Abstract
Upregulation of blood–brain barrier (BBB) P-glycoprotein expression causes central nervous system (CNS) pharmacoresistance. However, activation of BBB protein kinase C-β1 (PKC-β1) rapidly reduces basal P-glycoprotein transport activity. We tested whether PKC-β1 activation would reverse CNS drug resistance caused by dioxin acting through aryl hydrocarbon receptor. A selective PKC-β1 agonist abolished the increase in P-glycoprotein activity induced by dioxin in isolated rat brain capillaries and reversed the effect of dioxin on brain uptake of verapamil in dioxin-dosed rats. Thus, targeting BBB PKC-β1 may be an effective strategy to improve drug delivery to the brain, even in drug-resistant individuals.
Keywords: blood–brain barrier, capillaries, confocal microscopy, pharmacology, receptors
Introduction
Delivering drugs across the blood–brain barrier (BBB) to targets within the brain is a major challenge in the treatment of central nervous system (CNS) diseases, including brain cancer, neuro AIDS, and epilepsy (Pardridge, 2007). The ATP-driven drug efflux pump, P-glycoprotein, is localized to the luminal membrane of the brain capillary endothelium, which comprises the BBB. In this location, P-glycoprotein is an essential defense against neurotoxicants; however, it is also a primary obstacle to the delivery of small-molecule drugs to the CNS (Miller, 2010). Thus, an understanding of the signaling network that regulates activity of this BBB transporter may provide essential tools to improve CNS pharmacotherapy.
Endogenous metabolites and xenobiotics upregulate P-glycoprotein expression and transport activity at the BBB by targeting ligand-activated nuclear receptors, including, pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor (AhR; Bauer et al, 2004; Wang et al, 2010; Wang et al, 2011). Increased transporter protein expression leads to increased efflux transport activity and thus to selective tightening of the barrier to drugs that are P-glycoprotein substrates, that is, CNS drug resistance. Improving drug delivery to the brain may require effective modulation of P-glycoprotein transport activity. We recently identified two signaling pathways in rat brain capillaries that rapidly and reversibly reduce basal P-glycoprotein activity without altering transporter protein expression: vascular endothelial growth factor acting through Src kinase (Hawkins et al, 2010) and tumor necrosis factor-α acting through tumor necrosis factor receptor-1 and protein kinase C (PKC)-β1 (Rigor et al, 2010). Using in situ brain perfusion, both pathways were shown to signal increased brain accumulation of the P-glycoprotein substrate, verapamil.
The present study was focused on determining whether PKC-β1-based signaling can offset the increase in P-glycoprotein activity caused by induction of P-glycoprotein protein expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a widespread, persistent environmental pollutant that is a high-affinity ligand for AhR. We show that specific activation of PKC-β1 by the phorbol ester derivative, 2-deoxyphorbol-13-phenylacetate-20-acetate (dPPA), reversed the effect of TCDD on P-glycoprotein activity in (1) rat brain capillaries exposed to TCDD in vitro, (2) brain capillaries from TCDD-dosed rats, and (3) intact TCDD-dosed animals. Thus, rapid signaling to P-glycoprotein can be used to enhance access of drugs to the CNS, even in a drug-resistant population.
Materials and methods
Materials
The dPPA was purchased from Enzo Life Sciences (Plymouth Meeting, PA). The TCDD stock solution was prepared by RTI International (Research Triangle Park, NC). (N-∈(4-nitrobenzofurazan-7-yl)-D-Lys8)-cyclosporine A (NBD-CSA) was custom –synthesized, as described previously (Schramm et al, 1995). PSC833 was kindly provided by Novartis (Basel, Switzerland). MK571 was obtained from Cayman Chemical (Ann Arbor, MI). Texas red (sulforhodamine 101 (free acid)), Ficoll, and all other chemicals were obtained from Sigma-Aldrich (St Louis, MO, USA). The [14C]-sucrose (specific activity 300 mCi/mmol) and [3H]-verapamil (specific activity 80 Ci/mmol) were obtained from American Radiolabeled Chemicals (St Louis, MO).
