Abstract
Background
This paper introduces the 7/5/2011al Pupil index (NPi), a sensitive measure of pupil reactivity and an early indicator of increasing intracranial pressure (ICP). This may occur in patients with severe traumatic brain injury (TBI), aneurysmal subarachnoid hemorrhage, or intracerebral hemorrhage (ICH).
Methods
134 patients (mean age 46 years, range 18–87 years, 54 women and 80 men) in the intensive care units at eight different clinical sites were enrolled in the study. Pupillary examination was performed using a portable hand-held pupillometer.
Results
Patients with abnormal pupillary light reactivity had an average peak ICP of 30.5 mmHg versus 19.6 mmHg for the normal pupil reactivity population (P = 0.0014). Patients with “nonreactive pupils” had the highest peaks of ICP (mean = 33.8 mmHg, P = 0.0046). In the group of patients with abnormal pupillary reactivity, we found that the first evidence of pupil abnormality occurred, on average, 15.9 hours prior to the time of the peak of ICP.
Conclusions
Automated pupillary assessment was used in patients with possible increased ICP. Using NPi, we were able to identify a trend of inverse relationship between decreasing pupil reactivity and increasing ICP. Quantitative measurement and classification of pupillary reactivity using NPi may be a useful tool in the early management of patients with causes of increased ICP.
Keywords: Intracranial pressure, Neurological Pupil index, pupillometer, traumatic brain injury
INTRODUCTION
Different neuroanatomical pathways are involved in the control of the pupil, and the integrity and functionality of these neurological pathways can often be ascertained through the analysis and interpretation of pupillary behavior. This makes the pupil size and the pupillary light reflex an important factor to be considered in many clinical conditions as described, for example, in the work of Loewenfeld.[15] In the management and prognosis of severe traumatic brain injury (TBI), abnormalities of pupillary response or anisocoria (pupil size asymmetries) are often associated with neurological deteriorations, and they are correlated with poor neurological outcome.[2,6,13,20] More specifically, the location of the pupillomotor nuclei in the dorsal midbrain and the efferent oculomotor nerve running from the midbrain to the superior orbital fissure is particularly important for assessing the onset of descending transtentorial herniation and brainstem compression. Depressed light reflex and anisocoria have been associated with such phenomena,[9,16] and they have been proposed as prognostic indicators of functional recovery after traumatic transtentorial herniation.[1,30] In addition to herniation and third nerve compression, it has been shown through blood flow imaging that pupillary changes in the neurological intensive care unit (ICU) are highly correlated with brainstem oxygenation and perfusion/ischemia.[28]
Furthermore, other investigators have used pupil size and reactivity as the fundamental parameters of more general outcome predictive models in conjunction with other clinical information such as age, mechanism of injury and Glasgow Coma Scale (GCS),[18,23] and have correlated such models with the presence and the location of intracranial mass lesions.[3] It has been shown that neurosurgeons have the tendency to triage patients into either conservative therapy or surgical evacuation of mass lesions, depending on the status of the pupils.[34] Patients who undergo prompt intervention (i.e., surgery, hyperventilation, hyperosmolar therapy) after a new pupillary abnormality have improved recovery potential.[7]
Common terminology employed in the clinical literature to describe the pupillary light reflex and pupil size includes “unilateral” or “bilateral nonreactive pupils”, “fixed” or “dilated pupils”, as well as “brisk”, “sluggish”, and “nonreactive” pupils. These subjective terms are often applied without a standard clinical protocol or definition. A more precise assessment of the pupil is problematic, since manual pupillary assessment as part of the clinical routine is subject to compounded sources of inaccuracies and inconsistencies, and is characterized by large inter-examiner variability.[14] Moreover, ambient light conditions can affect the validity of the visual assessment of the pupil and increase the inter-observer disagreement. These factors may include, for example, poor lighting conditions in the patient's room, the examiner's visual acuity, and the strength of the flashlight stimulus, as well as its distance and orientation with respect to the patient's eye.[40] In a recent study,[8,22] there was a 39% discrepancy in agreement amongst examiners.[39]
Intracranial pressure (ICP) has been monitored dating back to over 100 years ago.[37] Historically, it has been shown that high ICPs (>20 mmHg) in brain-injured patients result more frequently in poor neurological outcomes and death.[17,21,24,29] ICP monitoring is invaluable in directing therapy for the brain-injured patient.[21,31] High ICPs are associated with pupillary abnormalities of brain-injured patients.[19,29,33] A more sensitive means to track and classify pupillary changes would be of particular utility in the early evaluation of patients with suspected intracranial pathology and elevated ICPs, before imaging studies are completed and ICP monitors are placed. This paper introduces the concept of Neurological Pupil index (NPi) as a means to assess pupillary response and investigate trends in the ICP of severely brain-injured patients.
