Abstract
When ovariectomized Fischer female rats are hormonally primed with 10 μg estradiol benzoate, a 5 min restraint experience rapidly inhibits lordosis behavior. Addition of progesterone to the hormonal priming prevents this restraint-induced inhibition. In prior work, we reported evidence that progesterone receptors (PR) may contribute to this protective effect of progesterone. In the current manuscript, we provide evidence that progesterone metabolites may also contribute to progesterone’s ability to reduce the effects of restraint. Ovariectomized female rats were hormonally primed with 10 μg estradiol benzoate followed 2 days later with 4.0 mg/kg of the progesterone metabolite, allopregnanolone. Allopregnanolone, administered either 4 hr or 2 hr before the restraint experience, was as effective as progesterone in reducing the lordosis-inhibitory effects of restraint. In the second experiment, progesterone metabolism was blocked with 50 mg/kg of the 5α-reductase inhibitor, finasteride. Surprisingly, finasteride did not prevent progesterone from reducing the effects of restraint. In a third experiment, we tested the possibility that allopregnanolone acted through metabolism to dihydroprogesterone. Rats were treated with allopregnanolone or with allopregnanolone plus the 3α-hydroxysteroid dehydrogenase inhibitor, indomethacin. Indomethacin did not prevent allopregnanolone from reducing the effects of restraint. Mechanisms are discussed whereby cross-talk between PR-mediated and metabolite-mediated events may converge in producing progesterone’s attenuation of the effect of restraint.
Keywords: ovariectomized female rats, hormonal priming, allopregnanolone, finasteride, indomethacin, stress
Introduction
In naturally cycling female rats, gonadal hormones synchronize behavioral and ovulatory events to optimize reproductive fitness. In ovariectomized rats, estradiol can induce a subset of the female’s reproductive behaviors, such as lordosis (Blaustein, 2008; Frye et al., 1998; Sodersten, 1981), but (except at high doses) does not induce the full repertoire of behaviors that characterize the naturally cycling rat with endogenous estradiol and progesterone priming (Erskine, 1989; Mani et al., 1994b; Ogawa et al., 1994). Progesterone’s interaction with the intracellular progesterone receptor (PR) and consequent genomic events are important for the hormone’s facilitation of sexual behavior (Blaustein, 2008; Ogawa et al., 1994). However, progesterone’s contribution to reproduction is not limited to this classical ligand-dependent PR activation (Mani and Portillo, 2010; Schumacher et al., 2007; Tetel et al., 2009). Moreover, membrane progesterone receptors also contribute to the control of reproduction by activation of a variety of intracellular signaling cascades (Apostolakis et al., 2000; Schumacher et al., 2007). In addition, progesterone can be metabolized by 5α-reductase into 5α-dihydroprogesterone (5α-DHP) and then into allopregnanolone by 3α-hydroxysteroid dehydrogenase (3α-HSD) (Rupprecht, 2003; Schule et al., 2011). Of these metabolites, considerable attention has been paid to allopregnanolone which appears to contribute to the enhancement of female sexual behavior (Beyer et al., 1995; Frye et al., 1998; Frye and Vongher, 1999) and may, in addition, contribute to progesterone’s anxiolytic effects (Auger and Forbes-Lorman, 2008; Frye et al., 2008; Walf et al., 2006).
Progesterone has been shown to exert anxiolytic action in a variety of animal models and, in many cases, progesterone’s antianxiety effects have been attributed to allopregnanolone and its positive modulation of the GABAergic system (Barbaccia, 2004; Eser et al., 2008; Majewska et al., 1986). We have proposed that this antianxiety effect of progesterone may be an important component of progesterone’s ability to enhance female rodent sexual behavior (Truitt et al., 2003; White and Uphouse, 2004). Seventy-eighty percent of Fischer inbred rats show high L/M ratios after priming with 10 μg estradiol benzoate (personal observations). This makes the Fischer strain ideally suited for studying effects of progesterone on the sexual behavioral response to restraint. For example, a mild 5 min restraint reduces lordosis behavior of ovariectomized Fischer females, primed only with estradiol benzoate, while females primed with estradiol benzoate and progesterone are unaffected by the restraint (Truitt et al., 2003; White and Uphouse, 2004). However, it is not known whether classical intracellular PR-mediated or nonclassical effects of progesterone are responsible for progesterone’s attenuation of the response to restraint.
In a prior study, when progesterone was administered 4 hr, 60 min, or 30 min before restraint, at least 60 min of progesterone priming were required for attenuation of the effects of restraint; and the antiprogestin, RU486, reduced the effect of progesterone (Hassell et al., 2011). In addition, the nonmetabolizable progestin, medroxyprogesterone (Lee et al., 1999), mimicked the effect of progesterone. These findings were most consistent with a requirement for PR-mediation, but these studies did not completely rule out progesterone metabolites such as allopregnanolone since (in some cases) lordosis-facilitating effects of allopregnanolone can also be attenuated by RU486 (Beyer et al., 1995). Furthermore, hormonal priming of ovariectomized rats with 10 μg estradiol benzoate and a dose of progesterone as low as 25 μg effectively prevented the effect of restraint (White and Uphouse, 2004). This requirement for a relatively low dose of progesterone prompted us to further consider the potential contribution of progesterone metabolites.
In the current experiments, we tested the hypothesis that progesterone metabolites, in particular allopregnanolone, contribute to progesterone’s attenuation of the effects of mild stress. If the progesterone metabolite, allopregnanolone, is important to this protection, the 5α-reductase inhibitor, finasteride (Finn et al., 2006), should prevent protective effects of progesterone and allopregnanolone should substitute for progesterone in reducing effects of restraint. Moreover, if allopregnanolone is indeed an effective agent, the effects of allopregnanolone should not be blocked by treatment with the 3α-HSD inhibitor, indomethacin (Penning, 1985). Portions of these data were presented at the Society for Neuroscience meeting in 2010.