Animals
All experiments were performed in compliance with NIH animal care and use guidelines, and approved by the NIEHS Animal Care and Use Committee. Male retired breeder Sprague–Dawley rats (6 to 9 months, Taconic, Germantown, NY) for in vitro experiments, and male Sprague–Dawley adult rats (250 to 300g, Charles River Laboratories, Raleigh, NC) for in situ brain perfusion experiments, were housed in temperature-controlled rooms under a 12-h light/12-h dark cycle and given ad libitum access to food and water. For in vivo dosing, TCDD dissolved in corn oil (Sigma-Aldrich) was administrated as a single 5 μg/kg dose by intraperitoneal injection. Controls received an equal volume of corn oil. After 48 hours, rats were either killed and brain capillaries isolated for transport experiments or underwent in situ brain perfusion.
Capillary Isolation and Transport Assay
Detailed procedures for capillary isolation were described previously (Hartz et al, 2004). Briefly, white matter, meninges, midbrain, choroid plexus, surface blood vessels, and olfactory lobes were removed under a dissecting microscope and brain tissue homogenized. Ficoll (30%) was added to an equal volume of brain homogenate, and capillaries were isolated by centrifuging at 5,800g for 20 minutes. Capillary pellets were washed with 1% bovine serum albumin in phosphate-buffered saline and passed through a syringe column filled with glass beads. Capillaries were released by gentle agitation and used immediately.
Confocal microscopy-based transport assays with isolated rat brain capillaries have been described previously (Hartz et al, 2004). For in vitro dosing experiments, brain capillaries in phosphate-buffered saline were exposed to TCDD for 3 hours without or with dPPA (30 minutes). Fluorescent substrates, NBD-CSA for P-glycoprotein (Hartz et al, 2004) or Texas red for Mrp2 (Bauer et al, 2008), were added and luminal substrate accumulation was measured after 1 hour. In dosing experiments, brain capillaries isolated from control and TCDD-dosed rats were incubated with NBD-CSA for 1 hour without or with 30 minutes exposure to dPPA.
In Situ Brain Perfusion
In situ brain perfusion was performed as described previously (Hawkins et al, 2007). Rats were anaesthetized with ketamine cocktail and heparinized. The common carotid arteries were exposed by midline incision at the neck, and perfused with oxygenated Ringer's solution (37 °C) at 3 ml/min. The [14C]-sucrose (0.5 μCi/ml) or [3H]-verapamil (0.1 μCi/ml) was infused into the circuit via syringe pump at 0.5 ml/min for 20 minutes. Samples of perfusate were collected from the cannulae at the end of each experiment. Brains were removed, stripped of meninges, midbrain, and choroid plexuses, and minced. Tissue and perfusate samples were solubilized (Hyamine Hydroxide, MP Biomedicals, Santa Ana, CA), neutralized (30% acetic acid), and counted in 4 mL liquid scintillation cocktail. Data are expressed as brain-to-perfusate disintegrations per minute (Rbr; Hawkins et al, 2007).
Results and Discussion
This laboratory has established a method to determine transport activity of P-glycoprotein and other ABC transporters in freshly isolated brain capillaries using fluorescent substrates, confocal microscopy, and digital image analysis (Hartz et al, 2004). For P-glycoprotein, we measure steady-state (60 minutes) luminal accumulation of a fluorescent CSA derivative, NBD-CSA. In brain capillaries, luminal NBD-CSA accumulation is concentrative, metabolism-dependent, and specific, that is, sensitive to inhibition by PSC833, a specific inhibitor of P-glycoprotein.
