Abstract
Objective
To investigate the possibility of suppressing bladder overactivity by electrical activation of somatic afferent nerves in the foot.
Materials and Methods
Cats with intact spinal cord were studied under α-chloralose anesthesia. Bladder pressure was recorded via a urethral catheter. Foot stimulation was applied via surface pad electrodes attached to the skin of the front or hind foot.
Results
Reflex micturition was inhibited by electrical stimulation of the hind foot at either low (5 Hz) or high (20 Hz) frequencies, but stimulation of the front foot was not effective. On average bladder capacity during a saline infusion cystometrogram (CMG) was significantly (P<0.05) increased to 153.2±18.2% and 136.9±14.3% of the control bladder capacity by stimulation at frequencies of 5 Hz and 20 Hz, respectively. Intravesical infusion of 0.25% acetic acid (AA) induced bladder overactivity and reduced bladder capacity to 20.3±8.9% of the control capacity measured during saline infusion. Foot stimulation inhibited the AA-induced bladder overactivity recorded under isovolumetric conditions, and significantly (P<0.05) increased bladder capacity during acetic acid infusion. However, it only restored the small bladder capacity caused by AA irritation to about 40-50% of the control bladder capacity measured during saline infusion. The effect of foot stimulation did not persist after termination of stimulation during repeated CMG tests.
Conclusions
This study demonstrated the potential of non-invasive transcutaneous electrical stimulation of somatic nerves in the foot to inhibit reflex bladder activity and treat overactive bladder symptoms.
Keywords: Bladder, Foot, Cat, Electrical stimulation, Transcutaneous
INTRODUCTION
It is well known that electrical stimulation of somatic afferent pathways in the pudendal nerve 1-4, posterior tibial nerve 5,6, or sacral spinal roots 7,8 can inhibit bladder activity in both humans and animals, and is clinically effective in treating overactive bladder symptoms. Stimulation of the sacral S3 spinal root is currently a FDA approved therapy for the lower urinary tract disorders including bladder overactivity, urgency, frequency, and incontinence 7,8. Although the mechanisms underlying neuromodulation are uncertain, this type of therapy has become popular because lower urinary tract dysfunctions in some patients are difficult to manage with medications 9.
However, sacral and pudendal neuromodulation requires surgery to implant a stimulator (InterStim®, Medtronic Inc.) and electrodes1,2,7,8. Meanwhile, the standard treatment using tibial nerve neuromodulation includes 30 min stimulation once per week for 12 consecutive weeks through a percutaneously inserted needle electrode cephalad to the medial malleolus (Urgent PC® stimulator, Uroplasty Inc.) 10. It requires skilled medical staff to insert the needle electrode close to the nerve during each clinical visit. If the initial 12 week treatment is effective, a maintenance treatment (once every 2-3 weeks) is usually required 11. Thus, current neuromodulation treatments are effective to suppress bladder overactivity, but they require surgery or repeated clinical visits that are expensive and inconvenient. A non-invasive neuromodulation method to treat overactive bladder could significantly increase the acceptance of neuromodulation treatment by more patients and reduce the high medical cost of the treatment.
Several non-invasive neuromodulation approaches have been investigated previously in an attempt to treat bladder overactivity, including intra-vaginal 12 or intra-anal 13 simulation using ring electrodes located on a vaginal/anal plug, dorsal penile/clitoral nerve stimulation using transcutaneous electrical stimulation applied to the penis, or the perigenital/perianal skin area 4,14,15,16. However, these approaches targeted very inconvenient locations causing discomfort and difficulty in maintaining the electrodes in place for an extended time period.
Since previous studies 1-8,10,11 have indicated that bladder activity could be modulated by somatic afferent input, in this study we explored the possibility that bladder overactivity could be suppressed by activation of afferent nerves in the foot or hand. This was tested in anesthetized cats by applying electrical stimulation through surface electrodes on the front or hind foot while monitoring reflex bladder activity. Stimulation of the foot or hand is non-invasive, easily accessible, and convenient, which could be a widely acceptable treatment for bladder overactivity if proven to be effective.
