Abstract
Intravital or multiphoton microscopy and laser-speckle imaging have become popular because they allow live monitoring of several processes during cerebral ischemia. Available rodent models have limitations for these experiments; e.g., filament occlusion of the proximal middle cerebral artery (MCA) is difficult to perform under a microscope, whereas distal occlusion methods may damage the MCA and the peri-arterial cortex. We found that placement of a 10% FeCl3-soaked filter paper strip (0.3 × 1 mm2) on the duramater over the trunk of the distal MCA through a cranial window for 3 minutes induced intraarterial thrombus without damaging the peri-arterial cortex in the mouse. This caused a rapid regional cerebral blood flow decrease within 10 minutes and total occlusion of the MCA segment under the filter paper in 17±2 minutes, which resulted in a typical cortical infarct of 27±4 mm3 at 24 hours and moderate sensorimotor deficits. There was no significant hemispheric swelling or hemorrhage or mortality at 24 hours. Reperfusion was obtained in half of the mice with tissue plasminogen activator, which allowed live monitoring of clot lysis along with restoration of tissue perfusion and MCA flow. In conclusion, this relatively simple and noninvasive stroke model is easy to perform under a microscope, making it suitable for live imaging and thrombolysis studies.
Keywords: focal ischemia, intravital microscopy, laser-speckle imaging, stroke model, thrombolysis
Introduction
Animal models of cerebral infarction are an indispensable step towards a better understanding of the pathophysiological mechanisms of cerebral ischemic injury and development of therapeutic interventions (Green, 2008). Several species and protocols have been used to gain further knowledge in this area; rodents, especially, have been very instrumental (Ginsberg and Busto, 1989). Several models of permanent or temporary and proximal or distal middle cerebral artery (MCA) occlusion have been developed (Ginsberg and Busto, 1989). A large infarct and high mortality rate caused by brain swelling are disadvantages of proximal MCA occlusion models, whereas damage to the duramater and peri-arterial cortex, as well as high cost in some cases (e.g., photothrombosis with laser) limit the use of distal MCA models (Brint et al, 1988; Buchan et al, 1992; Chen et al, 1986; Kaneko et al, 1985). Recently, intravital or multiphoton microscopy and laser-speckle imaging have become popular because they allow live monitoring of several pathophysiological processes during cerebral ischemia. However, proximal MCA occlusion is difficult to perform under a microscope, whereas most of the distal occlusion models induce spreading depression independently of ischemia owing to direct physical damage to the peri-arterial cortex caused by clipping or suturing the MCA. Therefore, there is a need for a new model of focal cerebral ischemia that is easy to perform under a microscope, does not inflict surgical trauma to the duramater and cortex, and has low mortality.
Ferric chloride (FeCl3)-induced thrombus formation is a widely used experimental model of both arterial and venous thrombosis in various species, including rodents (Lockyer and Kambayashi, 1999). Ferric chloride-induced thrombosis was first reported by Kurz et al (1990) as a small animal model of arterial thrombosis for studying novel antithrombotic agents. In this model, a blood vessel is briefly exposed to FeCl3 (Denis et al, 1998; Smyth et al, 2001; Weiss et al, 2002), which rapidly results in the formation of a thrombus (Kurz et al, 1990) with a morphology similar to that found in humans (Farrehi et al, 1998). To our knowledge, FeCl3 has never been applied to the MCA to induce cerebral ischemia, although it was used to produce intracarotid thrombi for studies with antithrombotic agents (Farrehi et al, 1998; Hagedorn et al, 2010; Wang et al, 2005; Wang and Xu, 2005).
In this report, a novel focal cerebral ischemia model produced by topical application of FeCl3 to the trunk of the distal MCA is introduced. The model is practical, nontraumatic, has low mortality, and is suitable for studies using intravital microscopy. Furthermore, it provides an opportunity to directly study thrombolysis under the microscope, a subject that may have considerable clinical relevance but has been little investigated experimentally.
Materials and methods
Animals
Swiss albino (n=55, weighing 21 to 38 g) or C57/Bl6 (n=2, 21 to 26 g) and SV129 (n=2, 20 to 24 g) mice were housed under diurnal lighting conditions (12-hour darkness and 12-hour light) and fasted overnight, but allowed free access to water before the experiment. Animal housing, care, and application of experimental procedures were all carried out in accordance with the institutional guidelines and approved by the Hacettepe University Animal Experiments Local Ethics Committee (2005/30-5).
Surgery and Middle Cerebral Artery Occlusion with Ferric Chloride
Mice were anesthetized with intraperitoneal administration of 50 mg/kg ketamine (Ketalar, Pfizer, Istanbul, Turkey) and 10 mg/kg xylazine (Alfazyne 2%, Alfasan International, Woerden, Netherlands), and with 1% isoflurane supplement if required. They were allowed to breathe spontaneously with oxygen support. Body temperature was monitored by a rectal probe and maintained at 37°C±0.1°C using a homoeothermic blanket control unit (Harvard Apparatus, Kent, UK). Pulse rate and tissue oxygen saturation (V3304 Digital Table-Top Pulse Oxymeter, Surgivet, Waukesha, WI, USA) were monitored during the procedure. Animals were placed in a stereotaxic frame (Stoelting, Wheat Lane Wood Dale, IL, USA) after deep anesthesia was obtained. Surgery was performed under an operating biomicroscope (Carl Zeiss AG, Jena, Germany) at 4 × to 25 × magnification.
