Abstract
Understanding variations in lipoprotein cholesterol levels throughout the menstrual cycle is important because there may be clinical implications regarding the appropriate timing of measurement and implications on the design and interpretation of studies in women of reproductive age. Our objective was to review the evidence comparing lipoprotein cholesterol levels throughout the menstrual cycle among premenopausal women. Overall, lipoprotein cholesterol levels were observed to vary in response to changing estrogen levels. Taken together, the evidence suggests that total cholesterol and LDL-C tend to be highest during the follicular phase and to decline during the luteal phase, with HDL C highest around ovulation. Based on these findings, the menstrual cycle phase should be taken into account when evaluating lipoprotein cholesterol levels among reproductive-aged women. Measuring cholesterol levels during menses is recommended for consistent comparisons as this phase can be more reliably identified than other phases, although women within National Cholesterol Education Program acceptable ranges, but near the boundaries when tested during menses, should undergo additional tests.
Keywords: cholesterol, estrogen, lipoprotein, menstrual cycle
According to the National Cholesterol Education Program (NCEP) guidelines, total blood cholesterol levels greater than or equal to 200 mg/dl put the average US adult at elevated risk for coronary heart disease (CHD) [1]. High lipoprotein cholesterol is one of the primary risk factors for heart disease, the leading cause of death among women [2]. Interestingly, the prevalence of cardiovascular disease (CVD) among women aged 20–39 years is half of that for men in the same age group (women: 7.8%; men: 15.9%). The sex disparity in CVD narrows with increasing age as the prevalence among women increases, leading researchers to consider estrogen as a potential modifying factor in CVD risk. As increasing evidence shows that estrogen levels impact various metabolic systems in the body, guidelines may need to be tailored separately to men and women. However, NCEP blood cholesterol guidelines are not sex-specific, despite the differences in health and risk factors between men and women.
Based upon the increased risk for CVD for postmenopausal women when compared with premenopausal women, hormone therapy (HT) was recommended to postmenopausal women as a means of cardioprotection [3–6]. Concurrently, randomized controlled clinical trials were implemented to further examine the cardioprotective effects of HT. While the Women’s Health Initiative (WHI) trial [7] and the Heart and Estrogen/Progestin Replacement Study (HERS) [8] found an antiatherogenic lipid profile among women using exogenous estrogens, estrogen plus progestin therapy was associated with increased rates of CHD. Although these studies may not demonstrate a direct relationship between lipid levels and CHD due to potential confounding by age and duration and indication of treatment, these results support the cardioprotective properties of HT, and especially estrogen-only HT. Furthermore, while estrogen-only HT showed the largest improvements in lipid levels among postmenopausal women [7–10], there is also evidence to suggest that exogenous estrogens in the form of oral contraceptives also improve the lipid profile among premenopausal women, although results depend upon the formulation and dose [11–14]. The effects of endogenous estrogens on the lipid profile are less clear, although there are strong biological mechanisms to support a beneficial role of estrogen in lipoprotein metabolism [15,16]. Conversely, progesterone is thought to oppose the stimulatory effects of estrogen or have a neutral effect on lipoprotein metabolism [13]. Thus, any antiatherogenic effect of reproductive hormones on lipid levels is usually attributed to estrogen.
Despite the increases in CHD among post-menopausal women on HT, questions regarding the role of endogenous estrogens on lipoproteins in premenopausal women remain. Understanding the impact of endogenous estrogens is critical for proper management of lipid levels, which are an important contributor to atherosclerosis. Furthermore, the effects of endogenous and exogenous estrogens may vary, and evaluation of the role of endogenous estrogens on lipoprotein metabolism may help to elucidate the role of estrogen in protecting premenopausal women against CHD. Lastly, understanding variations in lipoprotein cholesterol levels is important because there may be clinical implications regarding the appropriate timing of measurement during the cycle and implications on the design and interpretation of studies in women of reproductive age.
Therefore, our objective was to review the epidemiological evidence comparing lipoprotein cholesterol levels across the menstrual cycle among premenopausal women, highlight results from a recent large prospective study and discuss potential clinical implications regarding these findings.
Epidemiological evidence
Numerous epidemiological studies have evaluated the role of endogenous estrogens in lipoprotein metabolism. These studies yielded conflicting results, with some observing fluctuating plasma lipid levels throughout the menstrual cycle [17–32], while others found a lack of variation in lipid levels over the menstrual cycle [33–40]. These differences could be due to study design differences including small sample size, follow-up for only a single menstrual cycle and/or timing of sample collection, heterogeneity in markers of ovulation or inconsistencies in the timing of lipoprotein and hormone measurements. Various methods of timing of lipoprotein cholesterol measurements to menstrual cycle phase were implemented, including determining hormone levels by basal body temperature methods [22,37], ovulation charts [18] or blood samples [18,22,23,25,27–29,34,35,37,41]. Most studies typically only compared lipoprotein cholesterol levels between the follicular and luteal phases of the cycle, and did not estimate associations between hormone levels and lipoproteins at multiple points during the cycle. Many studies found that only certain component measures of lipoprotein cholesterol (total cholesterol [TC], HDL-C, LDL-C or triglycerides) differ between cycle phases.