Materials and Methods
Materials
Estradiol benzoate, progesterone, finasteride (N-tert-Butyl-3-oxo-4-aza-5α-androst-1-en-17β-carboxamide), allopregnanolone (3α-hydroxy-5α-pregnan-20-one), indomethacin (1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-3-indoleacetic acid), dimethyl sulfoxide (DMSO) and sesame seed oil were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Propylene glycol was obtained from Eastman Kodak Company (Rochester, NY). Isoflurane (AErrane®) was purchased from Henry Schein (Melville, NY). Decapicone® restrainers were from Braintree Scientific, Inc. (Braintree, MA). Other supplies came from Fisher Scientific (Houston, TX).
General Methods
Animals, housing and surgical procedures
Adult Fischer (F-344) female rats, purchased from Charles River Laboratories (Wilmington, MA), were housed 2 or 3 per cage in polycarbonate shoebox cages in a colony room maintained at 25 oC and 55% humidity, with lights on from 12 midnight to 12 noon. Food and water were available ad lib. When 80 to 90 days of age, females were anesthetized with AErrane® and ovariectomized as previously described (White and Uphouse, 2004). Approximately two weeks later, rats were used in the experiments. Females were sexually naïve at initiation of the experiments. All procedures were conducted according to PHS policy and were approved by the IACUC at Texas Woman’s University.
Restraint procedures
Restraint procedures were as previously described (Uphouse et al., 2007). The female was placed head first into a Decapicone® so that her nose was flush with the small opening at the tip of the cone. The base of the cone was gathered around the female’s tail and secured tightly with tape. The process of wrapping the female required between 30 and 60 sec. The wrapped female was set aside for 5 min of restraint.
Testing for sexual receptivity
Sexual behavior testing, as previously described (Uphouse et al., 1992), was initiated within 1 to 3 hr after colony lights off. Experimenter visibility was aided by red lighting. In a pretest for sexual receptivity, females were placed into the home cages of sexually experienced Sprague-Dawley male rats. Sprague-Dawley males are more sexually active than Fischer males (personal observations) and, therefore, more suitable for the testing procedures we use. The mounting behavior of Fischer males is slower than that of Sprague-Dawley males so that L/M data cannot be readily acquired for each of the required testing intervals. After the female was placed into the male’s cage, behavior was monitored for 10 min or until the male had accomplished 10 mounts; rats with a pretest lordosis to mount (L/M) ratio of 0.7 or higher were used to examine the effects of restraint. Immediately after the 5 min restraint, rats were placed again in the male’s cage and sexual behavior was monitored for 15 consecutive min. L/M ratios, lordosis quality and proceptivity (hopping and darting), as previously described (White and Uphouse, 2004), were recorded. The L/M ratio was computed as the number of lordosis responses to the male’s mounts divided by the number of mounts by the male. Lordosis quality was scored on a scale of 1 to 4. A lordosis response with minimal arching of the back was given a quality score of 1.0; an increased angle of the back’s arch was scored as 2.0. Complete arching of the back was scored as 3.0 and an exaggerated response, accompanied by elevation of the female’s front feet, was scored as 4.0. A female was scored as proceptive when she showed at least one hop/dart sequence within a test interval.
Statistical procedures
Data were evaluated in two separate ways. First, pretest effects of treatment for all animals were evaluated with univariate ANOVA (when multiple groups were included) or Student’s t-test (when only two groups were included in the experiment). This procedure was performed to determine if treatments had influenced sexual behavior prior to the restraint experience. Only rats with a pretest L/M ratio ≥0.7 were then included in the restraint procedures. The pretest behavior of this subset of females was used as the pre-restraint profile for comparison to post-restraint data. For effects of restraint, the L/M ratios, lordosis quality scores, and number of mounts by the male were grouped into the pre-restraint interval and five-min intervals after restraint; data were then evaluated by repeated measures ANOVA with time after restraint as the repeated factor. Post-hoc comparisons were made with Dunnett’s test for within group time comparison to the pre-restraint interval or with Tukey’s test for comparison between groups, within time interval. Proceptive behavior was compared with Chi-Square procedures. Statistical analyses were conducted with SPSS 17 for Macintosh or SPSS 15 for PC. Independent pair-wise comparisons were performed manually (Zar, 1999). An alpha level of 0.05 was required for rejection of the null hypothesis.
Specific Experiments
Experiment 1: Effects of allopregnanolone on the response to restraint
Ovariectomized rats were hormonally primed with 10 μg estradiol benzoate (EB) (in sesame seed oil; s.c.; 0.1 ml/rat) 53-54 hr before restraint. Such hormonal priming of ovariectomized Fischer females produces high levels of lordosis, but females remain vulnerable to the effects of restraint (White and Uphouse, 2004). Allopregnanolone (4 mg/kg in sesame seed oil, 1 ml/kg, s.c.) was injected 2 or 4 hr before restraint. The dose and timing for allopregnanolone was selected on the basis of prior experiments where the metabolite was effective in increasing lordosis behavior or male preference in ovariectomized, EB-primed rats (Frye et al., 1998; Picazo and Fernandez-Guasti, 1995). An additional group of rats (E-vehicle) received a sesame seed oil injection either 2 hr or 4 hr before restraint and data were combined for analysis.
After the pretest for sexual behavior, rats with a pretest L/M ratio of at least 0.7 were restrained for 5 min, returned to the male’s cage, and tested for 15 consecutive min. Pretest data for this subset of rats was defined as their pre-restraint data for comparison to behavior after restraint.
Experiment 2: Effects of finasteride on the response to restraint
Ovariectomized rats were hormonally primed with 10 μg EB followed 48 hr later with 50 μg progesterone or sesame seed oil. This dose of progesterone exceeds the minimal dose required to protect against the effect of restraint (White and Uphouse, 2004) and was used to optimize potential effects of the 5α-reductase inhibitor. Injections were given s.c. in a volume of 0.1 ml/rat. Two hr prior to injection with progesterone, rats were injected with either N-(2-methyl-2-propyl)-3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide (finasteride; dissolved in propylene glycol) or the propylene glycol vehicle. Finasteride was administered s.c. at a dose of 50 mg/kg in a volume of 1 ml/kg body weight. Under similar conditions, this dose of finasteride has been shown to alter measures of sexual behavior and to reduce brain levels of allopregnanolone (Frye et al., 1998). EO-vehicle rats were injected with sesame seed oil instead of progesterone and with propylene glycol instead of finasteride and were included as a positive control. Four to six hr after the progesterone (or sesame seed oil) injection, rats were pretested for sexual behavior. Only rats with a pretest L/M ratio of at least 0.7 continued in the experiment. These rats were restrained for 5 min and immediately placed back into the male’s cage for 15 consecutive min.