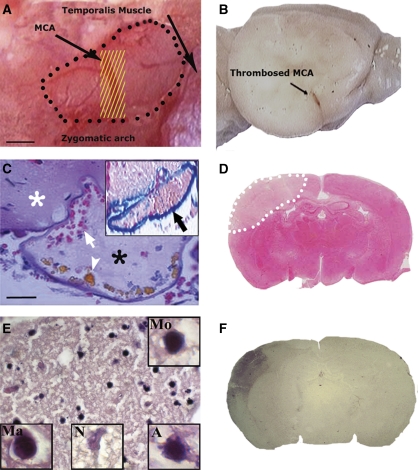
The scalp was opened, and the cranial sutures and bregma were exposed. The right temporal muscle was bluntly dissected until the squamous part of the temporal bone was exposed. The area just above the junction between the zygomatic arch and the squamous bone was thinned using a high-speed drill and cooled with saline. The trace of MCA was visualized through the thinned temporal bone. Attention was paid not to damage the MCA and the overlying duramater while the remaining thin bony film was lifted up by forceps (Figure 1A).
Figure 1.
Topical FeCl3 application onto the MCA for 3 minutes induced thrombosis and caused ischemia. (A) A filter paper strip soaked with FeCl3 (yellow lines) was placed over the trunk of the distal MCA through a small cranial window (marked with black dots) opened right after the zygomatic arch. The duramater was kept intact. (B) The thrombosed (brown discoloration) segment of the MCA on a formalin-fixed brain. (C) The thrombus within the MCA and the intact peri-arterial tissue (white asterisk). The lumen was filled with a loose fibrin mesh (*), platelets, and entrapped erythrocytes (white arrow) (H&E). FeCl3 caused hemosiderin deposition (arrowhead) and blue discoloration of the MCA wall (inset, Prussian blue reaction). (D) The resultant infarct (marked with white dots) 24 hours after FeCl3 application on a TTC-stained coronal section. (E) Typical ischemic microscopic changes in the cortex 3 days after ischemia. Insets illustrate a magnified monocyte (Mo), macrophage (Ma), necrotic red neuron showing karyolysis (N), and an apoptotic nucleus with blebs (A). (F) IgG immunostaining illustrates that the BBB is leaky in the ischemic area 72 hours after ischemia; however, vasogenic edema is not severe enough to cause hemispheric swelling. Panels D and F are from two separate mice. Scale bars: 300 μm in panel A and 20 μm in panel C. BBB, blood–brain barrier; FeCl3, ferric chloride; H&E, hematoxylin–eosin; MCA, middle cerebral artery; TTC, 2,3,4-triphenyltetrazolium chloride. The color reproduction of this figure is available on the html full text version of the manuscript.
A flexible probe (PF-318, Perimed, Stockholm, Sweden) was placed 2 mm posterior and 6 mm lateral to the bregma, away from large pial vessels to monitor the regional cerebral blood flow (rCBF) with laser-Doppler flowmetry (Periflux PF2B, Perimed) (n=29). In another group of mice, rCBF was followed by laser-speckle flowmetry (LSF) (n=27). After obtaining a stable 10-minute epoch of the preischemic rCBF, a piece of FeCl3-saturated filter paper (0.3 × 1 mm2) was placed over the intact duramater along the trace of the MCA right after the zygomatic arch, and rCBF was continuously monitored during the next 2 hours. We left the duramater intact because the space between the dura and the MCA is <40 μm (Helmchen and Denk, 2005), rendering the MCA vulnerable to surgical injury. Importantly, we observed that the paper strip must be in close contact with the duramater without any space remaining in between to induce clot formation, and hence, the cranial window must allow optimal placement of the paper strip to ensure tight contact. As the available literature was limited to the direct application of FeCl3 to peripheral arteries without an intervening barrier such as the duramater, we tried to find the optimum FeCl3 concentration to induce thrombosis in the MCA by applying 10, 20, or 30% FeCl3 in different sets of mice.
Intravital Imaging of Clot Formation
Fluorescein isothiocyanate-Dextran-70S (0.5 mg in 0.1 mL saline; Sigma, St Louis, MO, USA) was injected through the femoral artery to visualize vessels and to monitor clot formation in the distal MCA through the cranial window. Fluorescent images of the MCA were obtained using a Nikon SMZ 1000 (Nikon, Kawasaki, Kanagawa, Japan) microscope under × 40 magnification. After acquisition of a basal image, topical FeCl3 was applied as described above and images were taken with 1-minute intervals for 20 minutes.