MATERIALS AND METHODS
Patients
Patients were enrolled from a total of eight different neurological/critical care ICU sites. The study was approved by the institutional review boards at each of the eight sites, and informed consent was obtained from the family members prior to enrolment in the study. The patients had the following diagnoses: subdural hematoma (SDH), subarachnoid hemorrhage (SAH), epidural hematoma (EDH) induced from TBI, aneurysmal SAH, or spontaneous intracerebral hemorrhage (ICH). These patients typically require ICP monitoring and often exhibit a high potential for early changes in brain volume. A total of 172 patients were originally enrolled. Thirty-eight of these were excluded because of ocular injuries or malformation of the ocular structures found after enrolment, or due to premature withdrawal from the study (i.e., family decision to withdraw support). In total, there were 134 patients on whom the study was performed. The mechanisms of injury are listed in Table 1, and include TBI, aneurysmal SAH, and spontaneous ICH. Mean age was 46 years, with a range of 18–87 years. There were 54 women and 80 men. Enrolment criteria required the subject to have at least one reactive pupil at the time of enrolment, and for the evidence of brain injury to be suggestive of raised ICP on admission computed tomography (CT) scan (i.e., a CT scan showing either compressed/absent cisterns, a midline shift ≥3 mm, or an intracranial mass >25 cm3). Patients were followed for 72 hours after the initiation of measurements. This time period was selected because this is when patients are at greatest risk for deterioration in their neurological status. ICP was monitored continuously during this 72-hour period. Pupil examination with the pupillometer and the corresponding ICPs were recorded every 30 minutes. Five patients required barbiturates to manage their intracranial injuries. In these five patients, barbiturate levels were monitored for the duration of the trial, as these drugs can cause poorly reactive pupils. For these five patients, data that were taken during the period of action of the drug were excluded from analysis.
Table 1.
A table of patient mechanism of brain injury
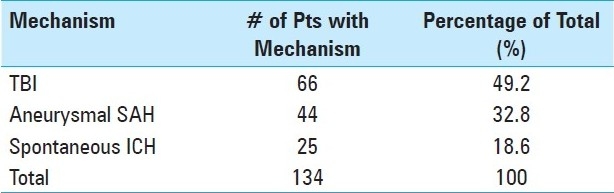
Pupillometer and the Neurological Pupil index
The NeurOptics pupillometer (NeurOptics, Irvine, CA, USA), used in the study to evaluate pupil size and reactivity, is a hand-held infrared system which automatically tracks and analyzes pupil dynamics over a 3-second time period. A detachable headrest facilitates the correct and consistent placement of the pupillometer in front of the eye [Figure 1]. A pupil light measurement begins with a flash of light of fixed intensity and duration effectively stimulating the pupil light constriction reflex. The measurement lasts 3.2 seconds, allowing for a full or partial recovery of the pupil size after the light constriction. The pupil is tracked at over 30 frames per second. The device has been specifically designed to minimize possible inter-observer variability in the pupillary evaluation. As compared to other pupillometers, the NeurOptics pupillometer demonstrates the best inter-observer agreements, and higher inter- and intra-observer repeatability.[32] The reliability and robustness of the pupil tracking algorithms and the corresponding pupil measurement precision and accuracy have been evaluated and described.[8,12,22,33]
Figure 1.