Experiment 3: Effect of indomethacin on the response to allopregnanolone
Ovariectomized rats were hormonally primed with 10 μg EB 53-54 hr before restraint. Two hr before restraint, rats were injected with 4 mg/kg allopregnanolone as in experiment 1. One to two min before injection with allopregnanolone, rats were injected s.c. with 6 mg/kg indomethacin (in 10% DMSO/ 90% propylene glycol; 1 ml/kg) or the DMSO/propylene glycol vehicle. Procedures with indomethacin were based on earlier studies (Avitsur and Yirmiya, 1999; Beyer et al., 1999). After the pretest for sexual behavior, rats with an L/M ratio of at least 0.7 were restrained for 5 min and then tested for 15 consecutive min.
Results
Experiment 1: Effects of allopregnanolone on the response to restraint
Behavior during the pretest
A total of 40 rats were used in the experiment. Relative to E-Vehicle rats, allopregnanolone slightly, but significantly, increased L/M ratios in the pretest (F2,37 = 6.08, p≤0.005) and increased the proportion of rats that showed proceptivity (Chi square = 6.18, df = 2, p≤0.05) (See Table 1).
TABLE 1.
EFFECTS OF ALLOPREGANANOLONE AND THE RESPONSE TO RESTRAINT
| PRETEST BEHAVIOR (all animals)* | E-VEHICLE | E-ALLO (2 HR) | E ALLO (4 HR) | Total |
|---|---|---|---|---|
| Initial Pretest n | 17 | 8 | 15 | 40 |
| Pretest Mean (S.E.) L/M Ratio | 0.76 (0.065) | 1.00 (0) | 0.99 (0.006) | |
| Pretest Mean (S.E.) Lordosis Quality | 2.7 (0.126) | 2.95 (0.038) | 2.94 (0.045) | |
| Number (%) Proceptive in Pretest | 7 (41.2%) | 7 (87.5%) | 11 (73.3%) | |
| Number (%) with Pretest L/M≥0.7 | 14 (82.4%) | 8 (100%) | 15 (100%) | 37 |
| Number missing mounts after restraint | 0 | 1 | 0 | 1 |
| BEHAVIOR OF RESTRAINED RATS (rats with pretest L/M ratio ≥0.7)** | ||||
| Final n for Restraint Analysis | 14 | 7 | 15 | 36 |
| Number (%) Proceptive before Restraint | 6 (42.9%) | 6 (85.7%) | 11 (73.3%) | |
| Number (%) Proceptive after Restraint | 2 (14.3%) | 3 (42.9%) | 9 (60%) |
behavior of all animals before restraint
only rats with pretest L/M ≥0.7 were included in the restraint procedures; pretest behavior of this subset of rats is included in describing the data before restraint.
Effects of restraint
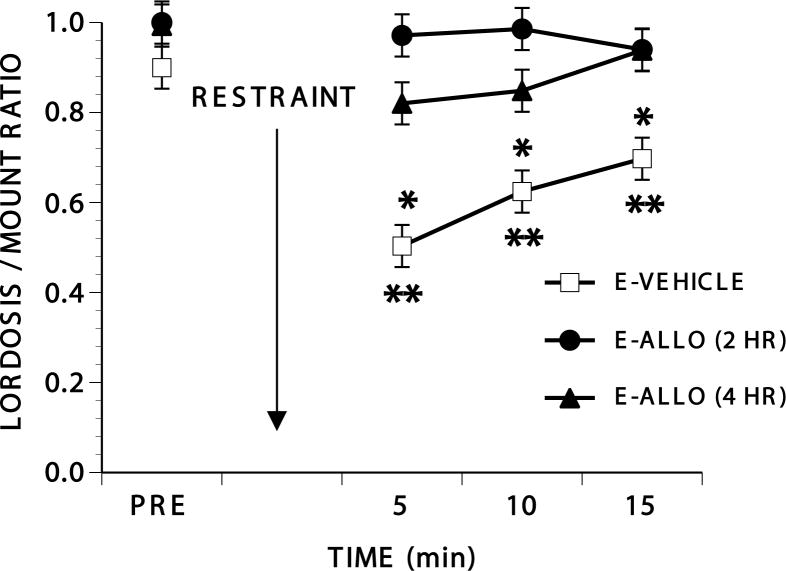
Rats hormonally primed with EB and vehicle showed a decline in lordosis behavior after restraint and allopregnanolone prevented this decline (Figure 1; Three E-Vehicle rats had L/M ratios in their pretest that were below 0.7 and were excluded from the study of restraint.). There were significant effects of treatment (F2,33 = 10.36, p≤0.001), time after restraint (F3,99 = 7.45, p≤0.001), and the time by treatment interaction (F6,99 = 2.25, p≤0.05). Group differences resulted from lower L/M ratios after restraint in E-Vehicle-treated rats relative to both groups of allopregnanolone-treated rats. E- Vehicle-treated rats were significantly different from their pre-restraint interval at every interval after restraint (Dunnett’s q99,4 2.10, p 0.05) and were different from both allopregnanolone groups at every interval after restraint (Tukey’s q99,3≥3.40, p≤0.05). Allopregnanolone-treated rats never differed from their pre-restraint values.
Figure 1.
Effects of allopregnanolone on the response to restraint
Ovariectomized rats were injected with 10 μg EB and restrained 53-54 hr later. Allopregnanolone (4 mg/kg in sesame seed oil, s.c.) was injected 4 hr [E-ALLO (4 hr)] or 2 hr [(E-ALLO (2 hr)] before restraint. Additional rats (E-VEHICLE) were injected with sesame seed oil instead of allopregnanolone. After a pretest (PRE) for sexual receptivity, rats were restrained for 5 min and immediately tested for 15 consecutive min. N’s for E-VEHICLE, E-ALLO (2 hr) and E-ALLO (4 hr), respectively, were 14, 7, and 15. Data are the mean ± S.E. L/M ratios for each of the test intervals. * Indicates significant differences from the pretest L/M ratio. ** Indicates significant differences between rats primed only with EB and those primed with EB and allopregananolone administered either 2 or 4 hr before restraint (all p≤0.05).
