Abstract
To clarify the involvement of GluR2 and GluR3 subunits of AMPA receptor in orofacial neuropathic pain, we studied changes in nocifensive behavior and extracellular-signal regulated kinase (ERK) phosphorylation followed by infraorbital nerve (ION)-partial transection model applied to GluR2 or GluR3 delta7 knock-in (KI) mice. In these animals, last seven amino acids of GluR2 or GluR3 subunit, the binding sites of interacting protein, are deleted in vivo. Head-withdrawal threshold to mechanical stimulation of the whisker pad skin ipsilateral to ION-partial transection was significantly reduced at 1, 3, 5, 7, 11 and 14 days after transection compared with that before transection in wild-type mice. In the GluR2 and GluR3 delta7 KI mice, the head-withdrawal threshold did not change following ION-partial transection. The number of pERK-LI cells examined in Vc and C1–C2 in wild-type mice after the non-noxious stimulation was larger than that of GluR2 and GluR3 delta7 KI mice.
The present findings suggest that GluR2 and GluR3 subunits of AMPA receptor play roles in the trigeminal nerve injury-mediated enhancement of Vc and C1–C2 neuronal excitability, and hyperalgesia.
Keywords: Infraorbital nerve injury, Phosphorylation of extracellular, signal-regulated kinase, AMPA receptor, Neuropathic pain
Noxious stimulation of the primary nociceptive neurons that innervate orofacial area activates second-order neurons in the trigeminal spinal subnucleus caudalis (Vc) and upper cervical spinal cord (C1–C2) [10,12,34]. The neuronal excitability of these neurons is significantly enhanced after peripheral inflammation [9,13,20]. This increase in excitability of Vc nociceptive neurons after injection of algesic agents into the temporomandibular joint can be suppressed by intrathecal administration of the AMPA receptor (AMPAR) antagonist, and NMDA receptor antagonist. These findings emphasize the role of AMPAR and NMDA receptors in modulating nociceptive transmission in Vc.
Emerging evidence indicates that AMPAR membrane trafficking plays an important role in synaptic plasticity in CNS [18,29]. Many reports indicate important role of AMPAR-interacting proteins in regulation of receptor trafficking at synaptic sites [1,3,19,21,22,24,28,31,37]. In particular, GluR2 and its interacting proteins appear to play essential roles in cerebellar Purkinje cell long-term depression (LTD) [32,33]. Recently, it is documented that the AMPAR binding proteins, PICK1 and NSF, dynamically regulated the synaptic delivery of GluR2-containing receptors during the calcium-permeable AMPAR plasticity and thus regulated the calcium permeability of AMPARs at excitatory synapse [6].
All subunits of AMPARs are also highly expressed in the spinal cord dorsal horn (DH), and involved in somatosensory processing there [4]. Numerous GluR2 subunits were present in the gray matter of DH, with the strongest existence in laminae I–II, whereas GluR3 subunits are strongly distributed throughout the laminae III–IV, with the low existence in laminae I–II [27]. It has also been reported that AMPAR is present in the presynaptic terminals of primary afferent neurons in the DH and involved in the modulation of DH nociceptive neuronal excitability [8]. The activation of presynaptic receptors inhibits neurotransmitter release at synapses between primary afferents and DH secondary neurons via primary afferent depolarization [5,16]. These findings indicate the role of AMPAR subunits in the modulation of nociceptive transmission in the DH.
All four AMPAR subunits, in particular GluR1 and GluR2, are abundantly expressed in the spinal dorsal horn, sequential genetic studies using AMPAR subunit knockout (KO) mice have revealed that GluR2 enhances nociceptive plasticity, resulting in increased inflammatory hyperalgesia. Moreover, GluR1 and GluR3 KO mice show subtle abnormalities to inflammatory pain [8,39]. The role of the GluR2 and GluR3 in development and maintaining of the trigeminal neuropathic pain remains unclear. We have examined how the reduced expression of these subunits affects excitability of the nociceptive neurons. To monitor the changes in the neuronal excitability, we have measured the levels of the phosphorylation of the extracellular signal-regulated kinase (ERK) in naïve and GluR2 and GluR3 delta7 KI mice following partial ION-transection.
