Abstract
Prophylactic neuroprotection against stroke could reduce stroke burden in thousands of patients at high risk of stroke, including those with recent transient ischemic attacks (TIAs). Prolyl hydroxylase inhibitors (PHIs), such as deferoxamine (DFO), reduce stroke volume when administered at high doses in the peristroke period, which is largely mediated by the hypoxia-inducible transcription factor (HIF-1). Yet, in vitro experiments suggest that PHIs may also induce neuroprotection independent of HIF-1. In this study, we examine chronic, prophylactic, low-dose treatment with DFO, or another iron chelator deferasirox (DFR), to determine whether they are neuroprotective with this paradigm and mediate their effects through a HIF-1-dependent mechanism. In fact, prophylactic administration of low-dose DFO or DFR significantly reduces stroke volume. Surprisingly, DFO remained neuroprotective in mice haploinsufficient for HIF-1 (HIF-1+/−) and transgenic mice with conditional loss of HIF-1 function in neurons and astrocytes. Similarly, DFR was neuroprotective in HIF-1+/− mice. Neither DFO nor DFR induced expression of HIF-1 targets. Thus, low-dose chronic administration of DFO or DFR induced a prolonged neuroprotective state independent of HIF-1 function. As DFR is an orally administered and well-tolerated medication in clinical use, it has promise for prophylaxis against stroke in patients at high risk of stroke.
Keywords: HIF-1, hypoxia, ischemia, stroke
Introduction
Over 700,000 strokes and 240,000 transient ischemic attacks (TIAs) occur in the United States each year. Unfortunately, these patients incur a 9% to 14% risk of a recurrent stroke within 90 days of their sentinel event, with the majority occurring within the first month and the highest risk occurring within the first few days (Hill et al, 2004; Johnston et al, 2007; Kleindorfer et al, 2005). Similarly, patients with intracranial artery stenosis (Thijs and Albers, 2000) or atrial fibrillation are at high risk of stroke. In contrast to the current emphasis on acute treatment of stroke, prophylactic delivery of neuroprotective agents could establish a neuroprotective state and provide adequate delivery of neuroprotective agents before the onset of ischemia (Fisher et al, 1994; Savitz and Fisher, 2007). Given the large number of patients at high risk of stroke, this unique approach has the potential to reduce stroke burden in tens of thousands of patients each year. Yet, few studies delivered neuroprotectants over an extended time frame to determine whether they remain neuroprotective and are tolerated over a course of several weeks, which would be needed in patients at high risk of stroke.
Prolyl hydroxylase inhibitors (PHIs), like deferoxamine (DFO), are neuroprotectants that induce the abundance of hypoxia-inducible factor 1 (HIF-1). Hypoxia-inducible factor 1 is a transcription factor critically involved in the ability of cells and the organism to adapt to hypoxia. Among the several dozen targets that are induced by HIF-1 are proteins that enhance angiogenesis, increase glycolysis, and inhibit mitochondrial-derived ROS production (Semenza, 2000, 2008). Prolyl hydroxylase inhibitors, like DFO, increase HIF-1 target expression and effectively reduce stroke volumes (Baranova et al, 2007; Siddiq et al, 2005). In the case of DFO, its protection is seen when it is administered at high doses (300 mg/kg) in the peristroke time period and HIF-1 function in neurons is critical for this protective effect of DFO (Baranova et al, 2007). As such, there is great interest in utilizing PHIs to induce HIF-1 function for acute stroke treatment. Yet, some in vitro studies suggest that PHIs, including DFO, also provide neuroprotection in the absence of HIF-1 function (Niatsetskaya et al, 2010; Siddiq et al, 2009). It is unknown if DFO and similar compounds are effective for prophylactic neuroprotection against stroke and if HIF-1 mediates these putative neuroprotective properties.
In this study, we demonstrate that prolonged prophylactic treatment with the iron chelator DFO at a low dose (40 mg/kg per day) significantly reduces stroke volume, which lasts for at least a month. Similarly, the iron chelator deferasirox (DFR) (10 to 20 mg/kg per day) also reduces stroke volume when administered for prolonged time frames. Interestingly, while haploinsufficiency of HIF-1 (HIF-1+/−) reduces or eliminates the hypoxic-induced expression of HIF-1 targets, the neuroprotection provided by low-dose chronic DFO or DFR is not reduced in HIF-1+/− mice. Similarly, in transgenic mice with conditional loss of HIF-1 function in both neurons and astrocytes, DFO remains effective at reducing stroke volume. Hypoxia-inducible factor 1 target abundance is not induced by either DFO or DFR, when select targets were examined by quantitative polymerase chain reaction (PCR) or when explored using microarray analysis. With microarray analysis, DFO and DFR induced very few transcripts in the brain that were largely independent target profiles. Thus, low-dose, prolonged DFO and DFR treatment induces HIF-1-independent neuroprotective mechanisms. Given the fact that DFR is well tolerated and is administered orally, this agent could be valuable in establishing prophylactic neuroprotection in patients at high risk of stroke.
Materials and methods
Temporary Middle Cerebral Artery Occlusion Stroke Model in Mice
All methods using mice were performed to minimize pain or discomfort to the mice and were approved by our university committee devoted to the ethical use of animals in research. Mice were anesthetized with isoflurane (Ohmeda Fluotec 4; Eagle Eye Anesthesia Inc., Jacksonville, FL, USA; 30% O2 and 70% nitrous oxide). The animal's temperature was maintained at 37°C by a heating pad and feedback control system (FHC, Bowdoin, ME, USA). A laser Doppler probe (Perimed, North Royalton, OH, USA) was fixed in place on the skull 5 mm lateral and 2 mm posterior to Bregma. A coated filament was placed into the external carotid artery lumen and advanced to the middle cerebral artery (MCA) with concurrent recording of laser Doppler cerebral blood flow to ensure that the cerebral blood flow decreased to below 25% of baseline. After 45 to 50 minutes, the filament was removed, the external carotid artery was permanently ligated, the temporary CCA ligation was reversed, the Doppler probe was removed, and the animal was allowed to recover in a temperature-controlled environment (Thermocare, Incline Village, NV, USA). The volume of the infarct was determined 24 hours later by staining the brain with 2,3,5-triphenyltetrazolium chloride. Only those mice in which a reduction in cerebral blood flow below 25% of baseline was achieved, with a return to >80% of baseline with filament removal, were included in data analysis. To account for the effects of edema, stroke volumes were calculated indirectly (Lin et al, 1993). All mice meeting the above criteria were included in the analysis and in all cases, the person performing the MCA occlusion (MCAO) and measuring the stroke volumes was blinded to the treatment groups.
Blood Pressure, Blood Oxygen Saturation, pH, and Cell Count
In a subset of animals, these indices were monitored via placement of a catheter in the femoral artery. Briefly, after being separated from the femoral vein and nerve, the femoral artery was ligated distally. The proximal end was clamped temporarily. A plastic catheter was placed into the proximal femoral artery. A pressure monitor BP-1 (World Precision Instruments, Sarasota, FL, USA) was used to measure the blood pressure throughout the operation. Blood samples were collected from the catheter into a heparinized calibrated pipets (44.7 μL; VWR International, Westchester, PA, USA) to measure blood gases. In addition, 100 μL of blood was used to quantify red and white blood cell counts.