Restraint reduced the proportion of E-Vehicle rats showing proceptivity and this was partially attenuated by allopregnanolone (Chi square = 6.42, df = 2, p≤0.04; Table 1). However, since such a small proportion of E-Vehicle rats showed proceptivity before restraint, restraint may simply have amplified this pre-restraint group difference.
Lordosis quality (F3,96 = 3.58, p≤0.02) and mounts (F3,102 = 19.67, p≤0.001) declined significantly with time, but no other effects were significant. The mean ± S.E. lordosis quality for rats treated with EB and vehicle or with EB and allopregnanolone 2 or 4 hr before restraint, respectively, were 2.67 ± 0.08, 2.89 ± 0.11 and 2.87 ± 0.08. Mean ± S.E. number of mounts per test interval, respectively, were 8.07 ± 0.53, 7.5 ± 0.51 and 6.59 ± 0.71.
Experiment 2: Effects of finasteride on the response to restraint
Behavior during the pretest
A total of 53 rats were used in the experiment. Whether or not rats received finasteride, progesterone slightly increased L/M ratios (F2,30 = 4.72, p≤0.02), lordosis quality (F2,50 = 3.99, p≤0.025), and proceptivity (Chi square = 8.98, df = 2, p≤0.02) (Table 2). There were no differences between rats given progesterone and vehicle and those given progesterone and finasteride.
TABLE 2.
EFFECTS OF FINASTERIDE AND THE RESPONSE TO RESTRAINT
| PRETEST BEHAVIOR (all animals)* | EO VEHICLE | EP VEHICLE | EP FINASTERIDE | Total |
|---|---|---|---|---|
| Initial Pretest n | 12 | 16 | 25 | 53 |
| Pretest Mean (S.E.) L/M Ratio | 0.81 (0.065) | 0.93 (0.036) | 0.96 (0.0199) | |
| Pretest Mean (S.E.) Lordosis Quality | 2.54 (0.167) | 2.79 (0.095) | 2.86 (0.047) | |
| Number (%) Proceptive in Pretest | 5 (41.7%) | 12 (75%) | 22 (88%) | |
| Number (%) with Pretest L/M ≥0.7 | 8 (66.7%) | 15 (93.8%) | 24 (96.0%) | 47 |
| Number missing mounts after restraint | 1 | 0 | 4 | 5 |
| BEHAVIOR OF RESTRAINED RATS (rats with pretest L/M ratio ≥0.7)** | ||||
| Final n for Restraint Analysis | 7 | 15 | 20 | 42 |
| Number (%) Proceptive before Restraint | 4 (57.1%) | 12 (80%) | 19 (95%) | |
| Number (%) Proceptive after Restraint | 4 (57.1%) | 8 (53.3%) | 12 (60%) |
behavior of all animals before restraint
only rats with pretest L/M ≥0.7 were included in the restraint procedures; pretest behavior of this subset of rats is included in describing the data before restraint.
Effects of restraint
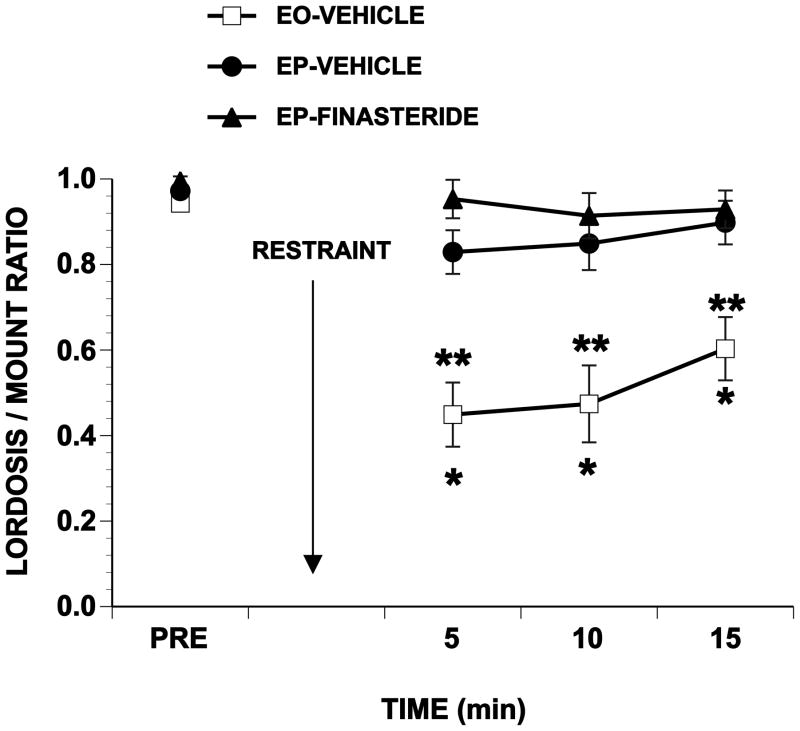
Eleven rats were excluded from analysis of restraint because of either low pretest L/M ratios or the absence of mounts after restraint (Table 2). For the remaining rats, restraint significantly reduced L/M ratios of EO-Vehicle rats and progesterone protected against the effect of restraint (Figure 2). Finasteride did not prevent this protection from taking place. There were significant effects of hormonal treatment (F1,39 = 14.46, p≤0.001), time after restraint (F3,117 = 20.18, p≤0.001) and their interaction (F6,117 = 5.55, p≤0.001). L/M ratios of EO-Vehicle-treated rats were different from their pre-restraint values at each test interval after restraint and were different from the other two groups at each post-restraint interval (Tukey’s q117,4≥4.05, all p≤0.05). L/M ratios of EP rats (whether treated with vehicle or finasteride) were never different from their pre-restraint ratios and never differed from each other (all p > 0.05).
Figure 2.
