Abstract
The ability of the brain to locally augment glucose delivery and blood flow during neuronal activation, termed neurometabolic and neurovascular coupling, respectively, is compromised in Alzheimer's disease (AD). Since perfusion deficits may hasten clinical deterioration and have been correlated with negative treatment outcome, strategies to improve the cerebral circulation should form an integral element of AD therapeutic efforts. These efforts have yielded several experimental models, some of which constitute AD models proper, others which specifically recapture the AD cerebrovascular pathology, characterized by anatomical alterations in brain vessel structure, as well as molecular changes within vascular smooth muscle cells and endothelial cells forming the blood–brain barrier. The following paper will present the elements of AD neurovascular dysfunction and review the in vitro and in vivo model systems that have served to deepen our understanding of it. It will also critically evaluate selected groups of compounds, the FDA-approved cholinesterase inhibitors and thiazolidinediones, for their ability to correct neurovascular dysfunction in AD patients and models. These and several others are emerging as compounds with pleiotropic actions that may positively impact dysfunctional cerebrovascular, glial, and neuronal networks in AD.
Keywords: amyloid-β, cholinesterase inhibitor, fibrosis, oxidative stress, pioglitazone, transforming growth factor-β 1, transgenic mice
Introduction
Alzheimer's disease (AD) is the most common cause of dementia in the elderly. It is characterized by a gradual decline in memory and cognition, which has been correlated with synaptic dysfunction and loss, and eventually to neuronal death (Querfurth and LaFerla, 2010). The disease is confirmed postmortem on detection of amyloid-β (Aβ) plaques and neurofibrillary tangles in the brain. These are respectively composed of aggregated Aβ derived from the amyloid precursor protein (APP) and of abnormally hyperphosphorylated τ, a microtubule-associated protein involved in cytoskeletal stabilization (Wang et al, 2007). Cytokines, chemokines, and free radicals emanating from activated glial cells recruited at the site of plaques promote an inflammatory and oxidative state (Akiyama et al, 2000; Butterfield et al, 2001). Already at the preclinical stage, the AD brain is characterized by a reduced blood supply at rest (Farkas and Luiten, 2001; Hirao et al, 2005; Bateman et al, 2006; Vicenzini et al, 2007; Claassen et al, 2009) and altered perfusion to activated areas, i.e., altered neurovascular coupling or functional hyperemia (Hock et al, 1997; Mentis et al, 1998; Rombouts et al, 2000; Machulda et al, 2003; Rosengarten et al, 2006). It is debated whether the latter is the result of decreased demand by diseased tissue or of a compromised cerebral vasculature that is unable to optimally increase cerebral blood flow (CBF) in activated areas. The CBF insufficiency poses a threat for homeostasis and protein synthesis underlying learning (Iadecola, 2004), and could activate hypoxia-sensitive pathways culminating in the upregulation of proteins, such as Aβ and transforming growth factor-β 1 (TGF-β1) (Krupinski et al, 1996; Bennett et al, 2000; Sun et al, 2006), which have been shown to be detrimental for cerebrovascular structure and function (Wyss-Coray et al, 1997, 2000; Iadecola et al, 1999; Gaertner et al, 2005; Tong et al, 2005; Han et al, 2008). In parallel with or as a consequence of cerebrovascular dysfunction, AD patients feature decreased basal cerebral glucose utilization (CGU), particularly in parietotemporal and posterior cingulate cortex (Langbaum et al, 2009), and altered neurometabolic coupling (Melrose et al, 2009) that would be expected to instigate a brain energy crisis. Dysfunctional neurons, astrocytes, and vascular cells that together coordinate the perfusion response are most likely at the root of impaired neurovascular and neurometabolic coupling in AD. Much of this knowledge is derived from in vitro and in vivo experimental models, and these will be presented and reviewed. Finally, we will critically evaluate two drug classes with recently demonstrated efficacy against AD neurovascular dysfunction, and evaluate their usefulness in improving clinical outcome or response to AD therapy.
Neurovascular dysfunction in Alzheimer's disease
Chronic brain hypoperfusion (Farkas and Luiten, 2001; Hirao et al, 2005; Bateman et al, 2006; Vicenzini et al, 2007; Claassen et al, 2009) and altered neurovascular coupling (Hock et al, 1997; Mentis et al, 1998; Rombouts et al, 2000; Machulda et al, 2003; Rosengarten et al, 2006) are prominent features of AD. Resting CBF in human is ∼50 ml/100 g brain per minute. In AD patients, CBF is reduced by 10% to 30% in temporoparietal, frontal, and posterior cingulate cortex, and in hippocampus relative to age-matched elderly subjects, and these decreases correlate with dementia severity (for review, see Farkas and Luiten (2001)). Further, the decreases in resting CBF have been shown to be powerful correlates of progression to AD (Hirao et al, 2005) and of response to treatment (Yoshida et al, 2007). The localized CBF increase associated with mental tasks is equally altered relative to that of age-matched control patients, being either reduced (Mentis et al, 1998; Machulda et al, 2003; Rosengarten et al, 2006) or simultaneously reduced in some brain areas, while increased in others, perhaps in compensation (Rombouts et al, 2000; Scarmeas et al, 2004; Drzezga et al, 2005; Peters et al, 2009). For example, CBF was significantly reduced in medial temporal lobe structures of AD patients during color picture encoding, but increased in the cerebellum (Rombouts et al, 2000). In Peters et al (2009), AD patients showed reduced CBF responses in various fronto-temporal areas during the encoding of short word lists as well as during the recognition phase of the task, but an increased response in the fusiform gyrus, a structure thought to be involved with orthographic processing. The hemodynamic changes seem to become established very early in the pathological process, as they are manifest in populations at risk of developing AD, i.e., individuals with mild cognitive impairment or those expressing the ɛ4 allele of apolipoprotein E, the most consistent genetic factor linked to increased AD risk and lower age of onset (Poirier et al, 1993; Strittmatter et al, 1993). In these groups, neurovascular coupling is either impaired (Lind et al, 2006) or increased in a compensatory manner as in AD patients, being characterized by a larger response magnitude, and neuronal recruitment to achieve performance at the level of healthy elderly controls (Bookheimer et al, 2000; Yetkin et al, 2006; reviewed in Wermke et al (2008)). As shown by Bookheimer et al (2000), the local perfusion increase during verbal recall was greater in cognitively normal apolipoprotein E ɛ4 relative to apolipoprotein E ɛ3 carriers, involved a larger number of brain regions, and correlated with cognitive decline in the apolipoprotein E ɛ4 subjects two years later.
The Default Mode Network
In the last decade, a new concept of brain activation has emerged, allowing a more comprehensive understanding of the altered perfusion patterns in AD patients and in susceptible populations (Raichle et al, 2001; Wermke et al, 2008). According to this view, increases in perfusion during specific cognitive tasks are accompanied by decreases in other areas not required for the task, and it is thought that the deactivation of the latter regions permits resource reallocation toward task-relevant areas. The structures that are deactivated during goal-directed activities are otherwise tonically active, functionally interconnected, and part of a synchronized resting-state network (Sorg et al, 2007). One of the well-studied resting-state networks is known as the default mode network, comprising the posterior cingulate/precuneus, lateral parietal cortex, and medial prefrontal cortex, a network exhibiting high activity at rest, but suspended (deactivated on positron emission tomography and functional magnetic resonance imaging studies) during challenging cognitive activities, such as memory encoding (Raichle et al, 2001; for review, see Sperling et al (2010)). Evidence suggests that the ability to deactivate resting-state networks, including the default mode network, is already impaired in mild cognitive impairment and, to a greater extent, in AD patients (Rombouts et al, 2000; Lustig et al, 2003; Drzezga et al, 2005; Celone et al, 2006) (Figure 1). It has even been postulated that detecting changes in deactivation patterns may be particularly useful in early diagnosis or have predictive value, as these patterns were able to discriminate between a subgroup of mild cognitive impairment subjects who progressed to AD and those who remained cognitively intact during a two-year follow-up period (Drzezga et al, 2005). The extent to which vascular versus neuronal factors contribute to these impairments is still unsettled, and complicated by the altered status of astrocytes, key mediators of the hyperemic response (Haydon and Carmignoto, 2006). Evidence supports that all components of the tripartite neurogliovascular unit (Hamel, 2006) are affected, and are likely the basis of altered neurovascular coupling patterns in AD.
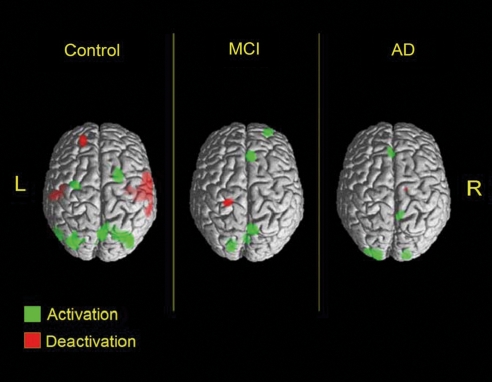
Figure 1.
The concept of brain deactivation in Alzheimer's disease (AD). The ability to deactivate task-irrelevant auditory cortices during active spatial navigation was decreased in subjects with mild cognitive impairment (MCI), and to a greater extent, in those with AD. Altered regional cerebral blood flow (CBF) patterns were measured with O15-positron emission tomography (PET). Reproduced from Drzezga et al (2005).
The Neurogliovascular Unit
During brain activation, the control of CBF relies on the concerted action of neurons, astrocytes, and vascular cells making up a tripartite entity called the neurogliovascular unit (Iadecola, 2004; Hamel, 2006). Signals generated by neurons are ultimately transduced by cerebrovascular smooth muscle to locally adjust blood flow for the duration of the activation. The nature of the vasoactive mediators released during neurovascular coupling continues to be an area of intense investigation. It appears to depend on the type, frequency, and duration of the incoming afferent input and on its processing by the locally activated neuronal network. Vasoactive messengers released can be of neuronal, astroglial, and vascular origin (reviewed in Koehler et al (2009) and Cauli and Hamel (2010)). In addition, it is believed that diffuse projections from subcortical nuclei can modulate the neurovascular coupling response, either by influencing neuronal output or by directly causing vascular diameter change via contacts with intracerebral arterioles expressing relevant receptors. For example, cortical perfusion can be directly increased by stimulating the cholinergic basal forebrain (Biesold et al, 1989), the main source of cholinergic innervation to the neocortex. On stimulation, perivascular cortical afferents release acetylcholine (ACh) onto endothelial M5 muscarinic ACh receptors (Elhusseiny and Hamel, 2000; Yamada et al, 2001). This leads to nitric oxide (NO)-mediated vasodilation and CBF increase (Vaucher and Hamel, 1995; Zhang et al, 1995; Kocharyan et al, 2008). The cholinergic system, which degenerates in AD (Whitehouse et al, 1981; Tong and Hamel, 1999), is central to attention (reviewed in Klinkenberg et al (2010)) and memory processes (reviewed in Micheau and Marighetto (2011)). Given the complex relationship among neurogliovascular components, and their interdependence in mounting an appropriate CBF response, a compromise in one or more of these elements could affect the functionality of the entire unit.
