Abstract
Experimental studies indicate that the 5-HT4 receptor activation influence cognitive function, affective symptoms, and the development of Alzheimer's disease (AD). The prevalence of AD increases with aging, and women have a higher predisposition to both AD and affective disorders than men. This study aimed to investigate sex and age effects on 5-HT4 receptor-binding potentials in striatum, the limbic system, and neocortex. Positron-emission tomographic scans were conducted using the radioligand [11C]SB207145 in a cohort of 30 healthy subjects (mean age 44 years; range 20 to 86 years; 14 men and 16 women). The output parameter, BPND, was modeled using the simplified reference tissue model, and partial volume correction was performed with the Muller–Gartner method. A decline with age of 1% per decade was found only in striatum. Women had a 13% lower 5-HT4 receptor binding in the limbic system. The lower limbic 5-HT4 receptor binding in women supports a role for 5-HT4 receptors in the sex-specific differences in emotional control and might contribute to the higher prevalence of affective diseases and AD in women. The relatively stable 5-HT4 receptor binding with aging contrasts others in subtypes of receptors, which generally decrease with aging.
Keywords: aging, gender, imaging, partial volume, receptor, serotonin
Introduction
Serotonergic neurotransmission is involved in the modulation of sleep, mood, aggression, neuroticism, sexual activity, and impulsivity, which may differ between genders and change with aging. In addition, depression (reviewed by Meyer, 2007) and anxiety (reviewed by Nutt, 2005) have been linked to serotonergic disturbances. The lifetime prevalence for both mood and anxiety disorders is nearly 14% in the western population, and the prevalence is twice as high in women compared with men (Alonso et al, 2004). Alzheimer's disease (AD) has also been linked to serotonergic disturbances (Salmon, 2007), and women have higher prevalence of the disease that causes severe personal, social, and economic burdens to societies worldwide.
Lowering the serotonin level by acute tryptophan depletion has larger memory-impairing effects in women (Sambeth et al, 2007), but in vivo positron-emission tomographic (PET) studies of sex differences of markers of the serotonin system have shown diverging results: lower 5-HT2A receptor binding in women was initially reported (Biver et al, 1996), but was not confirmed in larger samples (Adams et al, 2004; Frokjaer et al, 2009). Higher 5-HT1A receptor binding has been described in women in some (Costes et al, 2005; Jovanovic et al, 2008) but not all studies (Cidis Meltzer et al, 2001; Stein et al, 2008). Cerebral serotonin-transporter binding has not been consistently shown to depend on sex (Jovanovic et al, 2008; Kalbitzer et al, 2009; Meyer et al, 2004).
PET studies have primarily shown a decline or unchanged levels of serotonergic markers with normal aging: the 5-HT2A receptors decline most pronouncedly, with 17% per decade without partial volume (PV) correction (Sheline et al, 2002) and 6% per decade with PV correction (Adams et al, 2004). A decline with age is described for the 5-HT1A receptors in both genders (Moller et al, 2007; Tauscher et al, 2001), in women only (Costes et al, 2005) and men only (Cidis Meltzer et al, 2001; Rabiner et al, 2002). Studies involving the serotonin transporter have described localized decreases with age in varying regions (Kalbitzer et al, 2009; Meyer et al, 2001; Reimold et al, 2008).
The development and validation of the new PET tracer [11C]SB207145 has now made it possible to quantify 5-HT4 receptor binding in vivo in humans (Marner et al, 2009). The 5-HT4 receptor is a Gs protein-coupled 5-HT receptor, and its stimulation results in increased neuronal excitability (Bockaert, 2004). Experimental studies have suggested several beneficial effects from central 5-HT4 receptor agonism: better cognitive performance (King et al, 2008), fast treatment response in depression (Lucas et al, 2007), modulation of acetylcholine release (Matsumoto et al, 2001), and beneficial effects on the accumulation of β-amyloid have been shown (Cho and Hu, 2007). No sex or age effects on the 5-HT4 receptor density have been described in the human postmortem studies (Bonaventure et al, 2000; Reynolds et al, 1995; Varnas et al, 2003). In an earlier PET study with [11C]SB207145 in a smaller younger cohort, we found that 5-HT4 receptor binding declined with age, and a post hoc analysis suggested that women have lower binding in the hippocampus than men (Marner et al, 2010). The aim of this study was to evaluate age and sex effects on 5-HT4 receptor binding in a larger cohort of healthy subjects, also including older individuals. Three brain regions were included in the study: striatum, limbic system, and neocortex.