Deferoxamine and Deferasirox Treatment
ALZET microosmotic pumps (Durect Corporation, Cupertino, CA, USA) were used to deliver continuous DFO to the intraperitoneal space. An ALZET pump filled with DFO (DFO mesylate salt; Sigma, St Louis, MO, USA) saline solution was inserted into the peritoneal cavity. After the closure of the muscle and skin layer separately by sutures, the mice were allowed to recover in temperature-controlled incubator (Thermocare). These pumps were used for 1 day up to 4 weeks. In control mice, ALZET pumps were placed and filled with saline.
To treat mice with DFR, 6- to 8-week-old mice were acclimated 4 to 5 days with Bio-Serv 50 mL graduated water bottles filled with 50% apple juice (in water). The DFR was dissolved in the 50% apple juice to encourage consumption. A 125-mg DFR tablet was dissolved into 50% apple juice and fed to the mice at 5 to 20 mg/kg daily dose for 1 day up to 4 weeks. Control mice were fed 50% apple juice and water.
RNA Isolation, cDNA Synthesis, Quantitative Polymerase Chain Reaction, and Microarray
For mice subjected to hypoxia (8% O2 for 5 hours), DFO, or DFR treatment, we collected brain tissue immediately after the hypoxic exposure or after the drug treatment. As for the DFO continuously treated mice, we collected the brain tissue at 5 days, 14 days, and 4 weeks after the pumps insertion. Brain tissue was collected with tissue punches from the cortex that was located in a MCA distribution, an area that is typically involved in the stroke volume after transient MCAO. The brain tissue was collected from DFR-treated mice 14 days after initiating drug treatment. Brain tissue was dounce homogenized, RNA extracted, cDNA synthesized, and quantitative PCR was performed with the same methodology as described in the earlier work (Rempe et al, 2007). Briefly, by using RNA easy columns as per the manufacturer's (Qiagen, Valencia, CA, USA) recommendations, we collected total RNA. All the samples were treated with DNase. The RNA pellet was stored at −80°C until cDNA synthesis. Synthesis of cDNA was performed with SuperScript III (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations. We used 600 to 1000 ng of total RNA and random hexamers for the synthesis of cDNA. Control reactions were performed without reverse transcriptase. An ABI prism 7300 (Applied Biosystems, Foster City, CA, USA) sequence detector real-time PCR thermocycler was used for measuring relative abundance of transcript levels of the genes of interest. All samples were measured in duplicate and standard curves of known concentrations of different genes were used. β-Actin or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as internal controls. The primer and probe sequences for erythropoietin (EPO), vascular endothelial growth factor (VEGF), hexokinase 2 (HK2), glucose transporter 1 (GLUT-1), Nip3, Nix, HIF exon2, and monocarboxylase transporter 4 (MCT4) are listed in Supplementary Table S1 and are the same as those used in our earlier work (Rempe et al, 2007).
For microarray analysis, total RNA was shipped on dry ice to Asuragen (Austin, TX, USA) for quality validation (Agilent Bioanalyzer, Agilent Technologies, Santa Clara, CA, USA), preparation of Cy3-labeled cRNA (Illumina TotalPrep, Ambion/Applied Biosystems, Austin, TX, USA), hybridization of cRNA to Illumina MousRef8 BeadChip arrays, array scanning, and computation of background-subtracted fluorescence intensities and probabilities that probe signals were greater than background. The MouseRef8 array targets ∼25,600 well-annotated RefSeq transcripts and over 19,100 unique genes. Data were quantile normalized with Partek Genomics Suite software and exported to a Microsoft Excel spreadsheet for further analysis (see below). For those targets in which their expression abundance was confirmed by quantitative PCR, the primer and probe sequences of these targets are listed in Supplementary Table S1.
Protein Lysates, Immunoblots, and Enzyme-Linked Immunosorbent Assays
We collected whole-cell lysates from brain tissue. Briefly, 300 μL Radio-Immunoprecipitation Assay (RIPA) buffer with protease inhibitor (Sigma) and Phosstop (Roche, Indianapolis, IN, USA) were added at the recommended concentrations. The brain tissue was homogenized by dounce homogenizer. The tissue was sonicated under 0.5 power for 5 seconds three times (Sonicator 3000, Misonix, QSonica, Newtown, CT, USA). The samples were then centrifuged at 13,000 g for 15 minutes and the supernatant was collected. Protein concentration was determined using a modified Lowry method (Bio-Rad, Hercules, CA, USA). In some cases, 200 μg of protein lysate was used to measure the protein concentration of EPO or VEGF by enzyme-linked immunosorbent assay by following the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA).
To be used for Western blots, protein was diluted into buffer containing sodium dodecyl sulfate and β-mercaptoethanol, electrophoresis was performed using polyacrylamide gels. The protein was transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Temecula, CA, USA) and placed in blocking buffer (5% dry milk, 0.1% BSA, 0.1% Tween, 34 mmol/L NaCl, Tris, pH 7.5) for 1 hour at room temperature. The membranes were subsequently placed in diluted first antibody: anti-Phospho-Akt (Ser473) Antibody (1:1000; Cell Signaling Technologies, Danvers, MA, USA), anti-Akt Antibody (1:1000; Cell Signaling), anti-Phospho-p44/42 mitogen activated protein kinase (MAPK) antibody (1:1000; Cell Signaling), anti-p44/42 MAPK antibody (1:1000; Cell Signaling), anti-cAMP response element-binding (CREB)-1 antibody (1:1000; Upstate Biotechnologies; Millipore, Billerica, MA, USA), or antibody specific for β-actin at 4°C overnight. Horseradish peroxidase-conjugated secondary antibodies in appropriate dilution were used. The immunoblots were developed with chemiluminescence (Perkin-Elmer Life Sciences, Waltham, MA, USA) and exposed to X-Omat film (Eastman Kodak Co., Rochester, NY, USA). For each set of blots, the membranes were stripped and probed with multiple antibodies.
HIF-1α Heterozygous Knockout Mice (HIF-1+/−) and hGFAPcre∷HIF-1F/F Mice
HIF-1α heterozygous knockout mice (HIF-1+/−) were used to evaluate the role of HIF-1α in DFO-induced neuroprotection. The HIF-1+/− mice were derived from floxed HIF-1α mice, which were kindly supplied by Dr Randall Johnson (Ryan et al, 2000). The HIF-1α floxed mice were crossed with Synapsin Cre mice (Zhu et al, 2001). Although the Synapsin Cre mice were designed to be neuronal specific, germline recombination occurs in a certain percentage of the progeny (Rempe et al, 2006). Thus, we used progeny of these mice to accomplish germline recombination. Once recombination of the floxed HIF-1α allele was confirmed by PCR, the mice were breed with C57Bl6 mice to remove Synapsin Cre from their genotype. The selected HIF-1+/−progeny were subsequently bred with C57Bl6 mice for at least 10 generations. When using HIF-1+/− mice, littermate HIF-1+/+ mice were used for the control group to avoid any effects of genetic drift of our mouse colony. Similarly, to achieve loss of HIF-1 function in astrocytes and neurons in the cortex, the HIF-1α floxed mice were crossed with hGFAPcre mice (Zhuo et al, 2001) to form HIF-1F/F∷GFAPcre mice. Littermate controls (HIF-1F/F) were used as the control group.