Neuronal Dysfunction
Neurons are the initiators of the functional hyperemic response. In AD, their function is threatened by pathological cascades associated with Aβ, hyperphosphorylated τ, free radicals, inflammatory factors, and perhaps still unidentified factors that lead to neurodegeneration (Querfurth and LaFerla, 2010). Presynaptic and postsynaptic markers are decreased, trophic factors, neurotransmitters, and their respective receptors are likewise reduced, as is the neuronal glucose transporter-3 (Simpson et al, 1994), and disturbances in axonal transport linked with neurofibrillary tangles and microtubule disarrangement are evident (Querfurth and LaFerla, 2010). The basal forebrain cholinergic system degenerates (Whitehouse et al, 1981; Sassin et al, 2000) along with noradrenaline-containing neurons of the locus coeruleus (Tomlinson et al, 1981), and there are decreases in levels of dopaminergic, serotoninergic, and somatostatin markers in various brain areas (Rossor et al, 1980; Arai et al, 1984). The glutamatergic system is also affected, with excessive activation of glutamate receptors resulting in neuronal Ca2+ overload and possibly, excitotoxic death (Hynd et al, 2004). Interestingly, neuronal hyperexcitability has also been documented in an AD mouse model overexpressing mutated human APP. This was measured using in vivo electroencephalogram in freely behaving 3- to 7-month-old animals (Palop et al, 2007). The ensuing compensatory inhibitory drive, consisting of GABAergic sprouting and strengthened inhibitory postsynaptic currents, resulted in profoundly altered neuronal networks (Palop et al, 2007). In AD, such cellular and synaptic changes may underlie the reduced capacity to disengage the default mode network during complex cognitive tasks. Namely, abnormal GABAergic function and altered ability for cross-modal inhibition would interfere with the brain deactivation required for successful memory encoding. Evidence for a loss of inhibitory neuronal function has been reported in AD (Bélanger et al, 2010), leading to the hypothesis that cognitive and neurovascular dysfunction may be attributed, in part, to an imbalance between neuronal excitation and inhibition (Palop and Mucke, 2010).
Vascular Dysfunction
Profound alterations in the AD cerebral vasculature are believed to contribute to perfusion irregularities during neuronal activation (Mancardi et al, 1980; Roher et al, 1993; Vinters et al, 1994; Kalaria and Pax, 1995). These cerebrovascular changes may be important early factors in the aberrant hyperemic responses, as suggested by their presence in populations that are prone to develop AD, namely the elderly, subjects with chronic cardiovascular disorders (hypertension, hypercholesterolemia, and diabetes), stroke, and head trauma patients, and more so in groups exhibiting several of these factors at once (Luchsinger et al, 2005). The molecular mediators underlying cerebrovascular alterations in these populations and in AD are not completely elucidated, but strong evidence implicates increased levels of the Aβ and TGF-β1 peptides (Krupinski et al, 1996; Wyss-Coray et al, 1997; Grammas and Ovase, 2002; Peterson, 2005). In AD, aggregated forms of Aβ1−40 and Aβ1−42 settle within vessel walls of small to medium arteries, leading to cerebral amyloid angiopathy (CAA) (Roher et al, 1993). The deposition of amyloid in circumferential bands is consistent with smooth muscle cell synthesis (Roher et al, 1993); however, initial deposits on the abluminal side of the smooth muscle also suggest a neuronal origin. As the amyloid makes its way toward capillaries and arterioles, faulty clearance across the blood–brain barrier, and increased arterial stiffness hindering vessel pulsations that drive perivascular Aβ drainage would result in abluminal deposition (Herzig et al, 2006; Weller et al, 2008). Interestingly, CAA may also be secondary to profibrotic vascular remodeling mediated by TGF-β1 (Wyss-Coray et al, 1997). The TGF-β1-induced upregulation of basement membrane components, such as collagen and perlecan that bind Aβ (Castillo et al, 1997), could promote CAA by enhancing vessel ‘adhesiveness.' With severe CAA, amyloid gradually replaces the vascular smooth muscle, impairing vasomotor function, weakening the vessel wall, and increasing the risk of cerebral hemorrhage (Christie et al, 2001; Boche et al, 2008; Han et al, 2008).
However, before any visible CAA, the animal literature suggests that soluble Aβ potently deregulates cerebrovascular function by activating a free radical cascade that substantially reduces vasodilator half-life and imparts oxidative damage to cerebrovascular enzymes and receptors (Price et al, 1997; Iadecola et al, 1999; Tong et al, 2005; Park et al, 2005, 2008). Soluble Aβ also potentiates constrictions to endothelin-1 (ET-1) when applied to isolated human cerebral arteries collected after rapid autopsies, seemingly via a proinflammatory cascade (Townsend et al, 2002; Paris et al, 2003). Moreover, the cholinotoxic properties of soluble Aβ (Auld et al, 2002; Aucoin et al, 2005) may be responsible for the cholinergic deafferentation of intracerebral microvessels in AD (Tong and Hamel, 1999). This perivascular denervation could explain some of the reduced hyperemic responses, particularly during tasks demanding attention, which recruit the basal forebrain. In turn, our studies in transgenic mice overproducing TGF-β1 have shown that the peptide interferes with cerebrovascular dilatory and contractile capacity by altering levels or activities of enzymes and downstream signaling cascades in the vessel wall (Tong et al, 2005; Tong and Hamel, 2007; Nicolakakis et al, 2011; Papadopoulos et al, 2010). Within cerebrovascular cells, several molecular changes could be directly responsible for altered hemodynamic responses. These include downregulation of the endothelial glucose transporter-1 (Simpson et al, 1994) and changes in endothelial receptors regulating brain Aβ influx and efflux at the level of the blood–brain barrier; upregulation of the receptor for advanced glycation end products that brings Aβ into the brain and downregulation of the low-density lipoprotein receptor-related protein that transports Aβ into the circulation (Deane et al, 2003). In AD vascular smooth muscle cells, increased levels of serum response factor, and myocardin, which regulate contractile protein expression, have been associated with a hypercontractile phenotype and reduced activity-driven CBF increase (Chow et al, 2007).
Astrocytic Dysfunction
Astrocytes are considered intermediaries in neurovascular coupling, bridging neuronal activity with perfusion through their ensheathment of neuronal synapses on one hand, and vascular appositions on the other (Haydon and Carmignoto, 2006). It can be appreciated that dysfunction in the astrocytic compartment should therefore affect the evoked hyperemic response. Indeed, in AD, several pathological cascades are induced within astrocytic cells, including expression of the β-site APP cleaving enzyme 1 that generates Aβ (Hartlage-Rübsamen et al, 2003). This suggests that astrocytes can transition from an amyloid clearing (Wyss-Coray et al, 2003) to an amyloid-generating activity with detrimental consequences for neurons and vascular cells. Moreover, there is indication that basic astrocytic communication through Ca2+ signaling is affected (Takano et al, 2007; Kuchibhotla et al, 2009). In a transgenic mouse model of AD, astrocytes exhibited higher resting intracellular Ca2+ levels viewed with in vivo multiphoton imaging. Astrocytes also exhibited a greater number of spontaneous Ca2+ oscillations that were relatively rare in wild-type (WT) animals, and these spontaneous events were of higher amplitude and unrelated to neuronal activity, as evaluated in the presence of the sodium channel blocker tetrodotoxin (Kuchibhotla et al, 2009). The astrocytic hyperactivity was initiated by a single astrocyte in proximity to an Aβ plaque, and subsequently spread as an intercellular Ca2+ wave to distant astrocytes up to ∼200 μm away at speeds of ∼23 μm/s (Kuchibhotla et al, 2009). This suggested that hyperactive astrocytic networks could underlie global cortical and neurovascular coupling alterations in AD. Other studies have found that astrocytic end feet, which immediately contact the vasculature, exhibit loss of the water channel aquaporin 4 and of the inward rectifying voltage-gated potassium channel Kir4.1 (Wilcock et al, 2009). The latter is important for removal of rising extracellular K+ from actively firing neurons, and for regulation of potassium signaling that can initiate diameter changes in the local microcirculation (Filosa et al, 2006). Finally, it is believed that chronic astroglial secretion of inflammatory mediators can induce damage in neuronal and vascular cells that respectively initiate and execute the hyperemic response. It is, however, also possible that inflammatory activity within astrocytes could interfere with neurovascular coupling via defective synthesis and release of vasomediators, such as epoxyeicosatrienoic acids (EETs) (Koehler et al, 2009). The combined changes would contribute to a pathological astroglial phenotype that is expected to profoundly affect neurovascular function.
Experimental models of Alzheimer's disease neurovascular dysfunction
Artery Occlusion/Stenosis
One of the earliest attempts to investigate the role of the cerebral circulation in AD pathogenesis was through artery occlusion experiments in rodents (reviewed in Farkas et al (2007)). Ligation of the common carotid arteries bilaterally, referred to as two-vessel occlusion, produced a significant CBF drop, astrocyte activation, and neurodegenerative changes in the brain that were associated with learning and memory impairments in behavioral tests (de la Torre et al, 1992; reviewed in Farkas and Luiten (2001)). Although an attractive attempt to infer causality between hypoperfusion and dementia, the two-vessel occlusion paradigm often suffered from the confounding effect of pyramidal cell damage or death and of cholinergic deficits. It also failed to mimic the gradual and chronic CBF decline that characterizes AD patients. More recent attempts to develop four-vessel occlusion in rats highlighted the importance of choosing the right type of vessel for ligation and age of the animal, as these could dictate neuropathological and behavioral outcomes (Barros et al, 2009). For example, occlusion of the vertebral arteries and of the common carotid arteries 1 week later resulted in hippocampal neurodegeneration, retinal lesion, and cognitive deficits in young rats 40 days after four-vessel occlusion. However, occlusion of the two vertebral and two internal carotid arteries led to a mild learning deficit in old rats only, and spared overall hippocampal cell density and retinal integrity (Barros et al, 2009). Accumulation of APP and its proteolytic products had been shown after ischemic and axonal injury, as a result of disrupted axonal transport (Smith et al, 2003). Indeed, increases in APP immunoreactivity were observed in astrocytic processes and dystrophic axons of the cortex and hippocampus of rats that had undergone repeated, reversible occlusion of the middle cerebral artery or two-vessel occlusion. However, no Aβ plaque deposition was observed (Kalaria et al, 1993). More recently, transient middle cerebral artery occlusion (2 hours) in rats led to focal cerebral ischemia and to the accumulation, 1 week later, of APP and Aβ into diffuse aggregates in the corpus callosum, thalamus, and cortical areas adjacent to the infarct. Nine months later, these aggregates persisted only in the thalamus, and had turned into dense, plaque-like deposits. However, they could not be stained with Congo red or Thioflavine S (van Groen et al, 2005). These two routinely used histological dyes detect amyloid-like proteins displaying the β-pleated sheet conformation that is typical of mature Aβ plaques. At present, the occlusion models remain good tools with which to address the role of chronic hypoperfusion in AD and in other dementias where CBF is impaired, provided confounding factors can be adequately controlled. With the recent adaptation of these techniques to mice, which involves the insertion of microcoils in the common carotids to induce stenosis rather than complete occlusion (Miki et al, 2009), it will be possible to evaluate additional effects of CBF reduction in transgenic AD mouse models (see Transgenic mouse models). Indeed, exposure of transgenic APP-overexpressing mice to a hypoxic environment potentiated Aβ deposition and memory deficit (Sun et al, 2006), suggesting an aggravating role of hypoxic events on AD pathogenesis.
