | 0.022 |
| Baseline Drinking Days | 2.563 | 0.724 | 3.542 | 0.001 |
| 5-HTTLPR Genotype | −0.075 | 0.413 | −0.183 | 0.856 |
| Medication Groupa | 0.098 | 0.491 | 0.200 | 0.842 |
| Age of Onset of Alcohol Dependence (AD Onset)a | 0.544 | 0.707 | 0.769 | 0.443 |
| AD Onset × Medication Group | −1.117 | 0.838 | −1.333 | 0.185 |
| Block 2b | ||||
| Study Week | 0.026 | 0.046 | 0.564 | 0.573 |
| Medication Group × Genotype | −8.295 | 1.481 | −5.600 | <0.001 |
| AD Onset × Genotype | −5.396 | 1.458 | −3.700 | 0.001 |
| Study Week × Medication Group | 0.199 | 0.081 | 2.462 | 0.015 |
| Study Week × Genotype | 0.169 | 0.073 | 2.308 | 0.023 |
| Study Week × AD Onset | 0.277 | 0.111 | 2.491 | 0.014 |
| AD Onset × Genotype × Medication Group | 8.560 | 1.960 | 4.367 | <0.001 |
| Study Week × Medication Group × Genotype | −0.265 | 0.108 | −2.463 | 0.015 |
| Study Week × AD Onset × Medication Group | −0.546 | 0.140 | −3.895 | <0.001 |
| Study Week × AD Onset × Genotype | −0.383 | 0.138 | −2.776 | 0.007 |
| Study Week × AD Onset × Genotype × Medication Group | 0.518 | 0.184 | 2.822 | 0.006 |
Medication Group is a conditional effect for individuals who have late-onset alcohol dependence (AD); Age of Onset of AD is a conditional effect for individuals who are in the sertraline group.
The 2-way and 3-way interactions in block 2 are conditional effects for individuals coded zero on the predictor(s) not involved in the interaction (but included in the 4-way interaction).
Table 3.
Multilevel Regression Results for the Number of Heavy Drinking Days by Medication Group, Age of Alcohol Dependence Onset, and Genotype Group
| Predictor Variables | b | SE | t | p |
|---|---|---|---|---|
| Block 1 | ||||
| Sex | 0.817 | 0.463 | 1.764 | 0.080 |
| Age | −0.078 | 0.020 | −3.836 | 0.000 |
| Short Index of Problems Score | 0.018 | 0.024 | 0.754 | 0.452 |
| Lifetime Major Depression Diagnosis | 1.017 | 0.424 | 2.401 | 0.018 |
| Baseline Heavy Drinking Days | 1.578 | 0.620 | 2.543 | 0.013 |
| 5-HTTLPR Genotype | −0.067 | 0.405 | −0.167 | 0.868 |
| Medication Groupa | −0.351 | 0.442 | −0.795 | 0.428 |
| Age of Onset of Alcohol Dependence (AD)a | 0.434 | 0.682 | 0.637 | 0.525 |
| AD Onset × Group | −0.795 | 0.786 | −1.011 | 0.315 |
| Block 2b | ||||
| Study Week | 0.066 | 0.043 | 1.554 | 0.123 |
| Medication Group × Genotype | −3.716 | 1.524 | −2.438 | 0.016 |
| AD Onset × Genotype | −5.220 | 1.460 | −3.576 | 0.001 |
| Study Week × Medication Group | 0.281 | 0.096 | 2.934 | 0.004 |
| Study Week × Genotype | 0.137 | 0.078 | 1.766 | 0.079 |
| Study Week × AD Onset | 0.330 | 0.088 | 3.748 | <0.001 |
| AD Onset × Genotype × Medication Group | 8.402 | 2.116 | 3.972 | <0.001 |
| Study Week × Medication Group × Genotype | −0.356 | 0.125 | −2.850 | 0.006 |
| Study Week × AD Onset × Medication Group | −0.683 | 0.125 | −5.479 | <0.001 |
| Study Week × AD Onset × Genotype | −0.402 | 0.128 | −3.142 | 0.003 |
| Study Week × AD Onset × Genotype × Medication Group | 0.647 | 0.179 | 3.608 | 0.001 |
Medication Group is a conditional effect for individuals who have late-onset alcohol dependence (AD); Age of Onset of AD is a conditional effect for individuals who are in the sertraline group.