Effects of finasteride on the response to restraint
Ovariectomized rats were hormonally primed with 10 μg EB and injected 48 hr later with 50 μg progesterone (EP) or the sesame seed oil vehicle (EO). Two hr prior to injection with progesterone, rats were injected with finasteride (dissolved in propylene glycol) or the propylene glycol vehicle. Finasteride was administered s.c. at a dose of 50 mg/kg in a volume of 1 ml/kg body weight. N’s for EO-Vehicle, EP-Vehicle and EP-finasteride, respectively, were 7, 15 and 20. Four to six hr after progesterone, rats were pretested for sexual behavior, restrained for 5 min, and immediately tested for 15 consecutive min. Data are the mean ± S.E. L/M ratios for each of the test intervals. * Indicates significant differences from the pretest L/M ratio. ** Indicates significant differences between EO-Vehicle rats and those primed either with EB and progesterone or with EB and finasteride (all p≤0.05).
Prior to restraint, fewer EO-Vehicle rats showed proceptivity than rats treated with either EP-Vehicle or EP-Finasteride (Table 2), but group differences narrowly escaped statistical significance (Chi square = 5.53, df = 2, p≤0.063). There was no evidence for group differences after restraint (Chi square, p > 0.05).
There were no significant effects of treatment on lordosis quality (all p > 0.05); however, quality could not be assessed for 3 EO-Vehicle rats due to the absence of lordosis behavior in at least one time interval following restraint. For the remaining rats, the mean ± S.E. lordosis quality scores per interval for EO-Vehicle, EP-Vehicle, and EP-Finasteride rats, respectively, were 2.54 ± 0.06, 2.68 ± 0.06, and 2.81 ± 0.01. Similarly, although there was a time-dependent decline (over all groups) in the number of mounts received per interval (F3,132 = 22.03, p≤0.001), the number of mounts did not vary significantly across treatment groups; and there was not a significant interaction between number of mounts and time after restraint (all p > 0.05). The mean ± S.E. number of mounts per interval for EO-Vehicle, EP-Vehicle, and EP-Finasteride rats, respectively, were 6.81 ± 0.69, 8.43 ± 0.50 and 7.0 ± 0.40.
Experiment 3: Effect of indomethacin on the response to allopregnanolone
Since finasteride did not reduce effects of progesterone on restraint while allopregnanolone effectively substituted for progesterone, the third experiment was designed to examine the possibility that allopregnanolone’s effect resulted from its metabolism to dihydroprogesterone.
Behavior during the pretest
Twenty-one rats were used. There were no group differences in pretest, L/M, lordosis quality or proceptivity (all p > 0.05, Table 3).
TABLE 3.
EFFECTS OF INDOMETHACIN IN COMBINATION WITH ALLOPREGNANOLONE
| PRETEST BEHAVIOR (all animals)* | ALLOPREGNANOLONE | ALLOPREGNANOLONE AND INDOMETHACIN | Total |
|---|---|---|---|
| Initial Pretest n | 9 | 12 | 21 |
| Pretest Mean (S.E.) L/M Ratio | 0.94 (0.023) | 0.96 (0.019) | |
| Pretest Mean (S.E.) Lordosis Quality | 2.79 (0.078) | 2.87 (0.05) | |
| Number (%) Proceptive in Pretest | 1 (11.1%) | 4 (33.3%) | |
| Number (%) with Pretest L/M ≥0.7 | 9 (100%) | 12 (100%) | 21 |
| Number missing mounts after restraint | 1 | 2 | 3 |
| BEHAVIOR OF RESTRAINED RATS (rats with pretest L/M ratio ≥0.7)** | |||
| Final n for Restraint Analysis | 8 | 10 | 18 |
| Number (%) Proceptive before Restraint | 0 (0.0%) | 3 (30.0%) | |
| Number (%) Proceptive after Restraint | 1 (12.5%) | 0 (0.0%) | |
| Mean (S.E.) L/M Ratio before Restraint | 0.93 (0.024) | 0.95 (0.022) | |
| Mean (S.E.) L/M Ratio after Restraint | 0.81 (0.058) | 0.93 (0.055) |
behavior of all animals before restraint
only rats with pretest L/M ≥0.7 were included in the restraint procedures; pretest behavior of this subset of rats is included in describing the data before restraint.
Effects of restraint
Three rats did not receive sufficient mounts after restraint and were not included in the ANOVA for effects of restraint. For the remaining rats, there was no evidence that indomethacin reduced allopregnanolone’s ability to protect against the effects of restraint. There were no treatment effects on L/M ratios, lordosis quality, or proceptivity (all p > 0.05). Average pre- and post-restraint L/M ratios are shown in Table 3.
Discussion
The major objective of these experiments was to test the hypothesis that progesterone metabolites such as allopregnanolone contribute to progesterone’s ability to reduce the lordosis-inhibiting effects of 5 min restraint. Treatment of estradiol benzoate-primed ovariectomized rats with allopregnanolone clearly reduced effects of restraint. Because allopregnanolone can be metabolized via 3α-hydroxysteroid dehydrogenase to dihydroprogesterone, which can bind to the PR (Rupprecht, 2003; Rupprecht et al., 1993), the effect of indomethacin was critical in interpreting the protective effect of allopregananolone. Indomethacin inhibits 3α-hydroxysteroid dehydrogenase (Penning, 1985) and can reduce lordosis-facilitatory effects of allopregnanolone (Beyer et al., 1999). However, no effects of indomethacin were evident in the current experiment. Although we cannot rule out the possibility that the dose of indomethacin was too low, we attribute the absence of an effect of indomethacin as suggestive evidence that allopregnanolone, rather than dihydroprogesterone binding to the PR, was responsible for protection against the effects of restraint. Allopregnanolone does not interact with the intracellular PR (Raynaud et al., 1974; Smith et al., 1974) so these findings are consistent with the possibility that progesterone metabolites can protect against the effects of mild stress. Such metabolites do not, however, appear to be essential for progesterone’s protection because finasteride did not attenuate the effects of progesterone. The dose of finasteride used in the current experiment has been previously shown to reduce brain levels of allopregnanolone (Frye et al., 1998) but we cannot exclude the possibility that some allopregnanolone was produced, even in the presence of finasteride.