Exogenous Aβ Application
It was mainly work from the group of Iadecola that drew attention to the deleterious vasoactive effects of Aβ exposure in vivo. Until then, most demonstrations had been obtained from in vitro studies on isolated human or animal cerebral arteries treated with Aβ. Those studies had shown enhanced vasoconstrictions to serotonin (5-HT) and ET-1, and impaired endothelium-dependent relaxations to ACh and bradykinin, which were attributed either to oxidative (Price et al, 1997; Thomas et al, 1997) or to proinflammatory cascades (Townsend et al, 2002; Paris et al, 2003). Topical superfusion of soluble Aβ1−40 on the mouse neocortex yielded results that were in line with the in vitro findings. It led to an attenuation of resting CBF and of the CBF increase evoked by whisker stimulation or by endothelium-dependent vasodilators, as measured by laser Doppler flowmetry (LDF) (Niwa et al, 2000a, 2000b; Deane et al, 2003; Park et al, 2005; Takeda et al, 2009). These effects were abolished by free radical scavengers or by substitution of methionine with norleucin at residue 35 of Aβ1−40, a modification that prevents free radical generation by the peptide (Niwa et al, 2000b). The impairments were also abolished by gp91ds-tat, a peptide inhibitor of NADPH oxidase, the major source of superoxide (O2•−) in cerebral vessels. Further, no deficits were seen in mice lacking the gp91phox catalytic subunit of NADPH oxidase (Park et al, 2005). The collective findings supported an Aβ-induced oxidative stress hypothesis implicating NADPH oxidase-derived O2•−. At odds with this body of evidence was a study by the group of Rowan. The authors developed a method to simultaneously record electrophysiological and blood flow responses in rat CA1 after electrical stimulation of the Schaffer collateral/commissural pathway. High-frequency stimulation protocols triggered long-term potentiation and hippocampal blood flow increase, as measured with LDF. Intracerebroventricular injection of soluble Aβ1−40 dimers, Aβ1−42 or of the vasculotropic Aβ1−40 E22Q 30 minutes before high-frequency stimulation inhibited long-term potentiation, but had no effect on simultaneously recorded hyperemia or resting blood flow. In addition, a dose of Aβ1−42 capable of lowering baseline synaptic transmission by 25% did not alter baseline perfusion in the dorsal hippocampus (Hu et al, 2008). These findings challenged the view that soluble Aβ could initiate vascular dysfunction at concentrations inhibiting neuronal function. It also suggested that vascular deficits may act as secondary aggravating factors to the Aβ-induced neuronal pathology.
Transgenic Mouse Models
The advent of transgenic mice provided new opportunities to study the mechanisms of AD neurovascular dysfunction. Mice overexpressing mutated human APP or the Aβ-generating presenilin associated with rare early-onset AD exhibited AD neuropathology, cerebrovascular dysfunction, and memory impairments. This was especially true for mice harboring mutated human APP alone or combined with mutated human presenilin, whereas overexpression of the WT proteins in the first-generation transgenic models mostly failed to produce Aβ deposits (Mucke et al, 2000; reviewed in Dodart and May (2005)). Much progress has been afforded by transgenic research; however, the disappointing clinical trials that have derived from it (Abbott, 2008) remind us that the challenge remains to determine the relevance of transgenic mouse phenotypes to the human disease. Study interpretations should take into account differences between mouse and human physiology, and the potentially unknown alterations in physiology and behavior that may be initiated by insertion of human genes into the mouse genome. Other concerns include the difference between protein overexpression and natural protein upregulation or downregulation, as well as the importance of protein expression in a cell- and isoform-specific manner. Case in point is that neurons abundantly express APP695, whereas some APP models have been engineered to overexpress, in neurons, an alternatively spliced minigene encoding all three human APP isoforms, APP751, 770, 695 (Games et al, 1995; Mucke et al, 2000). With these caveats in mind, we highlight the following transgenic models that have greatly contributed to investigations of AD neurovascular dysfunction and to the development of therapies.
Amyloid precursor protein mice
Transgenic mice overexpressing mutated human APP with or without presenilin overproduce Aβ and develop cerebrovascular dysfunction as an early feature, often before several other neuropathological and behavioral AD symptoms (Iadecola et al, 1999; Niwa et al, 2000a, 2002a, 2002b; Park et al, 2005; Tong et al, 2005; Takeda et al, 2009). Young APP mice (2 to 3 months old) devoid of plaque pathology and neuronal loss display an attenuated perfusion response to endothelium-dependent vasodilators, and exaggerated CBF decrease to a constrictor, the thromboxane A2 analog U46619 (Iadecola et al, 1999). In addition, APP mice feature impaired autoregulatory ability, a homeostatic mechanism that ensures constant blood flow during changes in mean arterial pressure. Animals thus lack protection against ischemia (Niwa et al, 2002b; Takeda et al, 2009). The CBF increase to hypercapnia and endothelium-independent vasodilators such as SNAP (S-nitroso-N-acetylpenicillamine) or SNP (sodium nitroprusside) is either maintained (Iadecola et al, 1999; Niwa et al, 2000a; Christie et al, 2001; Shin et al, 2007) or impaired (Park et al, 2005; Han et al, 2008) in young Tg2576 mice, suggesting variable degree of smooth muscle dysfunction. However, the response to whisker stimulation is diminished, with the reduction being commensurate to levels of soluble brain Aβ1−40 and Aβ1−42 encountered in the various APP lines tested (Tg6209, Tg2123, and Tg2576) (Niwa et al, 2000a) (Table 1). However, a firm statement about the identity of the Aβ isoform responsible for the deficits could not be made. Furthermore, it was unclear in the latter study if neuronal dysfunction had a role. Quantitative radioautographical determination of CGU showed intact resting and evoked glucose usage in Tg2123 mice (Niwa et al, 2000a), but a subsequent study showed reduced basal glucose utilization in Tg2576 mice that are derived from a different background strain and produce higher Aβ levels (Niwa et al, 2002a). Recently, Takeda et al (2009) adjusted the magnitude of whisker stimulation so as to equalize somatosensory evoked potentials recorded at the site of CBF measurement. The authors were thus able to exclude that differences in neuronal activation accounted for the functional hyperemic deficit between 3-month-old WT and APP23 mice. Hence, the data in young transgenic APP mice support the deleterious effects of soluble Aβ on cerebrovascular function.
Table 1. Neurovascular coupling deficits during sensory stimulation in transgenic mouse models of AD or of the AD cerebrovascular pathology.
| Model | Age (months) | Deficit (%) | Technique | Reference |
|---|---|---|---|---|
| APP | ||||
| Tg6209 | 2–3 | 20a | LDF | Niwa et al (2000a) |
| Tg2123 | 2–3 | 47a | LDF | Niwa et al (2000a) |
| Tg2123 | 2–3 | 39 | IAP | Niwa et al (2000a) |
| Tg2576 | 2–3 | 60a | LDF | Niwa et al (2000a) |
| Tg2576 | 2–3 | 48a | LDF | Park et al (2005) |
| Tg2576 | 3–4 | 42a | LDF | Park et al (2008) |
| Tg2576 | 8 | NS | LSF | Shin et al (2007) |
| Tg2576 | 12–15 | 21a | LDF | Park et al (2008) |
| Tg2576 | 19 | 44a | LSF | Shin et al (2007) |
| J20 | 7.5 | 37 | LDF | This paper |
| J20 | 12 | 36 | LDF | Tong et al (2009) |
| J20 | ∼15 | 42 | LDF | Nicolakakis et al (2008) |
| APP23 | 3 | 73a | LDF | Takeda et al (2009) |
| APP23 | 6 | NS | fMRI | Mueggler et al 2003) |
| APP23 | 13 | NS | fMRI | Mueggler et al (2003) |
| APP23 | 25 | 54 | fMRI | Mueggler et al (2003) |
| TGF | ||||
| T64 | 6–8 | 38 | LDF | Ongali et al (2010) |
| T64 | 12 | 27* | LDF | Ongali et al (2010) |
| T64 | ∼18 | 24 | LDF | Nicolakakis et al (2011) |
| T64 | ∼18 | 27 | LDF | Papadopoulos et al (2010) |
| APP/TGF | ||||
| J20/T64 | 6–8 | NS | LDF | Ongali et al (2010) |
| J20/T64 | 12 | 31 | LDF | Ongali et al (2010) |
| J20/T64 | ∼18 | 41 | LDF | Ongali et al (2010) |
AD, Alzheimer's disease; APP, amyloid precursor protein; NS, not significant; IAP, quantitative 14C-labeled iodoantipyrine technique in awake mice; LDF, laser Doppler flowmetry; LSF, laser speckle flowmetry; TGF, transforming growth factor-β1.
Value is an estimate obtained from manuscript graph by comparing values between transgenic and age-matched WT mice (hence standard errors are not indicated). *P=0.057.