Materials and methods
Subjects
A total of 30 healthy subjects were included (mean age, 44 years; range, 20 to 86 years; 14 men). Subjects were recruited by public advertisements (N=26) or extracted from the civil registration system in Denmark (N=4). All subjects gave a written informed consent for participation. The study was approved by The Copenhagen Region Ethics Committee ((KF)01-274821 and (KF)01 2006-2, with amendments).
Exclusion criteria were significant medical history, drug or alcohol abuse, neurological or psychiatric disorders, mental disorder (ensured with DART45, which is a Danish version of the National Adult Reading Test (Nelson and O'Connell, 1978)), pregnancy, or head trauma. All subjects had a normal neurological examination and unremarkable brain magnetic resonance imaging (MRI) scans. Absence of psychiatric symptoms was ensured using the symptom check list revised (SCL-90-R; Derogatis, 1994) on the day of the PET scan. All subjects were scanned in the period from 2006 to 2009. A younger subset of the cohort (N=14) participated in two earlier studies wherein the quantification approach and test–retest variability were evaluated (Marner et al, 2009) and sensitivity to acute 5-HT release was measured (Marner et al, 2010).
MRI and Volumes of Interest
MRI was conducted on a Siemens Magnetom Trio 3T MR scanner. High-resolution 3D T1-weighted (matrix 256 × 256; 1 × 1 × 1 mm voxels) and 2D T2-weighted sequences were acquired. The T1-weighted MRIs were segmented into gray matter, white matter, and cerebrospinal fluid using Statistical Parametric Mapping (SPM5; Wellcome Department of Cognitive Neurology, London, UK). The T2-weighted images served for brain-masking purposes.
In all, 17 regions were automatically delineated on each subject's MRI in a user-independent manner with the Pvelab software package (Svarer et al, 2005; freely available on http://www.nru.dk/downloads):
Striatal regions (high 5-HT4 receptor binding): caudate nucleus and putamen.
Limbic regions (intermediate 5-HT4 receptor binding): hippocampus, amygdala, anterior cingulate gyrus, posterior cingulate gyrus, and thalamus.
Neocortical regions (low 5-HT4 receptor binding): orbitofrontal cortex, medial and inferior frontal gyri, superior frontal gyrus, insula, superior temporal gyrus, medial and inferior temporal gyri, sensory motor cortex, parietal cortex, and occipital cortex.
A region with negligible concentration of 5-HT4 receptors: cerebellum excluding vermis.
PET Imaging and Quantification of Nondisplaceable 5-HT4 Receptor Binding
PET scans were performed with an 18-ring GE-Advance scanner (General Electric, Milwaukee, WI, USA) operating in 3D acquisition mode, producing 35 image slices with an inter slice distance of 4.25 mm. The total axial field of view was 15.2 cm, with an approximate in-plane resolution of 6 mm. To minimize movement during the scan, a light headband fixation was used.
The scan was based on a 120 minute dynamic acquisition starting with a bolus injection of mean 491 MBq (range, 206 to 611 MBq) [11C]SB207145 given for more than 20 seconds. The mean mass dosage was 3.4 μg (range, 0.14 to 5.9 μg), the maximum upper dosage limit has been estimated to be 9.5 μg (occupancy <10% Madsen K et al, unpublished observations). The acquisition consisted of 38 time frames (6 × 5, 10 × 15, 4 × 30, 5 × 120, 5 × 300, and 8 × 600 seconds). After acquisition, attenuation- and decay-corrected recordings were reconstructed by filtered back projection using a 6 mm Hann filter.
Frames were aligned using AIR 5.2.5 (Woods et al, 1992) to correct for movements during the scan. Before alignment, each frame was filtered with a 12 mm Gaussian filter, and the rigid transformation of each frame to a selected single frame with sufficient structural information (frame 26: 15 to 20 minutes after injection) was estimated using the scaled least squares cost-function in AIR.