METHODS
1. Experimental setup
All protocols involving the use of animals in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh. Experiments were conducted in a total of 6 female cats (2.6 kg to 3.1 kg) under α-chloralose anesthesia (65 mg/kg, I.V. supplemented as necessary) after induction with isoflurane (2-3% in O2). Systemic blood pressure was monitored throughout the experiment via a catheter inserted in the right carotid artery. A tracheotomy was performed and a tube was inserted to keep the airway patent. A catheter for I.V. infusion was introduced into the right ulnar vein. The ureters were cut and drained externally. A double lumen catheter was inserted through the urethra into the bladder and secured by a ligature around the urethra. One lumen of the catheter was connected to a pump to infuse the bladder with either saline or 0.25% acetic acid (AA) at a rate of 0.5-2 ml/min, and the other lumen was connected to a pressure transducer to measure the pressure change in the bladder. After removal of the fur, surface self-adhesive pad electrodes (Grass F-E10ND, Astro-Med Inc., diameter: 1 cm) were attached to the skin area on the left hind foot (see Fig.1). Similarly, electrodes were also attached to the left front foot for stimulation.
Figure 1.
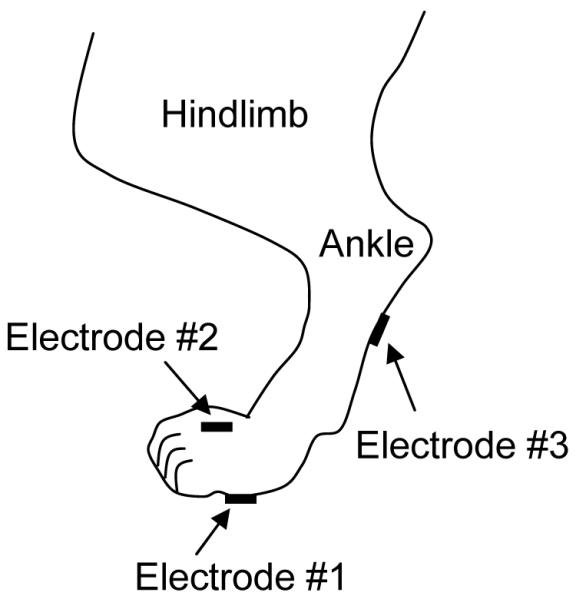
Electrode placement for electrical stimulation of the cat hind foot.
2. Stimulation protocol
Uniphasic rectangular pulses (0.2-1 ms pulse width) were delivered to the foot via the surface pad electrodes at a frequency of either 5 Hz or 20 Hz, based on our previous studies 3,4 that showed pudendal nerve stimulation inhibited bladder activity at 3-7 Hz, but excited bladder at 20 Hz. The threshold intensity to induce observable twitching of the toe was determined in each cat by a preliminary test at 5 Hz. Then, stimulation intensities at multiple thresholds of those to induce toe twitching were tested. Stimulation was applied via three combinations of electrodes as shown in Fig.1 in order to find the effective electrode locations for bladder inhibition. Electrode 1 severed as the cathode in electrode combinations 1-2 and 1-3, but electrode 2 was the cathode when electrode combination 2-3 was used.
In the first group of experiments, the bladder was infused with saline to a volume about 100-110% of the bladder volume (i.e. bladder capacity) to induce large amplitude (>30 cmH2O), rhythmic reflex bladder contractions, and then maintained under isovolumetric conditions. At this bladder volume, foot stimulation at 5 and 20 Hz was applied to determine the effective frequency for bladder inhibition. The stimulation duration was always longer than the period of at least two bladder contractions to clearly demonstrate an inhibitory effect.
In the second group of experiments, the effect of 5 or 20 Hz stimulation was tested during a cystometrogram (CMG) which consisted of a slow infusion of saline (0.5-2 ml/min) starting with an empty bladder to determine the bladder capacity. Two or three CMGs were preformed without stimulation to obtain the control bladder capacity and evaluate reproducibility. Then, foot stimulation was applied during repeated CMGs. The inhibitory effect was evaluated by measuring the increase in bladder capacity during the stimulation. Stimulation and infusion were stopped after the onset of the first micturition reflex contraction which had a large amplitude (>30 cmH2O) and long duration (>30 sec). Control CMGs were also performed between periods of stimulation to examine if there was any persistent post-stimulation carry-over effect. The bladder was emptied after each CMG and a 5-10 min rest period was inserted between CMGs to allow the bladder reflex to recover. The two stimulation frequencies were tested in a randomized order in different animals.
