Abstract
As it is often assumed that delayed cerebral ischemia (DCI) after subarachnoid hemorrhage (SAH) is caused by vasospasm, clinical trials often focus on prevention of vasospasm with the aim to improve clinical outcome. However, the role of vasospasm in the pathogenesis of DCI and clinical outcome is possibly smaller than previously assumed. We performed a systematic review and meta-analysis on all randomized, double-blind, placebo-controlled trials that studied the effect of pharmaceutical preventive strategies on vasospasm, DCI, and clinical outcome in SAH patients to further investigate the relationship between vasospasm and clinical outcome. Effect sizes were expressed in pooled risk ratio (RR) estimates with corresponding 95% confidence intervals (CI). A total of 14 studies randomizing 4,235 patients were included. Despite a reduction of vasospasm (RR 0.80 (95% CI 0.70 to 0.92)), no statistically significant effect on poor outcome was observed (RR 0.93 (95% CI 0.85 to 1.03)). The variety of DCI definitions did not justify pooling the DCI data. We conclude that pharmaceutical treatments have significantly decreased the incidence of vasospasm, but not of poor clinical outcome. This dissociation between vasospasm and clinical outcome could result from methodological problems, sample size, insensitivity of clinical outcome measures, or from mechanisms other than vasospasm that also contribute to poor outcome.
Keywords: delayed cerebral ischemia, meta-analysis, outcome, subarachnoid hemorrhage, systematic review
Introduction
Delayed cerebral ischemia (DCI) is a common complication of aneurysmal subarachnoid hemorrhage (SAH). Delayed cerebral ischemia can be reversible or it may progress to cerebral infarction. Cerebral infarction is associated with poor clinical outcome and death (Rabinstein et al, 2004). As vasospasm is strongly associated with DCI and clinical outcome, clinical trials in the last few decades focused on prevention of vasospasm with the aim to improve clinical outcome (Fergusen and Macdonald, 2007; Rosengart et al, 2007). Progress has been made. Meta-analysis of population-based studies of aneurysmal SAH found that mortality decreased by 50% in the last 2 decades, mostly due to improved in-hospital care (Lovelock et al, 2010). However, outcome remains poor, and some clinical trials designed to decrease vasospasm or DCI have not improved outcome after SAH (Macdonald et al, 2008). One reason for this may be that the role of vasospasm in the pathogenesis of DCI and clinical outcome is smaller than previously assumed, although the role of drug side effects and insensitive outcome measures has not been ruled out (Kreiter et al, 2009). To further investigate the relationship between vasospasm and clinical outcome, we pooled all randomized placebo-controlled trials that investigated pharmaceutical interventions as a preventive strategy in SAH patients.
Materials and methods
For this systematic review, the Cochrane Collaboration format was used (Higgins and Green, 2008).
Selection Criteria
Types of studies
All randomized, double-blind, placebo-controlled trials that studied the efficacy of pharmaceutical strategies aiming to prevent vasospasm, DCI, and poor outcome in SAH patients were included, regardless of type and dosage of drug and follow-up duration.
Types of outcome measures
Only trials that included vasospasm, DCI, and clinical outcome as outcome events were included. Trials that did not report on all three outcome events were excluded. A sensitivity analysis was performed for studies that had angiographic vasospasm, and not vasospasm diagnosed by transcranial Doppler ultrasound (TCD), as an outcome measure.
Definition of outcome measures
Radiographic vasospasm was defined as a focal or generalized reduction of cerebral arterial caliber on catheter cerebral angiography or increased cerebral blood flow velocities on TCD. As the included studies used various definitions of angiographic and TCD vasospasm, we used the definitions used by the investigators of the original studies. If the investigators of the original studies categorized angiographic vasospasm into no/mild, moderate, and severe vasospasm, we used the number of patients who had moderate-to-severe vasospasm as an outcome event. Transcranial Doppler ultrasound vasospasm was defined as flow velocity of at least 120 cm/second or peak flow velocity of >200 cm/second. If studies reported on both angiographic and TCD vasospasm, the data on angiographic vasospasm was used for our analyses. Clinical deterioration due to DCI was defined as the occurrence of focal neurologic impairment (such as hemiparesis, aphasia, apraxia, hemianopia, or neglect) or a decrease of at least two points on the Glasgow coma scale (either on the total score or on one of its individual components (eye, motor on either side, verbal)), lasting for at least 1 hour, not apparent immediately after aneurysm occlusion, and not attributable to other causes by means of clinical assessment, computed tomography or magnetic resonance imaging scanning of the brain, and appropriate laboratory studies (Vergouwen et al, 2010b). Poor clinical outcome was defined as severe disability, vegetative state, or death, as defined by the investigators of the individual studies, either measured with the modified Rankin Scale (mRS) or (extended) Glasgow Outcome Scale (GOS). If the investigators provided data on the individual categories of the used outcome scales, we considered a mRS of 3 to 6 or a GOS of 1 to 3 as poor clinical outcome. In those studies in which an inverted GOS was used, poor clinical outcome was readjusted to the original scale. For all included studies, we used data from the last blinded outcome measurement. If the study provided the percentage of patients with an outcome event, the actual numbers were calculated from the percentages.