Statistical Analysis
When comparing stroke volume between groups of mice, each treatment groups consisted of at least five mice, but contained up to 11 mice in some cases. In those treatment groups containing ⩾8 mice, a Shapiro–Wilk normality test was performed (Graphpad 5, La Jolla, CA, USA) to ensure a parametric distribution of the data. Thus, when comparing stroke volume among numerous groups, a one-way analysis of variance was used with Bonferroni corrections for post hoc comparisons. When stroke volume was compared in grouped comparisons, a two-way analysis of variance with Bonferroni corrections was used (see figure legends). All results in the text, tables, and figures are presented as an average±s.d. Statistical analysis of the quantitative PCR data was performed by comparing across groups (n=3 to 6 mice per group) and experimental conditions with either a one-way or two-way analysis of variance with Bonferroni corrections for post hoc comparisons.
After quantile normalization, the microarray data were analyzed in a Microsoft Excel Spreadsheet. As the primary objective of the microarray was to determine whether the treatment evoked the expression of HIF-1 targets, we maximized the sensitivity of detecting changes in target expression by choosing P values of ⩽0.01 (instead of the more stringent P values normally used for microarray studies) and ratios between treatment and control groups of 1.25. Thus, a target only needed to demonstrate an increase of 25% with a P value of 0.01 to be considered significant. The P values were determined by independent t-tests comparing the control samples (n=4 for each group) and the test samples (n=5 for each group). Again, to increase the sensitivity of observing changes in HIF-1 targets, no statistical corrections were used to correct for multiple comparisons.
Results
Low-Dose, Prolonged Administration of Deferoxamine Induces Long-Term Neuroprotection Against Stroke
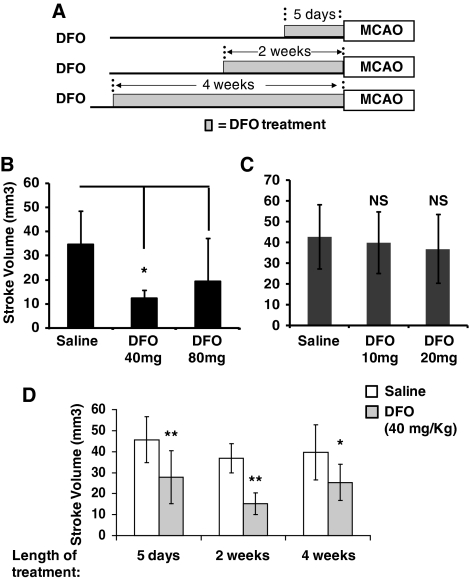
When administered within 6 hours of stroke onset, a 300 mg/kg single injection of DFO decreases stroke volume (Baranova et al, 2007). As DFO is administered to patients over an extended time course, we examined if prolonged treatment with DFO protects against stroke. To avoid possible toxicity with repeated administration of a high dosage of DFO, we first examined if 2 weeks of continuous infusion of DFO at 40 mg/kg per day or 80 mg/kg per day by osmotic pumps (ALZET) provides neuroprotection. Transient MCAO was performed in mice after treatment with prolonged DFO or saline (control) (Figure 1A) and the implanted pumps remained in place during and after MCAO. Two weeks of DFO (40 mg/kg per day) significantly reduced stroke volume, whereas 80 mg/kg per day of DFO had a smaller, nonsignificant effect (Figure 1B). Administration of lower doses of DFO (10 and 20 mg/kg per day) to mice for 2 weeks did not reduce stroke volume (Figure 1C). To examine whether treatment with 40 mg/kg per day of DFO also reduces stroke damage when administered for shorter or longer time periods, MCAO was performed in mice treated with DFO for 5 days and 4 weeks. Similar to the 2-week time point, treatment with DFO significantly diminished stroke volumes when administered for 5 days or 4 weeks (Figure 1D). These results suggest that DFO can provide neuroprotection against stroke when delivered continuously over an extended time frame. As DFO chelates irons, it has the potential to produce anemia and other toxic effects. These changes could influence stroke outcome. Thus, we measured the influence of DFO on blood oxygen saturation, blood pressure, pH, white blood cell count, and hemoglobin in a subset of mice. Four weeks of delivery of DFO did not influence these physiologic indices (Table 1).
Figure 1.
Low-dose, prolonged treatment with deferoxamine (DFO) reduces stroke volume. (A) A schematic diagram of the experimental protocols used demonstrating the different time periods of DFO treatment. (B) Two weeks of treatment with 40 mg/kg per day of DFO significantly reduced stroke volume, whereas the more modest reduction in stroke volume with 80 mg/kg per day was not significant (one-way analysis of variance (ANOVA) with Bonferroni's multiple comparison test, n=7 to 8 mice in each group). (C) Two weeks of treatment with 10 to 20 mg/kg per day of DFO did not reduced stroke volume (one-way ANOVA with Bonferroni's multiple comparison test, n=6 to 9 mice in each group). (D) Treatment with 40 mg/kg per day of DFO for 5 days, 2 weeks, or 4 weeks all significantly reduces stroke volume compared with saline controls (two-way ANOVA with Bonferroni's posttest analysis, n=5 to 9 mice in each group). For clarity of the comparisons, the same data for the 2-week time point that is plotted in (B) is also plotted in (C). (The error bars=s.d. in all figures; *P<0.05, **P<0.01, and ***P<0.001 in all figures).
Table 1. Physiological parameters of mice treated with DFO or saline (control).
| pH | pCO2 (mm Hg) | pO2 (mm Hg) | HCO3 (mmol/L) | WBC | HGB | BP (mm Hg) | |
|---|---|---|---|---|---|---|---|
| Saline | 7.3±0.01 | 40.6±5.1 | 152.3±10.6 | 19.4±1 | 7.1±1.1 | 15.4±1.4 | 105±5.96 |
| DFO | 7.29±0.01 | 42.5±5.2 | 158.2±8.3 | 19.5±2 | 5.6±0.3 | 15.9±0.5 | 108±10.5 |
BP, blood pressure; DFO, deferoxamine; HGB, hemoglobin; WBC, white blood cell count.