The portable pupillometer held up to a patient's eye during a measurement
The pupillary mechanism is characterized by different neuronal and mechanical nonlinearities.[27] Firstly, the retina operates over log light units until saturation occurs at both extremes of the illumination values. The pupil size effect is a midbrain mechanism acting at the level of the Edinger–Westphal nuclei, which results in different constriction gains as a function of the tonic pupil level. Latency also is a function of the tonic pupil operating level. Finally, and perhaps more importantly, the asymmetry of the smooth iris muscle length–tension curve produces different levels of muscle contraction for a given level of excitation as a function of pupil size.[35]
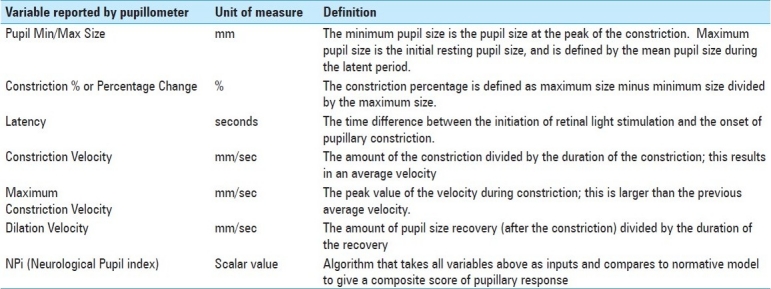
The NPi algorithm was developed to quantify pupillary reactivity and remove subjectivity from this assessment. Pupil variables including size, latency, constriction velocity, and dilation velocity are all parameters of the NPi algorithm. The pupil variables measured, and how these variables are calculated, for NPi are listed in Table 2. Each variable from an individual pupil measurement taken by the pupillometer is compared against the mean of a reference distribution of healthy subjects for the same variable, taking the difference and then standardizing it by the corresponding standard deviation. Finally, the set of all the standardized differences (or z-scores) are combined to fall into a scale set between 0 and 5. An NPi score ≥3 means that the pupil reactivity falls within the boundaries of the normative pupil behavior distribution (i.e., pupillary reaction to light is “brisk” or “normal”). An NPi value closer to 5 is considered more “brisk” than an NPi value closer to 3. An NPi score <3 denotes an abnormal pupillary light reflex (i.e., weaker than a normal pupil response, as defined by the multidimensional normative model, or “sluggish”), with a value of 1 being more abnormal than a value of 3.
Table 2.
A list of pupil size variables measured by the pupillometer and their corresponding descriptions
Analysis and clinical evaluation
Patients were monitored continuously for 72 consecutive hours. Pupil measurements were performed with the pupillometer by the clinical research coordinator or the bedside nurse in groups of four consecutive measurements (right pupil–left pupil–right pupil–left pupil) every 30 minutes, and ICP measurements were taken at the same time. Data from the pupillometer were not used in the treatment of the patients enrolled in the study, and the values of the NPi were not reported to the users. NPi was calculated off-line after the pupil data were acquired and downloaded from the device; in this way, users could not access the NPi score at the time of the measurement. Measurements were interrupted any time a patient required medical or surgical intervention. Thus, some patients in the study underwent periods where no pupil information was available. Each ICP measurement was averaged with the surrounding measurements in order to filter out sudden and temporally circumscribed events of high ICP. We focused on those events that lasted more than one single evaluation. We called this average “sustained ICP”. Each patient was thus represented by the peak of sustained ICP. Patients were grouped based on whether they received an abnormal NPi score at any point during the 72 hours. A one-tailed student's t-test was performed to test for group differences.