Poststroke Follow-Up
Mice recovering from anesthesia were allowed free access to food and water for 24 to 72 hours until killing. Body weights were recorded before surgery and every 24 hours, and the neurologic status was assessed according to a six-point scale: 0, no observable deficits; 1, forelimb flexion; 2, decreased resistance to lateral push; 3, circling behavior if pulled by the tail; 4, spontaneous circling behavior; and 5, no spontaneous motion (Huang et al, 1994).
Detection of Infarct Area and Volume
Mice were killed by decapitation after 24 or 72 hours of ischemia, and the ischemic area was evaluated by TTC (2,3,4-triphenyltetrazolium chloride) staining. In brief, after an overdose of chloral hydrate (50 mg/kg, intraperitoneal), the brains were quickly removed and placed in ice-cold saline for 5 minutes and then cut into 2-mm-thick coronal slices. Sections were incubated in TTC containing saline solution (2% Sigma) for 20 minutes and in 10% formalin overnight. The infarcted cortical area, outlined in white, was measured using the NIH 1.59 image analysis software (NIH, Bethesda, MD, USA) on the posterior surface of each coronal slice, and infarction volume was calculated by multiplying the sum of the infarct areas of five sequential sections of 2 mm (section thickness). The infarct was most prominent on sections two and three.
Evaluation of Blood–Brain Barrier Integrity and Microhemorrhage Formation
To show blood–brain barrier leakage, 3-μm-thick paraffin-embedded brain slices obtained from tissue plasminogen activator (tPA)-treated animals were deparaffinized and immunostained with goat anti-mouse IgG antibody (1:200, Vector Laboratories Inc., Burlingame, CA, USA). Diaminobenzidine was used as the chromogen.
The presence of microhemorrhages was evaluated by staining the paraffin-embedded slices using Perls' Prussian Blue Staining Kit (Diagnostic BioSystems, Serpentine, CA, USA) according to the manufacturer's instructions. Nuclei were counterstained with nuclear fast red. A positive control for hemorrhage was prepared by mechanically lesioning the cortex with a 26-G hypodermic needle and killing the animal 72 hours after surgery.
Histologic Demonstration of Thrombus Formation
Sagittal sections encompassing the occluded MCA were obtained from three mice. Sections of 3-μm thickness were prepared from paraffin-embedded blocks and stained with hematoxylin–eosin. Prussian blue reaction was used to show iron deposition within the MCA and the peri-arterial tissue. Fibrin stain was performed to examine the thrombus according to the method by Lendrum et al (1962). For this purpose, sections were first deparaffinized. After xylol and alcohol steps, they were consecutively incubated with Celestine blue solution, Mayer's hematoxylin, orange G solution, 1% acid fuchsine solution, and Macfarlane solution for 5 minutes in each. Reverse staining with light green was then performed. After washing in distilled water and dehydration with alcohol and xylol, sections were mounted with entellan.
Reperfusion Experiments with Tissue Plasminogen Activator
After establishment of a reproducible occlusion of the distal MCA with FeCl3, we investigated whether the clot formed could be lysed with tPA and reperfusion could be attained. Ten minutes after topical application of 10% FeCl3, intravenous tPA (Actilyse; INN alteplase, Boehringer Ingelheim, Ingelheim am Rhein, Germany) administration was started. Overall, 10% of the typical murine dose (10 mg/kg) (Kilic et al, 2000; Orset et al, 2007), which is ∼10 times higher than the human dose, was injected as a bolus and the remainder as a continuous infusion over a 30-minute interval using a syringe infusion pump (NE-1000, New Era Pump Systems, Farmingdale, NY, USA) (n=26). A 10-minute time point was chosen to allow enough time for clot formation and MCA occlusion based on the observation that rCBF decreased to ischemic values within 10 minutes after FeCl3 application (Figures 2 and 3). In the initial experiments (n=11), heparin (200 Units/kg, intravenous) was added to increase the successful reperfusion rate but it did not provide any noticeable benefit. Therefore, heparin was not used in subsequent experiments (n=15); the reperfusion rate was ∼50% with both protocols. Administering tPA 1 hour after 30% FeCl3 application failed to open the occluded artery in initial screening experiments (n=6) even when tPA was administered through a catheter placed into the ipsilateral carotid artery (n=3).
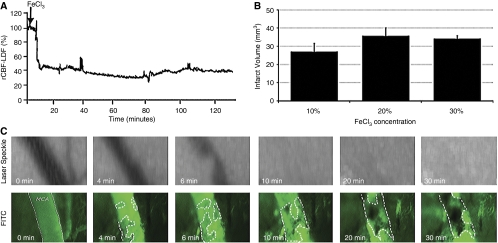
Figure 2.