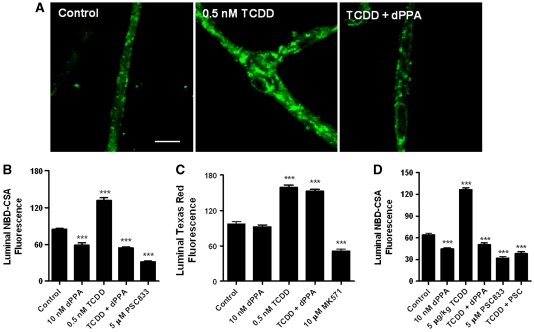
As shown previously (Hartz et al, 2004), NBD-CSA accumulated in the luminal space of control brain capillaries (Figure 1A); accumulation was sensitive to inhibition by PSC833 (Figure 1B). Exposing capillaries to 0.5 nmol/L TCDD for 3 hours more than doubled P-glycoprotein-mediated transport (Figures 1A and 1B). Exposing capillaries to 10 nmol/L dPPA (30 minutes), a specific PKC-β1 agonist, reduced basal P-glycoprotein activity (Figure 1B). These findings are in agreement with our recent reports (Rigor et al, 2010; Wang et al, 2011). Importantly, in capillaries exposed to TCDD and exhibiting increased P-glycoprotein activity, dPPA reduced transport activity to that of capillaries exposed to dPPA alone (Figures 1A and 1B).
Figure 1.
Activating protein kinase C (PKC)-β1 reverses 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced increases in P-glycoprotein transport activity in rat brain capillaries in vitro and ex vivo. (A) Representative confocal images of control capillaries and capillaries exposed to 0.5 nmol/L TCDD, 0.5 nmol/L TCDD plus 10 nmol/L 2-deoxyphorbol-13-phenylacetate-20-acetate (dPPA) in vitro. Capillaries were incubated for 3 hours in medium without and with TCDD. During the last 30 minutes, some of the capillaries were exposed to dPPA. At the end of the incubation period, (N-∈(4-nitrobenzofurazan-7-yl)-D-Lys8)-cyclosporine A (NBD-CSA; 2 μmol/L) was added to the medium and capillaries were imaged after 1 hour. White scale bar indicates 5 μm. (B) Effects of exposure to TCDD, dPPA, or TCDD plus dPPA on luminal NBD-CSA fluorescence. (C) Effects of exposure to TCDD, dPPA, or TCDD plus dPPA on luminal Texas red fluorescence. Protocol was the same as in A and B, except that Texas red was used to monitor changes in Mrp2 transport activity. (D) Effects of PKC-β1 activation on capillaries isolated from control and TCDD-exposed rats (single intraperitoneal dose, 5 μg/kg; tissue collected after 2 days). Protocol for isolated capillaries is same as in A. Shown are mean±s.e.m. for 8 to 12 capillaries from single preparations, each containing pooled brain tissue from 5 to 10 rats. Statistical comparisons (one-way analysis of variance): significantly different from control, ***P<0.001.
One could interpret the dPPA results as reflecting reduced P-glycoprotein activity or increased paracellular permeability. Indeed, exposing capillaries to a hypertonic solution, which opens tight junctions, does reduce luminal NBD-CSA accumulation (Hartz et al, 2004). We previously showed that neither tumor necrosis factor-α nor dPPA affected luminal accumulation of Texas red, a fluorescent organic anion that is transported by Mrp2 but not P-glycoprotein (Hartz et al, 2006; Rigor et al, 2010). Such transport is ATP dependent and concentrative and substantially reduced when capillaries are exposed to hypertonic solution. Figure 1C shows that irrespective of whether Mrp2-mediated transport was increased through TCDD exposure, dPPA was without effect. If dPPA increased junctional permeability, we would have expected to see reduced luminal accumulation of all actively transported substrates. This was not the case. Thus, PKC-β1 signaling specifically abolished the increase in P-glycoprotein transport activity caused by TCDD induction of transporter protein expression.