Lipoprotein cholesterol levels varied across the menstrual cycle. Specifically, TC and LDL-C were most often lower during the luteal phase, corresponding to the time of the menstrual cycle when estrogen and progesterone levels are high compared with the follicular phase. Furthermore, HDL-C levels were typically highest during the late follicular and periovulatory phases, a finding that tended not to be observed in studies that only compared measurements during the follicular and luteal phases. The increases in TC and LDL-C immediately prior to ovulation, and peak levels of HDL-C at ovulation, are of great physiological importance as cholesterol, and VLDL-C in particular, constitutes the precursor for steroid synthesis and needs to be dramatically increased should a pregnancy occur. Table 1 summarizes the results of studies that compared levels between phases. The mean changes in TC levels across the menstrual cycle varied between 4 and 10%, LDL-C between 4 and 12.5% and HDL-C by 11% across the studies evaluated. The mean intraindividual variability reported for TC ranged from 8 to 19%. Triglyceride levels did not vary cyclically throughout the cycle. Certain studies also reported various lipoprotein particle subfractions, although further work is needed in this area.
Table 1.
Summary of findings by selected studies evaluating cyclic changes in lipoprotein cholesterol levels across the menstrual cycle†.
| Study (year) | n (age in years) | Cycle, measurements | Changes from follicular to luteal phase (lipids and lipoprotein levels)‡ | Statistical tests used | Ref. |
|---|---|---|---|---|---|
| Barnett et al. (2004) | 44 (26.8 ± 4.1)§ | One cycle, two blood samples (follicular and luteal phases) | LDL-C: decrease (6.2%; p = 0.015) TC/HDL-C: decrease (5.1%; p = 0.0006) LDL-C/HDL-C: decrease (8.4%; p = 0.002) |
Paired t-test | [34] |
| Muesing et al. (1996) | 12 (27 ± 3)§ | Three cycles, four blood samples per cycle | LDL-C: decrease (4%; p < 0.01) LDL-C/HDL-C: decrease (6%; p < 0.005) |
ANOVA | [25] |
| Tonolo et al. (1995) | 16 (30 ± 1)§ | One cycle, two during menses and daily thereafter | TC: decrease (9.3%; p < 0.05) LDL-C: decrease (12.5%; p < 0.05) HDL-C: increase (11.4%; p < 0.05) ApoA-I: increase (p < 0.05) ApoB: NS |
Paired t-test and ANOVA | [29] |
| Lussier-Cacan et al. (1991) | 18 (23–38)¶ | Three cycles, blood samples taken at determined intervals | TC and TG: NS | ANOVA | [39] |
| Schijf et al. (1993) | 54 (28 ± 4.7)§ | One cycle, two blood samples (follicular and luteal phases) | TC: decrease (6.4%; p < 0.01) LDL-C: decrease (12%; p < 0.01) LDL-C/HDL-C: decrease (12.5%; p < 0.01) TC/HDL-C: decrease (7.3%; p < 0.01) ApoB: decrease (1.3%; p < 0.01) TG, HDL-C and apoA-I: NS |
Wilcoxon signed rank test | [41] |
| Azogui et al. (1992) | 18 (19–44)¶ | One cycle, three blood samples (early follicular, preovulatory and mid-luteal phases) | ApoA-I/HDL-C: increase (11%; p < 0.05) LDL-C, TC, TG, VLDL-C and apoB: NS |
Paired t-test | [33] |
| Elhadd et al. (2003) | 20 (34 ± 1)§ | One cycle, three blood samples (early and mid-follicular and luteal phases) | TC, LDL-C, HDL-C, TG, apoA-I, apoB and Lp(a): NS | Paired t-test | [36] |
| Jones et al. (1988) | 31 (20–40)¶ | Three cycles, two blood samples (mid-follicular and mid-luteal phases) | TC: decrease (6%; p < 0.05) TG and HDL-C: NS |
Paired t-test | [37] |
| Kim and Kalkhoff (1979) | 14 (33 ± 2)§ | Three cycles, blood samples taken every 3–5 days | TC: decrease (10%; p < 0.01) TG, LDL-C, HDL-C and HDL-C/LDL-C: NS |
Paired t-test | [22] |
| Barclay et al. (1965) | 11 (25–44)¶ | Approximately three cycles, 12 blood samples taken weekly | HDL-C2: increase at ovulation (49%; p = 0.05) | ANOVA | [30] |
| Basdevant et al. (1981) | 8 (25–30)¶ | 18 cycles, three blood samples (menses, follicular and luteal phases) | TC, TG and HDL-C: NS | Paired t-test | [47] |
| Mattsson et al. (1984) | 22 (18–35)¶ | One cycle, four blood samples | LDL-C: decrease (10%; p < 0.05) HDL-C: increase (11%; p < 0.05) TC/HDL-C: decrease (12%; p < 0.01) LDL-C/HDL-C: decrease (18%; p < 0.01) |
Paired one-sample Wilcoxon rank test | [48] |