FeCl3 application induced rapid clot formation within the MCA. (A) Laser-Doppler flowmetry (LDF) recording exhibits a rapid rCBF decrease in 10 minutes after FeCl3. (B) The infarct volumes detected at 24 hours after 10, 20, or 30% FeCl3 concentrations were not significantly different. (C) The kinetics of clot formation in the distal MCA visualized with FITC-Dextran-70S and simultaneous laser-speckle images of the MCA territory (scale bar: 50 μm). The clot formation started at multiple foci within the lumen (circled areas) 4 minutes after FeCl3 application, and the lumen was almost completely obliterated in 10 minutes while the clot continued to grow. Laser-speckle images illustrate that the MCA flow was not detectable after the tenth minute. FeCl3, ferric chloride; FITC, fluorescein isothiocyanate; MCA, middle cerebral artery; rCBF, regional cerebral blood flow.
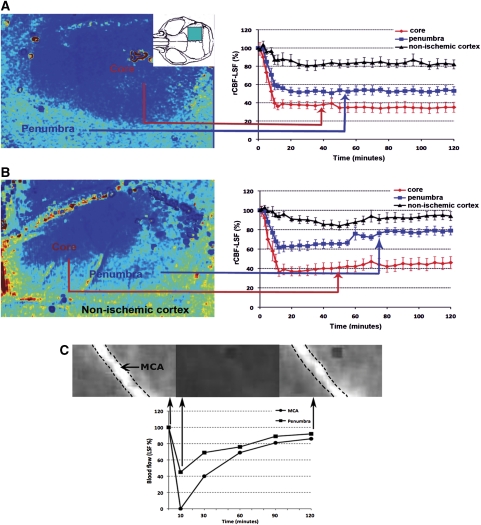
Figure 3.
Reperfusion can be obtained with tPA in half of the mice. Laser-speckle images illustrate the rCBF in the ischemic MCA area and in the nonischemic peri-lesional cortex in a (A) nonreperfused and in a (B) successfully reperfused animal after tPA. X axes show the time after FeCl3 application. tPA administration was initiated 10 minutes after FeCl3 application. Interestingly, reperfusion was achieved only in the periphery of the ischemic area throughout the 2-hour follow-up. The blue box in the inset indicates the area monitored with laser-speckle imaging. Percentage changes from the baseline rCBF measurements are illustrated. Means are shown with their s.e. LSF: laser-speckle flowmetry. (C) Tissue reperfusion was coincident with restitution of the blood flow within the MCA. Graph illustrates percentage flow changes from the baseline in the periphery (▪) and MCA trunk (•) after FeCl3 application. Tissue and MCA blood flow were monitored with LSF. tPA administration was initiated 10 minutes after FeCl3. Counters of the MCA were marked with dashed lines in the representative images captured before and at 10 and 120 minutes after FeCl3 application. FeCl3, ferric chloride; MCA, middle cerebral artery; rCBF, regional cerebral blood flow; tPA, tissue plasminogen activator. The color reproduction of this figure is available on the html full text version of the manuscript.
Laser-Speckle Flowmetry
The technique for LSF has previously been described in detail (Dunn et al, 2001). In brief, a CCD camera (Basler 602F, Basler Vision Technologies, Ahrensburg, Germany) was positioned above the head, and a laser diode (785 nm) was used to illuminate the skull surface in a diffuse manner. The penetration depth of the laser is ∼500 μm from the point laser light hits the skull. The field of imaging was adjusted using a variable magnification objective on the microscope (Nikon SMZ 1000, Nikon). In all, 10 consecutive raw speckle images were acquired at 100 Hz (an image set), processed by computing the speckle contrast using a sliding grid of 7 × 7 pixels, and averaged to improve the signal-to-noise ratio (Tom et al, 2008). Relative CBF images (percentage of baseline) were calculated by computing the ratio of a baseline image taken before the induction of ischemia, with an image at some later time point. To determine the changes in CBF, a region of interest was placed over different tissue areas. In case of cortical tissue measurements, the region of interest was placed away from cortical vessels. Cerebral blood flow changes in the MCA were quantified by placing the region of interest over the distal MCA identified visually from the speckle contrast images.
Statistical Analysis
Mean values in the text are given with their s.e. Values of the tissue pO2, pulse rate, rCBF, and infarct volume were compared by means of Kruskal–Wallis variance analysis, followed by Mann–Whitney's U-test. P<0.05 was considered to be significant.
Results
We performed a series of studies to determine the feasibility, reproducibility, and outcome of FeCl3-induced MCA thrombosis in 59 mice. Surgery time from skin incision to application of FeCl3 was ∼20 minutes. There was no surgery-related mortality.