The [11C]SB207145 was automatically co-registered to the MRI with the AIR algorithm using the mean of the first 20 minutes of the PET scan corresponding to a flow-weighted image. The quality of each co-registration was evaluated by visual inspection in three planes. Data were PV corrected using the Muller–Gartner method (Muller-Gartner et al, 1992), with a point spread function of 6 mm. The age and sex effects on 5-HT4 receptor binding were assessed with PV-corrected data as the PV effect results in an underestimation of counts in high-count voxels because of spill-over to neighboring voxels due to insufficient resolution of the PET scanner. Amounts of spill-out and spill-in of brain regions depend on brain structure and are therefore influenced by the increased occurrence of brain atrophy with age (Raz et al, 2005) and the well-documented structural sex differences (Cosgrove et al, 2007).
Regional time activity curves were constructed both with and without PV correction, and kinetic modeling was performed with the simplified reference tissue model using cerebellum as the reference region, as validated previously (Marner et al, 2009). The regional in vivo outcome measure, the binding potential, BPND, is defined as:
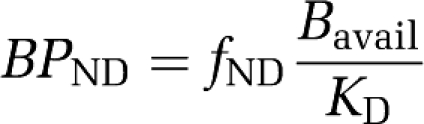 |
fND being the nonprotein-bound fraction of nondisplaceable binding in the brain tissue, Bavail the concentration of available receptors, and KD the dissociation constant. The kinetic modeling was performed using the PMOD software version 2.95, build 2 (PMOD Inc., Zürich, Switzerland). Volume-weighted means of BPND were calculated for striatum, limbic system, and neocortex.
Plasma Analysis: Metabolites and Free Fraction
Immediately before initiation of the scans, venous blood samples were drawn to measure the plasma-free fraction, fP, with equilibrium dialysis (N=26), as described previously (Kornum et al, 2009). Venous blood samples were drawn at 32 and 55 minutes after injection (N=21 and N=24, respectively), and the parent [11C]SB207145 compound and its radiolabeled metabolites were measured in plasma with high-performance liquid chromatography.
Statistics
To control for possible biases in age, body mass index, and plasma and tracer data, a linear analysis of covariance was used to test for sex differences and age effects on these variables (Table 1).
Table 1. Sex-specific values of demographic, tracer, and plasma data.
| Men | Women |
ANCOVA |
||
|---|---|---|---|---|
| Sex, P-value | Age, P-value | |||
| N | 14 | 16 | ||
| Age (years) | 41±20 | 47±21 | 0.47 | |
| BMI (kg/m2) | 27±4 | 27±7 | 0.70 | 0.53 |
| Injected mass per kg bodyweight (ng/kg) | 40±22 | 49±28 | 0.44 | 0.43 |
| Mean ligand concentration in cerebellum (fmol/ml) | 134±76 | 202±120 | 0.11 | 0.03 |
| fP (%), (N) | 26±6 (10) | 27±12 (13) | 0.74 | 0.94 |
| Parent compound in plasma, 32 minutes (%), (N) | 20±8 (10) | 20±6 (8) | 0.70 | 0.15 |
| Parent compound in plasma, 55 minutes (%), (N) | 13±3 (12) | 13±4 (9) | 0.97 | 0.81 |
ANCOVA, analysis of covariance; BMI, body mass index.
Values are mean±s.d. Sex differences and age effects are tested on parameters one by one in a linear ANCOVA.
As the primary investigation, a linear analysis of covariance was used to model the effect of age, sex, and their interaction on 5-HT4 receptor binding for each of the three brain regions. Interaction between age and sex was excluded from the analysis if not significant. Regional PV-corrected 5-HT4 receptor binding was the primary dependent variable. To evaluate the effect of PV correction, analyses were also performed without PV-corrected binding measures. Tests were two sided, and P values were considered significant when <0.05. Parameter estimates and s.d. were given when appropriate.
Results
The sex-specific values for demographic and tracer data are shown in Table 1. There was no significant effect of age and sex on demographic and tracer data, except for a significant increasing cerebellar mean concentration of unlabeled tracer with age (P=0.03, estimate 2.1 fmol/mL years±0.9).
Regional 5-HT4 receptor binding, with and without PV correction, and gray matter volumes are listed in Table 2. The regional distribution of the tracer is in concordance with previous studies of [11C]SB207145 (Marner et al, 2010) showing the binding pattern: neocortex<limbic system<striatum. No interaction was found between age and sex effects on regional 5-HT4 receptor binding, therefore, it was excluded from the analyses. Results of sex and age effects on regional 5-HT4 receptor binding are shown in Table 3.