Low-Dose, Prolonged Deferoxamine Administration Does Not Induce Hypoxia-Inducible Factor Targets in the Brain
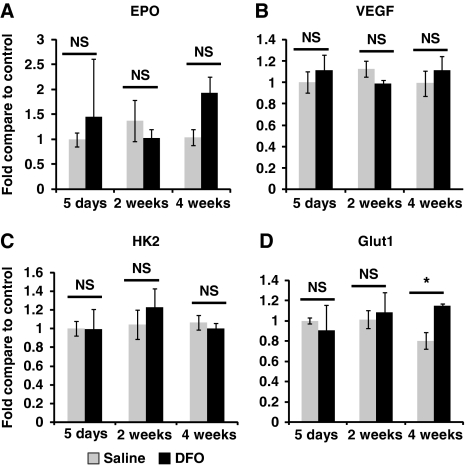
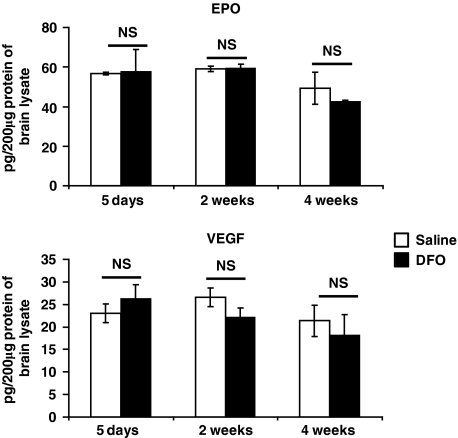
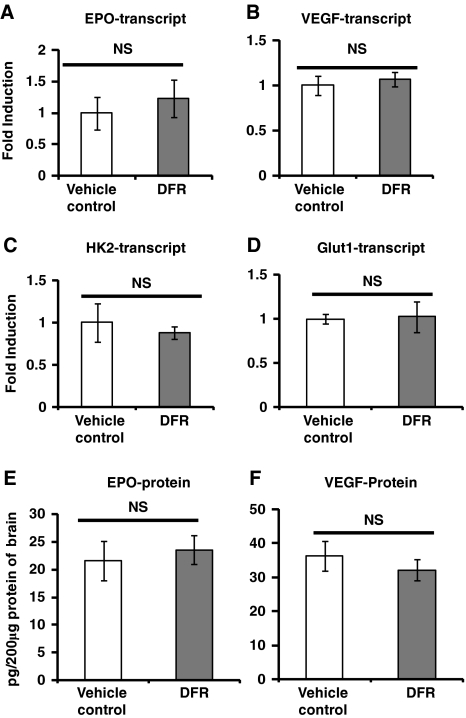
High-dose DFO induces HIF-1 protein abundance and HIF-1 target expression. Moreover, the protection mediated by DFO in acute stroke is diminished by selective loss of HIF-1 function in neurons (Baranova et al, 2007). Thus, we examined if HIF-1 targets are increased by chronic DFO treatment. Deferoxamine (40 mg/kg per day) was administered by osmotic pumps continuously for 5 days, 2 weeks, or 4 weeks. Deferoxamine administration for these time periods did not consistently induce the transcript abundance of the known HIF-1 targets VEGF, Glut-1, HK2 (Figure 2), or Nip3 (data not shown). In the case of Glut-1, there was a modest increase in their expression after 1 month of DFO treatment. Yet, no increases were observed at 5 days and 2 weeks of DFO administration, despite the fact that DFO reduced stroke volumes at these time points. In addition, EPO transcript abundance, which is primarily induced by the transcription factor HIF-2 (not HIF-1) (Chavez et al, 2006; Weidemann et al, 2009), was nonsignificantly increased after 4 weeks of DFO administration, but this trend was not observed at the other time points. As the neuroprotectants EPO and VEGF are targets of HIF-2 and HIF-1, respectively (Malhotra et al, 2006; Prass et al, 2003), we examined the protein abundance of EPO and VEGF in the brain using enzyme-linked immunosorbent assay. Prolonged low-dose DFO treatment did not change either EPO or VEGF protein abundance (Figure 3).
Figure 2.
Low-dose, prolonged treatment with deferoxamine (DFO) does not induce hypoxia-inducible factor (HIF) targets. (A–D) The HIF targets erythropoietin (EPO), vascular endothelial growth factor (VEGF), hexokinase 2 (HK2), and glucose transporter 1 (Glut-1) were not consistently induced by DFO (40 mg/kg per day) treatment for 5 days, 2 weeks, or 4 weeks (two-way analysis of variance (ANOVA) with Bonferroni's posttest analysis, n=6 mice in each group).
Figure 3.
Low-dose, prolonged treatment with deferoxamine (DFO) does not induce erythropoietin (EPO) or vascular endothelial growth factor (VEGF) protein abundance in brain as measured by enzyme-linked immunosorbent assay (ELISA) (two-way analysis of variance (ANOVA) with Bonferroni's posttest analysis, n=3 mice in each group).
Haploinsufficiency of Hypoxia-Inducible Factor 1 Function Does Not Reduce the Deferoxamine-Mediated Neuroprotection
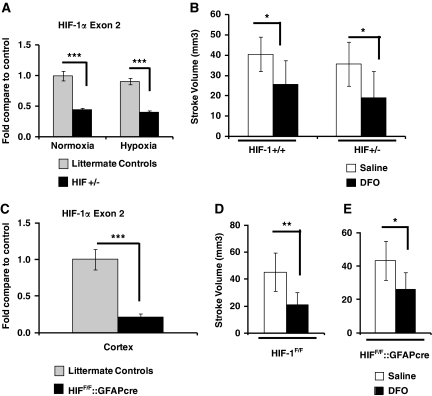
Loss of HIF-1 function selectively in neurons blocks the neuroprotection afforded by a single treatment with DFO (300 mg/kg) (Baranova et al, 2007). Similarly, the cardiac protection afforded by ischemic preconditioning is completely lost in mice with HIF-1 haploinsufficiency (HIF-1+/−) (Cai et al, 2008). We used HIF-1+/− mice, or littermate controls (HIF-1+/+), to address if haploinsufficiency of HIF-1 reduces DFO-mediated neuroprotection. First, to ensure that the function of HIF-1 is reduced in the brain of HIF-1+/− mice, we examined the transcript abundance of HIF-1α and its targets in mice. As expected, transcript abundance of HIF-1α exon2 was reduced by ∼50% in HIF-1+/− mice (Figure 4A). Exposure of littermate controls to 8% O2 for 5 hours, which are the conditions that induce protection with hypoxia preconditioning, induced the transcript abundance of several HIF-1 and HIF-2 targets including EPO, VEGF, Glut-1, HK2, and MCT4 (Supplementary Figure S1). Not surprisingly, hypoxia-induced expression of EPO, which is mainly regulated by HIF-2α (Chavez et al, 2006; Weidemann et al, 2009), was not reduced in HIF-1+/− mice. In contrast, the hypoxia-induced expression of known HIF-1 targets, such as Glut-1, HK2, and MCT4, were all significantly attenuated in HIF-1+/− mice. In fact, in the case of HK2 and MCT4, partial loss of HIF-1 function largely abrogated the hypoxia-induced increase in transcript abundance (Supplementary Figure S1). Thus, the hypoxic regulation of several HIF-1 targets was reduced in HIF-1+/− mice.
Figure 4.