Ferric Chloride-induced Middle Cerebral Artery Thrombosis
After several trials of different filter paper sizes and application durations, we found that topical application of a FeCl3-soaked filter paper strip on the duramater over the distal MCA for 3 minutes induced intraarterial thrombus without damaging the peri-arterial tissue as detected with hematoxylin–eosin-stained sections (Figure 1). A filter paper of 0.3-mm thickness covers the trunk of the MCA (∼0.1 mm) across, and a length of 1 mm was enough to attain total occlusion of the MCA, provided that the strip was in close contact with the duramater without any space in between. A part of the MCA exposed to FeCl3 exhibited brown discoloration on gross examination of the formalin-fixed brains (Figure 1B). Thrombus formation in the MCA lumen was verified histopathologically in all specimens examined (n=3 mice) (Figure 1C). In line with the literature characterizing the FeCl3-induced intraarterial thrombus (Dorffler-Melly et al, 2003; Lockyer and Kambayashi, 1999; Nesbitt et al, 2009), the entire lumen was filled with a fresh clot composed of a loose fibrin mesh, platelets, and entrapped erythrocytes, whereas FeCl3 caused blue discoloration of the MCA wall with Prussian blue reaction (Figure 1C, inset). The clot formation started at multiple foci within the lumen 3 minutes after FeCl3 application and the lumen was completely obliterated in 10 to 20 minutes (Figure 2C, see the below two paragraphs for detailed kinetics of MCA occlusion).
In initial screening experiments, 20 or 30% FeCl3 applications caused a rapid rCBF decrease within 10 minutes, and total occlusion was obtained in 19±12 minutes (n=4) and 14±11 minutes (n=7), respectively. The rCBF, detected by laser Doppler at a coordinate approximately targeting the core, was 24%±2% and 23%±5%, respectively, at the end of a 2-hour follow-up in these groups (Figure 2A). With 10% FeCl3 application, the rCBF decrease was more variable at this coordinate when detected with laser-Doppler flowmetry. However, an infarct of comparable size (27±4 mm3, n=3) to those induced by 20 or 30% FeCl3 application was detected at 24 hours (Figure 2B). There were no statistically significant differences between infarct volumes of the three groups.
Features of the Model
On the basis of these initial observations, 10% was considered to be the optimal FeCl3 concentration. The rCBF values recorded with laser-Doppler flowmetry are confined to one single point over the MCA territory, and hence, are vulnerable to sampling errors. Therefore, LSF was used in subsequent experiments with 10% FeCl3 to observe flow changes in the entire MCA-supplied cortex (n=27). Laser-speckle recordings showed a rapid decline in rCBF in 8±1 minutes and total occlusion was attained in 17±2 minutes (Figure 3). Two hours after FeCl3 application, rCBF in the MCA-supplied cortex reached 34%±5% of the baseline at the center and 52%±4% at the periphery when measured from a rectangular region of interest (0.05 mm2) away from vessels, in accordance with values reported after distal MCA occlusion with suture in the mouse (Ayata et al, 2004) (Figure 3).
Lesions developed in the MCA-supplied cortex exhibited the typical microscopic features of a focal cerebral infarct (Garcia et al, 1995) (Figure 1E). Despite evidence of blood–brain barrier damage as indicated by IgG leakage in the ischemic area (Figure 1F), vasogenic edema was not severe enough to induce a noticeable macroscopic brain swelling as indicated by the absence of a significant difference between the infarct sizes assessed with direct and indirect methods. There was no mortality 24 hours after ischemia (n=0/26). Only 3 out of 15 mice followed up for 72 hours died within the third day. All mice showed moderate sensorimotor deficits corresponding to the cortical infarct and characterized by forelimb flexion and decreased resistance to lateral push, which scored 2±0.3 on a 6-point scale used to assess neurologic dysfunction after stroke in the mouse (Huang et al, 1994). Neurologic deficit scores did not improve, and animals lost 29%±3% of their baseline weight during the 3-day follow-up.
All features of the model were reproduced equally well in two other mouse strains commonly used in stroke research (C57/Bl6 and SV129) and in mice having a wide range of body weight (20 to 38 g). As control, a saline-saturated filter paper strip was applied onto the MCA in two mice, which did not cause a decrease in rCBF, or intraarterial thrombus formation or an infarct.
Reperfusion with Tissue Plasminogen Activator
As detailed in the ‘Materials and methods' section, reperfusion with tPA was not feasible when 30% FeCl3 was used or when tPA administration was delayed for 1 hour after FeCl3 application possibly because size and retraction of the clot had reached a state difficult to be lysed by tPA (Sabovic et al, 1989; Sabovic and Blinc, 2000). Reperfusion was obtained with early (10 minutes after FeCl3) tPA administration in 12 out of 23 mice when thrombus was induced with 10% FeCl3. Two hours after tPA, rCBF reached 78%±4% of the baseline in the periphery and 47%±7% in the center of the ischemic area in these 12 mice. Interestingly, reperfusion was initially detected in the periphery of the ischemic area, whereas there was no significant rCBF increase in the core during the 2-hour follow-up period as previously reported in rats subjected to clot embolism and treated with tPA (Niessen et al, 2002). Although the quantitative accuracy of blood flow changes measured by LSF directly over an arterial branch is limited, we observed a clear restitution of blood flow within the MCA in 10 of the 12 reperfused brains (Figure 3C). In additional three mice, which were followed up with laser-Doppler flowmetry at a coordinate approximating the penumbra, the rCBF increased from 35%±7% to 67%±15% of the baseline 2 hours after intravenous tPA administration.