The ERK is known to be one of the mitogen-activated protein kinases that are activated by calcium influx in neurons and therefore used as a marker of neuronal activity [14]. In this report, we addressed the hypothesis that GluR2 and GluR3 subunits may play an important role in Vc and C1–C2 neuronal activity associated with orofacial neuropathic pain. We analyzed nocifensive behavior and pERK expression in Vc and C1–C2 in GluR2 and GluR3 delta 7 KI mice and found that the nocifensive threshold of KI mice were significantly higher than wild-type mice, and pERK expression was significantly decrease in KI mice compared to wild-type mice. These results suggest that GluR2 and GluR3 subunits are involved in the enhancement of the Vc and C1–C2 neuronal excitability, resulting in the trigeminal nerve injury-mediated hyperalgesia in orofacial region.
Adult male C57BL/6, wild-type and GluR2 and GluR3 delta7 KI mice (21–28 g) were used in these experiments. Genetic information about GluR2 and GluR3 delta7 KI mice was briefly described previously [32]. Each genotype was examined by regular three primer polymerase chain reaction (PCR) systems. PCR was performed on the isolated genomic DNA using taq DNA polymerase (TaKaRa Ex Taq™, Takara, Otsu, Japan) and primer sets (primer #21–23 for GluR2 delta7: #21, 5′-ACA GAG GAA GGT AGT GGA AGG GAG-3′; #22, 5′-CTT GGT TTG GTT GTT GGT CAT AGC-3′; #23, 5′-CTA GTG AAC CTC TTC GAG GGA C-3′; primer #31–33 for GluR3 delta7: #31, 5′-CCA ATA CTC CAC AGG GGC AAT TTA TC-3′; #32, 5′-CCG TTG ACT GTT TTG AAT CTC ACA CC-3′; #33, 5′-CTA GTG AAC CTC TTC GAG GGA C-3′). Size of the amplification product was estimated by gel electrophoresis for genotyping (210 bp for wild-type and 350 bp for GluR2 delta7; 440 bp for wild-type and 300 bp for GluR3 delta7). These mutant mice were viable and showed normal appearances.
The mice were housed under 12 h light/dark cycle conditions and had free access to food and water except during the experimental period. To minimize animal suffering, the number of animals used was based on the minimum required for statistically valid results. All methods and experimental approaches were approved by the Animal Care Committee of Nihon University and by the Committee for DNA transformation of Nihon University.
A total of 33 mice were used in this study (ION-transected mice: mechanical nocifensive behavior; n = 18, immunohistochemistry; n = 15). Mice were anesthetized with halothane (2%) mixed with oxygen during surgery. For the ION-partial transection, mice were placed on a warm mat and a small incision was made on the maxillary oral mucosa to expose the ION. The left ION was exposed and free from surrounding tissues, separated into deep and superficial branches, and the deep branch of ION was transected. After ION-partial transaction the incision was closed using 8–0 nylon sutures.
At least, 3 training sessions on successive days were given to provide baseline values of their head-withdrawal threshold before the ION-partial transection. Then, the ION-partial transected mice were examined for their head-withdrawal threshold to mechanical stimulation of the whisker pad skin ipsilateral and contralateral to the ION-partial transection by von Frey filaments. The head-withdrawal threshold was measured at day 0 (1 h before the ION-partial transection) and at 1, 3, 5, 7, 11 and 14 days after ION-partial transection.