In the third group of experiments, 0.25% AA was infused into the bladder to induce bladder irritation and overactivity. Then, experiments as described above were repeated in order to determine if the foot stimulation could also inhibit the irritation induced bladder overactivity.
3. Data analysis
For the analysis of rhythmic bladder activity, the area under bladder pressure curve was measured during the stimulation and was normalized to the measurement during the same time period before the stimulation. For repeated CMG recordings using saline or AA infusion, the bladder capacities were measured and normalized to the measurement of the first control CMG during saline infusion. Repeated measurements in the same animal during the same experiment were averaged. The normalized data from different animals are presented as means ± SE. One-sample Student’s t-test and paired Student’s t-test were used to detect statistical significance (P<0.05).
RESULTS
1. Foot stimulation during saline distension
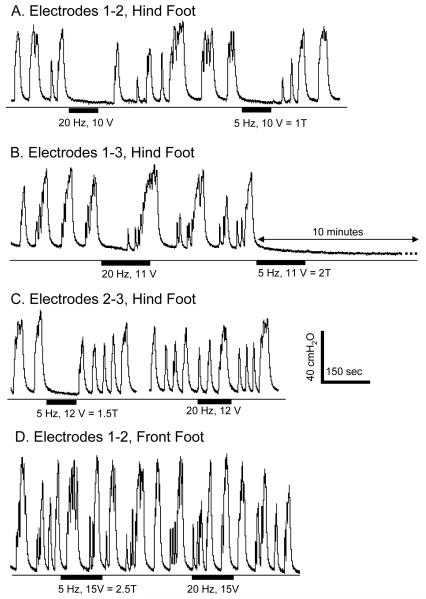
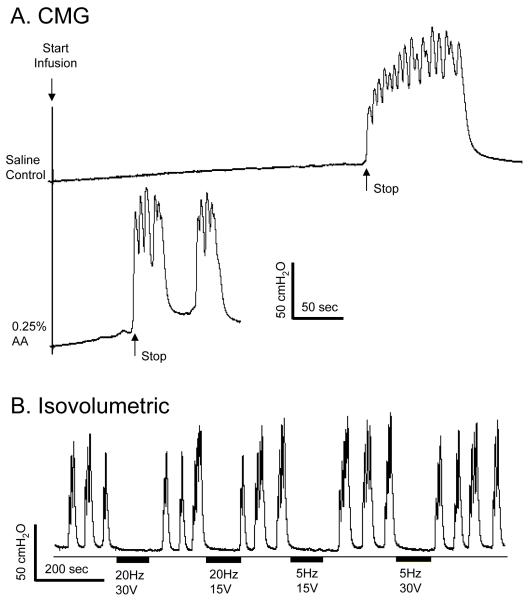
Under isovolumetric recording conditions with the bladder volume above the micturition volume threshold electrical stimulation of the hind foot inhibited reflex bladder activity (Fig.2 A-C), but stimulation of the front foot failed to induce any inhibitory effect at various stimulation intensities (Fig.2 D, N = 2 cats). Therefore, the remaining studies focused on hind foot stimulation which is termed foot stimulation in the following text and figures.
Figure 2.
Inhibition of isovolumetric bladder contractions by electrical stimulation applied to the hind foot via 3 different electrode configurations (A-C) as shown in Fig.1. Stimulation of the front foot was not effective in inhibiting the bladder (D). The thin lines under the pressure traces indicate zero pressure. Black bars under the pressure traces indicate the stimulation duration. Stimulation pulse width was 1 ms. T = threshold to induce toe twitching. Data were from 4 different cats.