| Woods et al. (1987) | 15 (24.2 ± 7.5)§ | One cycle, three blood samples (follicular, ovulatory and luteal phases) | TC, TG, VLDL-C, LDL-C and HDL-C: NS | ANOVA | [40] |
| Lebech and Kjaer (1989) | 37 (19–36)¶ | One cycle, three blood samples | TC, TG, LDL-C and HDL-C: NS | ANOVA | [38] |
| De Leon et al. (1992) | 29 (20–26)¶ | One cycle, blood samples collected every other day during the first and last weeks, daily during middle period | TC: decrease (4%; p < 0.05) TG: decrease (17%; p < 0.05) HDL-C: NS |
Paired t-test | [35] |
| Nduka and Agbedana (1993) | 14 (20–30)¶ | One cycle, 20 blood samples | TC: decrease (5%; p < 0.03) HDL-C: increase (11%; p < 0.03) |
Multivariate repeated measures | [26] |
| Oliver and Boyd (1953) | 12 (mean: 22) | One cycle, two blood samples per week for 5 weeks | TC: lowest point at ovulation (5% decrease from menses); no p-values presented | NA (only descriptive analysis performed) | [32] |
| Lyons Wall et al. (1994) | 12 (19–37)¶ | One cycle, 20 blood samples | HDL-C: increase (12%; p < 0.001) TC: increase (9%; p < 0.005) LDL-C: increase (11%; p < 0.025) TG: NS |
ANOVA | [24] |
| Larsen et al. (1996) | 19 (21–39)¶ | Two cycles, two blood samples per week for 9 weeks | TC: decrease (~8%; p < 0.001) LDL-C: decrease (~10%; p < 0.001) Changes from ovulation to late luteal phases: HDL-C: decrease (~8%; p < 0.001) |
Wilcoxon signed rank test | [23] |
| Adlercreutz and Tallqvist (1959) | 29 (20–41)¶ | One cycle, blood samples taken at timed visits | TC: decrease (p < 0.01) | ANOVA | [31] |
| Haines et al. (1997) | 47 control (33.7 ± 4.8)§ 43 study (35.1 ± 3.1)§ |
One cycle, two blood samples for control cycles and one blood sample for study cycles | Ovary not being stimulated: Lp(a): increase (5.6%; p < 0.05) TC, LDL-C, apoB, HDL-C, apoA-I and TG: NS Ovary hyperstimulation: Lp(a): increase (14.2%; p < 0.05) TC: decrease (6.9%; p < 0.001) LDL-C: decrease (11.7%; p < 0.0001) TG: increase (10.4%; p < 0.05) ApoB, HDL-C and apoA-I: NS |
Wilcoxon signed rank test | [49] |
| Ahumada Hemer et al. (1985) | 114 (mean: 24) | One cycle, one blood sample | TC: increase in the late follicular phase compared with early phase (p < 0.05) VLDL-C: increase (p < 0.05) LDL-C: decrease (p < 0.005) HDL-C and TG: NS |
ANOVA | [50] |
Selected only to show the varied findings by various authors.
As defined by the authors.
Mean ± standard deviation.
Range.
ANOVA: Analysis of variance; NA: Not applicable; NS: No significant change; TC: Total cholesterol; TG: Triglyceride.
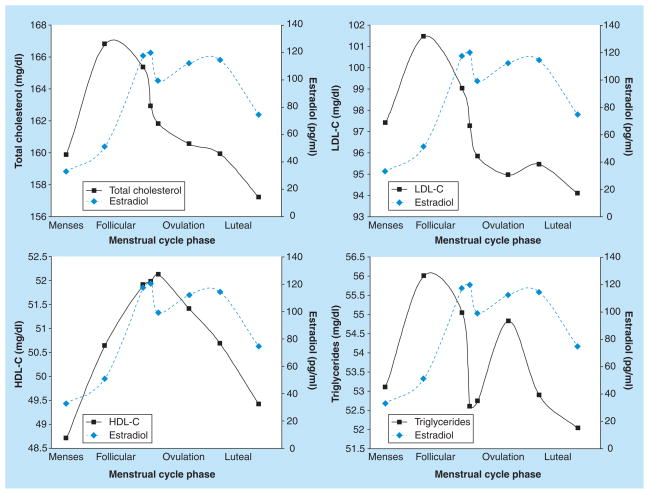
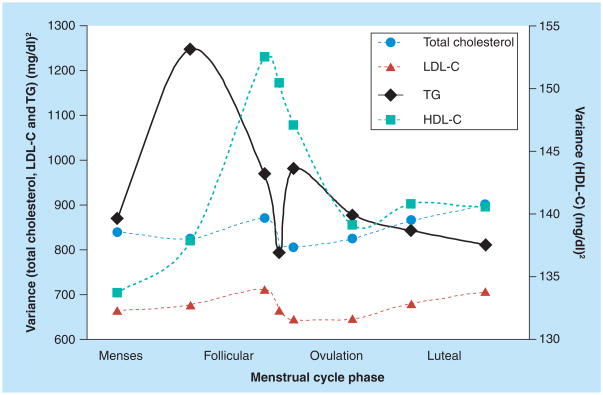
Not only do lipoprotein cholesterol levels differ between the follicular and luteal phases, but they have been shown to vary on a day-to-day basis throughout the cycle. In the largest study to date, hormones and lipoprotein cholesterol were measured at up to eight visits per cycle, for up to two cycles in a cohort of 259 healthy, regularly menstruating women [42]. Collection of these fasting blood samples was timed to specific phases of the menstrual cycle using fertility monitors. These multiple measurements enabled evaluation of the patterns of means and the variability of lipoprotein cholesterol levels across the cycle. As shown in Figure 1, TC and LDL-C follow a similar pattern across the menstrual cycle, with levels increasing rapidly after menses, peaking during the follicular phase and then declining throughout the luteal phase. The peak levels of TC and LDL-C were observed during the follicular phase prior to the rise and peak of estrogen, with TC and LDL-C levels declining during the luteal phase, corresponding to rising and peak concentrations of estrogen and progesterone. HDL-C levels were highest around ovulation, corresponding to high levels of estrogen, whereas triglyceride levels varied without a consistent pattern across the cycle. Interestingly, variability in lipoprotein cholesterol measurements fluctuated across the cycle (Figure 2). Minimum variation in TC, LDL-C and triglycerides was observed during menses and around ovulation, with HDL showing the most variability around ovulation. Many of the other studies reviewed observed similar variation in lipoprotein levels (standard errors or variance) during the follicular and luteal phases of the cycle, while some did not report the levels of variability. Among the studies reporting measurements during menses [18,24,27,29,39], most detected a decrease in the variability of TC levels [18,24,39], while an increase in variability in HDL levels around ovulation was observed in one other study [29].