The mice at day 7 after ION-partial transection were transcardially perfused with 1% paraformaldehyde (PFA) (50 ml) followed by 4% PFA in 0.1 M phosphate buffer (pH 7.4, 50 ml) at 5 min after applying non-noxious mechanical stimulation (2 g by von Frey filament for 10 min, n = 15) to the whisker pad. Since the mean head-withdrawal thresholds after ION-partial transection in ipsilateral side in wild-type, GluR2 and GluR3 delta7 KI mice were 0.005, 5.6 and 2.13 g respectively, we decided 2 g pressure was the intense non-noxious stimulus. The bilateral medulla and C1–C2 were removed and post-fixed with 4% PFA for 3 days at 4 °C. The tissues were then transferred to 20% sucrose (w/v) in phosphate-buffered saline (PBS) overnight for cryoprotection. Sections (30 μm-thick/section/19 sections) were cut from the brain stem and collected in PBS. The sections were reacted with rabbit anti-Phospho-p44/42 MAP kinase (Thr202/Tyr204) antibody (1:1000; Cell Signaling Technology, Danvers, MA) for 72 h at 4 °C. Next, the sections were then incubated in biotinylated goat anti-rabbit IgG (1:600; Vector Labs, Burlingame, CA) for 2 h at room temperature. After washing, the sections were incubated in peroxidase-conjugated avidin–biotin complex (1:100; Vector Labs) for 1 h at room temperature. After washing in 0.05 M Tris Buffer (TB), the sections were incubated in 0.035% 3,3-diaminobenzidine-tetra HCl (DAB, Tokyo Chemical Industry), 0.2% nickel ammonium sulfate, and 0.05% peroxide in 0.05 M TB (pH 7.4). The sections were washed in PBS, serially mounted on gelatin-coated slides, dehydrated in alcohols and cover slipped. The pERK-LI cells were drawn under a light microscope with an attached camera lucida drawing tube (Neurolucida 2000 Micro-BrightField, Colchester, UT). The number of pERK-LI cells was counted from every fourth section. The total number of pERK-LI cells from nineteen of every fourth section was calculated and the mean number of pERK-LI cells (/19 sections/mouse) was obtained from each animal.
Double immunofluorescence histochemistry was also carried out to determine if pERK is expressed in neurons. Free-floating tissue (30 μm) sections were rinsed in PBS, 3% normal goat serum in PBS for 2 h, and then incubated in rabbit anti Phospho-p44/42 MAPK antibody (1:300, Cell Signaling Technology) for 72 h at 4 °C and rats anti-NeuN antibody (1:1000, Chemicon, Temecula, CA) for 1 h and secondary antibodies conjugated with Alexa Fluor 488 and 568 (1:100; Molecular Probes) for 1 h at room temperature in a dark room. Sections were mounted on slides and cover slipped in PermaFluor (Sigma). Photographs were taken with a confocal laser microscope (LSM 510, Zeiss, Germany).
Results are presented as mean ± SEM and one-way analysis of variance with Tukey test was used for the behavioral and immunohistochemical data. Differences were considered as significant at p < 0.05.
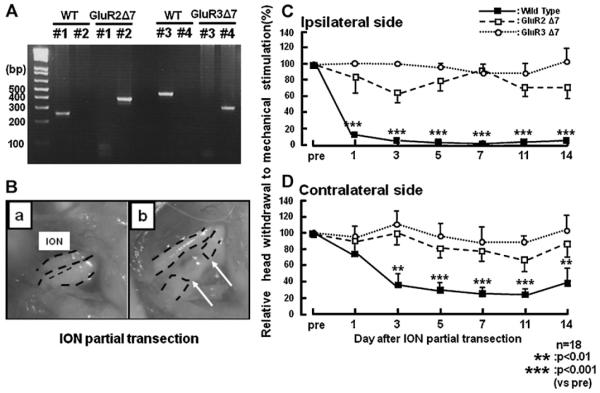
To identify the genotype of each mice PCR-based genotyping analysis was performed as previously described (Fig. 1A) [6]. The exposed left ION (Fig. 1B, a) was separated into two parts and the deep branch was transected (Fig. 1B b).
Fig. 1.