Interestingly, and in accord with the in vitro and in vivo superfusion data (see Exogenous Aβ application), endothelial dysfunction does not occur in young APP mice co-overexpressing the O2•− scavenger, superoxide dismutase (SOD), or receiving neocortical SOD application (Iadecola et al, 1999). Similarly, there is no impairment in the evoked CBF response to whisker stimulation or endothelium-dependent (ACh, bradykinin, and calcium ionophore A23187) and -independent (SNAP) vasoactive stimuli in young APP mice lacking the gp91phox NADPH oxidase catalytic subunit (Park et al, 2005). Our own work in young APP mice (line J20; Mucke et al, 2000) corroborates the cerebrovascular dysfunction induced by soluble Aβ through oxidative stress. Dilatory responses to ACh and calcitonin gene-related peptide were diminished by ∼50%, and there was reduced availability of NO, a vasodilator gas constitutively released by the endothelium and responsible, along with opposing effects from ET-1, for establishing vascular tone (Tong et al, 2005). Brief incubation of arterial segments with SOD or catalase that respectively eliminate O2•− and hydrogen peroxide radicals, quickly restored NO bioavailability and ACh-mediated dilatations. Similarly, the ACh response was recovered in cerebral arteries from old APP mice (over 18 months old) incubated with the NADPH oxidase inhibitor, apocynin (Hamel et al, 2008). The interaction between O2•− and NO yields peroxynitrite (ONOO-), detectable in the young APP vasculature as a nitrotyrosine immunoreactive product (Park et al, 2004; Tong et al, 2005). This oxidizing intermediate is capable of inflicting nitrosative stress on proteins like SOD (Guo et al, 2003) and on KATP channels that mediate calcitonin gene-related peptide relaxation in mouse cerebral arteries (Tong et al, 2009). Hence, eliminating O2•− can restore cerebrovascular function and prevent a self-perpetuating cycle of oxidative damage. Remarkably, and as a testament to the reversibility of this process, cerebrovascular rescue has been achieved even in aged APP mice (12 to 16 months old) after genetic (Park et al, 2008) or pharmacological interventions with antioxidants (Nicolakakis et al, 2008). The reversibility of cerebrovascular dysfunction at an advanced age when Aβ deposition, cholinergic denervation, and mnemonic impairment have developed is particularly relevant for AD patients who currently receive diagnosis at a late stage.
The above evidence suggests that cerebrovascular dysfunction in young APP mice lacking CAA is due to a free radical cascade triggered by soluble Aβ (Figure 2A). In older mice, the combined actions of soluble and deposited Aβ further impair vasodilatory and contractile capacity by damaging, and eventually destroying, vascular smooth muscle (Mueggler et al, 2003; Han et al, 2008; Park et al, 2008) (Figures 2A and 2B). In these mice, the effects of soluble Aβ can still be appreciated in CAA-free segments of the vasculature, when Aβ deposition is patchy and has not progressed to the entire arterial segment (Beckmann et al, 2003; Han et al, 2008; Tong et al, 2009) (Figure 2C). After this stage, both endothelium-dependent and -independent responses are impaired. Hence, Aβ-directed therapy to deplete soluble Aβ only partly restores function, suggesting irreversible CAA-induced damage (Han et al, 2008). However, the prevailing view of soluble Aβ vasoactivity has been challenged by studies showing the absence of cerebrovascular deficits after Aβ superfusion (Hu et al, 2008). Further, in 6- to 8-month-old Tg2576 mice, cerebrovascular responses to ACh and whisker stimulation were not different from those of WT littermates, and only became attenuated at 14 or 19 months of age (Christie et al, 2001; Shin et al, 2007). Authors credited the discrepancies to the noninvasiveness of their procedures that avoided exposing the cortex, in contrast to previous open cranial window preparations. However, Han et al (2008) had used a closed cranial window technique to reveal impaired dilatations to ACh, SNAP, and hypercapnia in the same 6-month-old Tg2576 mice (Figure 2B). Work from our laboratory on the J20 APP model has shown impaired hyperemic responses to whisker stimulation at 12 and ∼15 months of age using an intact skull preparation (Table 1) (Nicolakakis et al, 2008; Tong et al, 2009). APP mice carrying the vasculotropic London APP mutation further complicate the relationship between cerebrovascular function and Aβ. In these mice, resting CBF and hypercapnia measured by LDF were normal despite extensive CAA at 24 months of age (Van Dorpe et al, 2000). A comparative study of models and techniques might ultimately shed more light on these discrepancies.
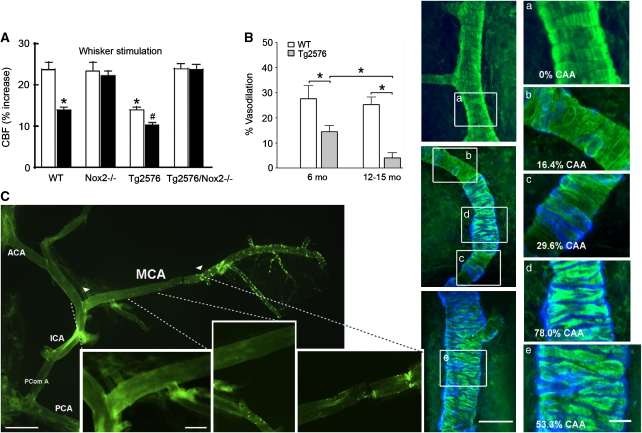
Figure 2.
The role of soluble and insoluble Aβ in cerebrovascular dysfunction of amyloid precursor protein (APP) mice. (A) The cerebral blood flow (CBF) increase to whisker stimulation is impaired in 3- to 4-month-old Tg2576 mice lacking Aβ deposits, and more so in 12-month-old Tg2576 animals, as assessed through open cranial window and laser Doppler flowmetry (LDF). Deficits were absent in Tg2576 mice missing the Nox2 catalytic NADPH oxidase subunit. This implicates soluble Aβ-induced oxidative stress in the dysfunctions. White bars, 4 months old; Black bars, 12 months old; WT, wild type; *, different from WT; #, different from young Tg2576 mice. Reproduced from Park et al (2008) by The National Academy of Sciences of the USA. (B) Hypercapnia-induced dilatation is impaired in 6-month-old Tg2576 mice lacking cerebral amyloid angiopathy (CAA), and further impaired in 12- to 15-month-old animals with increasing CAA severity and smooth muscle cell disarrangement, the latter illustrated and quantified in panels (a–e). CAA: methoxy-X04 stained blue; smooth muscle cells: phalloidin stained green. Scale bars: left, 50 μm; right, 20 μm. Reproduced from Han et al (2008) with permission conveyed through Copyright Clearance Center, Inc. (C) Reduced cerebrovascular responsiveness in the proximal CAA-free segment of the middle cerebral artery (MCA) (delineated by arrowheads, with first, second, and third subsegments magnified in the three panels) suggests effects of soluble Aβ in a 12-month-old J20 APP mouse. Scale bars: left, 0.5 cm; right, 0.2 cm. ACA, anterior cerebral artery; ICA, internal carotid artery; PComA, posterior communicating artery; PCA, posterior cerebral artery. Reprinted from Tong et al (2009) with permission from Elsevier.
Transforming growth factor-β1 mice
Investigations of the AD cerebrovascular pathology have been centered on CAA, especially in light of its undesirable increase during Aβ immunotherapy and the associated risk of cerebral hemorrhage in clinical trials (Boche et al, 2008). Amyloid precursor protein mice have been instrumental in such research. However, they do not reproduce the cerebrovascular fibrosis that also characterizes AD, and that some have hypothesized to be a factor in cerebrovascular Aβ deposition (Wyss-Coray et al, 1997). Transgenic mice overexpressing constitutively active TGF-β1 in astrocytes (TGF mice) were generated to clarify the role of TGF-β1 increase in AD, and more importantly, to address the cerebrovascular pathology related to the basement membrane (Wyss-Coray et al, 1995). The latter is thickened (Mancardi et al, 1980; Vinters et al, 1994) as a result of accumulation of collagen IV (Kalaria and Pax, 1995) and that of other matrix proteins, a phenomenon associated with high levels of TGF-β1 in AD vessels (Grammas and Ovase, 2002). Transforming growth factor mice feature increased expression of vascular growth factors (vascular endothelial growth factor; connective tissue growth factor), and accumulation of perlecan, fibronectin, laminin, and collagen in the vascular basement membrane that contribute to its thickening (Wyss-Coray et al, 1995, 2000; Tong et al, 2005; Nicolakakis et al, 2011). Not only do some of these proteins have the capacity to bind Aβ, and potentially initiate CAA (Castillo et al, 1997), but also their accumulation in capillary basement membranes could hinder substrate delivery and waste elimination across the blood–brain barrier. In line with this idea, TGF mice feature increased capillary basement membrane thickness and endothelial cell degeneration (Wyss-Coray et al, 2000), resting hypoperfusion throughout the brain (Gaertner et al, 2005), impaired neurovascular coupling during whisker stimulation (Nicolakakis et al, 2011) (Table 1), and reduced basal CGU (Galea et al, 2006). The hemodynamic and metabolic changes may be related to glial activation in the TGF mouse brain (Wyss-Coray et al, 1995; Lacombe et al, 2004; Nicolakakis et al, 2011), but are mainly believed to have a vascular etiology, in view of the unaltered neuronal indices in old transgenic animals relative to age-matched WT littermates (18 to 22 months old). At this age, TGF mice featured preserved cortical cholinergic innervation, intact CGU increase during whisker stimulation, as measured by 18F-fluorodeoxyglucose-positron emission tomography, and preserved spatial memory in the Morris water maze (Nicolakakis et al, 2011). Mechanisms of TGF-β1-induced vascular dysfunction included reduced levels of endothelial nitric oxide synthase responsible for basal and stimulus-induced NO synthesis, decreased COX-2 protein levels, and deregulation of the contractile ET-1 signaling pathway (Tong et al, 2005; Tong and Hamel, 2007; Papadopoulos et al, 2010; Nicolakakis et al, 2011). In contrast to APP mice, in vitro and in vivo antioxidant treatments were unable to restore vascular reactivity in arteries from young and old TGF mice. Further, protein levels of the O2•− marker SOD2 were unchanged in TGF cerebral vessels (Tong et al, 2005; Nicolakakis et al, 2011). This collectively argued against oxidative stress as a deleterious factor in cerebrovascular function of TGF mice.
The lack of neuronal impairments in the TGF model demonstrated that neurovascular dysfunction could occur in the absence of neuronal compromise. This pointed to the potentially important role that TGF-β1-induced cerebrovascular alterations could play in AD neurovascular dysfunction. Further, TGF mice offered new insight into the hypoperfusion–dementia link. The lack of cholinergic, metabolic, and cognitive anomalies in aged TGF mice, despite chronic hypoperfusion that should negatively affect these parameters (Craft et al, 2005; Ruitenberg et al, 2005), suggested either insufficient CBF impairment or its role as an aggravating factor in an ongoing pathogenic process. The lack of mnemonic deficits may also have been attributable to a TGF-β1 neuroprotective role (Tesseur et al, 2006; Caraci et al, 2008; Cheng et al, 2009), although TGF-β1 neurodegenerative effects have also been reported (Salins et al, 2008; Town et al, 2008). Until this issue is clarified, TGF mice should be viewed as alternative models to classic artery occlusion paradigms that are invasive and often result in neurodegeneration and white-matter damage (Farkas et al, 2007; Barros et al, 2009; Miki et al, 2009), undesirable confounds in the hypoperfusion–dementia relationship. The TGF model should thus aid in the search for therapies against cerebrovascular dysfunction associated with TGF-β1 elevations and structural changes in AD.