Table 2. Regional BPND values with and without PV correction and the corresponding regional gray matter volumes.
| Uncorrected BPND | PV-corrected BPND | Gray matter volume (ml) | |
|---|---|---|---|
| Neocortex | 0.36±0.07 | 0.69±0.08 | 350±60 |
| Limbic system | 0.57±0.09 | 0.70±0.09 | 23±3 |
| Striatum | 2.2±0.3 | 3.2±0.4 | 7±1 |
PV, partial volume.
Values are mean±s.d.
Table 3. Linear ANCOVA analyses with regional 5-HT4 receptor binding as a dependent variable, and age and sex as explanatory variables.
|
Uncorrected 5-HT4 receptor binding |
PV-corrected 5-HT4 receptor binding |
|||||
|---|---|---|---|---|---|---|
| Estimate±s.e. | P value | R2 | Estimate±s.e. | P value | R2 | |
| Neocortex | ||||||
| Age | −0.0020±0.0005 | 0.0009 | 0.00096±0.0007 | 0.20 | ||
| Sex | −0.028±0.022 | 0.21 | 0.39 | −0.073±0.029 | 0.017 | 0.21 |
| Limbic system | ||||||
| Age | −0.0021±0.0006 | 0.001 | −0.00016±0.0008 | 0.84 | ||
| Sex | −0.064±0.025 | 0.016 | 0.42 | −0.095±0.031 | 0.0048 | 0.27 |
| Striatum | ||||||
| Age | −0.0099±0.0020 | <0.0001 | −0.0085±0.0031 | 0.010 | ||
| Sex | −0.13±0.08 | 0.11 | 0.52 | −0.21±0.12 | 0.10 | 0.31 |
ANCOVA, analysis of covariance; PV, partial volume.
PV corrected values are the primary outcomes. Analyses are performed for each region one by one, and sex differences are analyzed with men as reference.
Effects of Sex on 5-HT4 Receptor Binding
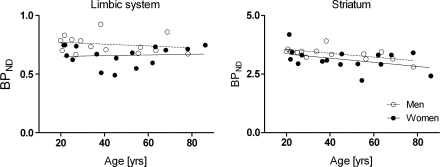
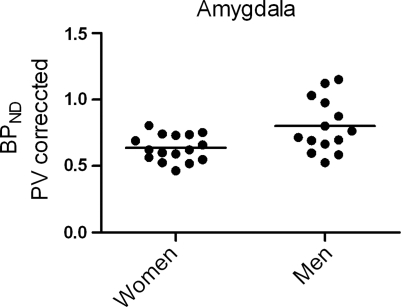
In all, 13% lower 5-HT4 receptor binding was found in women compared with men in the limbic system (see Figure 1A). The finding was similar without PV correction of data (11%), and the finding is also significant after Bonferroni correction for multiple comparisons (P=0.014 with PV correction and P=0.048 without). A post hoc analysis of limbic subregions showed that the difference was most pronounced, with 19% in the amygdala (P=0.0056 without PV correction and P=0.012 with; see Figure 2).
Figure 1.
The association between regional partial volume (PV)-corrected 5-HT4 receptor binding for men and women separately. There is no interaction between sex and age. Mean 13% lower limbic 5-HT4 receptor binding is found in women compared with men. There is a decline with age of 1% per decade in striatal 5-HT4 receptor binding.
Figure 2.
A post hoc analysis of limbic subregions showed that the sex difference was most pronounced in the amygdala with 19% (P=0.012).
A borderline tendency of 6% reduction in striatum of 5-HT4 receptor binding in women compared with men was found both with and without PV correction of data (see Figure 1B). For the neocortex, a significant reduction of 10% was found in PV-corrected data only. Thus, a similar pattern was found in striatum and neocortex, but after Bonferroni correction for multiple comparisons, there were no significant gender differences found neither for striatum nor for neocortex. Post hoc analyses of neocortical subregions showed significant reductions of 9% to 13% in women both with and without PV correction of data in orbitofrontal cortex, insula, and superior temporal gyrus (P values range from 0.005 to 0.04, uncorrected).