The 2-way and 3-way interactions in block 2 are conditional effects for individuals coded zero on the predictor(s) not involved in the interaction (but included in the 4-way interaction).
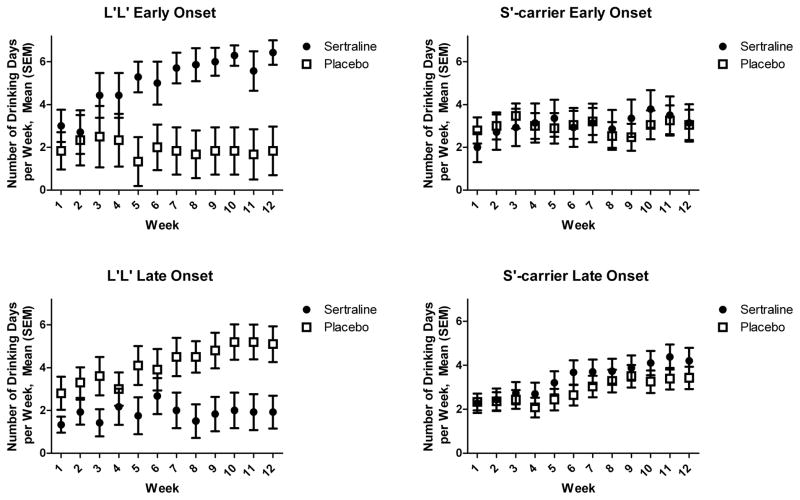
Second, we added a study week predictor and the relevant two-way, three-way and four-way interactions. During the first week of treatment, all patients showed a substantial decrease in drinking from baseline values (see Figure 1 and Table 1). For DDs, there was a significant 4-way interaction, such that the 3-way interaction of Medication Group, Age of Onset, and 5-HTTLPR Genotype varied across Time. In L′ homozygotes, the effects of Medication Group varied across Time and Age of Onset (P <0.001). In week 12, LOAs treated with sertraline had fewer DDs than those receiving placebo (Mean Δ = 3.01, se = 1.13, P = 0.009, Cohen’s d = 0.48), whereas EOAs treated with sertraline had more DDs than those receiving placebo (Mean Δ = 5.28, se = 0.98, P < 0.001, Cohen’s d = 0.98). Among S′-carriers, the Time × Medication Group × Age of Onset interaction was not significant (b = 0.03, se = 0.12, P = 0.82).
Figure 1.
Number of Drinking Days by Study Week, Age of Onset of Alcohol Dependence, Medication Group, and 5-HTTLPR Genotype. Values are mean (±SEM) and reflect drinking behavior during the week identified on the x-axis. As in the analyses, the figures include the intent-to-treat sample and imputed values for missing data. The decrease in drinking days from baseline to week 1 is not shown in the figure, but can be ascertained using data from Table 1.
Table 1.