Early tPA infusion starting at the tenth minute of FeCl3 application, limited the growth of the thrombus into the branches originating from the MCA segment under the paper strip, and possibly owing to this and dissolution of the potential microemboli by tPA, the infarct volume (10.2±1.3 mm3) was smaller in these mice than in untreated animals even when tPA failed to reopen the MCA. As expected, successfully reperfused mice had smaller infarct volumes (5.2±0.7 mm3 versus 10.2±1.3 mm3) and less severe neurologic deficits (1.1±0.1 versus 1.7±0.2) than did mice in whom reperfusion could not be attained despite tPA. Bleeding to the infarct area was rare possibly because tPA was administered soon after ischemia; hence, only a few microhemorrhages was barely noticeable by Perls' staining in two mice from each group.
Physiologic parameters were within normal limits in all mice throughout the experiments and there were no significant differences between the groups (Table 1).
Table 1. Physiologic parameters.
| Groups | Tissue oxygen saturation (%) | Pulse rate (b.p.m.) |
|---|---|---|
| Untreated | ||
| 10% FeCl3 (n=4) | 94±1 | 230±20 |
| 20% FeCl3 (n=4) | 93±1 | 236±11 |
| 30% FeCl3 (n=7) | 94±1 | 271±17 |
| tPA-treated (10% FeCl3) | ||
| Reperfused (n=12) | 97±1 | 243±21 |
| Nonreperfused (n=11) | 97±1 | 252±25 |
b.p.m., beats per minute; FeCl3, ferric chloride; tPA, tissue plasminogen activator.
All mice were kept normothermic at 37°C±0.1°C.
There were no significant differences between groups.
Discussion
In this study, we developed a focal cerebral ischemia model induced by topical application of FeCl3 onto the distal MCA in the mouse. Ferric chloride rapidly produced an intraarterial thrombus that caused complete occlusion of the MCA. The resultant infarct was small and brain swelling was not substantial; accordingly, the model had a low mortality rate. The model seems to be suitable for intravital and multiphoton microscopy experiments because it is easy to perform under a microscope and does not require the use of specialized surgical techniques or equipment. Importantly, it does not induce additional trauma other than ischemia to the brain tissue and microvascular bed.
Various distal MCA occlusion methods are available for studying focal cerebral ischemia in rodents. However, occlusion induced by electrocoagulation (Backhauss et al, 1992; Tyson et al, 1984) or by placing a snare ligature or surgical clip around the MCA can injure the duramater and cortex, induce spreading depression, and poses a risk of subarachnoid hemorrhage. Photochemical MCA occlusion, which involves irradiation of several branches of the distal MCA with laser after intravenous administration of the photosensitizing dye Rose Bengal (Watson et al, 1985; Yao et al, 1996), is not depended on platelet-derived or plasmatic coagulation-derived thrombosis and can damage the microvascular bed irradiated (Kleinschnitz et al, 2008). Furthermore, it requires relatively expensive equipment. The FeCl3 model offers some advantages over these models because it does not injure the duramater, the cortical tissue around the MCA, and the microvasculature. The chemical injury it induces is limited to the small MCA segment where it is topically applied. Moreover, it is inexpensive and easy to perform under a microscope. Its main disadvantage is that thrombolysis is required to attain recirculation, and that this is not always successful as commonly seen after tPA applications in stroke models (Kilic et al, 2000; Niessen et al, 2002). In that regard, the model mimics the clinical thrombolytic treatment and may be used to further our understanding of thrombolysis in patients with stroke, whereas it may not be suitable for neuroprotection studies requiring a dependable reperfusion. However, reperfusion is also hard to achieve when a suture or clip is applied to occlude the distal MCA, especially if the occlusion is prolonged. Electrocoagulation per se does not allow reperfusion at all. Similar to the FeCl3 model, the blood clot embolization model also requires thrombolysis for recanalization (Kaneko et al, 1985; Kudo et al, 1982). The latter model is further complicated by the highly variable distribution of emboli. Recently, a novel distal MCA occlusion model produced by microinjection of thrombin into the MCA lumen has been reported (Orset et al, 2007). The thrombin model, based on producing a clot within the distal MCA, shares the same features with our model and offers the same advantages, except that thrombin is more expensive compared with FeCl3, preparation of the micropipette requires special equipment, and expertise is required for the pneumatic intraarterial microinjection. In summary, FeCl3-induced MCA thrombosis seems to offer several advantages over the existing distal MCA occlusion models for studies requiring intravital microscopy, such as laser-speckle or multiphoton imaging. In addition, it looks promising to study thrombolysis directly under a microscope. The laser-speckle technique, which enables high spatiotemporal resolution imaging of blood flow changes over a large cortical area (Dunn et al, 2001), displayed slow restitution of blood flow within the trunk of recanalizing MCA with attendant rCBF increase starting from the periphery of the ischemic area as previously observed with perfusion-weighted magnetic resonance imaging studies in rats in which cerebral embolism was treated with tPA (Niessen et al, 2002). These observations suggest that the FeCl3-induced thrombosis model combined with intravital imaging may provide a better understanding of arterial recanalization and associated tissue perfusion changes after thrombolytic therapy, a subject that has been partly neglected experimentally despite the unsatisfactory success in clinical trials.