Search strategy for identification of studies
Two of the authors (NE and MDIV) conducted a systematic search of the Pubmed database and the RefMan software (Thomson Reuters, Version 12, New York, NY, USA) up to March 2010 (week 9) for the variable ‘SAH'. The search was limited to ‘randomized trials' and ‘human studies'. We also searched for additional studies in previous meta-analyses that investigated the efficacy of pharmaceutical interventions to prevent vasospasm and DCI.
Assessment of risk of bias
Two authors (NE and MDIV) independently assessed risk of bias by reviewing methodological quality of the included studies for allocation concealment and blinding.
Statistics
Data were processed in Review manager 5.0.24, as supplied by the Cochrane Collaboration. Effect sizes were expressed in (pooled) risk ratio (RR) estimates. Statistical uncertainty was expressed in 95% confidence intervals (CI). Pooled data were interpreted to be heterogeneous in case the probability value of the χ2-test was ≤0.20. If no heterogeneity could be demonstrated, we used a fixed-effects model. Otherwise a random-effects model was used. Funnel plots were made to investigate potential publication bias.
Results
The initial search yielded 733 articles, of which 697 were excluded after review of titles and abstracts. Publications were usually excluded because the study was not a randomized trial or it did not include the relevant outcome events. The remaining 37 studies were randomized, double-blind, placebo-controlled trials of pharmaceutical preventive strategies in patients with aneurysmal SAH, although some studies included patients with spontaneous SAH and no identified source of hemorrhage. Finally, 14 trials with 4,235 patients had radiographic vasospasm, DCI, and clinical outcome as outcome events and were included in the analysis (Chou et al, 2008; Findlay et al, 1995; Haley Jr et al, 1993a, 1993b, 1997; Kassell et al, 1996; Macdonald et al, 2008; Neil-Dwyer et al, 1987; Ono et al, 1984; Petruk et al, 1988; Philippon et al, 1986; Shibuya et al, 1992; Springborg et al, 2007; Tseng et al, 2005, 2007; Vergouwen et al, 2009). A total of 2,612 patients were randomized to pharmaceutical treatment and 1,623 patients to placebo. Funnel plots were not suggestive of publication bias (data not shown). Characteristics of the included studies are listed in the Table 1.
Table 1. Characteristics of included studies.