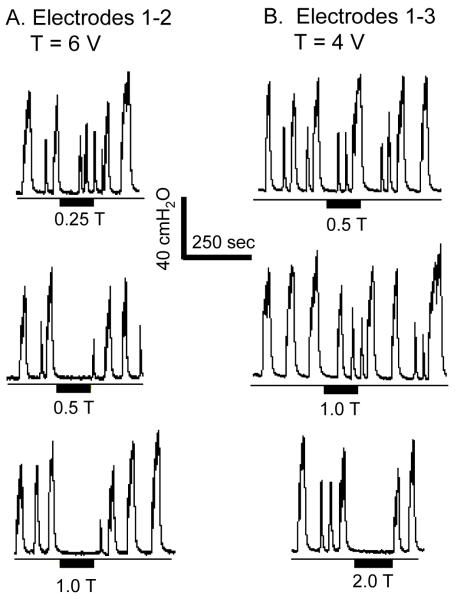
Under isovolumetric conditions foot stimulation at both 5 Hz and 20 Hz inhibited the large amplitude, rhythmic bladder contractions with electrode combinations 1-2 (Fig. 2A, N = 3 cats) and 1-3 (Fig. 2B, N = 5 cats); whereas with electrode combination 2-3 which was tested in 1 cat, only 5 Hz stimulation was effective (Fig. 2C). 5 Hz stimulation also induced bladder inhibition lasting 5-10 minutes after the stimulation was terminated (Fig. 2B). This long-lasting effect was not observed with 20 Hz stimulation. 5 Hz stimulation at intensities of 3-15 V which ranged from 0.5-2.5 times the threshold to induce toe twitching completely inhibited the isovolumetric bladder contractions (N = 5 cats), while 20 Hz stimulation at intensities (5-15 V) ranging from 1-2.5 times threshold to induce toe twitching elicited a similar inhibition (90.8±9.1%, P<0.05) of the reflex contractions (N = 5 cats). In 2 cats 5 Hz stimulation at an intensity as low as one half of the threshold to induce toe twitching was effective in completely inhibiting isovolumetric bladder contractions with electrodes 1-2 (Fig. 3A), but not with electrodes 1-3 (Fig. 3B). Lower stimulation intensities elicited partial inhibition consisting of either reduced amplitude bladder contractions (Fig. 3A at 0.25 T) or a significantly delayed large amplitude bladder contraction (Fig. 2B at 20 Hz).
Figure 3.
Inhibition of isovolumetric bladder contractions by 5 Hz electrical stimulation of the foot. A. Complete inhibition was achieved at one half of the intensity threshold (T = 6 V) for inducing toe movement with electrodes 1-2. B. Complete inhibition was achieved at 2 times of the intensity threshold (T = 4 V) for inducing toe movement with electrodes 1-3. The thin lines under the pressure traces indicate zero pressure. Black bars under the pressure traces indicate the stimulation duration. Stimulation pulse width was 1 ms. Data in A and B were from the same animal.
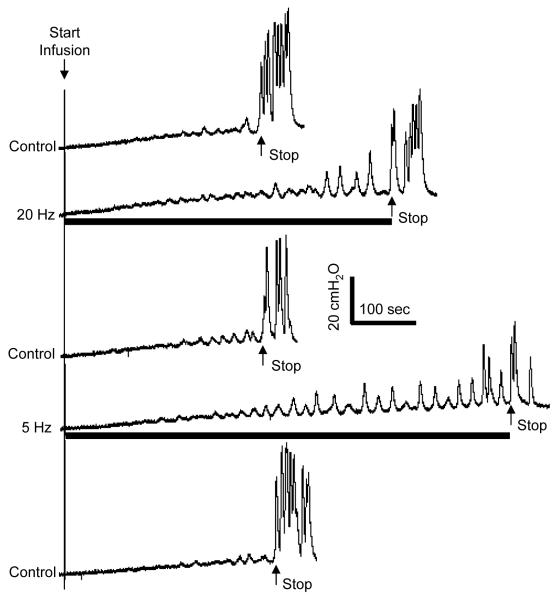
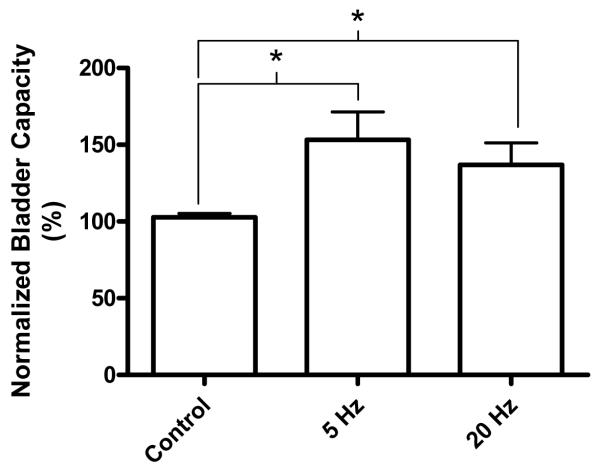