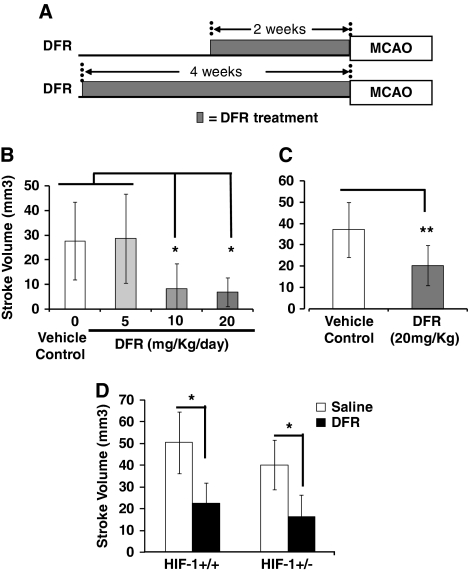
Deferoxamine (DFO) remains protective when hypoxia-inducible factor 1 (HIF-1) function is reduced in all tissue (HIF-1+/− mice) or lost selectively in neurons and astrocytes (HIF-1F/F∷hGFAPcre mice). (A) As expected, the abundance of HIF-1 transcript (exon2) is significantly reduced to ∼50% of control levels in brain cortex of HIF-1+/− mice (two-way analysis of variance (ANOVA) with Bonferroni's posttest analysis, n=4 mice in each group). (B) Despite the loss of HIF-1 transcript, low-dose, prolonged DFO (40 mg/kg per day for 2 weeks) significantly reduces stroke volume in HIF-1+/− mice and their littermate controls (two-way ANOVA with Bonferroni's posttest analysis, n=8 to 11 mice in each group). (C) HIF-1 transcript in the cortex of HIF-1F/F∷hGFAPcre mice is reduced by 79% compared with sibling controls (HIF-1F/F mice) (independent t-tests, n=10 to 14 mice in each group). (D) DFO significantly reduces stroke volume in HIF-1F/F mice (retain HIF-1 function) (independent t-tests, n=5 to 7 mice in each group). (E) DFO significantly reduces stroke volume with loss of HIF-1 function in neurons/astrocytes in HIF-1F/F∷hGFAPcre mice (independent t-tests, n=5 to 7 mice in each group).
As hypoxia-induced HIF-1 targets were reduced in HIF-1+/− mice, we used these mice to determine whether partial loss of HIF-1 function reduced neuroprotection mediated by prolonged DFO administration. Similar to the results reported above (Figure 1), 2 weeks of DFO treatment reduced stroke volume in HIF-1+/− and wild-type littermate controls (HIF-1+/+) (Figure 4B). Deferoxamine reduced the stroke volume of HIF-1+/+ and HIF-1+/− mice by 36% and 46%, respectively. Thus, there was no suggestion of partial loss of protection in the HIF-1+/− mice.
Selective Loss of Hypoxia-Inducible Factor 1 Function in Neurons and Astrocytes Does Not Reduce Deferoxamine-Mediated Neuroprotection
To further delineate HIF-1's potential role in mediating the neuroprotective properties of chronic DFO administration, we determined whether loss of HIF-1 function in neurons and astrocytes reduced the protection mediated by DFO. To conditionally knockout HIF-1 function in neurons and astrocytes, we used HIF-1F/F transgenic mice (Ryan et al, 2000), which contain a floxed HIF-1 transgene. These HIF-1F/F mice were breed with mice, which express cre recombinase in both astrocytes and neurons (Zhuo et al, 2001). To control for the effective recombination of floxed allele, we measure the HIF-1 transcript abundance in cortical punch biopsies (opposite hemisphere) in HIF-1F/F∷hGFAPcre mice and sibling controls when the brain tissue was collected for quantifying stroke volume. Hypoxia-inducible factor 1 transcript abundance was significantly reduced by 79% in HIF-1F/F∷hGFAPcre mice compared with HIF-1F/F sibling controls (Figure 4C). Despite this loss of HIF-1 transcript, DFO (40 mg/kg per day for 2 weeks) still significantly reduced stroke volume (Figure 4D). These data demonstrate that chronic low-dose DFO mediates neuroprotection independent of HIF-1 function.
Prolonged Administration of DFO Does Not Induce Phosphorylation of Akt, Extracellular Signal-Regulated Kinases (ERK), or CREB in the Brain or EPO Production in the Periphery
The data presented above strongly suggest that HIF-1-independent molecular mechanisms mediate the protection induced by prolonged low-dose DFO administration. Akt phosphorylation is critical in hypoxia-induced neuroprotection against OGD injury in vitro and is involved in the ischemic postconditioning against stroke in vivo. Yet, when examining the phosphorylation of Akt using a phosphorylation-specific antibody directed to Ser473, Akt phosphorylation was not altered by low-dose, prolonged DFO treatment. ERK is an important component of MAPK pathway and is activated by reactive oxygen species, which may be induced by DFO. Yet, phosphorylation of ERK was also not altered by DFO treatment (Supplementary Figure S2). Finally, iron chelation may increase cell viability by inducing CREB (Zaman et al, 1999). Yet, again no change in CREB abundance was observed in the DFO-treated mice (Supplementary Figure S2). As DFO has poor penetration of the blood–brain barrier into the brain, we also determined whether its protective effects could be mediated by inducing EPO in the periphery. Yet, DFO treatment did not alter the abundance of EPO protein in the kidney, liver, and serum (Supplementary Figure S3).
Prolonged Administration of Iron Chelator Deferasirox also Reduces Stroke Volume, Which Is Not Attenuated in HIF-1+/− Mice
Although DFO is neuroprotective, when used clinically, it must be administered subcutaneously and has several side effects limiting its clinical utility as a putative agent for prophylactic neuroprotection in patients. In contrast, DFR is an iron chelator that is administered orally and well tolerated (Cappellini, 2008). As such, we examined if DFR is neuroprotective when administered as a prophylactic agent before stroke. Mice were treated for 2 or 4 weeks with DFR and then underwent transient MCAO (Figure 5). Similar to that observed for DFO, treatment with DFR for 2 weeks significantly reduced stroke volume. When DFR was administered at 10 or 20 mg/kg per day, stroke volume was diminished (Figure 5B). In contrast, 5 mg/kg per day of DFR had no effect on stroke volume. Similarly, DFR (20 mg/kg per day) reduced stroke volume when administered for 1 month before MCAO (Figure 5C). To determine whether partial loss of HIF-1 function reduced the protection mediated by DFR, we also examined whether DFR protected HIF-1+/− mice against stroke. Similar to DFO, DFR-mediated protection was not diminished in HIF-1+/− mice compared with sibling controls (HIF-1+/+) (Figure 5D).
Figure 5.
Low-dose, prolonged administration of deferasirox (DFR) reduces stroke volume. (A) Schematic diagram of experimental paradigm. (B) DFR administered for 2 weeks reduces stroke volume when administered at 10 or 20 mg/kg per day, but not at 5 mg/kg per day (one-way analysis of variance (ANOVA) with Bonferroni's posttest analysis, n=6 to 10 mice in each group). (C) DFR (20 mg/kg per day) remains effective at reducing stroke volume when administered for 4 weeks (independent t-tests, n=9 to 12 mice in each group). (D) DFR (20 mg/kg per day for 2 weeks) significantly reduces stroke volume in HIF-1+/+ (sibling controls) and HIF-1+/− mice (two-way ANOVA with Bonferroni's posttest analysis, n=6 to 8 mice in each group). HIF-1, hypoxia-inducible factor 1.