Amyloid precursor protein/transforming growth factor-β1 mice
Transgenic mice simultaneously overproducing Aβ and TGF-β1 were created to investigate the relationship between cerebrovascular fibrosis and CAA (Wyss-Coray et al, 1997). This bitransgenic APP/TGF model not only reflected the combined Aβ and TGF-β1 elevations encountered in AD patients, but also provided new mechanistic insight into AD neurovascular dysfunction. In young APP mice lacking cerebrovascular and parenchymal Aβ deposits, genetic addition of TGF-β1 induced CAA (Wyss-Coray et al, 1997). This suggested that cerebrovascular fibrosis could be a CAA trigger. Conversely, it also suggested that approaches targeting vascular wall thickening could reverse or prevent CAA, an idea that could be exploited in vaccination trials. In addition, vascular reactivity experiments from our group on isolated APP/TGF cerebral arteries revealed lower tonic NO levels and impaired vasodilatory capacity in response to ACh and calcitonin gene-related peptide starting at an early age. These deficits were associated with changes in vascular remodeling and signaling pathways, similar to those in TGF mice (Ongali et al, 2010). Further, the impaired ACh-mediated dilatations could not be rescued by incubation of arteries with apocynin or SOD, suggesting resistance to antioxidant approaches. This observation may help guide therapies for neurovascular dysfunction in AD. Progressive neurovascular coupling deficits to whisker stimulation in APP/TGF mice surpassed those of TGF mice at ∼18 months of age (Papadopoulos et al, 2010; Nicolakakis et al, 2011), and reached a severity parallel to that of similarly aged APP mice (Shin et al, 2007; Nicolakakis et al, 2008) (Table 1). The hemodynamic dysfunction was thought to arise from neuronal, astrocytic, and vascular impairments driven by increasing soluble Aβ levels, particularly those of Aβ1−40 that continued to rise from 12 to 18 months of age, while those of Aβ1−42 had stabilized (Ongali et al, 2010). Our ongoing work on APP/TGF mice will clarify whether in vivo pharmacological interventions can restore arterial reactivity and neurovascular coupling, as well as improve the impaired cholinergic innervation, neurometabolic coupling, and spatial memory of the mice.
We have provided an overview of neurovascular function in transgenic models for which data were available. However, a similar evaluation would be particularly interesting in LaFerla's triple transgenic model (Oddo et al, 2003), which harbors human mutant APP, presenilin 1, and τ transgenes, and develops the neurofibrillary tangle pathology not seen in APP mice. The model could yield valuable insight on the potential effect of cytoskeletal and axonal transport deficits on neurovascular coupling. It could also elucidate the impact of hyperphosphorylated τ and neurofibrillary tangles on basal forebrain cholinergic neurons, which modulate neurovascular coupling. Significant axonal transport deficits and τ hyperphosphorylation were reported by Massaad et al (2010) in 8 and 12- to 16-month-old Tg2576 mice. Authors determined axonal transport rates by visualizing the rate of Mn2+ accumulation in olfactory bulb with magnetic resonance imaging, after application of a manganese chloride (MnCl2) solution to mouse nostrils.
Treatments for Alzheimer's disease neurovascular dysfunction
In APP mice, antioxidant approaches have proven beneficial against neurovascular dysfunction. Superfusion of the free radical scavenger MnTBAP on the somatosensory cortex restored neurovascular coupling during whisker stimulation in aged (12 to 15 months old) Tg2576 animals (Park et al, 2008). Similarly, we observed recovery of the functional hyperemic response in ∼8-month-old J20 APP mice after brief in vivo treatment with the SOD mimetic Tempol. In this experiment, the evoked CBF response to whisker stimulation was measured with LDF in the same animals before and after 10-day Tempol therapy. We found that the antioxidant significantly improved neurovascular coupling in every APP mouse tested (t=7.311, df=4, P<0.01), such that it completely normalized the impaired CBF response of APP mice (−37%, P<0.001; Table 1, Figure 3) to the level of WT littermates (Figure 3). There was no drug effect on the WT response (t=1.041, df=3, P=0.374). Such antioxidant approaches have not, to our knowledge, been verified in humans for their effect on evoked perfusion. But given their overall inefficacy in AD trials (Petersen et al, 2005), and on cerebrovascular function of TGF and APP/TGF mice, alternative therapies should be pursued. In the section that follows, we review two drug classes with reported benefits on brain hemodynamics and neurovascular coupling in AD patients. Their mechanisms of action have been elucidated to varying extent in the mouse models.
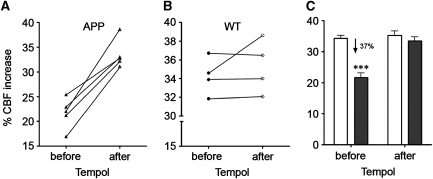
Figure 3.
Functional hyperemic rescue by Tempol in 7.5-month-old J20 amyloid precursor protein (APP) mice. (A) The neurovascular coupling response to whisker stimulation was improved in all APP mice tested before and after 10-day in vivo therapy with the antioxidant Tempol (1 mmol/L in drinking water, Sigma-Aldrich, St Louis, MO, USA), as measured with laser Doppler flowmetry (LDF), following the same protocols as in Nicolakakis et al (2008) (n=5, paired Student's t-test, GraphPad Prism 4, San Diego, CA, USA). (B) Responses of wild-type (WT) mice were mostly unaffected by treatment (n=4, paired Student's t-test). (C) Short Tempol therapy completely normalized the impaired CBF response of APP mice (gray bars, n=5) to that of WT littermates (white bars, n=4) (***P<0.001 for difference to all other groups, two-way analysis of variance with Newman–Keuls post hoc multiple comparison test (Statistica 9, StatSoft, Tulsa, OK, USA)). Error bars represent s.e.m. Results are expressed as the percent cerebral blood flow (CBF) increase during whisker stimulation relative to prestimulation baseline.
Cholinesterase Inhibitors
Cholinesterase inhibitors are the standard FDA-approved symptomatic therapy for mild-to-moderate cases of AD. Based on the premise that reduced cholinergic tone is partly responsible for attention deficits and cognitive decline in AD, these compounds were developed to prolong the synaptic life of ACh. Four are available, tacrine (Cognex, Parke-Davis Pharmaceuticals), donepezil (Aricept, Pfizer Inc.), rivastigmine (Exelon, Novartis Pharmaceuticals Corporation), and galantamine (Reminyl, Janssen Inc.), although the first of these is no longer used after liver-toxicity side effects. A Cochrane review found that the three compounds display a small but short-lived cognitive benefit (Birks, 2006). In light of renewed interest in the AD circulation, cholinesterase inhibitors were revisited and found to preserve or improve perfusion in AD patients undergoing therapy (Nakano et al, 2001; Nobili et al, 2002; Rosengarten et al, 2006; Bär et al, 2007; Yoshida et al, 2007) (Figure 4). In the study by Bär et al (2007), 5-week galantamine administration improved CO2 reactivity as measured in the middle cerebral artery of 17 AD patients with transcranial Doppler sonography. In contrast, galantamine had no effect on cerebral autoregulation and cortical hemoglobin decrease elicited in 13 AD patients during orthostatic hypotension. Hence, it may not offer protection against increased ischemic vulnerability during common activities such as standing up (Van Beek et al, 2010). Rosengarten et al (2006) found that 2-month donepezil therapy normalized the evoked flow response in the posterior cerebral artery during a reading task. Together, these studies suggested that some of the cognitive benefits of cholinesterase inhibition may arise from direct effects on the vasculature, as had been proposed by Claassen and Jansen (2006) in their cholinergic-vascular hypothesis. This idea was further supported by the finding that patients whose cognitive score had stabilized or improved with cholinesterase inhibitor therapy, the so-called ‘responders,' were also those who featured stabilized or increased perfusion, often within the first month of a 1-year treatment. Patients whose CBF had decreased during therapy were also those who had declined cognitively, the designated ‘nonresponders' (Nakano et al, 2001; Nobili et al, 2002; Yoshida et al, 2007). The latter data highlighted the importance of targeting perfusion deficits as a means to influence mental status. They also emphasized the value of CBF as a correlate and possible predictor of treatment outcome. However, the limited benefits currently afforded by cholinesterase inhibition also point to the need for more effective therapies and for earlier detection.
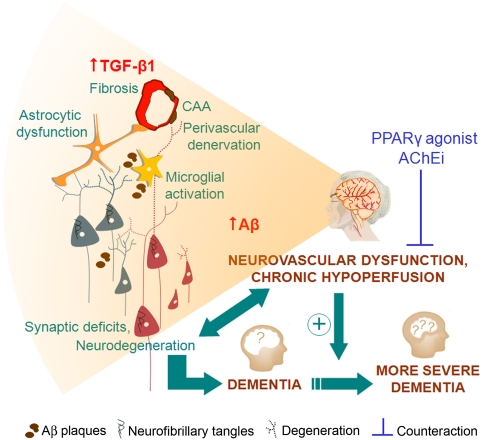
Figure 4.
Alzheimer's disease (AD) neurovascular dysfunction and chronic hypoperfusion are associated with diseased neuronal, astrocytic, and vascular networks. Countering neurovascular impairment with peroxisome proliferator-activated receptor γ (PPARγ) agonists, acetylcholinesterase inhibitors (AChEi), or other compounds may improve clinical outcome and delay progression to severe dementia. CAA, cerebral amyloid angiopathy; TGF-β1, transforming growth factor-β 1.
