Abstract
For many years, a tenet of cerebral metabolism held that glucose was the obligate energy substrate of the mammalian brain and that neuronal oxidative metabolism represented the majority of this glucose utilization. In 1994, Pellerin and Magistretti formulated the astrocyte–neuron lactate shuttle (ANLS) hypothesis, in which astrocytes, not neurons, metabolized glucose, with subsequent transport of the glycolytically derived lactate to fuel the energy needs of the neuron during neurotransmission. By considering the concentrations and kinetic characteristics of the nutrient transporter proteins, Simpson et al later supported the opposite view, in which lactate flows from neurons to astrocytes, thus leading to the neuron–astrocyte lactate shuttle (NALS). Most recently, a commentary was published in this journal attempting to discredit the NALS. This challenge has stimulated the present response in which we detail the inaccuracies of the commentary and further model several different possibilities. Although our simulations continue to support the predominance of neuronal glucose utilization during activation and neuronal to astrocytic lactate flow, the most important result is that, regardless of the direction of the flow, the overall contribution of lactate to cerebral glucose metabolism is found to be so small as to make this ongoing debate ‘much ado about nothing'.
Keywords: astrocytes, energy metabolism, glucose, lactate, mathematical modelling, neuronal–glial interaction
Let us begin this commentary by summarizing two contrasting perspectives on the modeling of cerebral energy metabolism and neuronal–glial interactions. One is based on the astrocyte–neuron lactate shuttle (ANLS) hypothesis as originally proposed by Pellerin and Magistretti (1994) and subsequently modeled by Aubert and colleagues (Aubert and Costalat, 2005; Aubert et al, 2007). This hypothesis maintains that astrocytes—and not neurons—are the primary sites of glucose uptake, glycolytic utilization, and export of lactate to neurons, especially upon brain activation. The alternative perspective is based on the model that was developed by Simpson et al (2007), to include the concentrations and kinetic characteristics of the blood–brain barrier, neuronal, and glial nutrient transporter proteins that specifically mediate brain glucose and lactate transport. On the basis of the application of this model, the authors concluded that the neuron metabolizes glucose and is the chief exporter of lactate (Simpson et al, 2007). This interpretation was subsequently reinforced by applying the model of Simpson et al (2007) to proton Magnetic Resonance Spectroscopy results obtained in the human brain during functional activation (Mangia et al, 2009), and the hypothesis was thus termed neuron–astrocyte lactate shuttle (NALS). Shortly thereafter, DiNuzzo et al (2010) combined the previous mathematical models of cerebral metabolism and nutrient transport (Aubert and Costalat, 2005; Aubert et al, 2007; Simpson et al, 2007) to elucidate the energetic significance of metabolite trafficking within the brain parenchyma, under different scenarios of astrocytic versus neuronal activation-derived sodium inflow, glycolytic and oxidative competence, and glucose transport capacity. Under the assumptions supported by current literature, the model by DiNuzzo et al (2010) confirmed a lactate shuttle from neurons to astrocytes, which nevertheless was secondary to direct neuronal glucose uptake.
Jolivet et al (2010) recently published a commentary entitled ‘Comment on recent modeling studies of astrocyte–neuron metabolic interactions', in which they severely criticize our modeling (DiNuzzo et al, 2010; Mangia et al, 2009; Simpson et al, 2007) in support of their own (Aubert and Costalat 2005; Aubert et al, 2007; Jolivet et al, 2009). Unfortunately, rather than stimulating interesting and timely debate, this latest commentary (Jolivet et al, 2010) neither discusses our studies in an appropriate context nor sheds new light on the question. Indeed, the commentary both misstates our analysis and disregards important findings of several other groups. Our purpose here is to highlight and clarify these inaccuracies such that the two models, which differ primarily in the cell-specific ‘sites' of glucose utilization and direction of lactate flow, can be fairly evaluated. Importantly, the significance of this scientific debate involves the identification of the cell type and sub-cellular structure(s) that predominantly consume glucose during activation, what adenosine triphosphate-requiring processes are activated to the greatest extent in what cell types, and how the increased energy demands are satisfied. We will also discuss the controversy in a broader context, especially regarding the significance of a brain lactate shuttle itself, irrespective of the direction of lactate flow.