Administration of Deferasirox Does Not Induce Transcript Abundance of Hypoxia-Inducible Factor 1 Targets
Multiple iron chelators, which act as PHIs inducing HIF-1 targets, reduce stroke volume (Baranova et al, 2007; Siddiq et al, 2005). Thus, although low-dose, prolonged DFO administration did not induce HIF-1 targets, we examined if prolonged administration of DFR for 2 weeks may induce HIF-1 targets. Similar to DFO, DFR does not significantly induce the transcript abundance of the HIF-1 targets HK2, Glut-1, or VEGF (Figure 6). Although DFR treatment increased the transcript abundance of EPO by 25%, this did not reach statistical significance (Figure 6A). Similar to DFO, DFR treatment did not induce EPO or VEGF protein abundance in the brain (Figures 6E and 6F).
Figure 6.
Low-dose, prolonged treatment with deferasirox (DFR) does not induce hypoxia-inducible factor (HIF) target transcripts in brain or erythropoietin (EPO)/vascular endothelial growth factor (VEGF) protein in brain. (A–D) The HIF targets EPO, VEGF, hexokinase 2 (HK2), and glucose transporter 1 (Glut-1) were not induced by treatment with 20 mg/kg per day of DFR for 2 weeks (independent t-tests, n=4 to 5 mice in each group). (E, F) DFR does not induce EPO or VEGF protein in brain (independent t-tests, n=6 mice in each group).
As several feedback mechanisms could reduce HIF-1 signaling if chronically activated, we also examined HIF-1 target expression 24 hours after initiating treatment with DFO or DFR. Neither DFO nor DFR induced HIF-1 targets at 24 hours after initiating treatment (Supplementary Figure S4). Finally, as DFR has poor penetration of the blood–brain barrier, but readily distributes to other organs such as the kidney and liver (Bruin et al, 2008), we examined if DFR may induce the expression of EPO protein in these organs and in serum. Yet, similar to DFO, DFR treatment for 2 weeks did not induce EPO protein in the kidney, liver, or serum (Supplementary Figure S5).
Prolonged Administration of Deferasirox Does Not Induce Phosphorylation of Akt, ERK, CREB, or Peripheral Erythropoietin
It is possible that DFR could induce a number of second messenger pathways that could increase viability of ischemic tissue. Yet, similar to DFO treatment, Akt phosphorylation, ERK phosphorylation, and CREB abundance was not altered by low-dose, prolonged DFR treatment (Supplementary Figure S6).
Hypoxia-Inducible Factor 1 Targets Are Not Induced in Brain Cortex on Microarray Analysis of Deferoxamine and Deferasirox-Treated Mice
As detailed above, using quantitative PCR of select HIF-1 targets, neither DFO nor DFR increase expression of HIF-1 targets. Yet, HIF-1 may enhance expression of several dozen transcripts. As such, DFO and DFR could alter a subset of these targets, which may be of particular importance in mediating neuronal protection. These targets could easily be missed if only a few HIF-1 targets were examined by qPCR. Thus, we explored changes in the transcript profiles in the brain cortex in mice treated with vehicle controls, DFR, or DFO. In the case of DFO, samples from the brain cortex of four control mice (saline delivered by ALZET microosmotic pump) and five DFO-treated mice were examined. Similarly, in the case of DFR-treated mice, samples from brain cortex of four control mice (mice consumed juice/water) and five DFR-treated mice were examined. After the data were quantile normalized, potential DFO or DFR-regulated targets were identified as those that were significantly different between control group and treatment group (independent t-test with P<0.01) and had a ratio of treatment to control of >1.25. This low ratio and less stringent P values were chosen to increase sensitivity for identifying HIF-1 targets regulated by DFO or DFR. Using these criteria, 59 and 37 transcripts were increased by DFO or DFR treatment, respectively (Supplementary Tables S2 and S3). None of these transcripts were identified as HIF-1 targets as based on prior microarrays studies (Greijer et al, 2005; Vengellur et al, 2003). Hypoxia-inducible factor 1 may also negatively regulate the expression of transcripts (Greijer et al, 2005). Potential targets that were negatively regulated by DFO and DFR were identified as those that were significantly different between control group and treatment group (independent t-test with P<0.01) and had a ratio of treatment to control of <0.75. Using these criteria, 68 and 36 transcripts were diminished by DFO or DFR treatment, respectively (Supplementary Tables S4 and S5). Yet, none of these targets were previously identified as being negatively regulated by HIF-1 (Greijer et al, 2005).
Multiple signaling pathways beyond HIF-1 and HIF-2 could contribute to the neuroprotection mediated by DFO and DFR. As such, we examined which targets were induced by DFO or DFR on the microarray analysis. In both DFO and DFR-treated mice, brain transcripts were only changed modestly. In the case of DFO, only two targets on the microarray were induced by twofold and only six targets increased by ⩾50% (Supplementary Table S2). Similarly, DFR induced only one target by twofold, and only four targets by ⩾50% (Supplementary Table S3). Given that DFO and DFR are both iron chelators, it might be expected that these two treatments would induce similar transcript profiles in the brain. Yet, there were no common targets in DFO and DFR-treated mice that met the criteria of being induced by 1.25-fold and were significantly different from controls (P<0.01). Similarly, there were no common targets with transcript expression that were diminished to <75% of their baseline values by both DFO and DFR (Supplementary Tables S4 and S5).
Quantitative PCR was used to examine the expression of 11 targets that microarray analysis predicted to be induced by at least 25% in the brains of DFO (six targets) or DFR (five targets)-treated mice (Supplementary Figure S7). While in general, these targets were modestly induced by the respective treatment, in most cases, the induction of the transcript was not statistically significant. In the case of DFO-treated mice, the transcript of protein kinase C and casein kinase substrate in neurons 3 was induced 50%, TRAF-type zinc-finger domain containing 1 was induced 30%, fibroblast growth factor 5 was induced 22%, and interleukin receptor 17D was induced only 15%. None of these changes were statistically significant (n=4 control and n=5 treatment). The abundance of Cobl-like 1 and β-actin were not changed by DFO treatment (data not shown) despite the fact that they were identified on the microarray as potential targets. In the case of DFR-treated mice, quantitative PCR demonstrated that early growth response 2 was induced almost twofold as predicted by the microarray. Yet, this difference did not reach significance (P<0.07; independent t-test). Deferasirox treatment induced dual specificity phosphatase 6 by 42%, which was significantly different than controls (P<0.01; independent t-test). Early growth response 4 was induced by 25% (not significant), whereas sh2 domain containing 3C and Glis1 (GLIS family zinc-finger 1) were not altered (data not shown).