Pretreatment Demographic and Clinical Measures1
| Placebo | Sertraline | Significance Level4 | |||||
|---|---|---|---|---|---|---|---|
| EOAs2 (n=25) | LOAs3 (n=46) | EOAs2 (n=21) | LOAs3 (n=42) | Treatment | Age of Onset | Interaction | |
| Demographic Features | |||||||
| Sex (% male) | 84.0 | 78.3 | 95.2 | 73.8 | 0.92 | 0.07 | 0.19 |
| Race (% white) | 100 | 89.1 | 90.5 | 90.5 | 0.60 | 0.24 | 0.13 |
| Age (years) | 42.2 (10.8) | 50.1 (7.5) | 44.1 (10.9) | 49.5 (9.3) | 0.81 | <0.001 | 0.45 |
| Education (years) | 14.0 (2.1) | 15.2 (2.4) | 14.4 (2.5) | 14.1 (2.0) | 0.13 | 0.22 | 0.07 |
| Clinical Features | |||||||
| Number of DSM-IV Criteria for AD5 | 6.2 (1.1) | 5.7 (1.1) | 6.3 (0.7) | 6.0 (1.0) | 0.32 | 0.02 | 0.64 |
| Drinking Days (%) | 62.7 (28.6) | 71.4 (25.3) | 70.2 (22.6) | 66.0 (27.8) | 0.84 | 0.57 | 0.18 |
| Heavy Drinking Days (%) | 56.8 (30.0) | 61.2 (28.0) | 57.8 (31.6) | 50.6 (30.1) | 0.19 | 0.84 | 0.29 |
| Mean Drinks Per Day | 6.7 (4.1) | 6.1 (3.8) | 7.7 (5.9) | 6.0 (4.3) | 0.74 | 0.17 | 0.47 |
| Short Index of Problems Score | 23.4 (7.5) | 20.8 (8.6) | 24.1 (8.0) | 19.1 (9.2) | 0.53 | 0.02 | 0.42 |
| Lifetime Major Depression (%) | 20.0 | 23.9 | 28.6 | 14.3 | 0.62 | 0.53 | 0.21 |
| Beck Depression Inventory Score | 6.7 (5.7) | 6.4 (5.6) | 7.2 (5.8) | 5.5 (6.5) | 0.68 | 0.35 | 0.52 |
Mean (SD) or percentage;
EOAs: Early-Onset Alcoholics;
LOAs: Late-Onset Alcoholics;
F-test for continuous measures and χ2 test for categorical measures;
Current alcohol dependence
During the first week of treatment, the number of HDDs also declined substantially in all groups compared with baseline values (see Figure 2 and Table 1). As with DDs, the 4-way interaction of Time, Medication Group, Age of Onset, and 5-HTTLPR Genotype was significant for the number of HDDs per week. The effects of Medication Group also varied across Time and Age of Onset for L′ homozygotes (P < 0.001). In week 12, whereas L′/L′ LOAs treated with sertraline reported marginally fewer HDDs than those receiving placebo (Mean Δ = 2.56, se = 1.30, P = 0.051, Cohen’s d = 0.38), EOAs receiving sertraline reported significantly more HDDs than those treated with placebo (Mean Δ = 5.56, se = 1.01, P < 0.001, Cohen’s d = 1.00). The Time × Medication Group × Age of Onset interaction was not significant among S′-allele carriers (b = 0.04, se = 0.12, P = 0.78).
Figure 2.
Number of Heavy Drinking Days by Study Week, Age of Onset of Alcohol Dependence, Medication Group, and 5-HTTLPR Genotype. Values are mean (±SEM) and reflect drinking behavior during the week identified on the x-axis. As in the analyses, the figures include the intent-to-treat sample and imputed values for missing data. The decrease in drinking days from baseline to week 1 is not shown in the figure, but can be ascertained using data from Table 1.
Other analyses excluded potential confounding by age and population group. Augmenting the models with two- and three-way interactions with age yielded no significant effects in the DD or HDD models and did not alter the age-of-onset interactions. Excluding the 11 patients who were not European American yielded slightly larger p-values for some effects, though the results were substantively unchanged.
We also analyzed the data without imputing missing values. Specifically, instead of summing the number of DDs and HDDs per week, which would assume missed values were non-drinking days, we examined the data at the daily level of analysis, thus letting the number of observations for each person vary. This approach, within the context of our random effects regression models, weights each individual’s contribution to the group comparison on the assumption that missing data are ignorable. Because of the binary nature of these outcomes we used a logit-link and Bernoulli sampling model. Results indicated significant 3-way Genotype × Medication Group × Age of Onset interactions for the DD (P = 0.001) and HDD (P = 0.001) models, but no 4-way interaction involving time. Similar to the results with the imputed data (but pertaining to the entire treatment period), among L′/L′ patients, LOAs treated with placebo were marginally more likely than sertraline-treated LOAs to drink on a given day (adjusted probabilities = 0.409 and 0.124, respectively; P = 0.085), whereas EOAs treated with sertraline were significantly more likely to drink on a given day than those treated with placebo (adjusted probabilities = 0.498 and 0.024, respectively; P < 0.001). There was no difference on HDDs between sertraline- and placebo-treated patients in L′/L′ LOAs (adjusted probabilities = 0.041 and 0.072, respectively; P = 0.47), but EOAs treated with sertraline were significantly more likely than placebo-treated EOAs to drink heavily on a given day (adjusted probabilities = 0.314 and 0.013, respectively; P < 0.001).