Although there are similarities between the two models, the model of Simpson et al (2007), as later applied to in vivo human data (Mangia et al, 2009) or included in integrative metabolic modeling (DiNuzzo et al, 2010), differs from the model by Aubert and colleagues (Aubert and Costalat, 2005; Aubert et al, 2007) in that it incorporates experimentally determined glucose and lactate transporter numbers and activities, and can thus more accurately predict mass transport directions. By determining the number of astrocytic cell surface GLUT1 proteins, we were able to compute that in order for the astrocyte to be the predominant lactate producer (i.e., ANLS is correct), astrocytic glucose transport capacity would need to be 12 fold greater than that measured experimentally. Another major difference between these models is that the ANLS hypothesis assumes that only astrocytes, not neurons, increase their glycolytic activity during brain activation. The commentary by Jolivet et al (2010) aims to discredit the NALS model by proposing that the two fundamental premises of the ANLS hypothesis are more representative of the current state of the field and offer several studies to support their position. A thorough survey of the literature suggests that such assertions are far from representative of the literature.
What is the current state of the literature on the question of the glycolytic response of neurons to activation? Jolivet et al (2010) claim that the consensus is that neurons are unable to increase their glycolytic activity in response to activation and are actually glycolytically inhibited by glutamate, and cite several in vitro studies (Herrero-Mendez et al, 2009; Patel and Brewer 2003; Porras et al, 2004). However, there are numerous weaknesses in these cited studies, which shed confusion on their interpretation. For example, the study of Patel and Brewer (2003) utilizes the mitochondrial poison p-trifluoromethoxyphenylhydrazone when determining 2-deoxyglucose transport activity, which precludes the ability to produce reliable data because of an absence of adenosine triphosphate that results from the inhibition of oxidative phoshorylation. The concept that glutamate specifically inhibits neuronal glycolysis (Porras et al, 2004) should be reviewed in light of studies by Maher and Simpson (1994) and Castro et al (2007), who found that glutamate did not affect 2-deoxyglucose uptake in cultured neurons. Glutamate was found to trigger the increase in surface expression of the neuronal glucose transporter protein, GLUT3, in cerebellar granule neurons, a process mediated by the adenosine monophosphate-dependent protein kinase and thus dependent on the energy state of the cell through increased adenosine monophosphate/adenosine triphosphate ratio (Weisova et al, 2009). Moreover, the molecular basis for activity-dependent increases in surface GLUT3, involving N-methyl--aspartate receptors, was recently characterized in primary cultured cortical and hippocampal neurons (Ferreira et al, 2011), thus proving that neurons can utilize a specific pathway for controlling energy supply during neuronal activity. Glutamate has been reported to induce either an increase (from ∼10% to ∼180%) or decrease (from ∼10% to ∼60%) of glucose utilization in astrocyte-enriched cell cultures (Dienel and Cruz, 2006). In addition, the absence of an upregulation of neuronal glycolysis was suggested by Almeida et al (2001) to explain the absence of lactate accumulation in neuronal cultures exposed to the respiratory inhibitor nitric oxide. However, nitric oxide-induced nitrosylative stress affects neurons more severely than astrocytes, as the effect of nitric oxide on glutathione metabolism and mitochondrial dysfunction is different in neurons and astrocytes. Specifically, astrocytic, but not neuronal, upregulation of glutathione synthesis is observed on nitric oxide exposure, and this is because neurons cannot increase the activity of glutamate cysteine ligase (Gegg et al, 2003 and reviewed by Banerjee et al, 2008).
Jolivet et al (2010) further support their contention that neurons are unable to activate glycolysis, by citing the study of Herrero-Mendez et al (2009), which indicates that the levels of PFK2/FBPase2 activity are reduced in the neuron when analyzed in vitro. PFK2/FBPase2 is the enzyme that regulates the level of Fru2,6-P2, a potent activator of PFK1. However, Fru2,6-P2 is not the exclusive regulator, as PFK1, and thus glycolysis, can also be stimulated by adenosine monophosphate, Rib1,5-P2, NH4+, K+, Pi, and Glc1,6-P2. In fact, early experiments showed that Rib1,5-P2 is a more powerful activator than Fru2,6-P2 during rapid activation of glycolysis in brain (Ogushi et al, 1990). Moreover, during ischemia, rapid activation of glycolysis is not accompanied by a change in the level of Fru2,6-P2, indicating that this metabolite is not critical for the upregulation of glycolysis (Ogushi et al, 1990; Pauwels and Trouet, 1984). Finally, the finding of apoptotic neuronal death after manipulation of the fraction of glucose channeled to glycolysis compared with the Pentose–Phosphate Pathway in basal conditions (Herrero-Mendez et al, 2009) cannot be used to infer conclusions under activated conditions at which the glucose consumption rate (CMRglc) is also increased.