In some cases, allopregnanolone’s positive effects on lordosis behavior have been seen within 5 to 30 min of intravenous (i.v.) or intracranial (i.c.) treatment (Beyer et al., 1995; Kubli-Garfias and Whalen, 1977) leading to speculation that allopregnanolone’s lordosis facilitation is mediated by rapid neurotransmitter events such as its positive modulation of the GABAA receptor (Frye and Vongher, 1999; Frye et al., 2006). In other cases, longer-term effects have been reported (Beyer et al., 1995; Gonzalez-Flores et al., 2006) and these effects may be blocked by RU486 (Beyer et al., 1995; Gonzalez-Mariscal et al., 1989). Therefore, it has been suggested that allopregnanolone can facilitate lordosis behavior through mechanisms that may include indirect activation of PR (Beyer et al., 1995; Gonzalez-Flores et al., 2010; Gonzalez-Flores et al., 2006). In the current studies, allopregnanolone was given s.c. 2 or 4 hr before restraint. In preliminary experiments, when estradiol-primed, ovariectomized females were injected with allopregnanolone 30 min before restraint, there was no evidence of protection against effects of the 5 min restraint (personal observations). Therefore, allopregnanolone’s protective action appears to require mechanisms beyond its rapid modulation of neurotransmitter function. However, since in many studies showing rapid effects of allopregnanolone investigators have utilized i.c. or i.v. administration, we cannot rule out a contribution of some of these rapid effects to the protective action of allopregnanolone in the current study.
Ligand-independent activation of PR, originally suggested as a mechanism for facilitatory effects of dopamine receptor agonists on lordosis behavior (Mani et al., 1994a), is an important pathway through which many signaling molecules may lead to activation of PR (Boonyaratanakornkit et al., 2008; Boonyaratanakornkit et al., 2001; Gonzalez-Flores et al., 2006) and has been suggested as a mechanism whereby reproductive events can be adaptively synchronized to environmental changes. Mechanisms mediating such PR effects include post-translational modification of PR or steroid receptor coactivators that are mediated through activation of, for example, PKC, PKA, or Src kinase/MAPK (Ballare et al., 2003; Boonyaratanakornkit et al., 2007; Li and Shang, 2007; Mani and Portillo, 2010; McKenna and O'Malley, 2002; Quiles et al., 2009; Tetel et al., 2009).
Allopregnanolone has been reported to activate PKA (Gonzalez-Flores et al., 2006; Mani et al., 2000) and MAPK (Etgen and Acosta-Martinez, 2003; Frye and Walf, 2008) and can increase Src kinase dependent Ca2+ influx (Blackmore, 2008). Of relevance to the current findings is that allopregnanolone’s facilitation of lordosis can be blocked by PKC inhibitors (Gonzalez-Flores et al., 2006) or MAPK (Gonzalez-Flores et al., 2004). Therefore, allopregnanolone may reduce the effects of restraint stress by activation of intracellular signaling events that then modulate PR action.
Such an indirect activation of PR by allopregnanolone would be consistent with the findings reported by Hassell et al. (2011) that implicated the PR in progesterone’s attenuation of the effects of restraint and by the current findings that allopregnanolone could substitute for progesterone but that finasteride did not block progesterone’s protective effect. Therefore, the collective findings implicate PRs in progesterone’s ability to protect against the lordosis-inhibiting effects of restraint on lordosis behavior. However, they also illustrate the potential for both ligand-dependent and ligand-independent PR-mediated responses in protecting against the effects of restraint. Given the importance of female reproductive behavior to survival of the species, such redundancy in protection against the lordosis-inhibiting impact of mild stress is not surprising. In fact, it is most likely that classical PR-mediated, nonclassical PR-mediated, as well as progesterone-mediated effects on neurotransmitter function, synchronously modulate the behavioral responses to mild stress.
We have previously suggested that 5 min restraint, by increasing release of 5-HT, leads to dual activation of 5-HT1A receptors (which inhibit lordosis behavior) and 5-HT2 receptors (which facilitate lordosis behavior and attenuate inhibitory effects of 5-HT1A receptor agonists) (Maswood et al., 1996; Uphouse, 2000; Uphouse et al., 2003). We have suggested that such dual modulation of sexual behavior would enhance the female’s overall fitness. Continuation of sexual behavior in the presence of a predator (such as an owl passing over) would likely reduce the female’s probability of surviving. However, it would be beneficial for the female to return to mating should the predator leave the environment.
The lordosis-inhibitory effect of 5-HT1A receptors appear to include inhibition of cAMP-mediated events (Uphouse et al., 2000); and the ability of a 5-HT2 receptor agonist to attenuate 5-HT1A receptor-mediated lordosis inhibition is attenuated by inhibition of PKC (Selvamani et al., 2007). Effects of progesterone and/or its metabolite, allopregnanolone, modulate these same signaling events. Progesterone treatment reduces extracellular 5-HT (Farmer et al., 1996) and attenuates 5-HT1A-receptor-mediated inhibition of lordosis (Truitt et al., 2003). Progesterone also increases cAMP (Mani et al., 2000) and inhibition of PKA attenuates progesterone’s facilitation of lordosis behavior (Gonzalez-Flores et al., 2006). Lordosis-facilitating effects of allopregnanolone are less vulnerable to PKA inhibition (Gonzalez-Flores et al., 2006) but may, instead, involve PKC (Frye and Walf, 2008; Gonzalez-Flores et al., 2006) and/or potentiation of the GABAergic system (Frye et al., 2006) which has also been reported to attenuate inhibitory effects of 5-HT1A receptors on lordosis behavior (Guptarak et al., 2004). Therefore, the collective evidence is consistent with the occurrence of considerable cross-talk among progesterone’s multiple signaling cascades and its ability to attenuate effects of mild stress on female rat sexual behavior.
RESEARCH HIGHLIGHTS.
Progesterone protects against the lordosis-inhibiting effects of a 5 min restraint experience.
Allopregnanolone substitutes for progesterone in protecting against the effects of the restraint.
Prevention of progesterone metabolism with finasteride does not reduce progesterone’s protective effects against restraint.