Deferoxamine and Deferasirox Protect Mice From Stroke Within 24 hours of Treatment
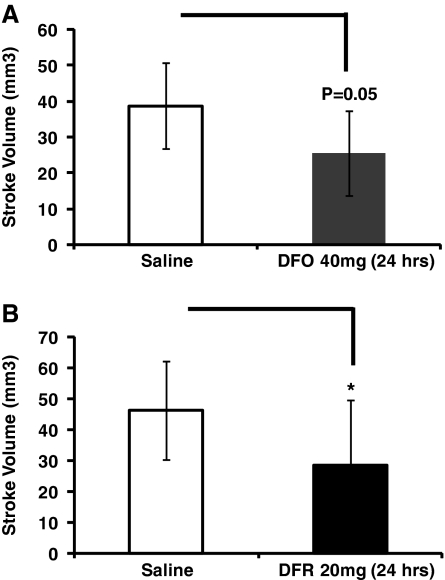
While a treatment with a neuroprotectant following TIA or minor stroke must maintain its efficacy over several weeks, recurrent stroke risk is greatest within the first few days following the sentinel event. As such, it is equally important that the neuroprotectant is effective early after beginning administration. To determine whether low-dose DFO and DFR are protective early after the onset of administration, we examined stroke volume at 24 hours after beginning treatment. Although somewhat less effective compared with longer time frames, stroke volume is reduced by both DFO and DFR within 24 hours of administration (Figure 7).
Figure 7.
Low-dose treatment with deferoxamine (DFO) or deferasirox (DFR) for 24 hours reduces stroke volumes. (A) Treatment with DFO (40 mg/kg) for 24 hours reduces stroke volume compared with saline controls (independent t-tests, n=7 to 8 mice in each group). (B) Treatment with DFR (20 mg/kg) for 24 hours significantly reduces stroke volume compared with mice control mice (independent t-tests, n=10 to 11 mice in each group).
Discussion
Acute stroke interventions have captivated the preponderance of research efforts in stroke. Yet, unfortunately, multiple neuroprotectants have failed to reduce stroke-induced disability (Labiche and Grotta, 2004). This failure is partially attributable to the inability to deliver sufficient quantities of neuroprotectants to minimally perfused brain tissue within a short time frame. Not surprisingly, delivery of neuroprotectants before, rather than after, stroke onset offers more protection in animal models (Cheng et al, 2004). While in most cases, preemptive delivery of neuroprotectants is impossible, it is potentially applicable to patients at high risk of stroke. For example, the risk of stroke within 90 days after TIA is 9% to 14% (Hill et al, 2004; Johnston et al, 2007; Kleindorfer et al, 2005), but it can be as high as 25% in some subgroups. Most of the risk of stroke following TIA is within the first 30 days (Johnston et al, 2007). Thus, if a prophylactic neuroprotection could be established for a period of several weeks after minor stroke or TIA, tens of thousands of patients may benefit yearly (Fisher et al, 1994; Savitz and Fisher, 2007).
Prolyl hydroxylase inhibitors induce HIF-1 and reduce stroke volume when administered acutely after stroke onset (Baranova et al, 2007; Nagel et al, 2010; Ratan et al, 2004; Siddiq et al, 2005). Recent work has implicated HIF-1 as mediating the protection afforded by DFO administration to mice in the peristroke period (Baranova et al, 2007). This study reported that after administration of a single dose of 300 mg/kg of DFO to mice induces HIF-1 targets in the brain including Glut-1 and VEGF (Baranova et al, 2007). This DFO-mediated induction of these targets was attenuated by loss of HIF-1 function in neurons. Similarly, loss of HIF-1 function in neurons also eliminated the DFO-induced neuroprotection, demonstrating the importance of neuronal HIF-1 for this DFO-induced protection. Another PHI, dimethyloxalylglycine (DMOG), also reduces stroke volume in rats subject to either transient MCAO or permanent MCAO (Nagel et al, 2010). In this study, DMOG induced HIF-1 protein, VEGF, and endothelial nitric oxide synthase abundance. DMOG-mediated protection was associated with enhanced blood flow to the ischemic hemisphere after permanent MCAO. The levels of HIF-1 protein were only partially correlated with DMOG neuroprotection. Thus, the role of HIF-1 in DMOG-mediated protection remains unclear.
We are not aware of other studies that administered DFO over prolonged time frames and examined neuroprotection. Yet, prolonged (2 weeks), intermittent administration of DFO was used to protect the retina against ischemia. Deferoxamine (200 mg/kg) was delivered as six doses over 2 weeks before onset of retinal ischemia (Zhu et al, 2008). Hypoxia-inducible factor 1 targets were induced in the retina by this experimental paradigm for DFO administration, suggesting that HIF-1 likely contributes to the retinal protection. In contrast to these results achieved in the retina and brain with high-dose DFO (200 to 300 mg/kg), chronic low-dose DFO administration does not induce the transcript abundance of HIF targets. Similarly, the protein abundance of the HIF targets EPO and VEGF is not altered. Furthermore, DFO remains protective in HIF-1+/− and mice with loss of HIF-1 function in neurons and astrocytes. When considering these divergent results, it is likely that DFO mediates protection of the brain or retina through multiple mechanisms depending on the dose and the time course of administration.
While high-dose DFO is thought to protect against stroke through the actions of HIF-1 in neurons (Baranova et al, 2007), recent in vitro studies suggest that DFO also protects neurons through mechanisms independent of HIF-1 or HIF-2 function (Siddiq et al, 2009). Instead, siRNA directed against prolyl 4-hydroxylase isoform 1 diminished neuronal death induced by oxidative stress through HIF-independent mechanisms (Siddiq et al, 2009). In addition, structurally diverse PHIs (including DFO) protect neurons in an in vitro model of Huntington's disease through a mechanism independent of HIF-1 function (Niatsetskaya et al, 2010). Yet, the molecular processes mediating this protective phenotype are unknown. As these neuroprotective mechanisms are better delineated, it will be interesting to determine whether similar molecular mechanisms contribute to low-dose chronic DFO or DFR-induced neuroprotection in vivo.
Similar to DFR and DFO, other medications, such as angiotensin receptor blockers, statins, and calcium channel blockers reduce stroke volume in animal models when administered for 2 to 4 weeks before stroke (Amin-Hanjani et al, 2001; Ito et al, 2002; Lukic-Panin et al, 2007). A recent clinical trial (PRoFESS trial) examined if chronic administration of an angiotensin receptor blocker to stroke patients provides neuroprotection with recurrent stroke (Diener et al, 2008). Unfortunately, angiotensin receptor blockers did not reduce disability with recurrent stroke. This could be related to the large percentage of small vessel strokes that occurred in these patients. It is unclear if angiotensin receptor blockers protect in this stroke subtype, as animal models of small vessel strokes are not widely used.
To our knowledge, this is the first study to examine the influence of DFR on stroke volume. Deferasirox is already in clinical use for patients with iron overload from repeated blood transfusions for blood dyscrasias. Deferasirox has advantages compared with DFO in that it is administered orally and better tolerated (Cappellini, 2008). Deferasirox is commonly administered to patients for several months demonstrating a good safety profile over prolonged time periods. As such, DFR has the potential to be used in patients at high risk of recurrent stroke to reduce ischemic damage of recurrent stroke. Moreover, there is a great need to identify agents to provide protection against brain ischemia in patients at high risk of stroke.