| Study | Vasospasm definition | DCI definition | Poor outcome definitiona | Adequate allocation concealment | Adequate blinding |
|---|---|---|---|---|---|
| Chou et al (2008) | Angiographic vasospasm defined as focal or generalized reduction of cerebral arterial caliber on conventional cerebral angiogram confirmed by a neuroradiologist and a neurocritical care physician TCD vasospasm defined as any peak systolic middle cerebral artery blood flow velocity >200 cm/second and a Lindegaard ratio of >3 | Unaccountable new focal neurologic deficit lasting >2 hours or any 2-point fall in modified Glasgow Coma Scale | mRS 3–6 | Unclear | Unclear |
| Findlay et al (1995) | Angiographic vasospasm defined as at least 50% luminal narrowing on cerebral angiogram 7–11 days after SAH | Unclear | GOS 1–3 | Unclear | Yes |
| Haley Jr et al (1993a, 1993b) | Angiographic vasospasm defined as moderate (25%–50%) to severe (>50%) vessel narrowing on cerebral angiogram 7–11 days after SAH TCD vasospasm defined as maximum mean MCA velocity >120 cm/second | Classic symptoms included onset at 5–12 days after SAH, worsening headache, stiff neck, and/or low-grade fever; insidious onset of confusion, disorientation, and/or decline in level of consciousness; and focal deficit, which may fluctuate in severity. Confirmation of vasospasm by angiography or transcranial Doppler ultrasonography is recommended, but not required | GOS 1–3 | Yes | Yes |
| Haley Jr et al (1997) | Angiographic vasospasm defined as moderate (25%–50%) to severe (>50%) vessel narrowing on cerebral angiogram 7–11 days after SAH TCD vasospasm defined as maximum mean MCA velocity >120 cm/second | Classic symptoms included onset at 5–12 days after SAH, worsening of headache, stiff neck, and/or low-grade fever; insidious onset of confusion, disorientation, and/or decline in level of consciousness; and focal deficit, which may fluctuate in severity. Confirmation of vasospasm by angiography or transcranial Doppler ultrasonography is recommended, but not required | GOS 1–3 | Unclear | Yes |
| Kassell et al (1996) | Angiographic vasospasm defined as moderate (25%–50%) to severe (>50%) vessel narrowing on cerebral angiogram 7–11 days after SAH TCD vasospasm defined as maximum mean MCA velocity >120 cm/second | Classic symptoms included onset at 5–12 days after SAH, worsening of headache, stiff neck, and/or low-grade fever; insidious onset of confusion, disorientation, and/or decline in level of consciousness; and focal deficit, which may fluctuate in severity. Confirmation of vasospasm by angiography or transcranial Doppler ultrasonography is recommended, but not required | GOS 1–3 | Unclear | Yes |
| Macdonald et al (2008) | Angiographic vasospasm was defined as a moderate (34%–66%) to severe (67%–100%) reduction in arterial diameter between baseline and day 9±2 DSA TCD vasospasm defined as a Lindegaard ratio ≥3, a mean middle or anterior cerebral artery flow velocity >200 cm/second, or an increase >50 cm/second/24 hours | Locally defined vasospasm on DSA or transcranial Doppler associated with neurologic worsening lasting for at least 2 hours. Neurologic worsening defined as a decline of at least 2 points in the modified Glasgow Coma Scale or an increase of at least 2 points in the abbreviated National Institutes of Health Stroke Scale. When patients were not evaluable neurologically, DIND was defined as clinical signs of vasospasm (e.g., unexplained fever, new neurologic deficit) with vasospasm on DSA or transcranial Doppler or when a new hypodensity was observed on a post-procedure CT scan | GOS 1–3 | Yes | Yes |
| Neil-Dwyer et al (1987) | Unclear | Unclear | Neurologic or intellectual impairment and death | Unclear | Unclear |
| Ono et al (1984) | Angiographic vasospasm was defined as moderate-to-severe vasospasm, not further defined | Unclear | Conscious but needs help to live, vegetative state, and death | Unclear | Yes |
| Petruk et al (1988) | Angiographic vasospasm was defined as a moderate (30%–50%) to severe (50% or more) narrowing of the lumen compared with its original diameter | A delayed ischemic deficit was determined to have occurred if the patient's neurologic status was noted on the case report form to have been stable or improving followed by a subsequent deterioration, with confirmation usually being sought from the discharge summary | GOS 1–3 | Yes | Yes |
| Philippon et al (1986) | Angiographic vasospasm was considered as moderate if arterial diameter was reduced by <50%, or severe if the diminution of caliber was >50% | Unclear | GOS 3–5 | Unclear | Unclear |
| Shibuya et al (1992) | Angiographically demonstrated vasospasm was classified into four grades: none; mild (minimal or mild change in vessel lumen); moderate (between mild and severe); and severe (threadlike and diffuse narrowing of vessels) | Neurologic deterioration, either temporary or permanent, was considered to be due to vasospasm when all other potential causes such as surgery, hydrocephalus, intracranial rebleeding, seizure, infection, cardiopulmonary dysfunction, electrolyte imbalance, and metabolic disturbances were excluded | GOS 1–3 | Yes | Yes |
| Springborg et al (2007) | TCD vasospasm was defined as a mean flow velocity in the MCA or ACA of ≥120 cm/second. A Lindegaard index between flow velocities in the MCA and ipsilateral ICA over 3.0 is recommended for diagnosis, but not required | Decline in Glasgow Coma Scale by ≥2 points or development of new focal motor deficits between post-hemorrhage days 5 and 12, with no other identifiable neurologic or systemic cause | GOS 1–3 | Yes | Yes |
| Tseng et al (2005, 2007) | TCD vasospasm defined as mean blood flow velocity >120 cm/second with Lindegaard ratio >3 | DCI defined as vasospasm-related if it was associated with severe vasospasm on TCD, which is defined as blood flow velocity >200 cm/second with Lindegaard ratio >3 | mRS 3–6 | Yes | Yes |
| Vergouwen et al (2009) | TCD vasospasm, distinguished in mild, moderate, and severe (mean blood flow velocity in MCA or ACA ≥120 cm/second and <160 cm/second, ≥160 cm/second and <200 cm/second, and ≥200 cm/second, respectively) | Development of focal neurologic impairment and/or a drop in the Glasgow Coma Scale by ≥2 points, after exclusion of other causes | GOS 1–3 | Yes | Yes |
ACA, anterior cerebral artery; CT, computed tomography; DCI, delayed cerebral ischemia; DIND, delayed ischemic neurologic deficit; DSA, digital subtraction angiography; GOS, Glasgow outcome score; ICA, internal cerebral artery; MCA, middle cerebral artery; mRS, modified Rankin scale; SAH, subarachnoid hemorrhage; TCD, transcranial Doppler.