Effects of Age on 5-HT4 Receptor Binding
Without correcting for the PV effect, a significant decline with age was found in all three regions corresponding to declines of 3% to 5% per decade. However, when correcting for the PV effect, a decline in 5-HT4 receptor binding with age was found in striatum only (see Figure 1B) corresponding to a decline of 1% per decade; this finding was significant after Bonferroni correction (P=0.04). Declines per decade are calculated as the change from 40 to 50 years.
Discussion
Sex Differences in 5-HT4 Receptor Binding
We found that women had 13% lower limbic 5-HT4 receptor binding than men (see Figure 1A). This is highly interesting because the limbic system has been linked historically to learning and memory, cognitive processing, and emotion. Further, post hoc analyses showed that the difference was most pronounced in the amygdala, which is highly involved in the control of emotions (Ehrlich et al, 2009), and was further found in the subregions of the neocortex that often are referred to as paralimbic: the orbitofrontal cortex, insula, and superior temporal gyrus, of which primarily the orbitofrontal cortex is involved in affective functions. Our observations support a role for 5-HT4 receptors in the sex-specific differences of emotional control, and the lower 5-HT4 receptor binding might contribute to the observed higher prevalence of affective diseases and AD in women, which persists even after controlling for the fact that women tend to live longer than men. An animal study has reported that 5-HT4 receptor agonism exerts a fast antidepressive response, with modification of key markers of antidepressant action: desensitization of 5-HT1A autoreceptors, increased tonus on hippocampal postsynaptic 5-HT1A receptors, and enhanced phosphorylation of the CREB protein and neurogenesis in the hippocampus (Lucas et al, 2007). Further, experimental studies have suggested 5-HT4 receptor agonists to represent a valuable pharmacological target for the treatment of AD because they may provide both symptomatic relief of cognitive impairments as well as neuroprotection by reducing β-amyloid generation and toxicity (reviewed by Lezoualc'h, 2007). However, human postmortem studies revealed no changes in 5-HT4 receptor affinity and density in AD in frontal and temporal cortex (Lai et al, 2003), whereas increased density of 5-HT4 receptors in frontal cortex and caudate nucleus was found in violent suicide victims (Rosel et al, 2004).
We thoroughly examined potential confounders that could have influenced the observed sex difference in this limited sample size. No differences were found in the fraction of nonprotein-bound tracer molecules (fP), metabolic rate, injected mass, or cerebellar concentration, indicating that bias from tracer availability did not explain the sex difference in limbic 5-HT4 receptor binding. Even though the BPND in limbic regions is moderate, the simplified reference tissue model yields low test–retest differences (Marner et al, 2009).
In this study, we were not able to examine whether differences in gonadal hormones and menstrual cycle phase had a role. However, no change in limbic 5-HT4 receptor binding was found with age, and no interaction was found between age and sex in any region, indicating that menopause does not affect the 5-HT4 receptor binding. Further, no difference in limbic 5-HT4 receptor binding was found between premenopausal and postmenopausal women (P=0.64, t-test with cutoff at 40 years).
The PV correction gave rise to an increased sex difference in 5-HT4 receptor binding, because the PV effect caused a larger underestimation of BPND in men. This is not surprising because it has been documented that men not only have greater brain volumes than women but also have greater volume of sulci, smaller gray/white matter ratios, and regional thinner cortical gray matter (Cosgrove et al, 2007), which all might contribute to an increased PV effect in men.
PET studies show, if anything, a pattern of higher levels of inhibitory receptors and lower levels of excitatory serotonergic receptors in women, and this is compatible with the finding in our study of lower 5-HT4 receptor binding in women.
Age Effects on 5-HT4 Receptor Binding
PV corrected data showed an age-related decrease only in striatal 5-HT4 receptor binding, 1% per decade. In agreement with our previous study (Marner et al, 2010), we found a decline of 3% to 5% per decade in BPND with age without PV correction in all investigated regions. Increasing atrophy with aging (Raz et al, 2005) increases the impact of the PV effect: the sulci widen and there is loss of gray matter, leading to increasing spill-out of counts to the cerebrospinal fluid and white matter especially in cortical regions. Particularly for PET scanners with medium-to-low spatial resolution, age effects cannot be reliably estimated without considering the PV effect, even though PV correction depends heavily on the MRI segmentation, co-registration, and size of point spread function, and the method introduces additional noise to the data.