(A) Genotype in each generation of mutant mice confirmed by PCR analysis on isolated genomic DNA. PCR was performed on the isolated genomic DNA using taq DNA polymerase and primer sets (#1 and #2 are for wild-type and GluR2 delta7 KI, #3 and #4 are for wild-type and GluR3 delta7 KI). Size of the amplification product was estimated by gel electrophoresis for genotyping (210 bp for wild-type and 350 bp for GluR2 delta7 KI by primer #1 and #2; 440 bp for wild-type and 300 bp for GluR3 delta7 KI by primer #3 and #4). (B) Photomicrographs of intact (a) and partial-transected ION (b). (C and D) Nocifensive behavior following mechanical stimulation of the whisker pad skin after ION partial transaction in the wild-type, GluR2 and GluR3 delta7 KI mice. ((C) Ipsilateral to ION partial transection; (D) contralateral to ION partial transection). Mechanical nocifensive behavior was measured before ION-partial transaction and at 1, 3, 5, 7 and 14 days after the transaction. The arrows in Bb indicate stump ends of ION. **p < 0.01, ***p < 0.001.
The mechanical head-withdrawal threshold in ION-partial transected wild-type, GluR2 and Glu3 delta7 KI mice was measured for 14 days after ION-partial transection (Fig. 1C and D). Head-withdrawal threshold to mechanical stimulation of the whisker pad skin ipsilateral and contralateral to the ION-partial transection were significantly decreased at 1, 3, 5, 7, 11 and 14 days after ION-partial transection compared to that of pre-transection in wild-type mice respectively. On the other hand, we could not observed any changes in head-withdrawal threshold to mechanical stimulation of the whisker pad skin in either GluR2 or GluR3 delta7 KI mice (Fig. 1C; pre vs. after ION-partial transaction, p < 0.05).
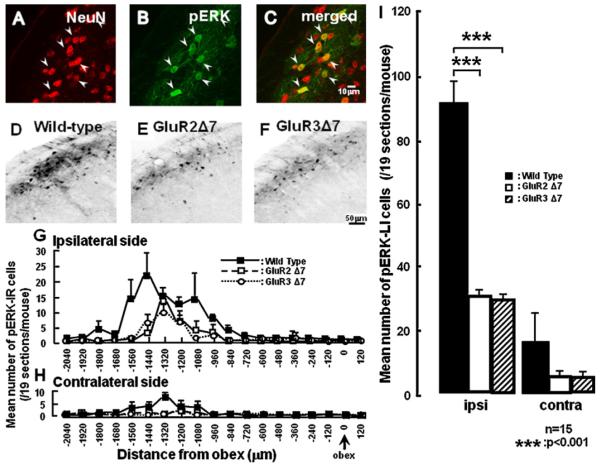
In the superficial laminae of Vc, all pERK-LI cells induced by non-noxious mechanical stimulation (2 g) of the whisker pad skin ipsilateral to the ION-partial transaction at day 7 also showed NeuN immunoreactivity (Fig. 2A–C). Many pERK-LI cells were expressed in the superficial laminae of the Vc and C1–C2 following non-noxious mechanical stimulation (2 g pressure) at 7 days after ION-partial transection in wild-type, GluR2 and GluR3 delta7 KI mice (Fig. 2D–F). The largest number of pERK-LI cells was observed at 1440 μm and 1320 μm caudal to the obex in the ipsilateral and contralateral Vc and C1–C2 respectively following non-noxious mechanical stimulation of the whisker pad skin in wild-type mice. The peak expression of pERK-LI cells in GluR2 and GluR3 delta7 KI mice ipsilateral and contralateral to transection was 1320 μm caudal to the obex (Fig. 2G). There were no peaks in GluR2 and GluR3 delta7 KI mice contralateral to transaction, and the small number of pERK-LI cell expression was observed (Fig. 2H). The number of pERK-LI cells in Vc and C1–C2 was significantly larger in wild-type mice compared with that of GluR2 and GluR3 delta7 KI mice at day 7 after ION-partial transection ipsilateral to ION-partial transection (Fig. 2I). We could not observe any differences in the number of pERK-LI cells between GluR2 and GluR3 delta7 KI mice.