Analyses
Of 14 studies eventually included in the meta-analysis, three reported on TCD vasospasm only (Springborg et al, 2007; Tseng et al, 2005; Vergouwen et al, 2009), five on both TCD and angiographic vasospasm (Chou et al, 2008; Haley et al, 1993b, 1997; Kassell et al, 1996; Macdonald et al, 2008), five on angiographic vasospasm only (Findlay et al, 1995; Ono et al, 1984; Petruk et al, 1988; Philippon et al, 1986; Shibuya et al, 1992), and in one study it was unclear (Neil-Dwyer et al, 1987). For TCD vasospasm, various definitions and cutoff values were used for mean and peak blood flow velocity, with or without a Lindegaard ratio >3 (Chou et al, 2008; Haley Jr et al, 1993b, 1997; Kassell et al, 1996; Macdonald et al, 2008; Springborg et al, 2007; Tseng et al, 2005; Vergouwen et al, 2009). In addition, for angiographic vasospasm, various definitions with different cutoff values and classifications for luminal narrowing and vasospasm severity were used (Table 1; Chou et al, 2008; Findlay et al, 1995; Haley Jr et al, 1993b, 1997; Kassell et al, 1996; Macdonald et al, 2008; Petruk et al, 1988; Philippon et al, 1986; Shibuya et al, 1992). The definition of vasospasm was unclear in two studies (Neil-Dwyer et al, 1987; Ono et al, 1984).
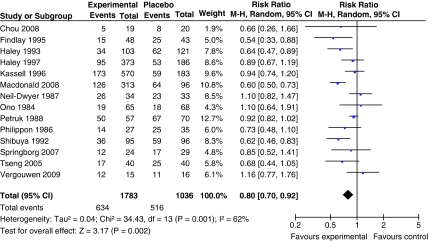
For the analysis of radiographic vasospasm, data from 2,819 patients were available (1,783 patients randomized to pharmaceutical treatment and 1,036 to placebo). The overall number of patients with radiographic vasospasm was 634 in the group of patients randomized to pharmaceutical treatment and 516 in the placebo group. Our meta-analysis including 14 trial arms demonstrated a significant and favorable effect of treatment, compared with the placebo, on angiographic vasospasm (pooled RR 0.80; 95% CI, 0.70 to 0.92). Heterogeneity between the trials was high (I2=62% and P=0.001; Figure 1). This justified our use of the random-effects meta-analysis model.
Figure 1.
Pooled RR estimates for patients on pharmaceutical treatment found to have radiographic vasospasm.
Regarding DCI, there was a wide variation of definitions (Table 1). This variety of definitions did not justify pooling the DCI data.