We used two techniques to show parallel results in rats dosed with TCDD: measurement of transport activity in brain capillaries ex vivo and measurement of blood-to-brain drug transport in vivo. First, we previously showed increased P-glycoprotein expression and transport activity in brain capillaries isolated from TCDD-dosed rats (Wang et al, 2011). In agreement with this finding, Figure 1D shows significantly increased P-glycoprotein transport activity in brain capillaries isolated from rats dosed with 5 μg/kg TCDD. This figure also shows that the TCDD-dependent increase in transport activity was abolished when capillaries from dosed rats were exposed to 10 nmol/L dPPA (Figure 1D).
Second, in situ brain perfusion is a well-established method for measuring net transport of molecules across the BBB (Smith and Allen, 2003). Transport is measured under conditions of constant flow and tracer substrate concentration, so that changes in brain accumulation reflect altered BBB-mediated transport rather than altered hemodynamics. Further, substrate is introduced directly into the cerebral circulation via the carotid artery without undergoing first-pass metabolism and hepatic and renal excretion; this point is particularly important because TCDD exposure induces expression of efflux transporters and enzymes in the liver (Wang et al, 2011). We previously used this in vivo technique in rats to show that: (1) TCDD dosing reduces transport of verapamil, a P-glycoprotein substrate, from vasculature to brain, a result consistent with increased P-glycoprotein expression (Wang et al, 2011); (2) PKC-β1 activation increases brain accumulation of verapamil, a result consistent with reduced transport activity (Rigor et al, 2010); and (3) neither TCDD dosing nor PKC-β1 activation affects tight junction permeability, as measured by brain sucrose accumulation (Wang et al, 2011; Rigor et al, 2010).
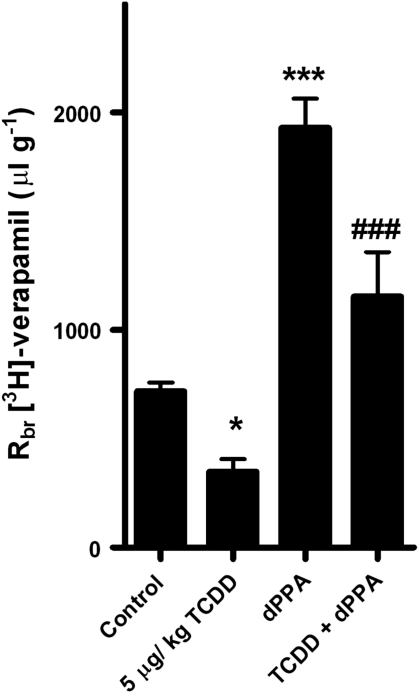
Figure 2 shows that dosing rats with 5 μg/kg TCDD reduced brain uptake of verapamil by about half when compared with controls. In control animals, addition of 100 nmol/L dPPA to the vascular perfusate increased by nearly threefold brain uptake of verapamil (Figure 2). These results confirm our previous findings (Rigor et al, 2010; Wang et al, 2011). As a point of reference, in the same experimental system, addition of 8 μmol/L CSA to the perfusate increases brain accumulation of verapamil fivefold (Hawkins et al, 2010; Rigor et al, 2010). Importantly, in TCDD-dosed animals, dPPA more than doubled brain uptake of verapamil (P<0.001; Figure 2). In fact, verapamil uptake in TCDD-dosed rats was nearly double than that of untreated controls (P<0.05). Thus, in rats with increased BBB P-glycoprotein expression and thus increased efflux transporter activity, activation of BBB PKC-β1 substantially improved delivery of a P-glycoprotein substrate to the brain.
Figure 2.
Activating protein kinase C-β1 restores brain uptake of verapamil in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-dosed rats. Rats received a single intraperitoneal injection of TCDD (5 μg/kg) or corn oil (controls). Two days later, common carotid arteries were perfused with Ringer's solution containing [3H]-verapamil or [3H]-verapamil plus 100 nmol/L 2-deoxyphorbol-13-phenylacetate-20-acetate; brain accumulation of the radiolabeled drug measured and the data expressed as brain-to-perfusate ratio (Rbr). Shown are mean±s.e.m. for 8 rats. Statistical comparisons (one-way analysis of variance): *significantly different from control, P<0.05; **significantly different from control, P<0.001; +++ significantly different from TCDD, P<0.001.