Fig. 2.
(A–C) Photomicrographs of NeuN positive cells (A), pERK-LI cells (B) and pERK-LI cells double stained with NeuN antibody (C) in the wild-type mice. (D–F) Photographs of pERK-LI cells in the wild-type mice (D) and GluR2 7 mutant mice (E) and GluR3 7 mutant mice (F) at 5 min after 2 g mechanical stimulation of the whisker pad skin at 7days after ION partial transaction. The photomicrographs in A–F were taken from sections at 1440 μm caudal to the obex. (G–I) Rostro-caudal distribution (G and H) of pERK-LI cells in the wild-type, GluR2 and GluR3 delta7 KI mice. ((G) Ipsilateral side with ION partial transection; (H) contralateral side with ION partial transection). (I) The mean number of pERK-LI cells in the both sides Vc and C1/C2 ION-transected mice. ***p < 0.001.
In order to evaluate the involvement of GluR2 and GluR3 subunits in orofacial allodynic pain, we studied the nocifensive behavior and ERK phosphorylation in Vc and C1–C2 neurons in ION-partial transected wild-type, GluR2 and GluR3 delta7 KI mice. The GluR2 and GluR3 delta7 KI mice used in the present study have deficits in membrane trafficking due to a missing interaction with their binding proteins, such as GRIP1/2 and PICK1 [6,32]. Therefore, these mutant mice are a valid model to investigate the functional significance of GluR2 or GluR3 membrane trafficking in Vc and C1–C2 neurons activated by noxious stimulation. We measured the head-withdrawal threshold to non-noxious mechanical stimulation of the whisker pad skin by von Frey filament to evaluate allodynic nocifensive behavior in ION-partial transected wild-type, GluR2 and GluR3 delta7 KI mice.
It has been reported that spinal nerve axotomy causes a marked increase in the excitability of primary afferent neurons [17,36]. The hyperexcitability of peripheral nerves also affects CNS neuronal activity, resulting in the central sensitization of the DH neurons [38]. In the trigeminal system, it has been shown that ION ligation causes the central sensitization of Vc neurons, and a variety of behavioral abnormalities in rats that includes face-grooming behavior and reduction in escape threshold to mechanical and/or heat stimulation of the face, which is thought to be involved in neuropathic pain [11,35]. In this study, we found the significant decrease in the head-withdrawal threshold following ION-partial transection in wild-type mice, whereas no changes in mechanical head-withdrawal threshold were observed in GluR2 and GluR3 delta7 KI mice, and also no differences in head-withdrawal threshold between GluR2 and GluR3 delta7 KI mice. Many pERK-LI cells were expressed in Vc and C1–C2 following non-noxious mechanical stimulation of the whisker pad skin at day 7 after ION-partial transaction in wild-type mice. The number of pERK-LI cells in Vc and C1–C2 was significantly larger in wild-type mice compared with that of GluR2 and GluR3 delta7 KI mice at day 7 after ION-partial transaction in ipsilateral to transection. It is well known that AMPA and NMDA receptors produce the functional interaction each other in dorsal horn neurons for sensory processing [2]. The functional deficit of AMPAR is thought to cause a decrease in the NMDA receptor activity. Together with previous results, our findings suggest that GluR2 and GluR3 subunits in AMPAR are indirectly involved in ERK phosphorylation via NMDA receptor mechanisms. We observed lowering of the head-withdrawal threshold but not ERK phosphorylation in the contralateral side to ION partial transection in the wild-type mice. It has been known that the ERK phosphorylation is one of possible mechanisms to enhance the nocifensive behavior following noxious stimulation of the peripheral structures. It is probable that other intracellular mechanisms may be involved in the change in head-withdrawal threshold in the contralateral side to ION transection. It has also been reported that Ca2+-permeable-AMPAR contributes to LTP in hippocampus [15,25] and in the spinal dorsal horn (DH) [7]. Recently, it have also reported that GluR2 subunit is involved in the induction of LTP and/or LTD in the superficial DH [39]. The LTP and/or LTD are thought to be involved in modulation of superficial DH neuronal excitability. These suggest that LTP and/or LTD also occur via GluR2 in Vc and C1–C2 following peripheral inflammation, resulting in modulation of Vc and C1–C2 neuronal activities.