RESULTS
Subjects whose pupil reactivity measurements scored in the abnormal range, as defined by the NPi scale, during the 72-hour period of the study, were characterized by higher peaks of ICP than subjects whose pupil reactivity was consistently normal. We divided our population of patients into three groups [Figure 2]. Group 1 included patients with pupil reactivity in the normal range (3–5) with regularity. Group 2 included patients with one or more occurrences of abnormal pupil reactivity (<3). We found that patients in Group 1 (normal NPi reactivity, n = 98) had a lower peak ICP, with a mean significantly lower than the patients in Group 2 (abnormal NPi reactivity, n = 28; mean Group 1 = 19.6 mmHg vs. mean Group 2 = 30.5 mmHg, P = 0.0014). If we restrict Group 2 to those patients who not only had abnormal reactivity, but also developed a nonreactive pupil, or only had occurrences of nonreactive pupils (n = 17), then the corresponding difference in ICP peak is even higher, as compared to the normal population. We identified these patients with nonreactive pupils as Group 3, the mean ICP of this group being 33.8 mmHg versus Group 1 mean ICP being 19.6 mmHg (P = 0.0046). The P-values were generated using a two-sample t-test, assuming unequal variances. In those subjects with abnormally reactive pupils, we found that the first event of abnormality occurred, on average, 15.9 hours prior to the time of the maximum ICP. Four subjects only had abnormalities that occurred after the time of the maximum ICP, and the rest ranged from 0 to 60 hours prior.
Figure 2.
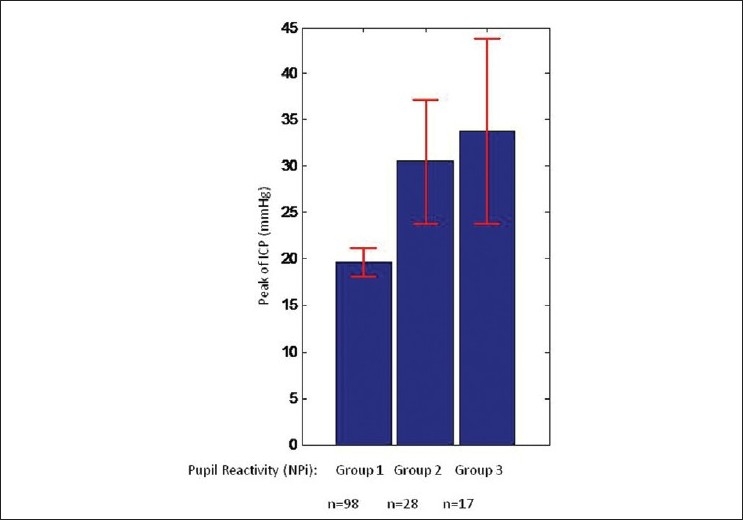
Peak of ICP was defined for each single patient as the maximum event of sustained ICP. The distribution of peak of ICP varied depending on the pupil NPi reactivity score. Those patients with normal pupil reactivity NPi (3–5, Group 1) had the lowest ICP. Those with one or more occurrences of abnormal NPi (<3, Group 2) had a significantly larger distribution of sustained ICP. Group 3, with the highest sustained ICPs, includes those patients who developed or had occurrences of a nonreactive pupil. Red error bars indicate 95% Confidence Interval (CI)
We present two case studies [Figure 3], such that we might delineate the finer details of the relationship between pupillometry and ICP. These studies illustrate how the pupillary reactivity defined by the NPi for a patient can change over time. Pupil reactivity, as reported by the NPi, is indicated in the two lower panels. The blue plot is for the left pupil and the red plot is for the right pupil. The progression of ICP is reported in the two top panels for each patient. Time is expressed in hours relative to the time of patient enrolment. The threshold between normal pupil reactivity and abnormal reactivity is indicated with a solid black horizontal line in the lower panels. Initially, both Patient A and Patient B had normal, reactive pupils.
Figure 3.