Moreover, contrary to the assertions of Jolivet et al (2010), there are numerous papers that support the notion that neurons upregulate glycolysis during activation (Gjedde and Marrett, 2001). In our 2009 paper, we claimed that ‘several studies aimed at assessing glycolytic or oxidative activity in synaptosomes prepared from adult brain support the notion that neuronal glycolysis increases markedly during activation (Kauppinen and Nicholls, 1986; Kauppinen et al, 1989; Erecinska et al, 1991).' In addition, incorporation of label from glucose into metabolic intermediates is increased during N-methyl--aspartate exposure, thus proving upregulation of neuronal glycolysis on neurotransmission (Bak et al, 2006, 2009). Furthermore, as stated in Jolivet et al (2010), it is not appropriate to derive conclusions on in vivo brain metabolism from studies conducted in culture, and on this we fully agree. Thus, we should examine in vivo studies for added support for increased neuronal glucose utilization during conditions of activation. In fact, a number of physiological and pathological situations associated with increased cerebral glucose utilization are characterized by increases in the neuronal glucose transporter, GLUT3, suggesting a natural adaptation to increased demand for neuronal glucose transport, for example, development, hypoxia/ischemia, and water deprivation/dehydration (reviewed in Vannucci et al, 1998). In conclusion, the argument that the current literature supports that neurons cannot upregulate glycolysis, as suggested by Jolivet et al (2010), is highly speculative at best.
The second point of disagreement is the extent of astrocytic glucose transport capacity. Jolivet et al (2010) attempt to use the simulations of Simpson et al (2007) to measure the amount of glucose entering the astrocytes versus the neuron. However, they used an incorrect equation in their calculation of rglc,astro=(j3−j5)/(j3−j5+j6). As both j3 and j5 represent transport into the astrocytes, these values should be summed and not subtracted as in our model of equilibrium exchange. They further dispute the values used in Mangia et al (2009) and in Simpson et al (2007) by comparing them with the values obtained from Barros et al (2009), which were derived from unstimulated tissue slices and therefore do not represent a valid comparison. The second study cited for negative comparison is that of Nehlig et al (2004), in which they estimated the percentage of glucose entering the astrocyte versus the neuron in vivo, making it far more relevant, although also only in the resting conscious, not stimulated, state. However, their interpretation of this study is incomplete. Nehlig et al (2004) did report a value of 53% of the label from glucose entering the astrocytic compartment; however, this must be compared with their concurrent value of 60% entering the neuron, a number that Jolivet et al (2010) failed to mention. Another limitation of the study (Nehlig et al, 2004) is that the authors only assayed tracks from identified cell bodies, which have lower rates of glucose utilization compared with synaptic and astrocytic processes in the neuropil. In the work of Mangia et al (2009), the fraction of glucose taken up by astrocytes was considered to be almost 20%, whereas in DiNuzzo et al (2010) the value was considered to be either 23% or 37%. Contrary to what was stated by Jolivet et al (2010), these values do not go against the available literature regarding the compartmentation of glucose capture between neurons and astrocytes. Indeed, although some studies have reported higher glucose uptake from astrocytic as compared with neuronal somata in acute cerebellar slices (90%, Barros et al, 2009) and in the sciatic nerve of the rat (Vega et al, 2003), other in vitro studies have shown that neurons take up the majority of glucose (Hassel et al, 1995; Qu et al, 2000). In addition, the only two studies performed in freely moving rats indicate that glucose is taken up by astrocytes that are appropriate to (Zielke et al, 2007) or slightly above (Nehlig et al, 2004) their volume fraction (Table 1). Furthermore, all these values reflect resting, and not functionally activated, conditions. While writing this commentary, a two-photon microscopy study of 6-NBDG (6-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose) (a fluorescent glucose analog) uptake in anesthetized rats (Chuquet et al, 2010) inferred that activation increases sugar transport in astrocytes but not in neurons. This inference, based on a very modest change in 6-NBDG uptake by astrocytes, must be tempered by the fact that GLUT1-mediated 6-NBDG transport is some 2,000 to 3,000 fold slower than -glucose transport (Speizer et al, 1985; Cloherty et al, 1995) and, therefore, not only more closely approximates transbilayer diffusion but also is unlikely to reflect transport changes on a physiological timescale. Furthermore, because the stereospecificity and glucose sensitivity of GLUT3-mediated 6-NBDG transports are not known, it is impossible to infer that the use of 6-NBDG reflects changes in neuronal -glucose transport. Finally, as 6-NBDG is not phosphorylated by hexokinase and thus cannot be accumulated by cells to levels exceeding those in the interstitium, explanations for altered astrocytic 6-NBDG uptake must invoke activation-induced, reversible alterations in cell surface transporter kinetics and/or expression or nonspecific membrane permeability—a phenomena that is not previously characterized in these cells.