Our recent studies have shown increased expression and activity of multiple ATP-driven efflux pumps in rodents exposed to ligands that activate three nuclear receptors, pregnane X receptor, constitutive androstane receptor, and AhR (Bauer et al, 2004; Wang et al, 2010; Wang et al, 2011). Certainly for P-glycoprotein, an important element of the BBB and a major obstacle to CNS pharmacotherapy with small-molecule drugs, increased expression suggests increased neuroprotection, but at the price of reduced CNS drug entry. The pregnane X receptor and constitutive androstane receptor ligands include endogenous substances, for example, bile acids, dietary constituents, and many therapeutic drugs, whereas potent AhR ligands are largely widespread and persistent environmental pollutants, for example, dioxins and polychlorinated biphenyls (Moore et al, 2003; White and Birnbaum, 2009). Given the potential for exposure to pregnane X receptor, constitutive androstane receptor, and AhR ligands, it is likely that BBB P-glycoprotein is already induced in a substantial percentage of the population.
The present data show that targeting a signaling pathway that acutely modulates P-glycoprotein activity within the BBB has the potential to restore the drug delivery to the brain in induced individuals. Targeting rapid signaling through PKC-β1 provides an effective way to transiently reduced P-glycoprotein activity, while maintaining other protective elements of the barrier. Certainly, use of the phorbol ester, dPPA, in patients is not practical. However, our unpublished experiments with rat brain capillaries suggest a wealth of rapid signaling events connecting PKC-β1 to P-glycoprotein. These include elements of lipid signaling and at least one protein phosphorylation cascade. Complete mapping of this signaling pathway should provide additional targets and pharmacological tools.
The authors declare no conflict of interest.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
References
- Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–419. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Lucking JR, Yang X, Pollack GM, Miller DS. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab. 2008;28:1222–1234. doi: 10.1038/jcbfm.2008.16. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol Pharmacol. 2004;66:387–394. doi: 10.1124/mol.104.001503. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Fricker G, Miller DS. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol Pharmacol. 2006;69:462–470. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Sykes DB, Miller DS. Rapid, reversible modulation of blood-brain barrier P-glycoprotein transport activity by vascular endothelial growth factor. J Neurosci. 2010;30:1417–1425. doi: 10.1523/JNEUROSCI.5103-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sic. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JT, Moore LB, Maglich JM, Kliewer SA. Functional and structural comparison of PXR and CAR. Biochim Biophys Acta. 2003;1619:235–238. doi: 10.1016/s0304-4165(02)00481-6. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug targeting to the brain. Pharm Res. 2007;24:1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- Rigor RR, Hawkins BT, Miller DS. Activation of PKC isoform beta(I) at the blood-brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain. J Cereb Blood Flow Metab. 2010;30:1373–1383. doi: 10.1038/jcbfm.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm U, Fricker G, Wenger R, Miller DS. P-glycoprotein-mediated secretion of a fluorescent cyclosporin analogue by teleost renal proximal tubules. Am J Physiol. 1995;268:F46–F52. doi: 10.1152/ajprenal.1995.268.1.F46. [DOI] [PubMed] [Google Scholar]
- Smith QR, Allen DD. In situ brain perfusion technique. Methods Mol Med. 2003;89:209–218. doi: 10.1385/1-59259-419-0:209. [DOI] [PubMed] [Google Scholar]
- Wang X, Hawkins BT, Miller DS. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J. 2011;25:644–652. doi: 10.1096/fj.10-169227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sykes DB, Miller DS. Constitutive androstane receptor-mediated upregulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. Mol Pharmacol. 2010;78:376–383. doi: 10.1124/mol.110.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Birnbaum LS. An overview of the effects of dioxins and dioxin-like compounds on vertebrates, asdocumented in human and ecological epidemiology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:197–211. doi: 10.1080/10590500903310047. [DOI] [PMC free article] [PubMed] [Google Scholar]