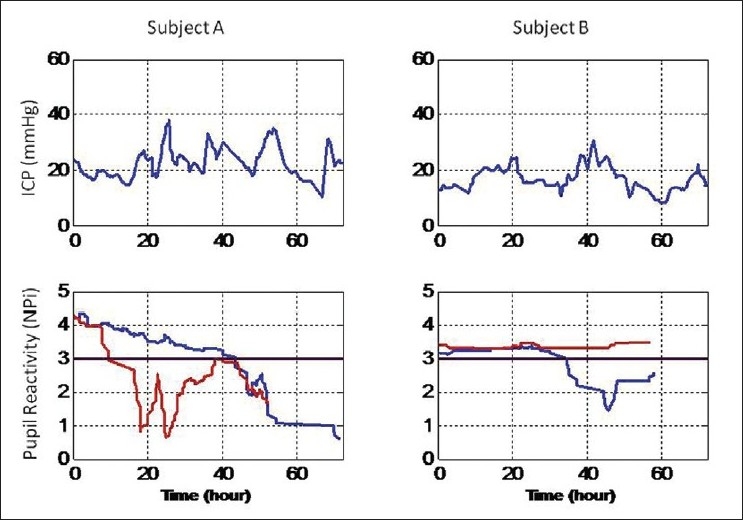
Temporal progression of pupil reactivity and ICP in two patients; ICP in the top panels, pupil reactivity (NPi) in the lower panels, red for the right pupil and blue for the left pupil (see text for clinical information). Normal range of pupil reactivity is for NPi values between 3 and 5. The threshold between normal and abnormal pupil reactivity is indicated with a solid back horizontal line in the lower panels
Patient A had a right temporal-occipital parenchymal hemorrhage; basal cisterns were absent or compressed, there was no midline shift, and a mass was present less than 4 cm3 in size. A CT scan performed at around hour 12 revealed an increasing low density in the right parietal and occipital lobes, suggesting increasing edema in the area of underlying injury. The reactivity of the right pupil (see the NPi red plot, Figure 3, Patient A, lower panel) dropped below normal, 2 hours before the increasing edema was reported with the CT scan. The ICP fluctuated around 20 mmHg during the first 18 hours and it peaked to almost 40 mmHg after about 12 hours from the first sign of NPi right pupil abnormality (see ICP peak in the upper panel, Figure 3, Patient A, top panel). ICP decreased again at around hour 30 and the NPi pupil reactivity increased for a period of time above the normal threshold. A second prominent peak of ICP at around hour 55 was preceded by an abnormal drop of the NPi in both pupils (see hour 40–45, Figure 3, Patient A, lower panel, red and blue plots) and later by a new oval pupil on right (and soon after on left too), which revealed brainstem compression.
Patient B suffered a TBI with subdural hemorrhage distributed over the left, frontal, temporal and parietal lobes. The pre-enrolment CT scan revealed a left to right midline shift of 18 mm, and a mass size of 25 cm3 on the left lobe that later enlarged to 30 cm3. Cisterns were absent or compressed. ICP values were initially maintained below 20 mmHg using medical interventions and CSF diversion; it then increased slightly above 20 mmHg at around hour 20 (Figure 3, Patient B, top panel). It decreased again and it finally peaked at over 30 mmHg at around hour 40. Another CT scan performed at this time revealed a left to right midline shift of 11 mm and a pronounced effacement of the basal cisterns, which suggested impending herniation. Extensive edema was also seen throughout the left cerebral hemisphere, consistent with progression of post traumatic edema and/or infarct. A few hours before this CT scan and the second elevated peak of ICP, the left pupil NPi reactivity decreased below the normal threshold (see blue plot, Figure 3, Patient B, lower panel) and it remained abnormal for the rest of the study.
DISCUSSION
Evaluation of pupil size and its light reflex mechanism is an integral part of the protocol for the treatment and management of severely brain-injured patients in ICUs worldwide. The American Association of Neurological Surgeons and the Brain Trauma Foundation guidelines recommend that severe TBI patients be evaluated for asymmetry in pupil size or reactivity to light, as well as fixed and/or dilated pupils.[4] The oculomotor nerve supplies efferent fibers to the extraocular muscles of the eye. Pupillomotor fibers travel along the dorsal periphery of the oculomotor nerve and are more sensitive to mass effect. The parasympathetic oculomotor nuclei in the midbrain are especially sensitive to brain stem compression and ischemia, and thus can indicate an expanding supra-tentorial mass lesion with transmission of the associated pressure and subsequent onset of herniation. The TBI literature provides much evidence that alterations of the pupil light reflex, size of the pupil, or anisocoria, are all closely correlated with the outcome of TBI.[3,33,34] Dilation of a pupil and subsequent herniation are late events and frequently irreversible.[34] A more sensitive means to track pupillary changes, in order to detect potential ICP problems before a monitor is inserted, would be of particular utility for the triaging of patients.