We controlled for possible confounders that could have caused this outcome: body mass index, fP, metabolic rate, CFP, and injected mass were unaffected by age (see Table 1). However, the increasing cerebellar concentration of ligand could bias the measurement of BPND and give an overestimation of the decrease with aging. Thus, the discrete striatal age-related decrease with aging may be caused by higher nondisplaceable binding with aging. Even though the simplified reference tissue model yields low test–retest differences in striatum, the model has been found to underestimate BPND in the high-binding striatal regions (Marner et al, 2009). All the same, 5-HT4 receptor binding is relatively stable with aging compared with other subtypes of receptors. This speaks against a direct involvement of 5-HT4 receptors in the cognitive decline in normal aging, despite the beneficial effects of central 5-HT4 receptor agonism on memory and learning found in experimental studies.
Conclusion
In this study, we found a 13% lower 5-HT4 receptor binding in the limbic system in women compared with men, with the largest difference of 19% being observed in amygdala. Whether the sex difference in 5-HT4 receptors explains part of the observed difference in the prevalence of AD and affective disorders between men and women remains to be elucidated. We found a decrease with aging of 1% per decade in striatal 5-HT4 receptor binding only, suggesting that this receptor subtype differs from the more pronounced age-related decline of other serotonergic markers. Future studies of the 5-HT4 receptor in vivo should focus on associations between the 5-HT4 receptor binding and affective symptoms as well as cognitive performance in neuropsychiatric disorders, and investigations might contribute to the development of new treatment paradigms in affective and neurodegenerative diseases.
Acknowledgments
The John and Birthe Meyer Foundation is gratefully acknowledged for the donation of the Cyclotron and PET scanner.
The authors declare no conflict of interest.
Footnotes
This study was supported by The Lundbeck Foundation, Rigshospitalet, EU 7th Framework Programme Euripides (HEALTH-F5-2007-201380), and the Danish Medical Research Council.
References
- Adams KH, Pinborg LH, Svarer C, Hasselbalch SG, Holm S, Haugbol S, Madsen K, Frokjaer V, Martiny L, Paulson OB, Knudsen GM. A database of [(18)F]-altanserin binding to 5-HT(2A) receptors in normal volunteers: normative data and relationship to physiological and demographic variables. Neuroimage. 2004;21:1105–1113. doi: 10.1016/j.neuroimage.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, de Girolamo G, Graaf R, Demyttenaere K, Gasquet I, Haro JM, Katz SJ, Kessler RC, Kovess V, Lepine JP, Ormel J, Polidori G, Russo LJ, Vilagut G, Almansa J, Arbabzadeh-Bouchez S, Autonell J, Bernal M, Buist-Bouwman MA, Codony M, Domingo-Salvany A, Ferrer M, Joo SS, Martinez-Alonso M, Matschinger H, Mazzi F, Morgan Z, Morosini P, Palacin C, Romera B, Taub N, Vollebergh WA. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;420:21–27. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- Biver F, Lotstra F, Monclus M, Wikler D, Damhaut P, Mendlewicz J, Goldman S. Sex difference in 5HT2 receptor in the living human brain. Neurosci Lett. 1996;204:25–28. doi: 10.1016/0304-3940(96)12307-7. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Compan V, Dumuis A. 5-HT4 receptors. Curr Drug Targets CNS Neurol Disord. 2004;31:39–51. doi: 10.2174/1568007043482615. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Hall H, Gommeren W, Cras P, Langlois X, Jurzak M, Leysen JE. Mapping of serotonin 5-HT(4) receptor mRNA and ligand binding sites in the post-mortem human brain. Synapse. 2000;36:35–46. doi: 10.1002/(SICI)1098-2396(200004)36:1<35::AID-SYN4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Cho S, Hu Y. Activation of 5-HT4 receptors inhibits secretion of beta-amyloid peptides and increases neuronal survival. Exp Neurol. 2007;203:274–278. doi: 10.1016/j.expneurol.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Cidis Meltzer C, Drevets WC, Price JC, Mathis CA, Lopresti B, Greer PJ, Villemagne VL, Holt D, Mason NS, Houck PR, Reynolds CF, III, DeKosky ST. Gender-specific aging effects on the serotonin 1A receptor. Brain Res. 2001;895:9–17. doi: 10.1016/s0006-8993(00)03211-x. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes N, Merlet I, Ostrowsky K, Faillenot I, Lavenne F, Zimmer L, Ryvlin P, Le Bars D. A 18F-MPPF PET normative database of 5-HT1A receptor binding in men and women overaging. J Nucl Med. 2005;46:1980–1989. [PubMed] [Google Scholar]