Acknowledgments
Research supported by NIH HD28419, by NIH GM55380, and by a TWU institutional research support grant. Appreciation is given to Dr. Jutatip Guptarak for her critical reading of an earlier version of the manuscript. We thank Ms. Karolina Blaha-Black and Mr. Dan Wall for animal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apostolakis EM, Garai J, Lohmann JE, Clark JH, O'Malley BW. Epidermal growth factor activates reproductive behavior independent of ovarian steroids in female rodents. Mol Endocrinol. 2000;14:1086–98. doi: 10.1210/mend.14.7.0490. [DOI] [PubMed] [Google Scholar]
- Auger CJ, Forbes-Lorman RM. Progestin receptor-mediated reduction of anxiety-like behavior in male rats. PLoS One. 2008;3:e3606. doi: 10.1371/journal.pone.0003606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Yirmiya R. Cytokines inhibit sexual behavior in female rats: I. Synergistic effects of tumor necrosis factor alpha and interleukin-1. Brain Behav Immun. 1999;13:14–32. doi: 10.1006/brbi.1999.0555. [DOI] [PubMed] [Google Scholar]
- Ballare C, Uhrig M, Bechtold T, Sancho E, Di Domenico M, Migliaccio A, Auricchio F, Beato M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol. 2003;23:1994–2008. doi: 10.1128/MCB.23.6.1994-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML. Neurosteroidogenesis: relevance to neurosteroid actions in brain and modulation by psychotropic drugs. Crit Rev Neurobiol. 2004;16:67–74. doi: 10.1615/critrevneurobiol.v16.i12.70. [DOI] [PubMed] [Google Scholar]
- Beyer C, Gonzalez-Flores O, Gonzalez-Mariscal G. Ring A reduced progestins potently stimulate estrous behavior in rats: paradoxical effect through the progesterone receptor. Physiol Behav. 1995;58:985–93. doi: 10.1016/0031-9384(95)00141-5. [DOI] [PubMed] [Google Scholar]
- Beyer C, Gonzalez-Flores O, Ramirez-Orduna JM, Gonzalez-Mariscal G. Indomethacin inhibits lordosis induced by ring A-reduced progestins: possible role of 3alpha-oxoreduction in progestin-facilitated lordosis. Horm Behav. 1999;35:1–8. doi: 10.1006/hbeh.1998.1457. [DOI] [PubMed] [Google Scholar]
- Blackmore PF. Progesterone metabolites rapidly stimulate calcium influx in human platelets by a src-dependent pathway. Steroids. 2008;73:738–50. doi: 10.1016/j.steroids.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu Rev Psychol. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Bi Y, Rudd M, Edwards DP. The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids. 2008;73:922–8. doi: 10.1016/j.steroids.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21:359–75. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–80. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: a review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Eser D, Baghai TC, Schule C, Nothdurfter C, Rupprecht R. Neuroactive steroids as endogenous modulators of anxiety. Curr Pharm Des. 2008;14:3525–33. doi: 10.2174/138161208786848838. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Acosta-Martinez M. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology. 2003;144:3828–35. doi: 10.1210/en.2003-0157. [DOI] [PubMed] [Google Scholar]
- Farmer CJ, Isakson TR, Coy DJ, Renner KJ. In vivo evidence for progesterone dependent decreases in serotonin release in the hypothalamus and midbrain central grey: relation to the induction of lordosis. Brain Res. 1996;711:84–92. doi: 10.1016/0006-8993(95)01403-9. [DOI] [PubMed] [Google Scholar]
- Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, Wiren KM. A new look at the 5alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12:53–76. doi: 10.1111/j.1527-3458.2006.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3alpha,5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Exploratory, anti-anxiety, social, and sexual behaviors of rats in behavioral estrus is attenuated with inhibition of 3alpha,5alpha-THP formation in the midbrain ventral tegmental area. Behav Brain Res. 2008;193:269–76. doi: 10.1016/j.bbr.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. Progesterone has rapid and membrane effects in the facilitation of female mouse sexual behavior. Brain Res. 1999;815:259–69. doi: 10.1016/s0006-8993(98)01132-9. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Activity of protein kinase C is important for 3alpha,5alpha-THP's actions at dopamine type 1-like and/or GABAA receptors in the ventral tegmental area for lordosis of rats. Brain Res Bull. 2008;77:91–7. doi: 10.1016/j.brainresbull.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Petralia SM. In the ventral tegmental area, progestins have actions at D1 receptors for lordosis of hamsters and rats that involve GABA A receptors. Horm Behav. 2006;50:332–7. doi: 10.1016/j.yhbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flores O, Beyer C, Gomora-Arrati P, Garcia-Juarez M, Lima-Hernandez FJ, Soto-Sanchez A, Etgen AM. A role for Src kinase in progestin facilitation of estrous behavior in estradiol-primed female rats. Horm Behav. 2010;58:223–9. doi: 10.1016/j.yhbeh.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flores O, Ramirez-Orduna JM, Lima-Hernandez FJ, Garcia-Juarez M, Beyer C. Differential effect of kinase A and C blockers on lordosis facilitation by progesterone and its metabolites in ovariectomized estrogen-primed rats. Horm Behav. 2006;49:398–404. doi: 10.1016/j.yhbeh.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flores O, Shu J, Camacho-Arroyo I, Etgen AM. Regulation of lordosis by cyclic 3',5'-guanosine monophosphate, progesterone, and its 5alpha-reduced metabolites involves mitogen-activated protein kinase. Endocrinology. 2004;145:5560–7. doi: 10.1210/en.2004-0823. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal G, Gonzalez-Flores O, Beyer C. Intrahypothalamic injection of RU486 antagonizes the lordosis induced by ring A-reduced progestins. Physiol Behav. 1989;46:435–8. doi: 10.1016/0031-9384(89)90016-4. [DOI] [PubMed] [Google Scholar]
- Guptarak J, Selvamani A, Uphouse L. GABAA-5-HT1A receptor interaction in the mediobasal hypothalamus. Brain Res. 2004;1027:144–50. doi: 10.1016/j.brainres.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Kubli-Garfias C, Whalen RE. Induction of lordosis behavior in female rats by intravenous administration of progestins. Horm Behav. 1977;9:380–6. doi: 10.1016/0018-506x(77)90073-3. [DOI] [PubMed] [Google Scholar]
- Lee TC, Miller WL, Auchus RJ. Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. J Clin Endocrinol Metab. 1999;84:2104–10. doi: 10.1210/jcem.84.6.5646. [DOI] [PubMed] [Google Scholar]