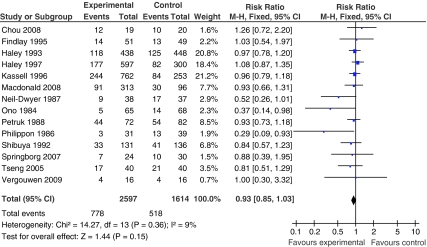
For clinical outcome, most studies used the GOS (Findlay et al, 1995; Haley Jr et al, 1993a, 1993b, 1997; Kassell et al, 1996; Macdonald et al, 2008; Petruk et al, 1988; Philippon et al, 1986; Shibuya et al, 1992; Springborg et al, 2007; Vergouwen et al, 2009) or mRS (Table 1; Chou et al, 2008; Tseng et al, 2005; Tseng et al, 2007). One study used an inverted GOS (Philippon et al, 1986). For the meta-analysis of poor clinical outcome, data from 4,211 patients were available (2,597 patients randomized to pharmaceutical treatment and 1,614 to placebo). The overall number of patients with poor outcome comprised 778 in the pharmaceutically treated group and 518 in the placebo group. Our meta-analysis including 14 trial arms did not show a significant effect of treatment, compared with the placebo, on poor clinical outcome (pooled RR 0.93; 95% CI, 0.85 to 1.03). Heterogeneity between the trials was not significant (I2=9% and P=0.36; Figure 2).
Figure 2.
Pooled RR estimates for patients on pharmaceutical treatment found to have poor clinical outcome.
Sensitivity analysis
In the sensitivity analysis that only included studies with angiographic vasospasm, and not TCD vasospasm, 10 studies were included with 3,994 patients. The pooled RR for angiographic vasospasm was 0.76 (95% CI 0.64 to 0.89) and for poor outcome 0.95 (95% CI 0.86 to 1.05).
Risk of Bias Assessment
For allocation concealment, risk of bias was low in seven studies (Haley Jr et al, 1993a, 1993b; Macdonald et al, 2008; Petruk et al, 1988; Shibuya et al, 1992; Springborg et al, 2007; Tseng et al, 2005, 2007; Vergouwen et al, 2009) and unclear in seven studies (Table 1; Chou et al, 2008; Findlay et al, 1995; Haley Jr et al, 1997; Kassell et al, 1996; Neil-Dwyer et al, 1987; Ono et al, 1984; Philippon et al, 1986). For blinding, risk of bias was low in 11 studies (Findlay et al, 1995; Haley Jr et al, 1993a, 1993b, 1997; Kassell et al, 1996; Macdonald et al, 2008; Ono et al, 1984; Petruk et al, 1988; Shibuya et al, 1992; Springborg et al, 2007; Tseng et al, 2005, 2007; Vergouwen et al, 2009) and unclear in 3 studies (Chou et al, 2008; Neil-Dwyer et al, 1987; Philippon et al, 1986). High risk of bias was not observed in any of the studies. The authors of this systematic review acknowledge potential risk of bias, because they are authors of two of the studies included in this systematic review (Macdonald et al, 2008; Vergouwen et al, 2009).
Discussion
This systematic review found that despite a decreased incidence of radiographic vasospasm, pharmaceutical treatment after SAH did not improve clinical outcome. A sensitivity analysis in which only those studies were included that had angiographic vasospasm as an outcome measure, and not TCD vasospasm, did not change our findings. The variety of definitions for clinical deterioration due to DCI did not justify pooling the DCI data.
Previous meta-analyses have questioned the relationship between vasospasm and clinical outcome (Dorhout Mees et al, 2007; Kramer and Fletcher, 2009; Zhang et al, 2010). However, most of these analyses focused on classes of drugs, and therefore the results might be related to pharmacological properties of the type of drug studied. The present systematic review differs from previous studies because we pooled all studies that analyzed the effect of any pharmaceutical intervention, regardless of the type of drug (Weyer et al, 2006). A systematic review that investigated the efficacy of the dihydropyridine calcium antagonist nimodipine showed that nimodipine reduces the incidence of DCI and improves clinical outcome without a statistically significant effect on vasospasm (Feigin et al, 1998). Potential explanations are that, in the nimodipine studies confirmation of vasospasm was not obtained when DCI developed, or that nimodipine exerts its beneficial effect through profibrinolytic properties or conversion of cortical spreading ischemia to cortical spreading hyperemia (Dreier et al, 1998; Vergouwen et al, 2007). Similar to the results of the present study, endothelin receptor antagonists reduced vasospasm and DCI, but not poor clinical outcome (Kramer and Fletcher, 2009). The effect of endothelin receptor antagonists on DCI is not unexpected as the included studies required radiographic confirmation of vasospasm in the diagnosis of DCI (Vergouwen, 2009). Tirilazad mesylate reduced DCI, but not cerebral infarction and poor clinical outcome (Zhang et al, 2010). Statin treatment had no effect on vasospasm, DCI, and clinical outcome (Vergouwen et al, 2010a). However, the statin treatment meta-analysis included only 190 patients, which makes it difficult to draw definitive conclusions about efficacy of statins and their effects on vasospasm, DCI, and clinical outcome.