Table 1. Experimentally observed fractional neuron and astrocyte glucose uptake.
| Reference |
Glucose uptake (%) |
Relative neuron:astrocyte uptake capacitya | System | |
|---|---|---|---|---|
| Neuron | Astrocyte | |||
| Barros et al (2009) | 10 | 90 | 1.0:17.1 | Cerebellar slices |
| Vega et al (2003) | 20 | 80 | 1.0:7.3 | Rat vagus nerve |
| Nehlig et al (2004)b | 53 | 47 | 1.0:1.6 | Freely moving rats |
| Zielke et al (2007) | 65 | 35 | 1.0:1.0 | Freely moving rats |
| Qu et al (2000) | 70 | 30 | 1.0:0.8 | Anesthetized rats |
| Hassel et al (1995) | 70 | 30 | 1.0:0.8 | Ex vivo |
These values take into account the different volume fractions of neurons (45%) and astrocytes (25%) in the cerebral cortex.
After normalization (error due to the experimental technique, it was 60% for neurons and 53% for astrocytes, which is >100%).
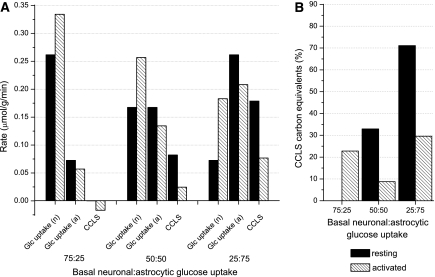
Most importantly, modeling results show that the neuronal/astrocytic ratio of glucose uptake at rest is not a critical parameter in predicting the direction and significance of lactate flow under increased neurotransmission (Figure 1). If we arbitrarily model a decrease in the basal neuronal to astrocytic glucose uptake ratio from 75:25 to 50:50 to 25:75, the direction (increase or decrease) of the glucose flux variation under activated conditions is unchanged, that is, increased neuronal glucose uptake and decreased astrocytic glucose uptake. The cell-to-cell lactate shuttle (CCLS) is either pushed toward increased neuronal–astrocyte lactate shuttle or toward reduced ANLS during neuronal activation (Figure 1B). Overall, our simulations show that net glucose uptake and utilization by individual cells is determined by cellular energy requirements. The high, resting product (Glc6-P) inhibition of neuronal hexokinase is substantially relaxed during increased adenosine triphosphate consumption due to rapid activation of PFK1 (Lowry and Passonneau, 1964).
Figure 1.
(A) Simulated rates of resting (solid bars) and activated (striped bars) substrate uptake in astrocytes and neurons, and contribution of cell-to-cell lactate shuttle (CCLS), for different conditions of basal neuronal: astrocytic glucose uptake. The suffix ‘a' or ‘n' stands for astrocytes or neurons, respectively. Here, by convention, we assume that if the lactate shuttle is from astrocytes to neurons (ANLS) the sign of the CCLS is positive, otherwise is negative. (B) Relative contribution of carbon equivalents derived from the CCLS compared with the total energy substrate flux in the specific cell compartment that is involved in lactate uptake (i.e., astrocytes in the 75:25 condition and neurons in the other conditions). In the 25:75 condition, the carbon equivalents derived from astrocytic lactate accounts for at most 30% of total neuronal energy substrates during activation, with the remaining coming directly from blood-borne glucose. In the condition of 75:25 neuronal versus astrocytic glucose uptake, the carbon equivalents derived from neuronal lactate accounts for ∼23% of total astrocytic energy substrates during activation. Note that the CCLS is neuronal–astrocyte lactate shuttle in the 75:25 condition, whereas it is ANLS in the other conditions. In addition, the bars are zero in the resting condition of the 75:25 case, as both neurons and astrocytes were found to export lactate in the interstitial space, and therefore no CCLS occurs between astrocytes and neurons. All simulations were performed using a 3:1 neuronal versus astrocytic stimulation ratio, and a 360-seconds stimulation (see DiNuzzo et al, 2010).
Several additional features of the model of Simpson et al (2007) were challenged by Jolivet et al (2010). First, they suggest that the model uses a single architectural geometry. This is not strictly true. Rather, the model uses relative cellular volumes, which are consistent with literature values, including those used by Aubert and Costalat (2005) (Hrabetova and Nicholson, 2004).