Pupillary evaluation in the clinical setting is often performed in a very subjective manner, with a pen flashlight for reactivity and a pupil gauge for pupil size. These methods are affected by the examiner's subjectivity and by pronounced inter-examiner variability.[8,14] The device used in the study is a hand-held portable infrared pupillometer which allows a reliable, reproducible, and objective measurement of pupillary reflexes and pupil sizes. More importantly, the numeric scale of the NPi, first introduced in this report, allows an automatic and rigorous interpretation and classification of the pupillary dynamics. The notion of normal or abnormal pupil reflex is automatically derived by comparing the reflex against an NPi model, which defines the behavior of the pupil light reflex mechanism. This reduces the subjectivity of the measurement.
In this study, we analyzed a group of patients who sustained severe intracranial injury and were monitored for at least 72 hours in eight different ICUs. ICP was continuously monitored. We found that patients who had continuous or even sporadic occurrences of abnormal pupil reactivity, as defined by NPi, were also characterized by peaks of sustained ICP significantly higher than those patients with normal pupillary activity. The analysis of the patients with increased ICP clearly demonstrates a statistically significant relationship between NPi <3 and increased ICPs. The abnormal NPi was also found to precede the ICP spike, thus providing useful predictive information, even in patients with an indwelling ICP monitor. This becomes important clinically when one plans therapy/studies based on the prediction of ICP problems, for example, in order to gain the benefits from an early decompressive craniectomy prior to ICP spikes both for TBI and hemispheric stroke.[11,26,36,38]
Increased ICP in a brain-injured patient is often difficult to detect in the field because of a lack of clinical information. The causes of poor neurological outcomes are innumerable, and include toxic and metabolic etiologies, cerebrovascular accidents, and TBI.[41] Recent studies have demonstrated that an accurate knowledge of pupillary function, or lack thereof, along with GCS and CT scan findings, are powerful predictors for outcome in patients with TBI.[5,10,25] The use of the pupillometer, and in particular the NPi, provides a rapid and non-invasive method for the screening and triage of patients with suspected increased ICP. Use of the NPi provides a more objective method of determining pupillary reactivity and may have clinical relevance in being able to guide neuroprotective and neurosurgical interventions. More solid correlations between NPi and increases in ICP must be established with further research to determine the validity of our interpretation of the NPi.
CONCLUSION
Classification of pupil reactivity according to the NPi eliminates ambiguity in clinical pupillary measurements. Our research shows that an abnormal NPi <3 is suggestive of trends in increased ICPs, significantly higher than those ICPs recorded in patients with normal pupillary reactivity, measured as having an NPi between 3 and 5. With more thorough future investigation, the NPi can provide a non-invasive means to track and predict problems with ICP, providing beneficial guidance for neurological therapy to improve patient outcome.
Acknowledgments
We would like to thank William Taylor, MD, Cynthia Kelbch, RN, and Kathleen E. Strege, RN (University of California, San Diego, CA); Alice Baker, MA, Latania Chura, CRC, and Lorraine Donison, BSN RN (Legacy Research, Portland, OR); Raj K. Narayan, MD, Suzanne Kempisty-Cliver, RN, and Nancy McMahon, RN (Mayfield Clinic, University of Cincinnati); and Charlotte B. Gilman, BSN RN, and Anne K. Hall, MS ANP (Virginia Commonwealth University, Richmond, VA), Peter Hutchinson, MD and Elizabeth Corteen (Addenbrooke's Hospital, Cambridge, UK), Domenic Esposito, MD (University of Mississippi Medical Centre), Grant Sinson, MD and Barb Alivo (Medical College of Wisconsin), and Joe Ordia, MD and Ellen Adamski, RN (Boston Medical Centre) for their diligent and professional work in collecting the data for this study. A special thanks to Michela Azzariti, MS (University of California at Berkeley) for her preliminary statistical analysis. We would also like to recognize Medtronic Neurosurgery for their financial support of this study.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/82/82248
Contributor Information
Jeff W. Chen, Email: jchen@lhs.org.