- Derogatis LR.1994Symptom Checklist-90-R. Administration, Scoring, and Procedures Manual3rd ed.Minneapolis, Minnesota: National Computer Systems [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, Erritzoe D, Madsen J, Paulson OB, Knudsen GM. Gender and the use of hormonal contraception in women are not associated with cerebral cortical 5-HT 2A receptor binding. Neuroscience. 2009;163:640–645. doi: 10.1016/j.neuroscience.2009.06.052. [DOI] [PubMed] [Google Scholar]
- Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, Halldin C, Nordstrom AL. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. 2008;39:1408–1419. doi: 10.1016/j.neuroimage.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Kalbitzer J, Frokjaer VG, Erritzoe D, Svarer C, Cumming P, Nielsen FA, Hashemi SH, Baare WF, Madsen J, Hasselbalch SG, Kringelbach ML, Mortensen EL, Knudsen GM. The personality trait openness is related to cerebral 5-HTT levels. Neuroimage. 2009;45:280–285. doi: 10.1016/j.neuroimage.2008.12.001. [DOI] [PubMed] [Google Scholar]
- King MV, Marsden CA, Fone KC. A role for the 5-HT(1A), 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol Sci. 2008;29:482–492. doi: 10.1016/j.tips.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Kornum BR, Lind NM, Gillings N, Marner L, Andersen F, Knudsen GM. Evaluation of the novel 5-HT4 receptor PET ligand [11C]SB207145 in the Göttingen minipig. J Cereb Blood Flow Metab. 2009;29:186–196. doi: 10.1038/jcbfm.2008.110. [DOI] [PubMed] [Google Scholar]
- Lai MK, Tsang SW, Francis PT, Esiri MM, Hope T, Lai OF, Spence I, Chen CP. [3H]GR113808 binding to serotonin 5-HT(4) receptors in the postmortem neocortex of Alzheimer disease: a clinicopathological study. J Neural Transm. 2003;110:779–788. doi: 10.1007/s00702-003-0825-9. [DOI] [PubMed] [Google Scholar]
- Lezoualc'h F. 5-HT4 receptor and Alzheimer's disease: the amyloid connection. Exp Neurol. 2007;205:325–329. doi: 10.1016/j.expneurol.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, Lambas-Senas L, Wiborg O, Haddjeri N, Pineyro G, Sadikot AF, Debonnel G. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55:712–725. doi: 10.1016/j.neuron.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Marner L, Gillings N, Comley RA, Baare WF, Rabiner EA, Wilson AA, Houle S, Hasselbalch SG, Svarer C, Gunn RN, Laruelle M, Knudsen GM. Kinetic modeling of 11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo. J Nucl Med. 2009;50:900–908. doi: 10.2967/jnumed.108.058552. [DOI] [PubMed] [Google Scholar]
- Marner L, Gillings N, Madsen K, Erritzoe D, Baare WF, Svarer C, Hasselbalch SG, Knudsen GM. Brain imaging of serotonin 4 receptors in humans with [11C]SB207145-PET. Neuroimage. 2010;50:855–861. doi: 10.1016/j.neuroimage.2010.01.054. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Togashi H, Mori K, Ueno K, Ohashi S, Kojima T, Yoshioka M. Evidence for involvement of central 5-HT(4) receptors in cholinergic function associated with cognitive processes: behavioral, electrophysiological, and neurochemical studies. J Pharmacol Exp Ther. 2001;296:676–682. [PubMed] [Google Scholar]
- Meyer JH. Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatr Neurosci. 2007;32:86–102. [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, Goulding V, Kennedy J, Wilson AA. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61:1271–1279. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- Moller M, Jakobsen S, Gjedde A. Parametric and regional maps of free serotonin 5HT1A receptor sites in human brain asfunction of age in healthy humans. Neuropsychopharmacology. 2007;32:1707–1714. doi: 10.1038/sj.npp.1301310. [DOI] [PubMed] [Google Scholar]