Second, they incorrectly conclude that our values for transporter density were derived from tissue culture. As stated above, we completely agree that values reflecting properties of metabolism in vivo cannot be reliably measured in vitro. Rather, our values (Simpson et al, 2007) are based on quantitative cytochalasin B binding measurements that were recorded in isolated rat brain membranes. Cytochalasin B is a potent competitive inhibitor of glucose transporters. Thus, measurements of -glucose-inhibitable cytochalasin B binding provide an accurate determination of total (GLUT1+GLUT3) glucose transporters in pmol/mg present within the membranes. To determine the relative concentrations of GLUT1 and GLUT3 in these membranes, we analyzed the same membranes by western Blot analysis and compared them with membranes previously calibrated for each transporter isoform. The calibration membranes were cerebellar granule cell membranes, for which we had determined the absolute concentrations of GLUTs 1 and 3 per mg membrane protein, respectively, using a 3H bis-mannose photolabel. The basis for these calculations is described in detail in Vannucci et al (1997) as referenced in Simpson et al (2007).
Third, Jolivet et al (2010) appear to take particular exception with our assigning a constant fraction of 1/12 of the glucose being diverted to nonoxidative processes in both neurons and astrocytes, including—but not limited to—glycogen. Such options also include protein and lipid glycosylation and nucleotide and amino-acid synthesis. This is entirely consistent with the numerous studies that have showed that 5 to 5.5 of the 6 carbons of glucose are oxidized to CO2 and the remaining carbon is tacitly assumed to be diverted to a nonoxidative fate (Shulman et al, 2001).
Fourth, other factors need to be considered when comparing the models of Mangia et al (2009) and Simpson et al (2007) with that of Aubert and Costalat (2005) and Aubert et al (2007). Initially, the model of Aubert and Costalat (2005) was explored under various sets of conditions of astrocytic versus neuronal stimulation. When analyzed under similar conditions, Aubert and Costalat (2005) and DiNuzzo et al (2010) yield similar predictions about the direction of lactate flow from neurons to astrocytes during activation, consistent with the findings of Mangia et al (2009). However, in the subsequent paper (Aubert et al, 2007), they chose one specific set of parameters that predicted only flow of lactate from astrocytes to neurons. Some of the assumptions used are contentious and poorly justified within the accompanying text. For example, they considered basal neuronal and astrocytic glucose uptake corresponding to 26% and 74%, respectively. These values were based on the assumption that the glycolytic capacity of neurons is five times lower than that of astrocytes, as mentioned in Table 2 of the supplementary information given by Aubert et al (2007). However, such an assumption is inconsistent with the evidence that expression of glycolytic enzymes in astrocytes and neurons are similar (Lovatt et al, 2007), and with the evidence that astrocytes, neurons, and synaptosomes have similar relative levels of hexokinase, indicating similar glycolytic capacity (Kao-Jen and Wilson, 1980; Snyder and Wilson, 1983; Wilson, 1972). Furthermore, they always assumed a very low oxidative capacity of astrocytes per unit volume, which is contrary to experimental evidence showing that astrocytic contribution to cerebral oxidative metabolism is at least equal to their volume fraction (see Hertz et al, 2007 and references therein).
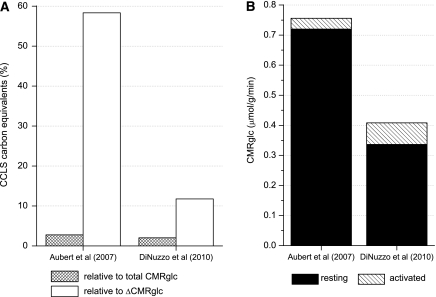
Notably, lactate transport is reversible and could occur in both directions between astrocytes and neurons, depending thermodynamically and kinetically on the relative values of the cytosolic and mitochondrial redox states in neurons and astrocytes (Cerdan et al, 2006). However, as pointed out by DiNuzzo et al (2010), irrespective of the direction of the lactate shuttle, when evaluated in terms of carbon equivalents, modeling results suggest that the lactate shuttle is of minor importance during activation when compared with the total brain glucose uptake. On the basis of the model by DiNuzzo et al (2010), the consumption rate of carbon equivalents derived from the CCLS during a 60-seconds stimulation is 0.0084 μmol/g/minute on average, and the direction of the lactate flow is from neurons to astrocytes. On the other hand, the basal brain glucose uptake derived from the model is 0.34 μmol/g/minute, and the increase of brain glucose consumption (ΔCMRglc) is ∼21% on average during stimulation, consistent with experimental measurements reported for the human brain (Gruetter et al, 2001 and references therein; Marrett and Gjedde, 1997; Newberg et al, 2005). Therefore, as summarized in Figure 2, the CCLS contribution corresponds to ∼2% of the total glucose consumption during activation and to 11% of the increase in brain glucose consumption. Although this issue was not specifically discussed in the papers by Aubert and colleagues (Aubert and Costalat 2005; Aubert et al, 2007), the relatively minor contribution of the CCLS was already inherent in the original Aubert and Costalat (2005) model. On the basis of the results shown in the Figure 3A, B of the paper by Aubert et al (2007), the carbons obtained on average from the CCLS during a 60-seconds stimulation is 0.021 μmol/g/min, and the direction of the lactate flow is from astrocytes to neurons. Considering that the basal glucose consumption reported in Aubert et al (2007) is 0.72 μmol/g/minute, with an average increase of 0.036 μmol/g/minute during stimulation (i.e., ΔCMRglc=∼5%), the CCLS contribution corresponds to ∼3% of the total glucose consumption during activation. Thus, when compared with ΔCMRglc, the CCLS contribution would be ∼58%, a result that is clearly incorrect as the increase of glucose consumption during activation is well above 5%.