Acknowledgments
The authors thank Dr Randall Johnson for generously providing the floxed HIF-1α mice and Dr Albee Messing for supplying the hGFAPcre mice. The authors thank Landa Prifti and Emily Terho for their excellent technical assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported in part by 1R01NS054192 (NINDS) and 1P01NS050315 (NINDS).
Supplementary Material
References
- Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Moskowitz MA. Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke. 2001;32:980–986. doi: 10.1161/01.str.32.4.980. [DOI] [PubMed] [Google Scholar]
- Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin GJ, Faller T, Wiegand H, Schweitzer A, Nick H, Schneider J, Boernsen KO, Waldmeier F. Pharmacokinetics, distribution, metabolism, and excretion of deferasirox and its iron complex in rats. Drug Metab Dispos. 2008;36:2523–2538. doi: 10.1124/dmd.108.022962. [DOI] [PubMed] [Google Scholar]
- Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res. 2008;77:463–470. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- Cappellini MD. Long-term efficacy and safety of deferasirox. Blood Rev. 2008;22 (Suppl 2:S35–S41. doi: 10.1016/S0268-960X(08)70007-9. [DOI] [PubMed] [Google Scholar]
- Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlof B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7:875–884. doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Jonas S, Sacco RL, Jones S. Prophylactic neuroprotection for cerebral ischemia. Stroke. 1994;25:1075–1080. doi: 10.1161/01.str.25.5.1075. [DOI] [PubMed] [Google Scholar]
- Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza GL, Meijer GA, van de Wiel MA, Belien JA, van Diest PJ, van der Wall E. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62:2015–2020. doi: 10.1212/01.wnl.0000129482.70315.2f. [DOI] [PubMed] [Google Scholar]
- Ito T, Yamakawa H, Bregonzio C, Terron JA, Falcon-Neri A, Saavedra JM. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke. 2002;33:2297–2303. doi: 10.1161/01.str.0000027274.03779.f3. [DOI] [PubMed] [Google Scholar]
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, Sidney S. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, Schneider A, Alwell K, Jauch E, Miller R, Moomaw C, Shukla R, Broderick JP. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720–723. doi: 10.1161/01.STR.0000158917.59233.b7. [DOI] [PubMed] [Google Scholar]
- Labiche LA, Grotta JC. Clinical trials for cytoprotection in stroke. NeuroRx. 2004;1:46–70. doi: 10.1602/neurorx.1.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.str.24.1.117. [DOI] [PubMed] [Google Scholar]
- Lukic-Panin V, Kamiya T, Zhang H, Hayashi T, Tsuchiya A, Sehara Y, Deguchi K, Yamashita T, Abe K. Prevention of neuronal damage by calcium channel blockers with antioxidative effects after transient focal ischemia in rats. Brain Res. 2007;1176:143–150. doi: 10.1016/j.brainres.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Savitz SI, Ocava L, Rosenbaum DM. Ischemic preconditioning is mediated by erythropoietin through PI-3 kinase signaling in an animal model of transient ischemic attack. J Neurosci Res. 2006;83:19–27. doi: 10.1002/jnr.20705. [DOI] [PubMed] [Google Scholar]
- Nagel S, Papadakis M, Chen R, Hoyte LC, Brooks KJ, Gallichan D, Sibson NR, Pugh C, Buchan AM. Neuroprotection by dimethyloxalylglycine following permanent and transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2010. pp. 1–12. [DOI] [PMC free article] [PubMed]
- Niatsetskaya Z, Basso M, Speer RE, McConoughey SJ, Coppola G, Ma TC, Ratan RR. HIF prolyl hydroxylase inhibitors prevent neuronal death induced by mitochondrial toxins: therapeutic implications for Huntington's disease and Alzheimer's disease. Antioxid Redox Signal. 2010;12:435–443. doi: 10.1089/ars.2009.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- Ratan RR, Siddiq A, Aminova L, Lange PS, Langley B, Ayoub I, Gensert J, Chavez J. Translation of ischemic preconditioning to the patient: prolyl hydroxylase inhibition and hypoxia inducible factor-1 as novel targets for stroke therapy. Stroke. 2004;35:2687–2689. doi: 10.1161/01.STR.0000143216.85349.9e. [DOI] [PubMed] [Google Scholar]
- Rempe D, Vangeison G, Hamilton J, Li Y, Jepson M, Federoff HJ. Synapsin I Cre transgene expression in male mice produces germline recombination in progeny. Genesis. 2006;44:44–49. doi: 10.1002/gene.20183. [DOI] [PubMed] [Google Scholar]
- Rempe DA, Lelli KM, Vangeison G, Johnson RS, Federoff HJ. In cultured astrocytes, p53 and MDM2 do not alter hypoxia-inducible factor-1\{alpha\} functi. J Biol Chem. 2007;282:16187–16201. doi: 10.1074/jbc.M702203200. [DOI] [PubMed] [Google Scholar]
- Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- Savitz SI, Fisher M. Prophylactic neuroprotection. Curr Drug Targets. 2007;8:846–849. doi: 10.2174/138945007781077382. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Mitochondrial autophagy: life and breath of the cell. Autophagy. 2008;4:534–536. doi: 10.4161/auto.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq A, Aminova LR, Troy CM, Suh K, Messer Z, Semenza GL, Ratan RR. Selective inhibition of hypoxia-inducible factor (HIF) prolyl-hydroxylase 1 mediates neuroprotection against normoxic oxidative death via HIF- and CREB-independent pathways. J Neurosci. 2009;29:8828–8838. doi: 10.1523/JNEUROSCI.1779-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq A, Ayoub IA, Chavez JC, Aminova L, Shah S, LaManna JC, Patton SM, Connor JR, Cherny RA, Volitakis I, Bush AI, Langsetmo I, Seeley T, Gunzler V, Ratan RR. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology. 2000;55:490–497. doi: 10.1212/wnl.55.4.490. [DOI] [PubMed] [Google Scholar]
- Vengellur A, Woods BG, Ryan HE, Johnson RS, LaPres JJ. Gene expression profiling of the hypoxia signaling pathway in hypoxia-inducible factor 1alpha null mouse embryonic fibroblasts. Gene Expr. 2003;11:181–197. doi: 10.3727/000000003108749062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, Kerdiles YM, Knaup KX, Rafie CA, Boutin AT, Stockmann C, Takeda N, Scadeng M, Shih AY, Haase VH, Simon MC, Kleinfeld D, Johnson RS. The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest. 2009;119:3373–3383. doi: 10.1172/JCI39378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman K, Ryu H, Hall D, O'Donovan K, Lin K-I, Miller MP, Marquis JC, Baraban JM, Semenza GL, Ratan RR. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21Waf1/cip1, and erythropoietin. J Neurosci. 1999;19:9821–9830. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Gidday JM. Deferroxamine preconditioning promotes long-lasting retinal ischemic tolerance. J Ocul Pharmacol Ther. 2008;24:527–535. doi: 10.1089/jop.2008.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.