Foot stimulation at both 5 Hz and 20 Hz also significantly (P<0.05) increased bladder capacity to 153.2±18.2% and 136.9±14.3%, respectively, of the control bladder capacity (Fig. 4-5). Stimulation intensities (3-12 V) about 1-2.5 times threshold to induce toe movement were effective (N = 5 cats, 3 cats: electrodes 1-2; 2 cats: electrode 1-3). There was no significant difference between 5 Hz and 20 Hz effects. The inhibitory effect of foot stimulation was rapidly reversible within 5-10 minutes after termination of the stimulation and was repeatable at about 20 min intervals. Repeated foot stimulation during multiple CMG recordings did not elicit a post-stimulation effect on bladder capacity (Fig. 4).
Figure 4.
Bladder capacity was increased by electrical stimulation of the foot at different frequencies (5 or 20 Hz). Stimulation intensity (12 V) was at 2 times of intensity threshold to induce toe movement. Stimulation pulse width was 1 ms. Electrodes 1-2 were used. The arrows indicate the start and stop of bladder infusion (2 ml/min). The black bars under the pressure traces indicate the stimulation duration.
Figure 5.
Bladder capacity was significantly increased by electrical stimulation of the foot at different frequencies (5 or 20 Hz). Stimulation intensity (3-12 V) was at 1-2.5 times of intensity threshold to induce toe movement. Stimulation pulse width was 1 ms. Data were from total 5 cats. Electrodes 1-2 were used in 3 cats, and electrodes 1-3 were used in another 2 cats. * indicates statistical significance (P<0.05).
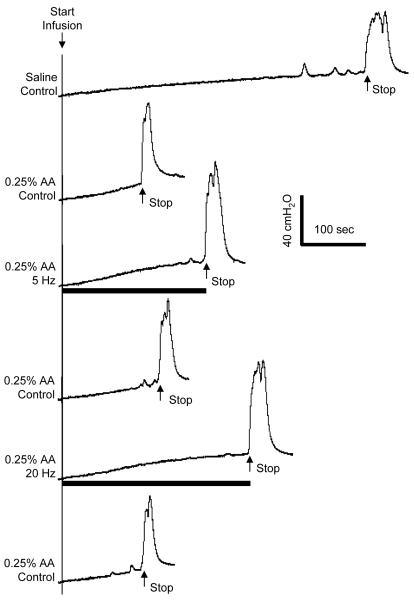
2. Foot stimulation during acetic acid (AA) irritation
When the bladder was filled with 0.25% AA bladder capacity was significantly reduced (Fig. 6A) and large amplitude contractions were elicited at small bladder volumes (Fig. 6B). Foot stimulation at either 5 Hz or 20 Hz completely inhibited the large amplitude, isovolumetric bladder contractions induced by AA irritation (Fig. 6B, N = 2 cats).
Figure 6.
Foot stimulation inhibited bladder overactivity induced by 0.25% acetic acid (AA). A. 0.25% AA irritated bladder, caused bladder overactivity, and reduced bladder capacity during CMG. The arrows indicate the start and stop of the infusion (2 ml/min). B. The overactive bladder contractions under isovolumetric condition at a smaller bladder volume as shown in A was inhibited by foot stimulation. The thin lines under the pressure traces indicate zero pressure. Black bars under the pressure traces indicate the stimulation duration. Stimulation pulse width was 0.2 ms. Electrodes 1-2 were used.