Secondary Outcome Measures
The 3-way interactions of Medication Group, Age of Onset, and 5-HTTLPR Genotype showed non-significant trends for both SIP score (F1,125 = 3.05, P = 0.083, partial η2 = 0.024) and GGTP concentration (F1,125 = 3.18, P = 0.077, partial η2 = 0.025), effects that were consistent with the analyses of drinking data. For SIP score, the interaction of Medication Group by Age of Onset was not significant among S′ carriers (F1,94 = 0.47, P = 0.50, partial η2 = 0.005), but was significant among L′ homozygotes (F1,30 = 6.84, P = 0.014, partial η2 = 0.186). Specifically, among EOAs, the mean SIP score at endpoint was 6.5 (SD = 9.6) for placebo and 24.0 (SD = 10.9) for sertraline; among LOAs, the scores were 15.9 (SD = 12.9) for placebo and 10.2 (SD = 9.9) for sertraline. For GGTP, there was not a significant interaction of Medication Group by Age of Onset among S′ carriers (F1,94 = 0.53, P = 0.82, partial η2 = 0.001), but there was a non-significant trend for the interaction among L′ homozygotes (F1,29 = 3.53, P = 0.070, partial η2 = 0.109). Among L′L′ EOAs, placebo-treated patients’ mean GGTP concentration at endpoint was 35.2 (SD = 30.7) compared with 165.8 (SD = 184.3) for sertraline-treated patients, while for L′L′ LOAs the concentrations were 92.0 (SD = 77.5) for placebo and 49.3 (SD = 28.9) for sertraline.
Safety and Tolerability
Three sertraline-treated patients experienced serious adverse events (AEs) resulting in hospitalization (1 with chest pain and 2 who relapsed to heavy drinking). Fourteen patients (9 sertraline and 5 placebo) required a reduction in dosage due to AEs and 7 patients (4 sertraline and 3 placebo) withdrew from the study due to AEs.
Treatment emergent AEs occurring in at least 5% of patients included: diarrhea (19.4%), sexual problems (13.4%), sleepiness/drowsiness (12.7%), nausea (10.4%), insomnia (9.7%), headache (5.2%), and agitation (5.2%). Of these, sleepiness/drowsiness [χ2(1) = 5.93, P = 0.015; sertraline = 20.6%, placebo = 5.6%] and sexual problems [χ2(1) = 9.54, P = 0.002; sertraline = 23.8%, placebo = 4.2%] differed significantly by Medication Group.
DISCUSSION
In this study, sertraline treatment resulted in greater reductions in drinking behavior from pretreatment levels in LOAs, but in EOAs greater reductions were seen with placebo treatment. Because the interaction effect occurred only in L′ homozygotes, these results provide only partial replication of prior studies of SSRIs in which alcoholism subtype was a moderator of treatment outcome. In the present study, trends in secondary outcome measures, including SIP score and GGTP concentration, were consistent with the effects on self-reported drinking.
To subtype patients, we used Age of Onset of DSM-IV AD based on the SCID (rather than cluster analysis7,8 or the age of onset of problem drinking elicited by a single question9). Although three studies have shown a moderating effect of Age of Onset or risk/vulnerability subtype on the response to SSRI treatment of AD, they differed in the simple main effects of treatment.7–9 Two studies7,9 showed that EOAs treated with an SSRI had poorer outcomes than those receiving placebo, while one8 showed a significant advantage of the SSRI in later-onset, lower risk/vulnerability patients. In the present study, both simple effects were statistically significant only in L′ homozygotes (i.e., sertraline resulted in better drinking outcomes than placebo in LOAs and worse drinking outcomes than placebo in EOAs). In view of our finding that 5-HTTLPR genotype moderated the response to sertraline, differences in the prevalence of the L′L′ genotype by study sample could explain the different simple effects in prior studies.