Peroxisome Proliferator-Activated Receptor γ Agonists
Agonists of the peroxisome proliferator-activated receptor γ form a class of oral antidiabetic drugs that were introduced in the late 1990s for the treatment of type 2 diabetes. Rosiglitazone (Avandia, GlaxoSmithKline) and pioglitazone (Actos, Takeda Pharmaceuticals, North America, Inc.) are the two prescribed thiazolidinedione peroxisome proliferator-activated receptor γ agonists that act by enhancing insulin sensitivity. However, their benefits in AD are believed to derive from their ability as nuclear receptor ligands to regulate transcription of a wide variety of oxidative, inflammatory, fibrotic, and neuronal survival genes, although transcription-independent effects may also be involved. Peroxisome proliferator-activated receptor γ agonists thus have the ability to improve neuronal, glial, and cerebrovascular networks in AD, and consequently to rescue brain hemodynamics (reviewed in Nicolakakis and Hamel (2010)) (Figure 4). Indeed, pioglitazone was recently shown to ameliorate cerebral perfusion in AD patients (Sato et al, 2009) and in transgenic mouse models (Nicolakakis et al, 2008, 2011). In Sato et al (2009), diabetic AD patients treated with pioglitazone for 6 months experienced regional CBF increase in the parietal lobe, concomitant with an improvement in cognition, whereas no such effects were seen in placebo-treated subjects. At the same time, fasting plasma insulin levels declined in pioglitazone-treated patients, indicating enhanced insulin sensitivity that could have led to some of the observed cognitive benefits. These findings are supported by data in aged APP mice treated with pioglitazone; cerebrovascular reactivity and evoked CBF and CGU responses were normalized, the latter respectively determined by LDF and 18F-fluorodeoxyglucose-positron emission tomography (Nicolakakis et al, 2008). However, despite these favorable effects on the cerebral circulation, and attenuation of oxidative stress and neuroinflammation, pioglitazone failed to rescue memory deficits. We originally attributed this failure to the short treatment duration (6 weeks), advanced aged of the mice (>15 months), and highly stringent conditions of the Morris water maze. Our more recent study in young APP mice treated for 3 months with a higher pioglitazone dose similarly failed to show notable cognitive improvement (Badhwar et al, 2010), suggesting that pioglitazone is unlikely to be a robust therapeutic approach to AD. Similarly, the latest pioglitazone pilot study in nondiabetic AD patients found no cognitive benefit (Geldmacher et al, 2011). In aged TGF mice, pioglitazone countered diminished arterial reactivity, glial activation, and neurovascular coupling deficits, despite persisting cerebrovascular fibrosis (Nicolakakis et al, 2011). This revealed additional mechanisms by which thiazolidinediones may restore cerebrovascular function in AD patients featuring TGF-β1 elevations, vascular wall thickening, and vascular antioxidant resistance. Despite cerebrovascular improvements, the collective literature casts doubt on the ultimate benefit of thiazolidinediones in AD. The magnitude of improvement reported in previous clinical trials with rosiglitazone (Watson et al, 2005; Risner et al, 2006; Gold et al, 2010) and pioglitazone (Hanyu et al, 2009; Sato et al, 2009) was not superior to that offered by cholinesterase inhibitors. Coupled to rosiglitazone cardiovascular side effects and potential removal from the North American market, we remain skeptical about the clinical value of thiazolidinediones. However, the safer cardiovascular profile of pioglitazone (Lincoff et al, 2007) may make it advantageous for subpopulations, for example diabetic AD patients, who could avoid cholinesterase inhibitor side effects in favor of drugs that also offer glycemic control.
Conclusion
Decreases in resting CBF and alterations in evoked responses, in particular the inability to silence task-irrelevant networks, are preclinical hemodynamic features that may be used to predict pathological conversion to AD from a presymptomatic state. They also correlate with therapeutic outcome, as shown with regards to cholinesterase inhibitors and peroxisome proliferator-activated receptor γ agonists. Such observations have prompted renewed interest in the AD circulation and the continued use of experimental models to explore and counter pathological changes in neuronal, astrocytic, and vascular compartments. Superfusion or overexpression of key vasoactive AD proteins has provided mechanistic insight and experimental evidence that therapeutic intervention is feasible, yet a disease-modifying treatment remains elusive, and earlier intervention may ultimately have greater impact in patients. Devising strategies to protect blood supply to an organ with very high energetic demands and lack of reserves should counter AD neurovascular dysfunction, and help delay disease progression and cognitive demise. Improved therapeutic end points may be better achieved by combined regimens, early intervention, and selection of subgroups of AD patients.
Acknowledgments
The authors thank Dr L Mucke (Gladstone Inst. of Neurological Disease and Department Neurology, UCSF, CA, USA) and the J David Gladstone Institutes for the hAPPSw,Ind and TGF-β1 transgenic mouse breeders, and Mrs A Badhwar for comments on the manuscript.
The authors received a research grant from Takeda Pharmaceuticals North America, Inc.
Footnotes
Work from our laboratory cited in the text was supported by research grants from the Canadian Institutes of Health Research (CIHR, MOP-84275), the Alzheimer Society of Canada, Takeda Pharmaceuticals North America, Inc., and a CIHR studentship (N.N.).
References
- Abbott A. The plaque plan. Nature. 2008;456:161–164. doi: 10.1038/456161a. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger SBS, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aucoin JS, Jiang P, Aznavour N, Tong XK, Buttini M, Descarries L, Hamel E. Selective cholinergic denervation, independent of oxidative stress, in a mouse model of Alzheimer's disease. Neuroscience. 2005;132:73–86. doi: 10.1016/j.neuroscience.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer's disease and the basal forebrain cholinergic system: relations to beta-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68:209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Arai H, Kosaka K, Iizuka R. Changes of biogenic amines and their metabolites in postmortem brains from patients with Alzheimer-type dementia. J Neurochem. 1984;43:388–393. doi: 10.1111/j.1471-4159.1984.tb00913.x. [DOI] [PubMed] [Google Scholar]
- Badhwar A, Lerch JP, Sled JG, Hamel E. Early treatment with pioglitazone in APP transgenic mice shows focal hippocampal volume increases related to improved cognitive performance. Alzheimer's Dementia. 2010;6 (Suppl July e7:P2–448. [Google Scholar]
- Bär KJ, Boettger MK, Seidler N, Mentzel HJ, Terborg C, Sauer H. Influence of galantamine on vasomotor reactivity in Alzheimer's disease and vascular dementia due to cerebral microangiopathy. Stroke. 2007;38:3186–3192. doi: 10.1161/STROKEAHA.107.492033. [DOI] [PubMed] [Google Scholar]
- Barros CA, Ekuni R, Moro MA, Pereira FM, Dos Santos Pereira MA, Milani H. The cognitive and histopathological effects of chronic 4-vessel occlusion in rats depend on the set of vessels occluded and the age of the animals. Behav Brain Res. 2009;197:378–387. doi: 10.1016/j.bbr.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Bateman GA, Levi CR, Schofield P, Wang Y, Lovett EC. Quantitative measurement of cerebral haemodynamics in early vascular dementia and Alzheimer's disease. J Clin Neurosci. 2006;13:563–568. doi: 10.1016/j.jocn.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Beckmann N, Schuler A, Mueggler T, Meyer EP, Wiederhold KH, Staufenbiel M, Krucker T. Age-dependent cerebrovascular abnormalities and blood flow disturbances in APP23 mice modeling Alzheimer's disease. J Neurosci. 2003;23:8453–8459. doi: 10.1523/JNEUROSCI.23-24-08453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger S, Belleville S, Gauthier S. Inhibition impairments in Alzheimer's disease, mild cognitive impairment and healthy aging: effect of congruency proportion in a Stroop task. Neuropsychologia. 2010;48:581–590. doi: 10.1016/j.neuropsychologia.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Bennett SAL, Pappas BA, Stevens WD, Davidson CM, Fortin T, Chen J. Cleavage of amyloid precursor protein elicited by chronic cerebral hypoperfusion. Neurobiol Aging. 2000;21:207–214. doi: 10.1016/s0197-4580(00)00131-7. [DOI] [PubMed] [Google Scholar]
- Biesold D, Inanami O, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006;25:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C, Nicoll JA. Consequence of Aβ immunization on the vasculature of human Alzheimer's disease brain. Brain. 2008;131:3299–3310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- Caraci F, Battaglia G, Busceti C, Biagioni F, Mastroiacovo F, Bosco P, Drago F, Nicoletti F, Sortino MA, Copani A. TGF-β1 protects against Aβ-neurotoxicity via the phosphatidylinositol-3-kinase pathway. Neurobiol Dis. 2008;30:234–242. doi: 10.1016/j.nbd.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Castillo GM, Ngo C, Cummings J, Wight TN, Snow AD. Perlecan binds to the β-amyloid proteins (Aβ) of Alzheimer's disease, accelerates Aβ fibril formation, and maintains Aβ fibril stability. J Neurochem. 1997;69:2452–2465. doi: 10.1046/j.1471-4159.1997.69062452.x. [DOI] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenerg. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in MCI and AD: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JS, Dubal DB, Kim DH, Legleiter J, Cheng IH, Yu GQ, Tesseur I, Wyss-Coray T, Bonaldo P, Mucke L. Collagen VI protects neurons against Aβ toxicity. Nat Neurosci. 2009;12:119–121. doi: 10.1038/nn.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van Nostrand W, Miano JM, Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer's phenotype. Proc Natl Acad Sci USA. 2007;104:823–828. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie R, Yamada M, Moskowitz M, Hyman B. Structural and functional disruption of vascular smooth muscle cells in a transgenic mouse model of amyloid angiopathy. Am J Pathol. 2001;158:1065–1071. doi: 10.1016/S0002-9440(10)64053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen JA, Diaz-Arrastia R, Martin-Cook K, Levine BD, Zhang R. Altered cerebral hemodynamics in early Alzheimer disease: a pilot study using Transcranial Doppler. J Alzheimers Dis. 2009;17:621–629. doi: 10.3233/JAD-2009-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen JA, Jansen RW. Cholinergically mediated augmentation of cerebral perfusion in Alzheimer's disease and related cognitive disorders: the cholinergic-vascular hypothesis. J Gerontol A Biol Sci Med Sci. 2006;61:267–271. doi: 10.1093/gerona/61.3.267. [DOI] [PubMed] [Google Scholar]
- Craft TK, Mahoney JH, Devries AC, Sarter M. Microsphere embolism-induced cortical cholinergic deafferentation and impairments in attentional performance. Eur J Neurosci. 2005;21:3117–3132. doi: 10.1111/j.1460-9568.2005.04136.x. [DOI] [PubMed] [Google Scholar]