There are several explanations for the observed dissociation between radiographic vasospasm and clinical outcome in this meta-analysis. First, the pharmaceutical treatments for vasospasm could have detrimental effects that counterbalance any benefits that they might have on clinical outcome (Kramer and Fletcher, 2009). Pulmonary complications, anemia, and hypotension occur more frequently after treatment with endothelin receptor antagonists and nicardipine, and these complications are independently associated with poor outcome (Kahn et al, 2006; Wartenberg et al, 2006). In addition, rescue therapy such as the induction of hypervolemia and hypertension can lead to cardiovascular complications, possibly resulting in worse clinical outcomes. Balloon angioplasty for treatment of vasospasm can lead to complications such as arterial dissection, rupture, stroke, and death. However, if rescue therapy or balloon angioplasty is actually effective, then it could obscure the beneficial effects of drug treatment by being used more in the placebo groups, and one would never show an association between prevention of vasospasm and outcome. Meta-analysis of trials of endothelin receptor antagonists found that these drugs increased side effects such as hypotension and pulmonary edema (Kramer and Fletcher, 2009). Second, sample sizes may be too low to show a statistically significant effect on clinical outcome. It has been estimated that >5,000 patients are needed to show a treatment effect size of 50% on the mRS at 3 months after SAH, assuming β=0.80 and α=0.05 (two-tailed; Kreiter et al, 2009). Third, the dichotomous GOS or mRS may be insensitive to clinically important effects of pharmaceutical treatments on outcome (Al-Khindi et al, 2010; Scott et al, 2010). Deficits in memory, executive function, and language are common cognitive sequelae of SAH and important contributors to poor functional outcome (Al-Khindi et al, 2010; Scott et al, 2010). Deficits in cognitive and functional performance are further complicated by depression, anxiety, fatigue, and sleep disturbances. Nevertheless, these sequelae are often not investigated in SAH studies. Fourth, there may not be a strong causal relationship between vasospasm and functional outcome. There is growing evidence that the pathogenesis of DCI is multifactorial. Microthromboembolism, cortical spreading ischemia, delayed effects of acute SAH-induced brain injury, and impaired cerebral autoregulation have been suggested to have a role in clinical outcome (Dreier et al, 2006; Vergouwen et al, 2008; Yundt et al, 1998).
In the studies that were included in the present systematic review, many different definitions for clinical deterioration due to DCI were used, which did not justify to aggregate and pool the studies in a meta-analysis. Often, included studies combined radiographic evidence of vasospasm with clinical features of cerebral ischemia, while multiple factors might contribute to DCI. Recently, a uniform definition of DCI has been proposed with the aim to standardize end points for clinical trials, simplify comparisons between studies, and aggregate study results in meta-analyses (Vergouwen et al, 2010b).
This study has some limitations. Angiography often was only performed in selected patients, a practice that would decrease the correlations among vasospasm, DCI, and clinical outcome. The definition and grading of radiographic vasospasm varied throughout the studies, and TCD as a diagnostic tool to measure vasospasm might add inaccuracy (Carrera et al, 2009). However, a sensitivity analysis that included studies with angiographic vasospasm only did not change the results. Finally, clinical outcome was measured with various scales and cutoffs for poor clinical outcome, and the timing of follow-up measurements differed among the included studies. However, these factors will have minimal effect because the GOS and mRS give almost identical results in patients with SAH (Macdonald et al, 2008). The timing of outcome assessment only matters if there is a change in the rates of recovery in the treated and untreated groups, which seems unlikely given that the treatments being applied are in the acute stage.
In conclusion, pharmaceutical treatments may decrease radiographic vasospasm in patients with SAH, but there is no effect on the clinical outcome. Future studies need to determine whether this dissociation between vasospasm and clinical outcome is due to methodological problems, sample size, insensitivity of clinical outcome measures, the effect of therapeutic strategies such as endovascular therapy, or because of mechanisms other than vasospasm also contributing to poor clinical outcome.
Nima Etminan is financially supported by an unrestricted grant from the Wilhem-Tonnis Foundation, Germany. Mervyn DI Vergouwen is financially supported by an unrestricted grant from the Niels Stensen Foundation, The Netherlands, and by a grant from the Netherlands Thrombosis Foundation (2010-4). Nima Etminan and RL Macdonald received research support from the Physicians Services Incorporated Foundation. Dr RL Macdonald is a consultant for Actelion Pharmaceuticals and Chief Scientific Officer of Edge Therapeutics.