GluR2 internalization is also known to be involved in pain abnormalities following CFA injection into the hind paw [23]. Furthermore, intrathecal administration of a selective blocker of GluR2 attenuates secondary mechanical allodynia following thermal injury, tight spinal nerve ligation or inter planter injection of carrageenan [30]. Together with these previous findings, the present results suggest that the membrane trafficking of GluR2 and GluR3 subunits of AMPAR in Vc is possible mechanism that the induction of orofacial neuropathic pain following trigeminal nerve injury. It has also been reported that GluR1 and GluR4 subtypes are distributed superficial DH neurons [26,27]. Although we do not have any direct evidence whether GluR1 and GluR4 subtypes are involved in nociceptive processing in VC and C1–C2, it is probable that these two subtypes may be involved in nociceptive processing in VC and C1–C2 based on these DH data.
Acknowledgments
This study was supported in part by Research Grants from Sato and Uemura Funds from Nihon University School of Dentistry, and a grant from the Dental Research Center, Nihon University School of Dentistry; Nihon University multidisciplinary research grant for KI; grants from the Ministry of Education, Culture, Sports, Science, and Technology to promote multidisciplinary research project “Translational Research Network on Orofacial Neurological Disorders” at Nihon University School of Dentistry; the Japan–Canada Joint Health Research Program 167458, NIH grant (DE04786), and CHIR grants (MOP-43095), (MOP-82831). We also thank Prof. A.M. Binshtok for commenting on this manuscript.
References
- [1].Dev KK, Nishimune A, Henley JM, Nakanishi S. The protein kinase C alpha binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. 1999;38:635–644. doi: 10.1016/s0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- [2].Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- [3].Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- [4].Engelman HS, Allen TB, MacDermott AB. The distribution of neurons expressing calcium-permeable AMPA receptors in the superficial laminae of the spinal cord dorsal horn. J. Neurosci. 1999;19:2081–2089. doi: 10.1523/JNEUROSCI.19-06-02081.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat. Rev. Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- [6].Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and, NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- [7].Gu JG, Albuquerque C, Lee CJ, MacDermott AB. Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature. 1996;381:793–796. doi: 10.1038/381793a0. [DOI] [PubMed] [Google Scholar]
- [8].Hartmann B, Ahmadi S, Heppenstall PA, Lewin GR, Schott C, Borchardt T, Seeburg PH, Zeilhofer HU, Sprengel R, Kuner R. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron. 2004;44:637–650. doi: 10.1016/j.neuron.2004.10.029. [DOI] [PubMed] [Google Scholar]
- [9].Honda K, Kitagawa J, Sessle BJ, Kondo M, Tsuboi Y, Yonehara Y, Iwata K. Mechanisms involved in an increment of multimodal excitability of medullary and upper cervical dorsal horn neurons following cutaneous capsaicin treatment. Mol. Pain. 2008;4:59. doi: 10.1186/1744-8069-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu JW, Sun KQ, Vernon H, Sessle BJ. Craniofacial inputs to upper cervical dorsal horn: implications for somatosensory information processing. Brain Res. 2005;1044:93–106. doi: 10.1016/j.brainres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [11].Imamura Y, Kawamoto H, Nakanishi O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Exp. Brain Res. 1997;116:97–103. doi: 10.1007/pl00005748. [DOI] [PubMed] [Google Scholar]
- [12].Imbe H, Iwata K, Zhou QQ, Zou S, Dubner R, Ren K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Implications for persistent temporomandibular pain. Cells Tissues Organs. 2001;169:238–247. doi: 10.1159/000047887. [DOI] [PubMed] [Google Scholar]
- [13].Iwata K, Imai T, Tsuboi Y, Tashiro A, Ogawa A, Morimoto T, Masuda Y, Tachibana Y, Hu J. Alteration of medullary dorsal horn neuronal activity following inferior alveolar nerve transection in rats. J. Neurophysiol. 2001;86:2868–2877. doi: 10.1152/jn.2001.86.6.2868. [DOI] [PubMed] [Google Scholar]