Kurz et al (1990) first described the use of FeCl3 to induce arterial thrombosis in rats. Our study shows that this method can be effectively applied to the MCA of small rodents to produce thrombus. The mechanism by which FeCl3 induces thrombosis is not well characterized. Iron induces formation of highly reactive oxidant species (Wang and Xu, 2005), which may induce endothelial damage and predispose the injured area to platelet adherence and aggregation, followed by coagulation activation and fibrin deposition. This produces a mixed thrombus containing fibrin, platelets, and red blood cells as observed in our study and reported by other laboratories (Lockyer and Kambayashi, 1999; Nesbitt et al, 2009).
There is a wide range of FeCl3 concentrations (2.5 to 65%) used to induce arterial thrombosis in the literature (Kurz et al, 1990; Wang and Xu, 2005). Application durations and filter paper sizes also vary; however, similar to our results, previous studies showed that direct exposure of FeCl3 to the adventitia of an artery caused total occlusion usually within 10 to 20 minutes (Elvers et al, 2010; Hagedorn et al, 2010; Kleinschnitz et al, 2010; Watson, 1998). These studies reported that total carotid artery thrombosis could be obtained by 10 to 15% FeCl3 concentration in the mouse, whereas higher FeCl3 concentrations have been used in the rat or for peripheral arterial thrombosis. We initially tested 20 and 30% FeCl3 concentrations, considering the potential diffusion limitation caused by the duramater overlying the MCA. Compared with 10% FeCl3, the rCBF decrease was faster with 20 and 30% FeCl3 concentrations; however, the ensuing infarct at 24 hours was not significantly larger than that in the 10% group. Conversely, the clot induced by 30% FeCl3 could not be lysed with tPA; therefore, we conclude that 10% FeCl3 should be used if reperfusion is required and higher concentrations could be preferred for inducing faster MCA occlusion.
In conclusion, a topical application of FeCl3 to the trunk of the distal MCA produces reproducible cortical infarcts. The model introduced is practical, nontraumatic, has low mortality, and is suitable for studies using intravital microscopy. Furthermore, it provides an opportunity to directly study thrombolysis under the microscope.
Acknowledgments
T Dalkara's work is supported by the Turkish Academy of Sciences. The authors thank Sevda Lüle for her excellent technical assistance.
The authors declare no conflict of interest.
References
- Ayata C, Dunn AK, Gursoy OY, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–755. doi: 10.1097/01.WCB.0000122745.72175.D5. [DOI] [PubMed] [Google Scholar]
- Backhauss C, Karkoutly C, Welsch M, Krieglstein J. A mouse model of focal cerebral ischemia for screening neuroprotective drug effects. J Pharmacol Toxicol Methods. 1992;27:27–32. doi: 10.1016/1056-8719(92)90017-u. [DOI] [PubMed] [Google Scholar]
- Brint S, Jacewicz M, Kiessling M, Tanabe J, Pulsinelli W. Focal brain ischemia in the rat: methods for reproducible neocortical infarction using tandem occlusion of the distal middle cerebral and ipsilateral common carotid arteries. J Cereb Blood Flow Metab. 1988;8:474–485. doi: 10.1038/jcbfm.1988.88. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Xue D, Slivka A. A new model of temporary focal neocortical ischemia in the rat. Stroke. 1992;23:273–279. doi: 10.1161/01.str.23.2.273. [DOI] [PubMed] [Google Scholar]
- Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Cullere M, Hynes RO, Wagner DD. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci USA. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorffler-Melly J, de Kruif M, Schwarte LA, Franco RF, Florquin S, Spek CA, Ince C, Reitsma PH, ten Cate H. Functional thrombomodulin deficiency causes enhanced thrombus growth in a murine model of carotid artery thrombosis. Basic Res Cardiol. 2003;98:347–352. doi: 10.1007/s00395-003-0416-9. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- Elvers M, Stegner D, Hagedorn I, Kleinschnitz C, Braun A, Kuijpers ME, Boesl M, Chen Q, Heemskerk JW, Stoll G, Frohman MA, Nieswandt B. Impaired alpha(IIb)beta(3) integrin activation and shear-dependent thrombus formation in mice lacking phospholipase D1. Sci Signal. 2010;3:ra1. doi: 10.1126/scisignal.2000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrehi PM, Ozaki CK, Carmeliet P, Fay WP. Regulation of arterial thrombolysis by plasminogen activator inhibitor-1 in mice. Circulation. 1998;97:1002–1008. doi: 10.1161/01.cir.97.10.1002. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Ho KL.1995Neuronal necrosis after middle cerebral artery occlusion in Wistar rats progresses at different time intervals in the caudoputamen and the cortex Stroke 26636–642.discussion 43 [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20:1627–1642. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- Green AR. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br J Pharmacol. 2008;153 (Suppl 1:S325–S338. doi: 10.1038/sj.bjp.0707594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn I, Schmidbauer S, Pleines I, Kleinschnitz C, Kronthaler U, Stoll G, Dickneite G, Nieswandt B. Factor XIIa inhibitor recombinant human albumin Infestin-4 abolishes occlusive arterial thrombus formation without affecting bleeding. Circulation. 2010;121:1510–1517. doi: 10.1161/CIRCULATIONAHA.109.924761. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- Kaneko D, Nakamura N, Ogawa T. Cerebral infarction in rats using homologous blood emboli: development of a new experimental model. Stroke. 1985;16:76–84. doi: 10.1161/01.str.16.1.76. [DOI] [PubMed] [Google Scholar]
- Kilic E, Hermann DM, Hossmann KA. Recombinant tissue-plasminogen activator-induced thrombolysis after cerebral thromboembolism in mice. Acta Neuropathol. 2000;99:219–222. doi: 10.1007/pl00007430. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Braeuninger S, Pham M, Austinat M, Nolte I, Renne T, Nieswandt B, Bendszus M, Stoll G. Blocking of platelets or intrinsic coagulation pathway-driven thrombosis does not prevent cerebral infarctions induced by photothrombosis. Stroke. 2008;39:1262–1268. doi: 10.1161/STROKEAHA.107.496448. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, Austinat M, Nieswandt B, Wiendl H, Stoll G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- Kudo M, Aoyama A, Ichimori S, Fukunaga N. An animal model of cerebral infarction. Homologous blood clot emboli in rats. Stroke. 1982;13:505–508. doi: 10.1161/01.str.13.4.505. [DOI] [PubMed] [Google Scholar]
- Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990;60:269–280. doi: 10.1016/0049-3848(90)90106-m. [DOI] [PubMed] [Google Scholar]
- Lendrum AC, Fraser DS, Slidders W, Henderson R. Studies on the character and staining of fibrin. J Clin Pathol. 1962;15:401–413. doi: 10.1136/jcp.15.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer S, Kambayashi J. Demonstration of flow and platelet dependency in a ferric chloride-induced model of thrombosis. J Cardiovasc Pharmacol. 1999;33:718–725. doi: 10.1097/00005344-199905000-00007. [DOI] [PubMed] [Google Scholar]
- Nesbitt WS, Westein E, Tovar-Lopez FJ, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- Niessen F, Hilger T, Hoehn M, Hossmann KA. Thrombolytic treatment of clot embolism in rat: comparison of intra-arterial and intravenous application of recombinant tissue plasminogen activator. Stroke. 2002;33:2999–3005. doi: 10.1161/01.str.0000038096.60932.f4. [DOI] [PubMed] [Google Scholar]
- Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, Agin V, Vivien D. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38:2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- Sabovic M, Lijnen HR, Keber D, Collen D. Effect of retraction on the lysis of human clots with fibrin specific and non-fibrin specific plasminogen activators. Thromb Haemost. 1989;62:1083–1087. [PubMed] [Google Scholar]
- Sabovic M, Blinc A. Biochemical and biophysical conditions for blood clot lysis. Pflugers Arch. 2000;440:R134–R136. doi: 10.1007/s004240000035. [DOI] [PubMed] [Google Scholar]
- Smyth SS, Reis ED, Vaananen H, Zhang W, Coller BS. Variable protection of beta 3-integrin--deficient mice from thrombosis initiated by different mechanisms. Blood. 2001;98:1055–1062. doi: 10.1182/blood.v98.4.1055. [DOI] [PubMed] [Google Scholar]
- Tom WJ, Ponticorvo A, Dunn AK. Efficient processing of laser speckle contrast images. IEEE Trans Med Imaging. 2008;27:1728–1738. doi: 10.1109/TMI.2008.925081. [DOI] [PubMed] [Google Scholar]
- Tyson GW, Teasdale GM, Graham DI, McCulloch J. Focal cerebral ischemia in the rat: topography of hemodynamic and histopathological changes. Ann Neurol. 1984;15:559–567. doi: 10.1002/ana.410150608. [DOI] [PubMed] [Google Scholar]
- Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu L. An optimized murine model of ferric chloride-induced arterial thrombosis for thrombosis research. Thromb Res. 2005;115:95–100. doi: 10.1016/j.thromres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- Watson BD. Animal Models of Photochemically Induced Brain, Ischemia and Stroke. Oxford: Blackwell Science; 1998. pp. 52–73. [Google Scholar]
- Weiss EJ, Hamilton JR, Lease KE, Coughlin SR. Protection against thrombosis in mice lacking PAR3. Blood. 2002;100:3240–3244. doi: 10.1182/blood-2002-05-1470. [DOI] [PubMed] [Google Scholar]
- Yao H, Ibayashi S, Sugimori H, Fujii K, Fujishima M. Simplified model of krypton laser-induced thrombotic distal middle cerebral artery occlusion in spontaneously hypertensive rats. Stroke. 1996;27:333–336. doi: 10.1161/01.str.27.2.333. [DOI] [PubMed] [Google Scholar]