L′ homozygotes are the group for which there is the greatest potential impact of 5-HT transporter blockade (e.g., by SSRIs), since the high activity L′ allele is associated with greater mRNA concentrations, protein density, and 5-HT uptake activity than the low activity (S′) allele.18–21 Blockade of the transporter could, therefore, produce the greatest increase in synaptic 5-HT concentrations in L′ homozygotes. An increase in synaptic 5-HT would stimulate post-synaptic 5-HT3 receptors. Because alcohol-induced release of dopamine in the nucleus accumbens contributes to ethanol reinforcement39,40 and 5-HT3 receptors synapse on these dopamine neurons,41–43 5-HT3 receptors could modulate the release of dopamine and the reinforcing effects of alcohol consumption42,43 Consistent with these effects, 5-HT3 antagonists reduce alcohol intake in animals and humans.10,11,41,44–48 It is possible that sertraline could differentially influence drinking because of age-of-onset-related differences in the expression or function of the 5-HT3 receptor.
In view of the findings reported here, the prevalence of the L′ allele in the general population could determine the impact of SSRI treatment on outcomes among alcoholics. Given estimates of the distribution of the LG, S, and LA alleles in the general U.S. population of 24%, 25% and 51%, respectively, in African-Americans49 and 14%, 36%, and 50%, respectively, in European-Americans,22,50 the overall prevalence of the L′L′ genotype is approximately 25%. The current U.S. prevalence of AD was estimated to be 3.8%,51 [i.e., 2.4% EOAs and 1.3% LOAs (B. Grant, personal communication; February 3, 2010)]. If one-quarter of those individuals are responsive to SSRI treatment, as much as 0.6% of the total U.S. population could be adversely affected and more than 0.3% could benefit from the medication.
The growing use of antidepressants underscores the importance of these findings. The rate of antidepressant treatment in the United States increased from 5.84% in 1996 to 10.12% in 2005, with nearly three-fourths of patients treated for a diagnosis other than depression.52 More than 10% of adults from the National Comorbidity Survey Replication received antidepressant treatment in the year preceding the study, with nearly three-quarters being treated by general medical providers, who were less likely than psychiatrists to treat individuals meeting criteria for mood or anxiety disorders.53 The high prevalence of AD and the widespread use of antidepressants suggest that the findings reported here are relevant to a substantial proportion of the U.S. population.
Strengths of this study include its design, which was prospective, randomized, and placebo-controlled, with analysis by intent to treat and conservative imputation of missing data. The study was based on prior findings and yielded results that underscore the need to individualize treatments for AD to take into account the patient’s clinical features and genotype. The findings differ from prior studies on the SSRI treatment of AD in that Age of Onset of AD moderated the response to medication only in a subgroup (i.e., L′ homozygotes), which implicates 5-HTTLPR genotype as a moderator of SSRI treatment effects. Differences in alcohol-related problem (i.e., SIP) scores and in liver enzyme (i.e., GGTP) concentrations, though modest, were consistent with the effects on drinking behavior.
Limitations of the study included a small initial sample size and a comparatively high rate of attrition, particularly in the L′L′ EOAs who were treated with sertraline. When missing data were imputed as heavy drinking days, there was an interaction of Genotype × Age of Onset × Medication Group that was significantly greater at the end of the study, when attrition was greatest. A less conservative approach to the treatment of missing data (i.e., random effects regression analysis) yielded a significant Genotype × Age of Onset × Medication Group interaction, but no effect of time. In view of the small sample size and differential attrition, the findings must be considered preliminary and their replication in larger samples is necessary. Subsequent studies will also benefit from additional efforts to reduce attrition from treatment to minimize the need for imputation of drinking data. Such studies should be large enough to permit stratification on both Age of Onset and Genotype, to ensure control of potential confounders and adequate numbers of patients in the L′ homozygote groups. Such studies are also needed to determine the extent to which the findings can be extended to other SSRIs.