- Muller-Gartner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, Davatzikos C, Frost JJ. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- Nelson HE, O'Connell A. Dementia: the estimation of premorbid intelligence levels using the New Adult Reading Test. Cortex. 1978;14:234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr. 2005;10:49–56. doi: 10.1017/s1092852900009901. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Messa C, Sargent PA, Husted-Kjaer K, Montgomery A, Lawrence AD, Bench CJ, Gunn RN, Cowen P, Grasby PM. A database of [(11)C]WAY-100635 binding to 5-HT(1A) receptors in normalmalevolunteers: normative data and relationship to methodological, demographic, physiological, and behavioral variables. Neuroimage. 2002;15:620–632. doi: 10.1006/nimg.2001.0984. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Reimold M, Batra A, Knobel A, Smolka MN, Zimmer A, Mann K, Solbach C, Reischl G, Schwarzler F, Grunder G, Machulla HJ, Bares R, Heinz A.2008Anxiety is associated with reduced central serotonin transporter availability in unmedicated patients with unipolar major depression: a [11C]DASB PET study Mol Psychiatry 13606–613.557 [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Mason SL, Meldrum A, De Keczer S, Parnes H, Eglen RM, Wong EH. 5-Hydroxytryptamine (5-HT)4 receptors in post mortem human brain tissue: distribution, pharmacology and effects of neurodegenerative diseases. Br J Pharmacol. 1995;114:993–998. doi: 10.1111/j.1476-5381.1995.tb13303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosel P, Arranz B, Urretavizcaya M, Oros M, San L, Navarro MA. Altered 5-HT2A and 5-HT4 postsynaptic receptors and their intracellular signalling systems IP3 and cAMP in brains from depressed violent suicide victims. Neuropsychobiology. 2004;49:189–195. doi: 10.1159/000077365. [DOI] [PubMed] [Google Scholar]
- Salmon E. A review of the literature on neuroimaging of serotoninergic function in Alzheimer's disease and related disorders. J Neural Transm. 2007;114:1179–1185. doi: 10.1007/s00702-007-0636-5. [DOI] [PubMed] [Google Scholar]
- Sambeth A, Blokland A, Harmer CJ, Kilkens TO, Nathan PJ, Porter RJ, Schmitt JA, Scholtissen B, Sobczak S, Young AH, Riedel WJ. Sex differences in the effect of acute tryptophan depletion on declarative episodic memory: a pooled analysis of nine studies. Neurosci Biobehav Rev. 2007;31:516–529. doi: 10.1016/j.neubiorev.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Mintun MA, Moerlein SM, Snyder AZ. Greater loss of 5-HT(2A) receptors in midlife than in late life. Am J Psychiatry. 2002;159:430–435. doi: 10.1176/appi.ajp.159.3.430. [DOI] [PubMed] [Google Scholar]
- Stein P, Savli M, Wadsak W, Mitterhauser M, Fink M, Spindelegger C, Mien LK, Moser U, Dudczak R, Kletter K, Kasper S, Lanzenberger R. The serotonin-1A receptor distribution in healthy men and women measured by PET and [carbonyl-11C]WAY-100635. Eur J Nucl Med Mol Imaging. 2008;35:2159–2168. doi: 10.1007/s00259-008-0850-x. [DOI] [PubMed] [Google Scholar]
- Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbol S, Frokjaer VG, Holm S, Paulson OB, Knudsen GM. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–979. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Verhoeff NP, Christensen BK, Hussey D, Meyer JH, Kecojevic A, Javanmard M, Kasper S, Kapur S. Serotonin 5-HT1A receptor binding potential declines with age asmeasured by [11C]WAY-100635 and PET. Neuropsychopharmacology. 2001;24:522–530. doi: 10.1016/S0893-133X(00)00227-X. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Pike VW, Hall H. Distribution of 5-HT4 receptors in the postmortem human brain—an autoradiographic study using [125I]SB 207710. Eur Neuropsychopharmacol. 2003;13:228–234. doi: 10.1016/s0924-977x(03)00009-9. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]