Figure 1.
Mean levels of total cholesterol, LDL-C, HDL-C, triglycerides and estrogen levels across the menstrual cycle among 259 women enrolled in the BioCycle Study.
Data taken from [42].
Figure 2.
Variance in lipoprotein cholesterol measurements during different phases of the menstrual cycle among 259 women enrolled in the BioCycle Study.
TG: Triglyceride.
Data taken from [42].
Owing to the observed day-to-day changes in both the mean and variability of lipoprotein cholesterol levels, studies have analyzed the association between estrogen and lipoprotein cholesterol levels across the cycle (Table 2). Positive correlations and associations between HDL and estrogen levels were observed. TC and LDL-C levels were inversely associated with estrogen levels, although findings were not statistically significant in all of the studies. In the most recent study, endogenous estrogen was also positively associated with TC and HDL-C and inversely associated with LDL-C in acute effects models (which consider hormones and lipoprotein cholesterol measured on the same day), and significantly and inversely associated with TC and LDL-C levels during the cycle in persistent effects models (which consider hormones measured at the visit immediately prior to measurement of lipoprotein cholesterol levels) [42]. These models took into account repeated measurements across the cycle, levels of other circulating reproductive hormones and other factors known to impact these associations, such as age and BMI [42]. Further adjustment for physical activity and dietary intake did not alter the results. These findings suggest that estrogen has a rapid effect on increasing TC and HDL-C levels and decreasing LDL-C levels, as well as nonacute effects on decreasing LDL-C, which lead to decreases in TC.
Table 2.
Selected studies evaluating the association between lipoprotein cholesterol levels and estrogen.
| Study (year) | n (age in years) | Cycle, measurements | Association between lipoprotein cholesterol levels and estrogen | Statistical tests used | Ref. |
|---|---|---|---|---|---|
| Lyons Wall et al. (1994) | 12 (19–37)† | One cycle, 20 fasting blood samples on alternate days (for 5 weeks); ovulation timed by hormone measurements; divided into six phases | HDL-C and estrogen: r = 0.75 (p < 0.01) during ovulation TC and estrogen: r = 0.27; across cycle: NS LDL-C and estrogen: r = -0.02; across cycle: NS Mean intraindividual variation: TC: 7.8% HDL-C: 8.3% LDL-C: 11% TG: 14.9% |
Linear regression | [24] |
| Lamon-Fava et al. (1989) | 60 Caucasian (26.2 ± 5.7)† 117 African– American (25.5 ± 5.2)† |
Cross-sectional | HDL-C (square root transform), estrogen (log transform): β = 0.163 (p < 0.03) adjusted for age, BMI, waist:hip ratio and ethnicity | Multiple linear regression | [51] |
| Gorbach et al. (1989) | 24 healthy (premenopausal) | Single follicular phase sample between days 4 and 6 | HDL-C and estrogen (partial r = 0.57; p = 0.02) VLDL-C and estrogen (partial r = 0.63; p = 0.01) LDL-C and estrogen (partial r = −0.77; p < 0.001) |
Multiple linear regression | [20] |
| Mumford et al. (2010) | 259 (27.3 ± 8.2)† | Two cycles, eight fasting blood samples per cycle timed using fertility monitors | TC and estrogen: β = −0.0173 (p < 0.0001) HDL-C and estrogen: β = 0.0186 (p < 0.0001; acute) LDL-C and estrogen: β= −0.0228 (p < 0.0001) TG and estrogen: β = −0.0410 (p < 0.0001) Mean intraindividual variation TC: 19%, 27.7 mg/dl |
Weighted linear mixed effects models | [42] |
| Reed et al. (2000) | 39 (premenopausal) | Three cycles (dietary intervention), blood samples drawn once per week | Amplitude of cycling 5.6 mg/dl from menses to follicular phases; intraindividual variation (range: 38.8 mg/dl) | Linear mixed models | [28] |
Mean ± standard deviation.