A clinician familiar with the DSM-IV criteria for AD can readily assess the age of onset of AD. Further, access to genotyping is becoming increasingly available in clinical settings. If replicated, these findings would support the routine use of the Age of Onset and 5-HTTLPR Genotype to predict which non-depressed patients with current AD are likely to show a good response to SSRI treatment and which are likely to show either no response or poorer outcomes than if no medication were prescribed.
Supplementary Material
Supplemental Figure A: Flow Sheet of Patient Screening, Randomization, and Study Completion.
Acknowledgments
Supported by NIH grants R01 AA13631, K24 AA13736, and M01 RR06192. Pfizer donated sertraline and placebo tablets for use in the study. The staff of the Clinical Research and Evaluation Unit of the UConn Alcohol Research Center was instrumental in the conduct of the study.
Footnotes
The authors have the following financial disclosures:
SA, HT, JC, and RF have no disclosures to make. During the past 3 years, HK has had consulting arrangements with the following pharmaceutical companies: Alkermes, Lundbeck, Gilead, and GlaxoSmithKline. Eli Lilly, Janssen, Schering Plough, Lundbeck, Alkermes, GlaxoSmithKline, Abbott, and Johnson & Johnson provide support to the Alcohol Clinical Trials Initiative (ACTIVE), from which HK also receives support. HK reports research support from Merck. HP reports consulting arrangements with Alkermes, AstraZeneca, Cephalon, Eli Lilly, Forest, GlaxoSmithKline, and Merck and research support from Alkermes, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest, Merck, and Ortho-McNeil. CO reports consulting arrangements with Pfizer Pharmaceuticals; research support from Pfizer and Nabi; and donations of nicotine replacement products and placebo from GlaxoSmithKline and Johnson & Johnson.
References
- 1.LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol Psychiatry. 1994;36(6):395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- 2.LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994;36(5):326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 3.Kranzler HR. Pharmacotherapy of alcoholism: gaps in knowledge and opportunities for research. Alcohol Alcohol. 2000;35(6):537–547. doi: 10.1093/alcalc/35.6.537. [DOI] [PubMed] [Google Scholar]
- 4.Naranjo CA, Kadlec KE, Sanhueza P, et al. Fluoxetine differentially alters alcohol intake and other consummatory behaviors in problem drinkers. Clin Pharmacol Ther. 1990;47(4):490–498. doi: 10.1038/clpt.1990.62. [DOI] [PubMed] [Google Scholar]
- 5.Kranzler HR, Burleson JA, Korner P, et al. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995;152(3):391–397. doi: 10.1176/ajp.152.3.391. [DOI] [PubMed] [Google Scholar]
- 6.Babor TF, Hofmann M, DelBoca FK, et al. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49(8):599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- 7.Kranzler HR, Burleson JA, Brown J, et al. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20(9):1534–1541. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 8.Pettinati HM, Volpicelli JR, Kranzler HR, et al. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24(7):1041–1049. [PubMed] [Google Scholar]
- 9.Chick J, Aschauer H, Hornik K. Efficacy of fluvoxamine in preventing relapse in alcohol dependence: a one-year, double-blind, placebo-controlled multicentre study with analysis by typology. Drug Alcohol Depend. 2004;74(1):61–70. doi: 10.1016/j.drugalcdep.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. JAMA. 2000;284(8):963–971. doi: 10.1001/jama.284.8.963. [DOI] [PubMed] [Google Scholar]
- 11.Kranzler HR, Pierucci-Lagha A, Feinn R, et al. Effects of ondansetron in early- versus late-onset alcoholics: a prospective, open-label study. Alcohol Clin Exp Res. 2003;27(7):1150–1155. doi: 10.1097/01.ALC.0000075547.77464.76. [DOI] [PubMed] [Google Scholar]
- 12.Buydens-Branchey L, Branchey MH, Noumair D. Age of alcoholism onset. I. Relationship to psychopathology. Arch Gen Psychiatry. 1989;46(3):225–230. doi: 10.1001/archpsyc.1989.01810030031004. [DOI] [PubMed] [Google Scholar]