Figure 2.
Quantification of intercellular lactate trafficking based on glucose consumption, according to different models, during a 60-seconds stimulation. (A) Both in the model by Aubert et al (2007) and DiNuzzo et al (2010), the amount of predicted cell-to-cell lactate shuttle (CCLS) is a small fraction of the concomitant CMRglc (3% and 2%, respectively). Only when compared with the functional variation of glucose flux during activated conditions (i.e., ΔCMRglc) the two models differ substantially, accounting for 58% or 11% of ΔCMRglc, respectively. (B) Simulated resting and activated CMRglc in the two models. The conclusion that lactate transfer represents a major contribution to energy substrate availability during activation is biased by the incorrect prediction about ΔCMRglc during activation obtained in Aubert et al (2007), which is only +5%. Note that the values pertaining to the work of Aubert et al (2007) were calculated based on the plots reported in their Figure 3A and B.
Finally, mathematical models of complex systems can provide useful guides to understanding physiologic events, and can also provide interesting intellectual exercises analyzing various potential scenarios, and for those so inclined our models are still available online. However, in the instance of lactate shuttling in cerebral metabolism, one might question the biological relevance and value of insisting that it must be one way or another, given its minimal contribution to overall glucose oxidation. The overriding goal is to understand the cellular basis of cell–cell interactions related to the energetics of brain activation, which is necessary for interpretation and modeling of metabolic imaging and spectroscopic studies in normal and disease states. If the modeling predicts metabolic activity in the wrong cell type(s), then the focus of research and interpretation of metabolic studies is misdirected. To date, all studies support that neurons have the highest energy demand of all neural cells and are most vulnerable to energy failure. Thus, it seems reasonable that neurons will, and do, use whatever fuel they can incorporate into their metabolic pathways, including ketone bodies and fatty acids, in addition to lactate and of course glucose.
Acknowledgments
We thank Dr Art From for helpful discussions.
The authors declare no conflict of interest.
Footnotes
This study was supported by NIH P41RR08079; P30 NS057091; the W. M. Keck Foundation and the MIND Institute (to CMRR, S Mangia); NIH DK 44888, DK 36081 (A Carruthers); DK075130 (IA Simpson); AHA 0575055N (SJ Vannucci).