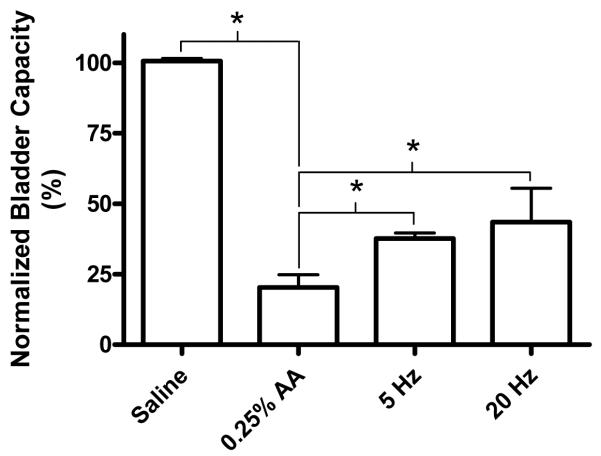
AA irritation significantly (P<0.05) reduced bladder capacity to 20.3±8.9% of the control bladder capacity measured during saline infusion (Fig. 7-8). Foot stimulation partially reversed the effect of AA irritation, increasing the bladder capacity to 37.6±2.1% and 43.5±12.0% of the saline control bladder capacity at frequencies of 5 Hz and 20 Hz, respectively (Figs. 7-8). There was no significant difference between 5 Hz and 20 Hz effects.
Figure 7.
Reduction in bladder capacity induced by 0.25% acetic acid (AA) was partially reversed by foot stimulation at different frequencies (5 or 20 Hz). Stimulation intensity (10 V) was at 1.25 times of intensity threshold to induce toe movement. Stimulation pulse width was 1 ms. Electrodes 1-2 were used. The arrows indicate the start and stop of bladder infusion (2 ml/min). The black bars under the pressure traces indicate the stimulation duration.
Figure 8.
Reduction in bladder capacity induced by 0.25% acetic acid (AA) was partially reversed by foot stimulation at different frequencies (5 or 20 Hz). Stimulation intensity (6-12 V) was at 1.25-3 times of intensity threshold to induce toe movement. Stimulation pulse width was 1 ms. Electrodes 1-2 were used in total 4 cats. * indicates statistical significance (P<0.05).
DISCUSSION
This study showed that electrical stimulation of the foot was effective in inhibiting reflex bladder activity and increasing bladder capacity in anesthetized cats. Although 5 Hz stimulation was superior to 20 Hz in some cases (Fig. 2 B-C and Fig. 3-5), there was on average no significant difference between these two frequencies in inhibiting reflex bladder activity (Fig. 5 and 8). These results provided urodynamic evidence indicating that electrical stimulation of the foot might be an effective treatment for bladder overactivity.
All three electrode combinations (Fig.1) are effective in inhibiting bladder activity (Fig.2), indicating that afferent activation of a specific nerve in the foot might not be required. There are two major nerves innervating the foot. Branches from the peroneal nerve run on the dorsal surface of the foot, while the tibial nerve mainly branches on the plantar surface of the foot. Electrical stimulation used in this study probably activated nerve branches from both peroneal and tibial nerves, indicating that specifically targeting the posterior tibial nerve as currently used in clinical settings 5,6,10,11 might not be necessary. Previous studies in both cat 17 and monkey 18 have shown that electrical stimulation of the peroneal nerve in the hindlimb also inhibits reflex bladder activity.
An advantage of foot stimulation is that the nerves in the foot are much closer to the skin surface than peroneal or posterior tibial nerve which is located deeper between the leg muscles. Therefore, electrical activation of the nerves on the foot requires only surface electrodes and is much easier than the posterior tibial nerve stimulation that is usually performed by inserting a needle electrode close to the nerve 6,10,11. However, it is worth noting that foot stimulation did not induce a long-lasting inhibitory effect during repeated CMG tests on the same day (Fig. 4). This is different from the clinical reports of posterior tibial nerve stimulation applied over the course of many weeks where the inhibitory effect could last weeks or months 6,10,11. This difference might occur due to different species, presence of anesthesia, or different stimulation parameters, as well as different stimulation locations.