References
- Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:e519–e536. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- Carrera E, Schmidt JM, Oddo M, Fernandez L, Claassen J, Seder D, Lee K, Badjatia N, Connolly ES, Jr, Mayer SA. Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2009;65:316–323. doi: 10.1227/01.NEU.0000349209.69973.88. [DOI] [PubMed] [Google Scholar]
- Chou SH, Smith EE, Badjatia N, Nogueira RG, Sims JR, Ogilvy CS, Rordorf GA, Ayata C. A randomized, double-blind, placebo-controlled pilot study of simvastatin in aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:2891–2893. doi: 10.1161/STROKEAHA.107.505875. [DOI] [PubMed] [Google Scholar]
- Dorhout Mees SM, Rinkel GJ, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, van GJ. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007;(3):CD000277. doi: 10.1002/14651858.CD000277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Korner K, Ebert N, Gorner A, Rubin I, Back T, Lindauer U, Wolf T, Villringer A, Einhaupl KM, Lauritzen M, Dirnagl U. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab. 1998;18:978–990. doi: 10.1097/00004647-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Rinkel GJ, Algra A, Vermeulen M, van Gijn J. Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology. 1998;50:876–883. doi: 10.1212/wnl.50.4.876. [DOI] [PubMed] [Google Scholar]
- Fergusen S, Macdonald RL. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;60:658–667. doi: 10.1227/01.NEU.0000255396.23280.31. [DOI] [PubMed] [Google Scholar]
- Findlay JM, Kassell NF, Weir BK, Haley EC, Jr, Kongable G, Germanson T, Truskowski L, Alves WM, Holness RO, Knuckey NW. A randomized trial of intraoperative, intracisternal tissue plasminogen activator for the prevention of vasospasm. Neurosurgery. 1995;37:168–176. [PubMed] [Google Scholar]
- Haley EC, Jr, Kassell NF, Apperson-Hansen C, Maile MH, Alves WM. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in North America. J Neurosurg. 1997;86:467–474. doi: 10.3171/jns.1997.86.3.0467. [DOI] [PubMed] [Google Scholar]
- Haley EC, Jr, Kassell NF, Torner JC. A randomized controlled trial of high-dose intravenous nicardipine in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. J Neurosurg. 1993a;78:537–547. doi: 10.3171/jns.1993.78.4.0537. [DOI] [PubMed] [Google Scholar]
- Haley EC, Jr, Kassell NF, Torner JC. A randomized trial of nicardipine in subarachnoid hemorrhage: angiographic and transcranial Doppler ultrasound results. A report of the Cooperative Aneurysm Study. J Neurosurg. 1993b;78:548–553. doi: 10.3171/jns.1993.78.4.0548. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S.2008Cochrane Handbook for Systematic Reviews of Interventions The Cochrane Collaboration; Chichester, UK: John Wiley & Sons, Ltd. Available at http://www.cochrane-handbook.org [Google Scholar]
- Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med. 2006;34:196–202. doi: 10.1097/01.ccm.0000194540.44020.8e. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Haley ECJ, Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg. 1996;84:221–228. doi: 10.3171/jns.1996.84.2.0221. [DOI] [PubMed] [Google Scholar]
- Kramer A, Fletcher J. Do endothelin-receptor antagonists prevent delayed neurological deficits and poor outcomes after aneurysmal subarachnoid hemorrhage? A meta-analysis. Stroke. 2009;40:3403–3406. doi: 10.1161/STROKEAHA.109.560243. [DOI] [PubMed] [Google Scholar]
- Kreiter KT, Mayer SA, Howard G, Knappertz V, Ilodigwe D, Sloan MA, Macdonald RL. Sample size estimates for clinical trials of vasospasm in subarachnoid hemorrhage. Stroke. 2009;40:2362–2367. doi: 10.1161/STROKEAHA.109.547331. [DOI] [PubMed] [Google Scholar]
- Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage. Population-based study and systematic review. Neurology. 2010;74:1494–1501. doi: 10.1212/WNL.0b013e3181dd42b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S, Pasqualin A. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- Neil-Dwyer G, Mee E, Dorrance D, Lowe D. Early intervention with nimodipine in subarachnoid haemorrhage. Eur Heart J. 1987;8 (Suppl K:41–47. doi: 10.1093/eurheartj/8.suppl_k.41. [DOI] [PubMed] [Google Scholar]