NS: No significant change; r: Correlation coefficient; TC: Total cholesterol; TG: Triglyceride.
Taken together, these studies show cyclic changes in lipoprotein cholesterol levels during the menstrual cycle, which are associated with circulating estrogen levels. The changes in lipids between different days of the menstrual cycle are fairly small, but should be taken into account when evaluating lipoprotein cholesterol levels among reproductive-aged women, particularly in light of the corresponding differences in lipid variability observed over the course of the menstrual cycle.
Biological rationale
Changes in lipoprotein cholesterol levels during the menstrual cycle in response to fluctuating estrogen levels are well supported by the available biological evidence. Initially, the rates of formation of all lipoprotein fractions increase to some degree under the influence of estrogen, but their removal rates are variably increased or decreased [15]. There is growing evidence that improvements in the atherogenic nature of the plasma lipid profile in response to endogenous or exogenous estrogen consist of increasing VLDL synthesis, which subsequently increases HDL and decreases LDL. These changes depend upon an elegant interaction of many components. The upregulation of the LDL receptors increases clearance of LDL-C, while upregulation of the ATP-binding cassette transporter and apoA-I increases HDL synthesis, and the suppression of hepatic class B scavenger receptors expression leads to decreased hepatic selective cholesterol uptake from HDL and further effects on LDL [43–45]. The evidence reviewed supports these findings in that endogenous estrogen improves the lipid profile by elevating HDL levels and lowering LDL levels. Alternatively, fluid retention and hemodilution during the luteal phase induced by rising progesterone levels could potentially account for a portion of the reduction in lipoprotein cholesterol levels during the luteal phase. However, some studies demonstrate that the cyclic changes in lipoprotein cholesterol levels are greater than the accompanying changes in plasma volume [18,27,46].
Clinical implications
Based on these findings, menstrual cycle phase should be taken into account when evaluating lipoprotein cholesterol levels among reproductive- aged women in order to improve interpretation in clinical settings and in future research.
Although the changes observed in mean levels by cycle phase were modest (only 5–8% on average), these differences have potential clinical implications for reproductive-aged women. In fact, women crossed clinical boundaries of acceptable lipoprotein cholesterol levels when tested at different phases of the menstrual cycle. Specifically, fewer women were classified as having high cholesterol when measured during the luteal phase compared with the follicular phase (TC: 7.9 vs 14.3%; LDL: 10.5 vs 17.8%) [42]. Based on these findings, the mid-follicular phase may be the best phase for measurement to reduce false negatives if we assume that management of a woman’s cholesterol should be based on a level outside the NCEP guidelines at any point during the cycle. While treatment decisions regarding the lipid profile may still require repeated samples above the recommended level, standardizing the timing of lipid measurements may improve the interpretability of results and consequently reduce the overall number of tests. Notably, the observed changes occurred among healthy women. It is possible that variability in lipoprotein cholesterol levels over the course of the menstrual cycle could be even greater among other groups of women.
While the best time to measure cholesterol during a woman’s cycle has yet to be established, measurements should be made at the same time each month for consistent comparisons. Even measurements taken a week or two apart may be quite different solely because of changing estrogen levels. Both women and physicians should take menstrual cycle phase into account when interpreting a woman’s cholesterol measurement. Measurements during menses consistently had the least amount of variability in several studies, and given the difficulties in timing clinic visits to other phases of the cycle, we recommend measuring cholesterol levels during menses to ensure consistent comparisons. However, due to menstrual cycle variability, any woman that is within NCEP acceptable ranges but near the boundaries during menses should undergo additional tests.
Conclusion
Overall, lipoprotein cholesterol levels have been observed to change over the menstrual cycle in response to changing reproductive hormone levels. TC and LDL-C tend to be highest during the follicular phase and to decline during the luteal phase. HDL-C is most often highest during the late follicular and periovulatory phases. Based on these findings, the menstrual cycle phase should be taken into account when evaluating lipoprotein cholesterol levels among reproductive-aged women. Testing during menses is recommended to facilitate consistent comparisons due to reduced variability during this time and because this menstrual cycle phase can be more reliably identified than others. Implementation of uniform timing of cholesterol testing in reproductive-aged women would improve interpretation in clinical settings as well as future studies. These findings are important in that they show that the standard of care based on men are not necessarily appropriate for women, and that women need to be studied directly. Thus, considering menstrual cycle phase in the development of clinical guidelines for reproductive-aged women could improve the current standard of care.