- [14].Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J. Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, Abramow-Newerly W, Roder J. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- [16].Lee CJ, Bardoni R, Tong CK, Engelman HS, Joseph DJ, Magherini PC, MacDermott AB. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron. 2002;35:135–146. doi: 10.1016/s0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- [17].Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J. Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- [18].Malinow R. AMPA receptor trafficking and long-term potentiation. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- [20].Okada-Ogawa A, Porreca F, Meng ID. Sustained morphine-induced sensitization and loss of diffuse noxious inhibitory controls in dura-sensitive medullary dorsal horn neurons. J. Neurosci. 2009;29:15828–15835. doi: 10.1523/JNEUROSCI.3623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Osten P, Khatri L, Perez JL, Kohr G, Giese G, Daly C, Schulz TW, Wensky A, Lee LM, Ziff EB. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- [22].Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- [23].Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J. Neurosci. 2009;29:3206–3219. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J. Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat. Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- [26].Polgar E, Al-Khater KM, Shehab S, Watanabe M, Todd AJ. Large projection neurons in lamina I of the rat spinal cord that lack the neurokinin 1 receptor are densely innervated by VGLUT2-containing axons and possess GluR4-containing AMPA receptors. J. Neurosci. 2008;28:13150–13160. doi: 10.1523/JNEUROSCI.4053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Polgar E, Watanabe M, Hartmann B, Grant SG, Todd AJ. Expression of AMPA receptor subunits at synapses in laminae I-III of the rodent spinal dorsal horn. Mol. Pain. 2008;4:5. doi: 10.1186/1744-8069-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- [29].Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- [30].Sorkin LS, Yaksh TL, Doom CM. Pain models display differential sensitivity to Ca2+-permeable non-NMDA glutamate receptor antagonists. Anesthesiology. 2001;95:965–973. doi: 10.1097/00000542-200110000-00028. [DOI] [PubMed] [Google Scholar]
- [31].Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, Weinberg RJ, Ziff EB. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- [32].Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- [33].Takamiya K, Mao L, Huganir RL, Linden DJ. The glutamate receptor-interacting protein family of GluR2-binding proteins is required for long-term synaptic depression expression in cerebellar Purkinje cells. J. Neurosci. 2008;28:5752–5755. doi: 10.1523/JNEUROSCI.0654-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Takeda M, Tanimoto T, Ito M, Nasu M, Matsumoto S. Role of capsaicin-sensitive primary afferent inputs from the masseter muscle in the C1 spinal neurons responding to tooth-pulp stimulation in rats. Exp. Brain Res. 2005;160:107–117. doi: 10.1007/s00221-004-1990-2. [DOI] [PubMed] [Google Scholar]
- [35].Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J. Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Woolf CJ, Ma Q. Nociceptors—noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- [37].Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- [38].Xu XJ, Zhang X, Hokfelt T, Wiesenfeld-Hallin Z. Plasticity in spinal nociception after peripheral nerve section: reduced effectiveness of the NMDA receptor antagonist MK-801 in blocking wind-up and central sensitization of the flexor reflex. Brain Res. 1995;670:342–346. doi: 10.1016/0006-8993(94)01360-t. [DOI] [PubMed] [Google Scholar]
- [39].Youn DH, Royle G, Kolaj M, Vissel B, Randic M. Enhanced LTP of primary afferent neurotransmission in AMPA receptor GluR2-deficient mice. Pain. 2008;136:158–167. doi: 10.1016/j.pain.2007.07.001. [DOI] [PubMed] [Google Scholar]