- Li S, Shang Y. Regulation of SRC family coactivators by post-translational modifications. Cell Signal. 2007;19:1101–12. doi: 10.1016/j.cellsig.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mani S, Portillo W. Activation of progestin receptors in female reproductive behavior: Interactions with neurotransmitters. Front Neuroendocrinol. 2010;31:157–71. doi: 10.1016/j.yfrne.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Allen JM, Clark JH, Blaustein JD, O'Malley BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994a;265:1246–9. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, Allen JM, Law SW, O'Malley BW, Clark JH. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994b;135:1409–14. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- Mani SK, Fienberg AA, O'Callaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O'Malley BW. Requirement for DARPP-32 in progesterone-facilitated sexual receptivity in female rats and mice. Science. 2000;287:1053–6. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- Maswood S, Andrade M, Caldarola-Pastuszka M, Uphouse L. Protective actions of the 5-HT2A/2C receptor agonist, DOI, on 5-HT1A receptor-mediated inhibition of lordosis behavior. Neuropharmacology. 1996;35:497–501. doi: 10.1016/0028-3908(95)00195-6. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW. Minireview: nuclear receptor coactivators--an update. Endocrinology. 2002;143:2461–5. doi: 10.1210/endo.143.7.8892. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Olazabal UE, Parhar IS, Pfaff DW. Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mRNA on reproductive behavior and progesterone receptor immunoreactivity in female rat. J Neurosci. 1994;14:1766–74. doi: 10.1523/JNEUROSCI.14-03-01766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM. Irreversible inhibition of delta 5-3-oxosteroid isomerase by 2-substituted progesterones. Biochem J. 1985;226:469–76. doi: 10.1042/bj2260469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picazo O, Fernandez-Guasti A. Anti-anxiety effects of progesterone and some of its reduced metabolites: an evaluation using the burying behavior test. Brain Res. 1995;680:135–41. doi: 10.1016/0006-8993(95)00254-n. [DOI] [PubMed] [Google Scholar]
- Quiles I, Millan-Arino L, Subtil-Rodriguez A, Minana B, Spinedi N, Ballare C, Beato M, Jordan A. Mutational analysis of progesterone receptor functional domains in stable cell lines delineates sets of genes regulated by different mechanisms. Mol Endocrinol. 2009;23:809–26. doi: 10.1210/me.2008-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud JP, Philibert D, Azadian-Boulanger G. Progesterone-Progestin receptors. Basic Life Sci. 1974;4:143–60. doi: 10.1007/978-1-4684-2889-6_10. [DOI] [PubMed] [Google Scholar]
- Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139–68. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglgansberger W, Holsboer F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–30. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- Schule C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R. Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu EE. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev. 2007;28:387–439. doi: 10.1210/er.2006-0050. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Lincoln C, Uphouse L. The PKC inhibitor, bisindolymaleimide, blocks DOI's attenuation of the effects of 8-OH-DPAT on female rat lordosis behavior. Behav Brain Res. 2007;179:99–106. doi: 10.1016/j.bbr.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HE, Smith RG, Toft DO, Neergaard JR, Burrows EP, O'Malley BW. Binding of steroids to progesterone receptor proteins in chick oviduct and human uterus. J Biol Chem. 1974;249:5924–32. [PubMed] [Google Scholar]
- Sodersten P. Estradiol-progesterone interactions in the reproductive behavior of female rats. In: Ganten D, Pfaff D, editors. Current Topics in Neuroendocrinology: Actions of Progesterone on the Brain. Springer-Verlag; New York: 1981. pp. 141–174. [Google Scholar]
- Tetel MJ, Auger AP, Charlier TD. Who's in charge? Nuclear receptor coactivator and corepressor function in brain and behavior. Front Neuroendocrinol. 2009;30:328–42. doi: 10.1016/j.yfrne.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt W, Harrison L, Guptarak J, White S, Hiegel C, Uphouse L. Progesterone attenuates the effect of the 5-HT1A receptor agonist, 8-OH-DPAT, and of mild restraint on lordosis behavior. Brain Res. 2003;974:202–11. doi: 10.1016/s0006-8993(03)02581-2. [DOI] [PubMed] [Google Scholar]
- Uphouse L. Female gonadal hormones, serotonin, and sexual receptivity. Brain Res Brain Res Rev. 2000;33:242–57. doi: 10.1016/s0165-0173(00)00032-1. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Caldarola-Pastuszka M, Montanez S. Intracerebral actions of the 5-HT1A agonists, 8-OH-DPAT and buspirone and of the 5-HT1A partial agonist/antagonist, NAN-190, on female sexual behavior. Neuropharmacology. 1992;31:969–81. doi: 10.1016/0028-3908(92)90097-9. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Hiegel C, Perez E, Guptarak J. Serotonin receptor involvement in effects of restraint on female rat lordosis behavior. Pharmacol Biochem Behav. 2007;86:631–6. doi: 10.1016/j.pbb.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphouse L, Maswood S, Jackson A. Factors elevating cAMP attenuate the effects of 8-OH-DPAT on lordosis behavior. Pharmacol Biochem Behav. 2000;66:383–8. doi: 10.1016/s0091-3057(00)00179-9. [DOI] [PubMed] [Google Scholar]
- Uphouse L, White S, Harrison L, Hiegel C, Majumdar D, Guptarak J, Truitt WA. Restraint accentuates the effects of 5-HT2 receptor antagonists and a 5-HT1A receptor agonist on lordosis behavior. Pharmacol Biochem Behav. 2003;76:63–73. doi: 10.1016/s0091-3057(03)00194-1. [DOI] [PubMed] [Google Scholar]
- Walf AA, Sumida K, Frye CA. Inhibiting 5alpha-reductase in the amygdala attenuates antianxiety and antidepressive behavior of naturally receptive and hormone-primed ovariectomized rats. Psychopharmacology (Berl) 2006;186:302–11. doi: 10.1007/s00213-005-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S, Uphouse L. Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Horm Behav. 2004;45:201–8. doi: 10.1016/j.yhbeh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Zar J. Biostatistical Analysis. Prentice Hall; Englewood Cliffs, New Jersey: 1999. [Google Scholar]