- de la Torre JC, Fortin T, Park GA, Butler KS, Kozlowski P, Pappas BA, de Socarraz H, Saunders JK, Richard MT. Chronic cerebrovascular insufficiency induces dementia-like deficits in aged rats. Brain Res. 1992;582:186–195. doi: 10.1016/0006-8993(92)90132-s. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Dodart J-C, May P.2005Overview on rodent models of Alzheimer's disease Curr Protoc Neurosci Chapter 9Unit 9.22 [DOI] [PubMed] [Google Scholar]
- Drzezga A, Grimmer T, Peller M, Wermke M, Siebner H, Rauschecker JP, Schwaiger M, Kurz A. Impaired cross-modal inhibition in Alzheimer disease. PLoS Med. 2005;2:e288. doi: 10.1371/journal.pmed.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhusseiny A, Hamel E. Muscarinic—but not nicotinic—acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: a possible role for the M5 receptor subtype. J Cereb Blood Flow Metab. 2000;20:298–305. doi: 10.1097/00004647-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Galea E, Feinstein DL, Lacombe P. Pioglitazone does not increase cerebral glucose utilisation in a murine model of Alzheimer's disease and decreases it in wild-type mice. Diabetologia. 2006;49:2153–2161. doi: 10.1007/s00125-006-0326-0. [DOI] [PubMed] [Google Scholar]
- Gaertner RF, Wyss-Coray T, Von Euw D, Lesné S, Vivien D, Lacombe P. Reduced brain tissue perfusion in TGF-beta 1 transgenic mice showing Alzheimer's disease-like cerebrovascular abnormalities. Neurobiol Dis. 2005;19:38–46. doi: 10.1016/j.nbd.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol. 2011;68:45–50. doi: 10.1001/archneurol.2010.229. [DOI] [PubMed] [Google Scholar]
- Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, Craft S, Landreth G, Linnamägi U, Sawchak S. Rosiglitazone monotherapy in mild-to-moderate Alzheimer's disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30:131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Cerebrovascular transforming growth factor-β contributes to inflammation in the Alzheimer's disease brain. Am J Pathol. 2002;160:1583–1587. doi: 10.1016/s0002-9440(10)61105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zou MH, Kirber M, Lieberthal W, Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol. 2003;285:H1396–H1403. doi: 10.1152/ajpheart.00096.2003. [DOI] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Hamel E, Nicolakakis N, Aboulkassim T, Ongali B, Tong XK. Oxidative stress and cerebrovascular dysfunction in mouse models of Alzheimer's disease. Exp Physiol. 2008;93:116–120. doi: 10.1113/expphysiol.2007.038729. [DOI] [PubMed] [Google Scholar]
- Han BH, Zhou ML, Abousaleh F, Brendza RP, Dietrich HH, Koenigsknecht-Talboo J, Cirrito JR, Milner E, Holtzman DM, Zipfel GJ. Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-β peptide, partial restoration via γ-secretase inhibition. J Neurosci. 2008;28:13542–13550. doi: 10.1523/JNEUROSCI.4686-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer's disease and mild cognitive impairment with diabetes mellitus. J Am Geriatr Soc. 2009;57:177–179. doi: 10.1111/j.1532-5415.2009.02067.x. [DOI] [PubMed] [Google Scholar]
- Hartlage-Rübsamen M, Zeitschel U, Apelt J, Gärtner U, Franke H, Stahl T, Günther A, Schliebs R, Penkowa M, Bigl V, Ro S. Astrocytic expression of the Alzheimer's disease β-secretase (BACE1) is stimulus-dependent. Glia. 2003;41:169–179. doi: 10.1002/glia.10178. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Herzig MC, Van Nostrand WE, Jucker M. Mechanism of cerebral β-amyloid angiopathy: murine and cellular models. Brain Pathol. 2006;16:40–54. doi: 10.1111/j.1750-3639.2006.tb00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao K, Ohnishi T, Hirata Y, Yamashita F, Mori T, Moriguchi Y, Matsuda H, Nemoto K, Imabayashi E, Yamada M, Iwamoto T, Arima K, Asada T. The prediction of rapid conversion to Alzheimer's disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage. 2005;28:1014–1021. doi: 10.1016/j.neuroimage.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Hock C, Villringer K, Müller-Spahn F, Wenzel R, Heekeren H, Schuh-Hofer S, Hofmann M, Minoshima S, Schwaiger M, Dirnagl U, Villringer A. Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer's disease monitored by means of near-infrared spectroscopy (NIRS)-correlation with simultaneous rCBF-PET measurements. Brain Res. 1997;755:293–303. doi: 10.1016/s0006-8993(97)00122-4. [DOI] [PubMed] [Google Scholar]
- Hu NW, Smith IM, Walsh DM, Rowan MJ. Soluble amyloid-β peptides potently disrupt hippocampal synaptic plasticity in the absence of cerebrovascular dysfunction in vivo. Brain. 2008;131:2414–2424. doi: 10.1093/brain/awn174. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Neurosci Rev. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Bhatti SU, Lust WD, Perry G. The amyloid precursor protein in ischemic brain injury and chronic hypoperfusion. Ann N Y Acad Sci. 1993;695:190–193. doi: 10.1111/j.1749-6632.1993.tb23050.x. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Pax AB. Increased collagen content of cerebral microvessels in Alzheimer's disease. Brain Res. 1995;705:349–352. doi: 10.1016/0006-8993(95)01250-8. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Sambeth A, Blokland A.2010Acetylcholine and attention Behav Brain Resdoi: 10.1016/j.bbr.2010.11.033 [DOI] [PubMed]
- Kocharyan A, Fernandes P, Tong XK, Vaucher E, Hamel E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J Cereb Blood Flow Metab. 2008;28:221–231. doi: 10.1038/sj.jcbfm.9600558. [DOI] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe P, Mathews PM, Schmidt S, Breidert T, Heneka MT, Landreth GE, Feinstein DL, Galea E. Effect of anti-inflammatory agents on transforming growth factor beta over-expressing mouse brains: a model revised. J Neuroinflammation. 2004;1:11–27. doi: 10.1186/1742-2094-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbaum JB, Chen K, Lee W, Reschke C, Bandy D, Fleisher AS, Alexander GE, Foster NL, Weiner MW, Koeppe RA, Jagust WJ, Reiman EM, Alzheimer's Disease Neuroimaging Initiative Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI) Neuroimage. 2009;45:1107–1116. doi: 10.1016/j.neuroimage.2008.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, Adolfsson R, Bäckman L, Nilsson LG, Petersson KM, Nyberg L. Reduced functional brain activity response in cognitively intact apolipoprotein E ɛ4 carriers. Brain. 2006;129:1240–1248. doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang M-X, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O'Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR. Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology. 2003;61:500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancardi GL, Perdelli F, Rivano C, Leonardi A, Bugiani O. Thickening of the basement membrane of cortical capillaries in Alzheimer's disease. Acta Neuropathol. 1980;49:79–83. doi: 10.1007/BF00692225. [DOI] [PubMed] [Google Scholar]
- Massaad CA, Amin SK, Hu L, Mei Y, Klann E, Pautler RG. Mitochondrial superoxide contributes to blood flow and axonal transport deficits in the Tg2576 mouse model of Alzheimer's disease. PLoS One. 2010;5:e10561. doi: 10.1371/journal.pone.0010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis MJ, Alexander GE, Krasuski J, Pietrini P, Furey ML, Schapiro MB, Rapoport SI. Increasing required neural response to expose abnormal brain function in mild versus moderate or severe Alzheimer's disease: PET study using parametric visual stimulation. Am J Psychiatry. 1998;155:785–794. doi: 10.1176/ajp.155.6.785. [DOI] [PubMed] [Google Scholar]
- Melrose RJ, Campa OM, Harwood DG, Osato S, Mandelkern MA, Sultzer DL. The neural correlates of naming and fluency deficits in Alzheimer's disease: an FDG-PET study. Int J Geriatr Psychiatry. 2009;24:885–893. doi: 10.1002/gps.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau J, Marighetto A.2011Acetylcholine and memory: a long, complex and chaotic but still living relationship Behav Brain Resdoi: 10.1016/j.bbr.2010.11.052 [DOI] [PubMed]
- Miki K, Ishibashi S, Sun L, Xu H, Ohashi W, Kuroiwa T, Mizusawa H. Intensity of chronic cerebral hypoperfusion determines white/gray matter injury and cognitive/motor dysfunction in mice. J Neurosci Res. 2009;87:1270–1281. doi: 10.1002/jnr.21925. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of Aβ1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueggler T, Baumann D, Rausch M, Staufenbiel M, Rudin M. Age-dependent impairment of somatosensory response in the APP23 transgenic mouse model of Alzheimer's disease. J Neurosci. 2003;23:8231–8236. doi: 10.1523/JNEUROSCI.23-23-08231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S, Asada T, Matsuda H, Uno M, Takasaki M. Donepezil hydrochloride preserves regional cerebral blood flow in patients with Alzheimer's disease. J Nucl Med. 2001;42:1441–1445. [PubMed] [Google Scholar]
- Nicolakakis N, Aboulkassim T, Aliaga A, Tong XK, Rosa-Neto P, Hamel E. Intact memory in TGF-β1 transgenic mice featuring chronic cerebrovascular deficit: recovery with pioglitazone. J Cereb Blood Flow Metab. 2011;31:200–211. doi: 10.1038/jcbfm.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolakakis N, Aboulkassim T, Ongali B, Lecrux C, Fernandes P, Rosa-Neto P, Tong XK, Hamel E. Complete rescue of cerebrovascular function in aged Alzheimer's disease transgenic mice by antioxidants and pioglitazone, a peroxisome proliferator-activated receptor gamma agonist. J Neurosci. 2008;28:9287–9296. doi: 10.1523/JNEUROSCI.3348-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolakakis N, Hamel E. The nuclear receptor PPARγ as a therapeutic target for cerebrovascular and brain dysfunction in Alzheimer's disease. Front Aging Neurosci. 2010;2:21. doi: 10.3389/fnagi.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Carlson GA, Iadecola C. Exogenous Aβ1-40 reproduces cerebrovascular alterations resulting from amyloid precursor protein overexpression in mice. J Cereb Blood Flow Metab. 2000b;20:1659–1668. doi: 10.1097/00004647-200012000-00005. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002b;283:H315–H323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol Dis. 2002a;9:61–68. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C. Aβ-1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci USA. 2000a;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili F, Koulibaly M, Vitali P, Migneco O, Mariani G, Ebmeier K, Pupi A, Robert PH, Rodriguez G, Darcourt J. Brain perfusion follow-up in Alzheimer's patients during treatment with acetylcholinesterase inhibitors. J Nucl Med. 2002;43:983–990. [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ongali B, Nicolakais N, Lecrux C, Aboulkassim T, Rosa-Neto P, Papadopoulos P, Tong XK, Hamel E. Transgenic mice overexpressing APP and transforming growth factor-β1 feature cognitive and vascular hallmarks of Alzheimer's disease. Am J Pathol. 2010;177:3071–3080. doi: 10.2353/ajpath.2010.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Synaptic depression and aberrant excitatory network activity in Alzheimer's disease: two faces of the same coin. Neuromol Med. 2010;12:48–55. doi: 10.1007/s12017-009-8097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos P, Ongali B, Hamel E. Selective in vivo antagonism of endothelin receptors in transforming growth factor-β1 transgenic mice that mimic the vascular pathology of Alzheimer's disease. Can J Physiol Pharmacol. 2010;88:652–660. doi: 10.1139/Y10-042. [DOI] [PubMed] [Google Scholar]