Future perspective
Although recent studies have elucidated the hormonal effects on lipoprotein metabolism, many questions remain. Of great interest is the role of androgens in this setting as androstendione and testosterone are both precursors of estrogen and thus covary with estrogen levels during the menstrual cycle. It is hypothesized that androgens oppose the stimulatory effects of estrogen, and future studies need to take circulating estrogens, progesterone and testosterone into account. This may further elucidate the suggestion of an association between polycystic ovary syndrome and CHD. In addition, thyroid hormones and insulin metabolism are intricately connected to lipoprotein metabolism, and future research should evaluate the interplay between these systems in a comprehensive manner. Furthermore, now that the importance of menstrual cycle phase has been introduced, the particular times in the cycle that should be measured remain unclear. It is unknown whether measurements at different phases are more or less correlated with long-term outcomes. Based on future studies of these effects, re-evaluation of the recommended TC levels for reproductive-aged women may be justified to take timing and menstrual cycle variability into account.
Executive summary.
The role of endogenous hormones on lipoprotein cholesterol levels has not yet been clearly defined.
Epidemiological evidence suggests that lipoprotein cholesterol levels vary during the menstrual cycle and are significantly associated with endogenous reproductive hormone levels. Total cholesterol and LDL-C tend to be highest during the follicular phase and decline during the luteal phase. HDL-C tends to be highest around ovulation.
Both women and physicians should take menstrual cycle phase into account when interpreting a woman’s cholesterol measurement. Cyclic variations also have implications on the design and interpretation of studies in women of reproductive age.
Measuring cholesterol levels during menses is recommended for consistent comparisons due to reduced variability in cholesterol levels during this phase and because this phase can be more reliably identified and scheduled than others. Any woman within the National Cholesterol Education Program acceptable ranges, but near the boundary when measured during menses, should undergo additional testing.
Footnotes
Financial & competing interests disclosure
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
• of considerable interest
- 1•.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. Outlines clinical recommendations for treatment of high cholesterol for both men and women. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics – 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.Adams MR, Kaplan JR, Manuck SB, et al. Inhibition of coronary artery atherosclerosis by 17-β estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10(6):1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 4.Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117(12):1016–1037. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 5.Rijpkema AH, van der Sanden AA, Ruijs AH. Effects of post-menopausal oestrogen-progestogen replacement therapy on serum lipids and lipoproteins: a review. Maturitas. 1990;12(3):259–285. doi: 10.1016/0378-5122(90)90007-s. [DOI] [PubMed] [Google Scholar]
- 6.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991;20(1):47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 9.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1995;273(3):199–208. [PubMed] [Google Scholar]
- 10.Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325(17):1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 11.Hedon B. The evolution of oral contraceptives. Maximizing efficacy, minimizing risks. Acta Obstet Gynecol Scand. 1990;152(Suppl 1):7–12. doi: 10.3109/00016349009156500. [DOI] [PubMed] [Google Scholar]
- 12.Krauss RM, Burkman RT. The metabolic impact of oral contraceptives. Am J Obstet Gynecol. 1992;167:1177–1184. doi: 10.1016/s0002-9378(12)90408-1. [DOI] [PubMed] [Google Scholar]
- 13.Gaspard UJ. Metabolic effects of oral contraceptives. Am J Obstet Gynecol. 1987;157:1029–1041. doi: 10.1016/s0002-9378(87)80128-x. [DOI] [PubMed] [Google Scholar]
- 14.Burkman RT. Lipid metabolism effects with desogestrel-containing oral contraceptives. Am J Obstet Gynecol. 1993;168:1033–1040. doi: 10.1016/0002-9378(93)90334-f. [DOI] [PubMed] [Google Scholar]
- 15•.Knopp RH, Paramsothy P, Retzlaff BM, et al. Sex differences in lipoprotein metabolism and dietary response: basis in hormonal differences and implications for cardiovascular disease. Curr Cardiol Rep. 2006;8(6):452–459. doi: 10.1007/s11886-006-0104-0. Discusses the implications of differences in lipoprotein metabolism between men and women. [DOI] [PubMed] [Google Scholar]