References
- Almeida A, Almeida J, Bolanos JP, Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci USA. 2001;98:15294–15299. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert A, Costalat R. Interaction between astrocytes and neurons studied using a mathematical model of compartmentalized energy metabolism. J Cereb Blood Flow Metab. 2005;25:1476–1490. doi: 10.1038/sj.jcbfm.9600144. [DOI] [PubMed] [Google Scholar]
- Aubert A, Pellerin L, Magistretti PJ, Costalat R. A coherent neurobiological framework for functional neuroimaging provided by a model integrating compartmentalized energy metabolism. Proc Natl Acad Sci USA. 2007;104:4188–4193. doi: 10.1073/pnas.0605864104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS. Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J Cereb Blood Flow Metab. 2006;26:1285–1297. doi: 10.1038/sj.jcbfm.9600281. [DOI] [PubMed] [Google Scholar]
- Bak LK, Walls AB, Schousboe A, Ring A, Sonnewald U, Waagepetersen HS. Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. J Neurochem. 2009;109:87–93. doi: 10.1111/j.1471-4159.2009.05943.x. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Vitvitsky V, Garg SK. The undertow of sulfur metabolism on glutamatergic neurotransmission. Trends Biochem Sci. 2008;33:413–419. doi: 10.1016/j.tibs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Barros LF, Courjaret R, Jakoby P, Loaiza A, Lohr C, Deitmer JW. Preferential transport and metabolism of glucose in Bergmann glia overPurkinje cells: a multiphoton study of cerebellar slices. Glia. 2009;57:962–970. doi: 10.1002/glia.20820. [DOI] [PubMed] [Google Scholar]
- Castro MA, Pozo M, Cortes C, Garcia Mde L, Concha II, Nualart F. Intracellular ascorbic acid inhibits transport of glucose by neurons, but not by astrocytes. J Neurochem. 2007;102:773–782. doi: 10.1111/j.1471-4159.2007.04631.x. [DOI] [PubMed] [Google Scholar]
- Cerdan S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, Garcia-Martin ML. The redox switch/redox coupling hypothesis. Neurochem Int. 2006;48:523–530. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Chuquet J, Quilichini P, Nimchinsky EA, Buzsáki G. Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J Neurosci. 2010;30:15298–15303. doi: 10.1523/JNEUROSCI.0762-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloherty EK, Sultzman LA, Zottola RJ, Carruthers A. Net sugar transport is a multistep process. Evidence for cytosolic sugar binding sites in erythrocytes. Biochemistry. 1995;34:15395–15406. doi: 10.1021/bi00047a002. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Astrocyte activation in working brain: energy supplied by minor substrates. Neurochem Int. 2006;48:586–595. doi: 10.1016/j.neuint.2006.01.004. [DOI] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Changes in glucose uptake rather than lactate shuttle take center stage in subserving neuroenergetics: evidence from mathematical modeling. J Cereb Blood Flow Metab. 2010;30:586–602. doi: 10.1038/jcbfm.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Chance B. Depolarization-induced changes in cellular energy production. Proc Natl Acad Sci USA. 1991;88:7600–7604. doi: 10.1073/pnas.88.17.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JM, Burnett AL, Rameau GA. Activity-dependent regulation of surface glucose transporter-3. J Neurosci. 2011;31:1991–1999. doi: 10.1523/JNEUROSCI.1850-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Beltran B, Salas-Pino S, Bolanos JP, Clark JB, Moncada S, Heales SJ. Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: implications for neuroprotection/neurodegeneration. J Neurochem. 2003;86:228–237. doi: 10.1046/j.1471-4159.2003.01821.x. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Marrett S. Glycolysis in neurons, not astrocytes, delays oxidative metabolism of human visual cortex during sustained checkerboard stimulation in vivo. J Cereb Blood Flow Metab. 2001;21:1384–1392. doi: 10.1097/00004647-200112000-00002. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab. 2001;281:E100–E112. doi: 10.1152/ajpendo.2001.281.1.E100. [DOI] [PubMed] [Google Scholar]
- Hassel B, Sonnewald U, Fonnum F. Glial-neuronal interactions asstudied by cerebral metabolism of [2-C-13]acetate and [1-C-13]glucose—an ex-vivo C-13 Nmr spectroscopic study. J Neurochem. 1995;64:2773–2782. doi: 10.1046/j.1471-4159.1995.64062773.x. [DOI] [PubMed] [Google Scholar]
- Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hrabetova S, Nicholson C. Contribution of dead-space microdomains to tortuosity of brain extracellular space. Neurochem Int. 2004;45:467–477. doi: 10.1016/j.neuint.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Jolivet R, Allaman I, Pellerin L, Magistretti PJ, Weber B. Comment on recent modeling studies of astrocyte-neuron metabolic interactions. J Cereb Blood Flow Metab. 2010;30:1982–1986. doi: 10.1038/jcbfm.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivet R, Magistretti PJ, Weber B. Deciphering neuron-glia compartmentalization in cortical energy metabolism. Front Neuroenergetics. 2009;1:4. doi: 10.3389/neuro.14.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao-Jen J, Wilson JE. Localization of hexokinase in neural tissue: electron microscopic studies of rat cerebellar cortex. J Neurochem. 1980;35:502–514. doi: 10.1111/j.1471-4159.1980.tb03706.x. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Nicholls DG. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem. 1986;158:159–165. doi: 10.1111/j.1432-1033.1986.tb09733.x. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Taipale HT, Komulainen H. Interrelationships between glucose metabolism, energy state, and the cytosolic free calcium concentration in cortical synaptosomes from the guinea pig. J Neurochem. 1989;53:766–771. doi: 10.1111/j.1471-4159.1989.tb11771.x. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. The relationship between substrates and enzymes of glycolysis in brain. J Biol Chem. 1964;239:31–42. [PubMed] [Google Scholar]
- Maher F, Simpson IA. Modulation of expression of glucose transporters GLUT3 and GLUT1 by potassium and N-methyl--aspartate in cultured cerebellar granule neurons. Mol Cell Neurosci. 1994;5:369–375. doi: 10.1006/mcne.1994.1044. [DOI] [PubMed] [Google Scholar]
- Mangia S, Simpson IA, Vannucci SJ, Carruthers A. The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem. 2009;109 (Suppl 1:55–62. doi: 10.1111/j.1471-4159.2009.06003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrett S, Gjedde A. Changes of blood flow and oxygen consumption in visual cortex of living humans. Adv Exp Med Biol. 1997;413:205–208. doi: 10.1007/978-1-4899-0056-2_22. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Wittendorp-Rechenmann E, Lam CD. Selective uptake of [14C]2-deoxyglucose by neurons and astrocytes: high-resolution microautoradiographic imaging by cellular 14C-trajectography combined with immunohistochemistry. J Cereb Blood Flow Metab. 2004;24:1004–1014. doi: 10.1097/01.WCB.0000128533.84196.D8. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Wang J, Rao H, Swanson RL, Wintering N, Karp JS, Alavi A, Greenberg JH, Detre JA. Concurrent CBF and CMRGlc changes during human brain activation by combined fMRI-PET scanning. Neuroimage. 2005;28:500–506. doi: 10.1016/j.neuroimage.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Ogushi S, Lawson JWR, Dobson GP, Veech RL, Uyeda K. A new transient activator of phosphofructokinase during Initiation of rapid glycolysis in brain. J Biol Chem. 1990;265:10943–10949. [PubMed] [Google Scholar]
- Patel JR, Brewer GJ. Age-related changes in neuronal glucose uptake in response to glutamate and beta-amyloid. J Neurosci Res. 2003;72:527–536. doi: 10.1002/jnr.10602. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ, Trouet A. Role of fructose 2,6-bisphosphate in the regulation of glycolysis in various types of cultivated brain-cell. Neurosci Lett. 1984;46:173–177. doi: 10.1016/0304-3940(84)90437-3. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras OH, Loaiza A, Barros LF. Glutamate mediates acute glucose transport inhibition in hippocampal neurons. J Neurosci. 2004;24:9669–9673. doi: 10.1523/JNEUROSCI.1882-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Waagepetersen HS, van Hengel M, Wolt S, Dale O, Unsgard G, Sletvold O, Schousboe A, Sonnewald U. Effects of thiopental on transport and metabolism of glutamate in cultured cerebellar granule neurons. Neurochem Int. 2000;37:207–215. doi: 10.1016/s0197-0186(00)00024-3. [DOI] [PubMed] [Google Scholar]
- Shulman R, Hyder F, Rothman D. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001;14:389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CD, Wilson JE. Relative levels of hexokinase in isolated neuronal, astrocytic, and oligodendroglial fractions from rat brain. J Neurochem. 1983;40:1178–1181. doi: 10.1111/j.1471-4159.1983.tb08111.x. [DOI] [PubMed] [Google Scholar]
- Speizer L, Haugland R, Kutchai H. Asymmetric transport of a fluorescent glucose analogue by human erythrocytes. Biochim Biophys Acta. 1985;815:75–84. doi: 10.1016/0005-2736(85)90476-6. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Clark RR, Koehler-Stec E, Li K, Smith CB, Davies P, Maher F, Simpson IA. Glucose transporter expression in brain: relationship to cerebral glucose utilization. Dev Neurosci. 1998;20:369–379. doi: 10.1159/000017333. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vega C, Martiel JL, Drouhault D, Burckhart MF, Coles JA. Uptake of locally applied deoxyglucose, glucose and lactate by axons and Schwann cells of rat vagus nerve. J Physiol. 2003;546:551–564. doi: 10.1113/jphysiol.2002.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisova P, Concannon CG, Devocelle M, Prehn JHM, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29:2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE. The localization of latent brain hexokinase on synaptosomal mitochondria. Arch Biochem Biophys. 1972;150:96–104. doi: 10.1016/0003-9861(72)90015-x. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ. Oxidation of (14)C-labeled compounds perfused by microdialysis in the brains of free-moving rats. J Neurosci Res. 2007;85:3145–3149. doi: 10.1002/jnr.21424. [DOI] [PubMed] [Google Scholar]