- Paris D, Humphrey J, Quadros A, Patel N, Crescentini R, Crawford F, Mullan M. Vasoactive effects of Aβ in isolated human cerebrovessels and in a transgenic mouse model of Alzheimer's disease: role of inflammation. Neurol Res. 2003;25:642–651. doi: 10.1179/016164103101201940. [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C. Aβ-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab. 2004;24:334–342. doi: 10.1097/01.WCB.0000105800.49957.1E. [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid β peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters F, Collette F, Degueldre C, Sterpenich V, Majerus S, Salmon E. The neural correlates of verbal short-term memory in Alzheimer's disease: an fMRI study. Brain. 2009;132:1833–1846. doi: 10.1093/brain/awp075. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Peterson MC. Circulating transforming growth factor β-1: a partial molecular explanation for associations between hypertension, diabetes, obesity, smoking and human disease involving fibrosis. Med Sci Monit. 2005;11:RA229–RA232. [PubMed] [Google Scholar]
- Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Price JM, Sutton ET, Hellermann A, Thomas T. Beta-amyloid induces cerebrovascular endothelial dysfunction in the rat brain. Neurol Res. 1997;19:534–538. doi: 10.1080/01616412.1997.11740853. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ME, Saunders AM, Altman JFB, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ. β-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Veltman DJ, Machielsen WC, Witter MP, Bierlaagh MA, Lazeron RH, Valk J, Scheltens P. Functional MR imaging in Alzheimer's disease during memory encoding. Am J Neuroradiol. 2000;21:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- Rosengarten B, Paulsen S, Molnar S, Kaschel R, Gallhofer B, Kaps M. Acetylcholine esterase inhibitor donepezil improves dynamic cerebrovascular regulation in Alzheimer patients. J Neurol. 2006;253:58–64. doi: 10.1007/s00415-005-0926-5. [DOI] [PubMed] [Google Scholar]
- Rossor MN, Emson PC, Mountjoy CQ, Roth M, Iversen LL. Reduced amounts of immunoreactive somatostatin in the temporal cortex in senile dementia of Alzheimer type. Neurosci Lett. 1980;20:373–377. doi: 10.1016/0304-3940(80)90177-9. [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- Salins P, He Y, Olson K, Glazner G, Kashour T, Amara F. TGF-beta1 is increased in a transgenic mouse model of familial Alzheimer's disease and causes neuronal apoptosis. Neurosci Lett. 2008;430:81–86. doi: 10.1016/j.neulet.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Sassin I, Schultz C, Thal DR, Rüb U, Arai K, Braak E, Braak H. Evolution of Alzheimer's disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol. 2000;100:259–269. doi: 10.1007/s004019900178. [DOI] [PubMed] [Google Scholar]
- Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T.2009Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease Neurobiol Agingdoi: 10.1016/j.neurobiolaging.2009.10.009 [DOI] [PubMed]
- Scarmeas N, Anderson KE, Hilton J, Park A, Habeck C, Flynn J, Tycko B. APOE-dependent PET patterns of brain activation in Alzheimer disease. Neurology. 2004;63:913–915. doi: 10.1212/01.wnl.0000137274.93125.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HK, Jones PB, Garcia-Alloza M, Borrelli L, Greenberg SM, Bacskai BJ, Frosch MP, Hyman BT, Moskowitz MA, Ayata C. Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain. 2007;130:2310–2319. doi: 10.1093/brain/awm156. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Chundu KR, Davies-Hill T, Honer WG, Davies P. Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's disease. Ann Neurol. 1994;35:546–551. doi: 10.1002/ana.410350507. [DOI] [PubMed] [Google Scholar]
- Smith DH, Uryu K, Saatman KE, Trojanowski JQ, McIntosh TK. Protein accumulation in traumatic brain injury. Neuromolecular Med. 2003;4:59–72. doi: 10.1385/NMM:4:1-2:59. [DOI] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Mühlau M, Calhoun VD, Eichele T, Läer L, Drzezga A, Förstl H, Kurz A, Zimmer C, Wohlschläger AM. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci USA. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer's disease. Neuromol Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci USA. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Han X, Deane R, Zlokovic B, Nedergaard M. Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer's disease. Ann NY Acad Sci. 2007;1097:40–50. doi: 10.1196/annals.1379.004. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sato N, Takeuchi D, Kurinami H, Shinohara M, Niisato K, Kano M, Ogihara T, Rakugi H, Morishita R. Angiotensin receptor blocker prevented β-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension. 2009;54:1345–1352. doi: 10.1161/HYPERTENSIONAHA.109.138586. [DOI] [PubMed] [Google Scholar]
- Tesseur I, Zou K, Esposito L, Bard F, Berber E, Van Can J, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal TGF-β signaling promotes neurodegeneration and Alzheimer's pathology. J Clin Investig. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, McLendon C, Sutton ET, Thomas G. Cerebrovascular endothelial dysfunction mediated by beta-amyloid. Neuroreport. 1997;8:1387–1391. doi: 10.1097/00001756-199704140-00014. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Irving D, Blessed G. Cell loss in the locus coeruleus in senile dementia of Alzheimer type. J Neurol Sci. 1981;49:419–428. doi: 10.1016/0022-510x(81)90031-9. [DOI] [PubMed] [Google Scholar]
- Tong XK, Hamel E. Regional cholinergic denervation of cortical microvessels and nitric oxide synthase-containing neurons in Alzheimer's disease. Neuroscience. 1999;92:163–175. doi: 10.1016/s0306-4522(98)00750-7. [DOI] [PubMed] [Google Scholar]
- Tong XK, Hamel E. Transforming growth factor-beta 1 impairs endothelin-1-mediated contraction of brain vessels by inducing mitogen-activated protein (MAP) kinase phosphatase-1 and inhibiting p38 MAP kinase. Mol Pharmacol. 2007;72:1476–1483. doi: 10.1124/mol.107.039602. [DOI] [PubMed] [Google Scholar]
- Tong XK, Nicolakakis N, Fernandes P, Ongali B, Brouillette J, Quirion R, Hamel E. Simvastatin improves cerebrovascular function and counters soluble amyloid-beta, inflammation and oxidative stress in aged APP mice. Neurobiol Dis. 2009;35:406–414. doi: 10.1016/j.nbd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Tong XK, Nicolakakis N, Kocharyan A, Hamel E. Vascular remodeling versus amyloid β-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer's disease. J Neurosci. 2005;25:11165–11174. doi: 10.1523/JNEUROSCI.4031-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, Duman RS, Flavell RA. Blocking TGF-β-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14:681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend KP, Obregon D, Quadros A, Patel N, Volmar CH, Paris D, Mullan M. Proinflammatory and vasoactive effects of Aβ in the cerebrovasculature. Ann NY Acad Sci. 2002;977:65–76. doi: 10.1111/j.1749-6632.2002.tb04799.x. [DOI] [PubMed] [Google Scholar]
- van Beek AH, Sijbesma JC, Jansen RW, Rikkert MG, Claassen JA. Cortical oxygen supply during postural hypotension is further decreased in Alzheimer's disease, but unrelated to cholinesterase-inhibitor use. J Alzheimers Dis. 2010;21:519–526. doi: 10.3233/JAD-2010-100288. [DOI] [PubMed] [Google Scholar]
- Van Dorpe J, Smeijers L, Dewachter I, Nuyens D, Spittaels K, Van Den Haute C, Mercken M, Moechars D, Laenen I, Kuiperi C, Bruynseels K, Tesseur I, Loos R, Vanderstichele H, Checler F, Sciot R, Van Leuven F. Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the London mutant of human APP in neurons. Am J Pathol. 2000;157:1283–1298. doi: 10.1016/S0002-9440(10)64644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, Puurunen K, Mäki HM, Sivenius J, Jolkkonen J. Transformation of diffuse β-amyloid precursor protein and β-amyloid deposits to plaques in the thalamus after transient occlusion of the middle cerebral artery in rats. Stroke. 2005;36:1551–1556. doi: 10.1161/01.STR.0000169933.88903.cf. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Hamel E. Cholinergic basal forebrain neurons project to cortical microvessels in the rat: electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J Neurosci. 1995;15:7427–7441. doi: 10.1523/JNEUROSCI.15-11-07427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicenzini E, Ricciardi MC, Altieri M, Puccinelli F, Bonaffini N, Di Piero V, Lenzi GL. Cerebrovascular reactivity in degenerative and vascular dementia: a transcranial doppler study. Eur Neurol. 2007;58:84–89. doi: 10.1159/000103642. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Secor DL, Read SL, Frazee JG, Tomiyasu U, Stanley TM, Ferreiro JA, Akers MA. Microvasculature in brain biopsy specimens from patients with Alzheimer's disease: an immunohistochemical and ultrastructural study. Ultrastructural Pathol. 1994;18:333–348. doi: 10.3109/01913129409023202. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson G, Cholerton B, Reger M, Baker L, Plymate S, Asthana S, Fishel M, Kulstad J, Green P, Cook D, Kahn S, Keeling M, Craft S. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-β peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermke M, Sorg C, Wohlschläger AM, Drzezga A. A new integrative model of cerebral activation, deactivation and default mode function in Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2008;35 (Suppl 1:S12–S24. doi: 10.1007/s00259-007-0698-5. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer's disease. Neuroscience. 2009;159:1055–1069. doi: 10.1016/j.neuroscience.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM, Rockenstein EM, Mucke L. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am J Pathol. 1995;147:53–67. [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E. Chronic overproduction of transforming growth factor-β1 by astrocytes promotes Alzheimer's disease-like microvascular degeneration in transgenic mice. Am J Pathol. 2000;156:139–150. doi: 10.1016/s0002-9440(10)64713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-βin vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, Mucke L. Amyloidogenic role of cytokine TGF-β1 in transgenic mice and in Alzheimer's disease. Nature. 1997;389:603–606. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic dilation of cerebral blood vessels is abolished in M5 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 2001;98:14096–14101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetkin FZ, Rosenberg RN, Weiner MF, Purdy PD, Cullum CM. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer's disease. Eur Radiol. 2006;16:193–206. doi: 10.1007/s00330-005-2794-x. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ha-Kawa S, Yoshimura M, Nobuhara K, Kinoshita T, Sawada S. Effectiveness of treatment with donepezil hydrochloride and changes in regional cerebral blood flow in patients with Alzheimer's disease. Ann Nucl Med. 2007;21:257–265. doi: 10.1007/s12149-007-0022-2. [DOI] [PubMed] [Google Scholar]
- Zhang F, Xu S, Iadecola C. Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience. 1995;69:1195–1204. doi: 10.1016/0306-4522(95)00302-y. [DOI] [PubMed] [Google Scholar]