Electrical stimulation of the front foot in this study failed to induce an inhibitory effect on reflex bladder activity, while stimulation of the hind foot was effective. This suggests that the spinal segmental distribution of the stimulated somatic afferent pathways is an important factor in the efficacy of this type of neuromodulation. In the cat the afferent projections from the hind foot into the spinal cord exhibit some segmental overlap in the lumbosacral spinal cord with the afferent projections from the lower urinary tract (LUT) 19, providing a greater possibility of spinal interactions between hind foot and LUT afferent pathways. Therefore, it is probable that the somatic afferent input from the hind foot inhibits the micturition reflex at the sacral spinal cord level. However, inhibition at a supraspinal site can not be excluded. A previous study in cats 20 showed that the inhibitory effect on bladder activity elicited by electrical stimulation of the nerves from hindlimb muscles was lost after chronic spinal cord transaction at the thoracic level, indicating a possible role of the supraspinal mechanisms in somato-vesical inhibition. The same study 20 also showed that activation of large myelinated afferent nerves (conduction velocity about 50m/s) induced the inhibition. Our experiments which showed that foot stimulation produced inhibition at stimulation intensities 1-2 times threshold support this conclusion. However another study 17 identified a role of small myelinated or unmyelinated hind limb afferent nerves in somato-vesical inhibition in the cat.
Recent studies in the cats 3,4,16 showed that the effect of pudendal nerve stimulation on bladder activity was dependent on the stimulation frequency. At 3-7 Hz, pudendal nerve stimulation significantly inhibited the bladder, but at 20-40 Hz it could excite the bladder. However, this frequency dependency was not observed in this study. Foot stimulation significantly inhibited the bladder at both 5 Hz and 20 Hz (Fig. 2-8), indicating that the underlying mechanisms of bladder modulation by foot stimulation and pudendal nerve stimulation might be different, even though in our cat model they appear to be equally effective in suppressing reflex bladder activity3,4. This similar inhibitory efficacy in cats of foot stimulation and pudendal nerve stimulation which has also been shown clinically to be effective in treating bladder overactivity 1,2 suggests that foot stimulation might also be a useful treatment for bladder overactivity in humans.
Foot stimulation inhibited not only the bladder activity induced by saline distention (Fig.2-5), but also the bladder overactivity induced by AA irritation (Fig. 6-8). However, the marked reduction in bladder capacity (80%) caused by AA irritation was only partially reversed by foot stimulation to about 40-50% of the saline control bladder capacity (Fig. 7-8). The ability of foot stimulation to inhibit bladder overactivity induced by AA irritation is clinically relevant, because AA irritation activates the C-fiber afferents that play an important role in the generation of bladder overactivity under pathological conditions 21. Pudendal nerve stimulation produced similar inhibitory effects in the AA model in cats 22.
Further animal studies are needed to explore a broader range of stimulus patterns and frequencies as well as different electrode placements on the foot stimulation to determine if efficacy can be improved particularly in regard to suppressing bladder overactivity induced by AA. Only continuous stimulation was used in this study, which might not be the optimal stimulus to induce and maintain the inhibitory effect. Intermittent stimulation with on and off periods might further enhance the inhibitory effect. Furthermore, different electrode placements which increase the probability of activating either cutaneous or muscle afferents should be tested to determine if one type of afferent pathway is more effective in controlling bladder activity.
This study demonstrated the potential of a non-invasive electrical stimulation method using electrodes applied to skin of the foot to inhibit bladder overactivity. However, it did not detect a prolonged inhibitory effect that persisted following the termination of the stimulation. Therefore continuous 24-hour stimulation of the foot might be necessary as required with the sacral neuromodulation technique 7,8. Toe movement and sensory responses might be a side effect of the treatment, but would be justified by the benefits of suppressing bladder dysfunction. In summary, the present study raises the possibility that a non-invasive, convenient method to treat overactive bladder symptoms could be developed utilizing transcutaneous stimulation of somatic nerves in the foot.
ACKNOWLEDGEMENTS
This study is supported by the NIH under grants DK-068566, DK-077783, and by the Christopher and Dana Reeve Foundation.