- 13.Buydens-Branchey L, Branchey MH, Noumair D, et al. Age of alcoholism onset. II. Relationship to susceptibility to serotonin precursor availability. Arch Gen Psychiatry. 1989;46(3):231–236. doi: 10.1001/archpsyc.1989.01810030037005. [DOI] [PubMed] [Google Scholar]
- 14.Swann AC, Johnson BA, Cloninger CR, et al. Relationships of plasma tryptophan availability to course of illness and clinical features of alcoholism: a preliminary study. Psychopharmacology (Berl) 1999;143(4):380–384. doi: 10.1007/s002130050962. [DOI] [PubMed] [Google Scholar]
- 15.Fils-Aime ML, Eckardt MJ, George DT, et al. Early-onset alcoholics have lower cerebrospinal fluid 5-hydroxyindoleacetic acid levels than late-onset alcoholics. Arch Gen Psychiatry. 1996;53(3):211–216. doi: 10.1001/archpsyc.1996.01830030029006. [DOI] [PubMed] [Google Scholar]
- 16.Serretti A, Kato M, De Ronchi D, et al. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry. 2007;12(3):247–257. doi: 10.1038/sj.mp.4001926. [DOI] [PubMed] [Google Scholar]
- 17.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 18.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 19.Praschak-Rieder N, Kennedy J, Wilson AA, et al. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11)C] DASB positron emission tomography study. Biol Psychiatry. 2007;62(4):327–31. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg BD, Tolliver TJ, Huang SJ, et al. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet. 1999;88(1):83–87. [PubMed] [Google Scholar]
- 21.Murphy DL, Lerner A, Rudnick G, et al. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4(2):109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- 22.Hu XZ, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierucci-Lagha A, Feinn R, Modesto-Lowe V, et al. Effects of rapid tryptophan depletion on mood and urge to drink in patients with co-morbid major depression and alcohol dependence. Psychopharmacology (Berl) 2004;171(3):340–348. doi: 10.1007/s00213-003-1588-6. [DOI] [PubMed] [Google Scholar]
- 24.Moreno FA, Rowe DC, Kaiser B, et al. Association between a serotonin transporter promoter region polymorphism and mood response during tryptophan depletion. Mol Psychiatry. 2002;7(2):213–216. doi: 10.1038/sj.mp.4000962. [DOI] [PubMed] [Google Scholar]
- 25.Roache JD, Wang Y, Ait-Daoud N, et al. Prediction of serotonergic treatment efficacy using age of onset and Type A/B typologies of alcoholism. Alcohol Clin Exp Res. 2008;32(8):1502–1512. doi: 10.1111/j.1530-0277.2008.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.First M, Spitzer R, Gibbon M. Patient Edition with Psychotic Screen (SCID-I/P W/ PSYSCREEN) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2001. Structured Clinical Interview for DSM-IC-TR Axis I Disorders, Research Version. [Google Scholar]
- 27.Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measureing Alcohol Consumption. Clifton, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 28.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 29.Kranzler HR, Abu-Hasaballah K, Tennen H, et al. Using daily interactive voice response technology to measure drinking and related behaviors in a pharmacotherapy study. Alcohol Clin Exp Res. 2004;28(7):1060–1064. doi: 10.1097/01.alc.0000130806.12066.9c. [DOI] [PubMed] [Google Scholar]
- 30.Feinn R, Tennen H, Cramer J, et al. Measurement and prediction of medication compliance in problem drinkers. Alcohol Clin Exp Res. 2003;27(8):1286–1292. doi: 10.1097/01.ALC.0000080670.59386.6E. [DOI] [PubMed] [Google Scholar]
- 31.Miller W, Tonigan J. NIAAA Project MATCH Monograph Series. Vol. 4. Bethesda, MD: National Institutes of Health; 1995. The Drinker Inventory of Consequences (DrInC) NIH Publ. No. 95–3911. [Google Scholar]
- 32.Feinn R, Tennen H, Kranzler HR. Psychometric properties of the short index of problems as a measure of recent alcohol-related problems. Alcohol Clin Exp Res. 2003;27(9):1436–1441. doi: 10.1097/01.ALC.0000087582.44674.AF. [DOI] [PubMed] [Google Scholar]