- 16.LaRosa JC. Women, lipoproteins and cardiovascular disease risk. Can J Cardiol. 1990;6(Suppl B):B23–B29. [PubMed] [Google Scholar]
- 17.Chu MC, Rath KM, Huie J, Taylor HS. Elevated basal FSH in normal cycling women is associated with unfavourable lipid levels and increased cardiovascular risk. Hum Reprod. 2003;18(8):1570–1573. doi: 10.1093/humrep/deg330. [DOI] [PubMed] [Google Scholar]
- 18.Cullinane EM, Yurgalevitch SM, Saritelli AL, Herbert PN, Thompson PD. Variations in plasma volume affect total and low-density lipoprotein cholesterol concentrations during the menstrual cycle. Metabolism. 1995;44(8):965–971. doi: 10.1016/0026-0495(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 19.Furberg AS, Jasienska G, Bjurstam N, et al. Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA study. Cancer Epidem Biomar Prev. 2005;14(1):33–40. [PubMed] [Google Scholar]
- 20.Gorbach SL, Schaefer EJ, Woods M, et al. Plasma lipoprotein cholesterol and endogenous sex hormones in healthy young women. Metabolism. 1989;38(11):1077–1081. doi: 10.1016/0026-0495(89)90043-7. [DOI] [PubMed] [Google Scholar]
- 21.Karpanou EA, Vyssoulis GP, Georgoudi DG, Toutouza MG, Toutouzas PK. Disparate serum lipid changes between normotensive and hypertensive women during the menstrual cycle. Am J Cardiol. 1992;70(1):111–113. doi: 10.1016/0002-9149(92)91402-p. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Kalkhoff RK. Changes in lipoprotein composition during the menstrual cycle. Metabolism. 1979;28(6):663–668. doi: 10.1016/0026-0495(79)90020-9. [DOI] [PubMed] [Google Scholar]
- 23.Larsen LF, Andersen HR, Hansen AB, Andersen O. Variation in risk indicators of cardiovascular disease during the menstrual cycle: an investigation of within-subject variations in glutathione peroxidase, haemostatic variables, lipids and lipoproteins in healthy young women. Scand J Clin Lab Invest. 1996;56(3):241–249. doi: 10.3109/00365519609088613. [DOI] [PubMed] [Google Scholar]
- 24•.Lyons Wall PM, Choudhury N, Gerbrandy EA, Truswell AS. Increase of high-density lipoprotein cholesterol at ovulation in healthy women. Atherosclerosis. 1994;105(2):171–178. doi: 10.1016/0021-9150(94)90047-7. Observed cyclic changes over one cycle with 20 blood samples and also evaluated correlations between lipoprotein cholesterol levels and estradiol. [DOI] [PubMed] [Google Scholar]
- 25.Muesing RA, Forman MR, Graubard BI, et al. Cyclic changes in lipoprotein and apolipoprotein levels during the menstrual cycle in healthy premenopausal women on a controlled diet. J Clin Endocrinol Metab. 1996;81(10):3599–3603. doi: 10.1210/jcem.81.10.8855808. [DOI] [PubMed] [Google Scholar]
- 26•.Nduka EU, Agbedana EO. Total cholesterol, high density lipoprotein cholesterol and steroid hormone changes in normal weight women during the menstrual cycle. Int J Gynaecol Obstet. 1993;41(3):265–268. doi: 10.1016/0020-7292(93)90554-a. Shows cyclic changes in lipoprotein cholesterol levels with 20 blood samples taken over one cycle. [DOI] [PubMed] [Google Scholar]
- 27.Pahwa MB, Seth S, Seth RK. Lipid profile in various phases of menstrual cycle and its relationship with percentage plasma volume changes. Clin Chim Acta. 1998;273(2):201–207. doi: 10.1016/s0009-8981(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 28•.Reed RG, Kris-Etherton P, Stewart PW, Pearson TA. Variation of lipids and lipoproteins in premenopausal women compared with men and postmenopausal women. DELTA (Dietary Effects on Lipoproteins and Thrombogenic Activity) Investigators. Metabolism. 2000;49(9):1101–1105. doi: 10.1053/meta.2000.8603. Compares cyclic changes during the menstrual cycle with variability in men and postmenopausal women, concluding that intraindividual variability is similar between premenopausal women and postmenopausal women and men. [DOI] [PubMed] [Google Scholar]
- 29.Tonolo G, Ciccarese M, Brizzi P, et al. Cyclical variation of plasma lipids, apolipoproteins, and lipoprotein(a) during menstrual cycle of normal women. Am J Physiol. 1995;269(6 Pt 1):E1101–E1105. doi: 10.1152/ajpendo.1995.269.6.E1101. [DOI] [PubMed] [Google Scholar]
- 30.Barclay M, Barclay RK, Skipski VP, et al. Fluctuations in human serum lipoproteins during the normal menstrual cycle. Biochem J. 1965;96:205–209. doi: 10.1042/bj0960205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adlercreutz H, Tallqvist G. Variations in the serum total cholesterol and hematocrit values in normal women during the menstrual cycle. Scand J Clin Lab Invest. 1959;11(1):1–9. doi: 10.3109/00365515909060400. [DOI] [PubMed] [Google Scholar]
- 32.Oliver MF, Boyd GS. Changes in the plasma lipids during the menstrual cycle. Clin Sci (Lond) 1953;12(2):217–222. [PubMed] [Google Scholar]