REFERENCES
- 1.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn. 2005;24:643–647. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 2.Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int. 2007;100:835–839. doi: 10.1111/j.1464-410X.2007.07082.x. [DOI] [PubMed] [Google Scholar]
- 3.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal cord injured cat. Exp Neurol. 2006;197:225–234. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Tai C, Shen B, Wang J, Chancellor MB, Roppolo JR, de Groat WC. Inhibitory and excitatory perigenital-to-bladder spinal reflexes in the cat. Am J Physiol Renal Physiol. 2008;294:F591–F602. doi: 10.1152/ajprenal.00443.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129:78–79. doi: 10.1016/s0022-5347(17)51928-x. [DOI] [PubMed] [Google Scholar]
- 6.Peters KM, MacDiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol. 2009;182:1055–1060. doi: 10.1016/j.juro.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 7.Nakib K, Siegel S. Neuromodulation versus neurotoxin for the treatment of refractory detrusor overactivity: for neuromodulation. Nat Clin Pract Urol. 2008;5:118–119. doi: 10.1038/ncpuro1033. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland SE, Lavers A, Carlson A, Holtz C, Kesha J, Siegel S. Sacral nerve stimulation for voiding dysfunction: one institution’s 11-year experience. Neurourol Urodyn. 2007;26:19–28. doi: 10.1002/nau.20345. [DOI] [PubMed] [Google Scholar]
- 9.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: Basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 10.van Balken MR. Percutaneous tibial nerve stimulation: the Urgent PC® device. Expert Rev Med Dev. 2007;4:693–698. doi: 10.1586/17434440.4.5.693. [DOI] [PubMed] [Google Scholar]
- 11.Pal FVD, van Balken MR, Heesakkers JPFA, Debruyne FMJ, Bemelmans BLH. Percutaneous tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: is maintenance treatment necessary? BJU International. 2006;97:547–550. doi: 10.1111/j.1464-410X.2006.06055.x. [DOI] [PubMed] [Google Scholar]
- 12.Lindstrom S, Fall M, Carlsson CA, Erlandson BE. The neurophysiological basis of bladder inhibition in response to intravaginal electrical stimulation. J Urol. 1983;129:405–410. doi: 10.1016/s0022-5347(17)52127-8. [DOI] [PubMed] [Google Scholar]
- 13.Godec C, Cass AS, Ayala GF. Bladder inhibition with functional electrical stimulation. Urol. 1975;6:663–666. doi: 10.1016/0090-4295(75)90791-8. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler JS, Walter JS, Zaszczurynski PJ. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol. 1992;147:100–103. doi: 10.1016/s0022-5347(17)37145-8. [DOI] [PubMed] [Google Scholar]
- 15.Walter JS, Wheeler JS, Robinson CJ, Wurster RD. Inhibiting the hyperreflexic bladder with electrical stimulation in a spinal animal model. Neurourol Urodyn. 1993;12:241–253. doi: 10.1002/nau.1930120306. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Liu H, Shen B, Roppolo JR, de Groat WC, Tai C. Bladder inhibition or excitation by electrical perianal stimulation in a cat model of chronic spinal cord injury. BJU Int. 2009;103:530–536. doi: 10.1111/j.1464-410X.2008.08029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato A, Sato Y, Schmidt R. Reflex bladder activity induced by electrical stimulation of hind limb somatic afferents in the cat. J Auto Nerv Sys. 1980;1:229–241. doi: 10.1016/0165-1838(80)90019-3. [DOI] [PubMed] [Google Scholar]
- 18.McGuire E, Morrissey S, Zhang S, Horwinski E. Control of reflex detrusor activity in normal and spinal injured non-human primates. J Urol. 1983;129:197–199. doi: 10.1016/s0022-5347(17)51982-5. [DOI] [PubMed] [Google Scholar]
- 19.Reighard J, Jennings HS. Anatomy of the cat. Holt, Rinehart and Winston; 1935. [Google Scholar]
- 20.McPherson A. The effects of somatic stimuli on the bladder in the cat. J Physiol. 1966;185:185–196. doi: 10.1113/jphysiol.1966.sp007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behavioural Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 22.Shen B, Wang J, Liu H, Subbaroyan J, DiUbaldi A, Wahlgren S, Roppolo JR, de Groat WC, Tai C. 2008 Neuroscience Meeting Abstract. Society for Neuroscience; Washington DC: 2008. Bladder activity modulated by transcutaneous pudendal nerve stimulation. [Google Scholar]