- Ono H, Mizukami M, Kitamura K, Kikuchi H. Ticlopidine: quo vadis? Subarachnoid hemorrhage. Agents Actions Suppl. 1984;15:259–272. [PubMed] [Google Scholar]
- Petruk KC, West M, Mohr G, Weir BK, Benoit BG, Gentili F, Disney LB, Khan MI, Grace M, Holness RO. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J Neurosurg. 1988;68:505–517. doi: 10.3171/jns.1988.68.4.0505. [DOI] [PubMed] [Google Scholar]
- Philippon J, Grob R, Dagreou F, Guggiari M, Rivierez M, Viars P. Prevention of vasospasm in subarachnoid haemorrhage. A controlled study with nimodipine. Acta Neurochir (Wien) 1986;82:110–114. doi: 10.1007/BF01456369. [DOI] [PubMed] [Google Scholar]
- Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, Atkinson JL, Wijdicks EF. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:1862–1866. doi: 10.1161/01.STR.0000133132.76983.8e. [DOI] [PubMed] [Google Scholar]
- Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–2321. doi: 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- Scott RB, Eccles F, Molyneux AJ, Kerr RS, Rothwell PM, Carpenter K. Improved cognitive outcomes with endovascular coiling of ruptured intracranial aneurysms. Neuropsychological outcomes from the International Subarachnoid Aneurysm Trial (ISAT) Stroke. 2010;41:1743–1747. doi: 10.1161/STROKEAHA.110.585240. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, Nagata I, Kikuchi H, Takemae T, Hidaka H. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. 1992;76:571–577. doi: 10.3171/jns.1992.76.4.0571. [DOI] [PubMed] [Google Scholar]
- Springborg JB, Moller C, Gideon P, Jorgensen OS, Juhler M, Olsen NV. Erythropoietin in patients with aneurysmal subarachnoid haemorrhage: a double blind randomised clinical trial. Acta Neurochir (Wien) 2007;149:1089–1101. doi: 10.1007/s00701-007-1284-z. [DOI] [PubMed] [Google Scholar]
- Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- Tseng MY, Hutchinson PJ, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute pravastatin treatment on intensity of rescue therapy, length of inpatient stay, and 6-month outcome in patients after aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:1545–1550. doi: 10.1161/STROKEAHA.106.475905. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD. Effect of endothelin-receptor antagonists on delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage remains unclear. Stroke. 2009;40:e714–e716. doi: 10.1161/STROKEAHA.109.565887. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke. 2010a;41:e47–e52. doi: 10.1161/STROKEAHA.109.556332. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Meijers JC, Geskus RB, Coert BA, Horn J, Stroes ES, van der PT, Vermeulen M, Roos YB. Biologic effects of simvastatin in patients with aneurysmal subarachnoid hemorrhage: a double-blind, placebo-controlled randomized trial. J Cereb Blood Flow Metab. 2009;29:1444–1453. doi: 10.1038/jcbfm.2009.59. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1761–1770. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Vermeulen M, de Haan RJ, Levi M, Roos YB. Dihydropyridine calcium antagonists increase fibrinolytic activity: a systematic review. J Cereb Blood Flow Metab. 2007;27:1293–1308. doi: 10.1038/sj.jcbfm.9600431. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Vermeulen M, van GJ, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, terBrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YB. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies. Proposal of a multidisciplinary research group. Stroke. 2010b;41:2391–2695. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, Parra A, Connolly ES, Mayer SA. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–623. doi: 10.1097/01.ccm.0000201903.46435.35. [DOI] [PubMed] [Google Scholar]
- Weyer GW, Nolan CP, Macdonald RL. Evidence-based cerebral vasospasm management. Neurosurg Focus. 2006;21:E8. doi: 10.3171/foc.2006.21.3.8. [DOI] [PubMed] [Google Scholar]
- Yundt KD, Grubb RL, Jr, Diringer MN, Powers WJ. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab. 1998;18:419–424. doi: 10.1097/00004647-199804000-00010. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang L, Liu M, Wu B. Tirilazad for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2010;2:CD006778. doi: 10.1002/14651858.CD006778.pub2. [DOI] [PubMed] [Google Scholar]