- 33.Azogui G, Ben-Shlomo I, Zohar S, Kook A, Presser S, Aviram M. High density lipoprotein concentration is increased during the ovulatory phase of the menstrual cycle in healthy young women. Gynecol Endocrinol. 1992;6(4):253–257. doi: 10.3109/09513599209024987. [DOI] [PubMed] [Google Scholar]
- 34.Barnett JB, Woods MN, Lamon-Fava S, et al. Plasma lipid and lipoprotein levels during the follicular and luteal phases of the menstrual cycle. J Clin Endocrinol Metab. 2004;89(2):776–782. doi: 10.1210/jc.2003-030506. [DOI] [PubMed] [Google Scholar]
- 35.De Leon RG, Austin KL, Richards L, Guerrero F. Lipid and hormonal profile of Panamanian women during the menstrual cycle. Int J Gynecol Obstet. 1992;39(3):219–226. doi: 10.1016/0020-7292(92)90660-b. [DOI] [PubMed] [Google Scholar]
- 36.Elhadd TA, Neary R, Abdu TA, et al. Influence of the hormonal changes during the normal menstrual cycle in healthy young women on soluble adhesion molecules, plasma homocysteine, free radical markers and lipoprotein fractions. Int Angiol. 2003;22(3):222–228. [PubMed] [Google Scholar]
- 37.Jones DY, Judd JT, Taylor PR, Campbell WS, Nair PP. Menstrual cycle effect on plasma lipids. Metabolism. 1988;37(1):1–2. doi: 10.1016/0026-0495(88)90020-0. [DOI] [PubMed] [Google Scholar]
- 38.Lebech AM, Kjaer A. Lipid metabolism and coagulation during the normal menstrual cycle. Horm Metab Res. 1989;21(8):445–448. doi: 10.1055/s-2007-1009258. [DOI] [PubMed] [Google Scholar]
- 39.Lussier-Cacan S, Xhignesse M, Desmarais JL, Davignon J, Kafrissen ME, Chapdelaine A. Cyclic fluctuations in human serum lipid and apolipoprotein levels during the normal menstrual cycle: comparison with changes occurring during oral contraceptive therapy. Metabolism. 1991;40(8):849–854. doi: 10.1016/0026-0495(91)90014-n. [DOI] [PubMed] [Google Scholar]
- 40.Woods M, Schaefer EJ, Morrill A, et al. Effect of menstrual cycle phase on plasma lipids. J Clin Endocrinol Metab. 1987;65(2):321–323. doi: 10.1210/jcem-65-2-321. [DOI] [PubMed] [Google Scholar]
- 41.Schijf CP, van der Mooren MJ, Doesburg WH, Thomas CM, Rolland R. Differences in serum lipids, lipoproteins, sex hormone binding globulin and testosterone between the follicular and the luteal phase of the menstrual cycle. Acta Endocrinol (Copenhagen) 1993;129(2):130–133. doi: 10.1530/acta.0.1290130. [DOI] [PubMed] [Google Scholar]
- 42•.Mumford SL, Schisterman EF, Siega-Riz AM, et al. A longitudinal study of serum lipoproteins in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle study. J Clin Endocrinol Metab. 2010;95(9):E80–E85. doi: 10.1210/jc.2010-0109. Largest study to date with multiple cholesterol and hormone measurements across two cycles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srivastava RA, Baumann D, Schonfeld G. In vivo regulation of low-density lipoprotein receptors by estrogen differs at the post-transcriptional level in rat and mouse. Eur J Biochem. 1993;216(2):527–538. doi: 10.1111/j.1432-1033.1993.tb18171.x. [DOI] [PubMed] [Google Scholar]
- 44.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271(5248):518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 45.Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med. 2006;84(4):276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 46.Adlercreutz H, Tallqvist G. Variations in the serum total cholesterol and hematocrit values in normal women during the menstrual cycle. Scand J Clin Lab Invest. 1959;11(1):1–9. doi: 10.3109/00365515909060400. [DOI] [PubMed] [Google Scholar]
- 47.Basdevant A, De Lignieres B, Bigorie B, Guy-Grand B. Estradiol, progesterone and plasma lipids during the menstrual cycle. Diabetes Metab. 1981;7(1):1–4. [PubMed] [Google Scholar]
- 48.Mattsson LA, Silfverstolpe G, Samsioe G. Lipid composition of serum lipoproteins in relation to gonadal hormones during the normal menstrual cycle. Eur J Obstet Gynecol Reprod Biol. 1984;17(5):327–335. doi: 10.1016/0028-2243(84)90111-4. [DOI] [PubMed] [Google Scholar]
- 49.Haines CJ, Cheung LP, Lam CW. Changes in atherogenic lipids and lipoproteins during natural and hyperstimulated cycles in healthy women. Fertil Steril. 1997;68(2):231–235. doi: 10.1016/s0015-0282(97)81507-5. [DOI] [PubMed] [Google Scholar]
- 50.Ahumada Hemer H, Valles de Bourges V, Juarez Ayala J, Brito G, Díaz-Sánchez V, Garza-Flores J. Variations in serum lipids and lipoproteins throughout the menstrual cycle. Fertil Steril. 1985;44(1):80–84. [PubMed] [Google Scholar]
- 51.Lamon-Fava S, Fisher EC, Nelson ME, et al. Effect of exercise and menstrual cycle status on plasma lipids, low density lipoprotein particle size, and apolipoproteins. J Clin Endocrinol Metab. 1989;68(1):17–21. doi: 10.1210/jcem-68-1-17. [DOI] [PubMed] [Google Scholar]