- 33.Wei L. An application of an urn model to the design of sequential controlled clinical trials. J Amer Statistic Assoc. 1978;73:559–563. [Google Scholar]
- 34.McCrady B, Dean L, DuBreuil R, et al. In: Relapse Prevention. Marlatt GA, Gordon JR, editors. New York: Guilford Press; 1985. [Google Scholar]
- 35.Monti P, Abrams D, Kadden R, et al. Treating Alcohol Dependence: A Coping Skills Training Guide. New York: Guilford Press; 1989. [Google Scholar]
- 36.Hu X, Oroszi G, Chun J, et al. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura M, Ueno S, Sano A, et al. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5(1):32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- 38.Covault J, Tennen H, Herman AI, et al. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biol Psychiatry. 2007;61:609–616. doi: 10.1016/j.biopsych.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 39.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13(5):177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 41.Fadda F, Garau B, Marchei F, et al. MDL 72222, a selective 5-HT3 receptor antagonist, suppresses voluntary ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 1991;26(2):107–110. doi: 10.1093/oxfordjournals.alcalc.a045088. [DOI] [PubMed] [Google Scholar]
- 42.Wozniak KM, Pert A, Linnoila M. Antagonism of 5-HT3 receptors attenuates the effects of ethanol on extracellular dopamine. Eur J Pharmacol. 1990;187(2):287–289. doi: 10.1016/0014-2999(90)90015-x. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimoto K, Yayama K, Sorimachi Y, et al. Possibility of 5-HT3 receptor involvement in alcohol dependence: a microdialysis study of nucleus accumbens dopamine and serotonin release in rats with chronic alcohol consumption. Alcohol Clin Exp Res. 1996;20(9 Suppl):311A–319A. [PubMed] [Google Scholar]
- 44.Hodge CW, Samson HH, Lewis RS, et al. Specific decreases in ethanol- but not water-reinforced responding produced by the 5-HT3 antagonist ICS 205–930. Alcohol. 1993;10(3):191–196. doi: 10.1016/0741-8329(93)90034-l. [DOI] [PubMed] [Google Scholar]
- 45.Dyr W, Kostowski W. Evidence that the amygdala is involved in the inhibitory effects of 5-HT3 receptor antagonists on alcohol drinking in rats. Alcohol. 1995;12(4):387–391. doi: 10.1016/0741-8329(95)00023-k. [DOI] [PubMed] [Google Scholar]
- 46.Tomkins DM, Le AD, Sellers EM. Effect of the 5-HT3 antagonist ondansetron on voluntary ethanol intake in rats and mice maintained on a limited access procedure. Psychopharmacology (Berl) 1995;117(4):479–485. doi: 10.1007/BF02246222. [DOI] [PubMed] [Google Scholar]
- 47.Knapp DJ, Pohorecky LA. Zacopride, a 5-HT3 receptor antagonist, reduces voluntary ethanol consumption in rats. Pharmacol Biochem Behav. 1992;41(4):847–850. doi: 10.1016/0091-3057(92)90237-a. [DOI] [PubMed] [Google Scholar]
- 48.Sellers EM, Toneatto T, Romach MK, et al. Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence. Alcohol Clin Exp Res. 1994;18(4):879–885. doi: 10.1111/j.1530-0277.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 49.Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32(9):2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- 50.Hu XZ, Rush AJ, Charney D, et al. Association between a functional serotonin transporter promoter polymorphism and citalopram treatment in adult outpatients with major depression. Arch Gen Psychiatry. 2007;64(7):783–792. doi: 10.1001/archpsyc.64.7.783. [DOI] [PubMed] [Google Scholar]
- 51.Grant BF, Dawson DA, Stinson FS, et al. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74(3):223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- 53.Mojtabai R, Olfson M. National patterns in antidepressant treatment by psychiatrists and general medical providers: results from the national comorbidity survey replication. J Clin Psychiatry. 2008;69(7):1064–1074. doi: 10.4088/jcp.v69n0704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure A: Flow Sheet of Patient Screening, Randomization, and Study Completion.