Zoe J. Gombart, Email: zgombart@lhs.org.
Shana Rogers, Email: slrogers@lhs.org.
Stuart K. Gardiner, Email: sgardiner@deverseye.org.
Sandy Cecil, Email: scecil@lhs.org.
Ross M. Bullock, Email: rbullock@med.miami.edu.
REFERENCES
- 1.Andrews BT, Pitts LH. Functional recovery after traumatic transtentorial herniation. Neurosurgery. 1991;9:227–31. doi: 10.1097/00006123-199108000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Braakman R, Gelpke GJ, Habbema JD, Maas AI, Minderhoud JM. Systematic selection of prognostic features in patients with severe head injury. Neurosurgery. 1980;6:362–70. [PubMed] [Google Scholar]
- 3.Chesnut RM, Gautille T, Blunt BA, Klauber MR, Marshall LF. The localizing value of asymmetry in pupillary size in severe head injury: Relation to lesion type and location. Neurosurgery. 1994;34:840–6. doi: 10.1227/00006123-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Chestnut RM, Ghajar J, Maas AI, Marion DW, Servadei F, Teasdale GM, et al. Management and prognosis of severe traumatic brain injury. New York: Brain Tumour Foundation, Inc; 2000. Early indicators of prognosis in severe traumatic brain injury. [Google Scholar]
- 5.Chieregato A, Martino C, Pransani V, Nori G, Russo E, Noto A, et al. Classification of a traumatic brain injury: The Glasgow Coma scale is not enough. Acta Anaesthesiol Scand. 2010;54:696–702. doi: 10.1111/j.1399-6576.2010.02234.x. [DOI] [PubMed] [Google Scholar]
- 6.Choi SC, Narayan RK, Anderson RL, Ward JD. Enhanced specificity of prognosis in severe head injury. J Neurosurg. 1988;69:381–5. doi: 10.3171/jns.1988.69.3.0381. [DOI] [PubMed] [Google Scholar]
- 7.Clusmann H, Schaller C, Schramm J. Fixed and dilated pupils after trauma, stroke, and previous intracranial surgery: Management and outcome. J Neurol Neurosurg Psychiatry. 2001;71:175–81. doi: 10.1136/jnnp.71.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du R, Meeker M, Bacchetti P, Larson MD, Holland MC, Manley GT. Evaluation of portable infrared pupillometer. Neurosurgery. 2005;57:198–203. doi: 10.1227/01.neu.0000163425.79170.cb. [DOI] [PubMed] [Google Scholar]
- 9.Goebert HW., Jr Head injury associated with a dilated pupil. Surg Clin North Am. 1970;50:427–32. doi: 10.1016/s0039-6109(16)39090-9. [DOI] [PubMed] [Google Scholar]
- 10.Hofbauer M, Kdolsky R, Figl M, Grünauer J, Aldrian S, Ostermann RC, et al. Predictive factors influencing the outcome after gunshot injuries to the head: A retrospective cohort study. J Trauma. 2010;69:770–5. doi: 10.1097/TA.0b013e3181c81d7d. [DOI] [PubMed] [Google Scholar]
- 11.Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. HAMLET investigators. Surgical decompression for space-occupying cerebral infarction (the hemicraniectomy after middle cerebral artery infarction with life-threatening oedema trial [HAMLET]): A multicentre, open randomized trial. Lancet Neurol. 2009;8:326–33. doi: 10.1016/S1474-4422(09)70047-X. [DOI] [PubMed] [Google Scholar]
- 12.Hults KN, Knowlton SL, Oliver JW, Wolfson T, Gamst A. A study of pupillary assessment in outpatient neurosurgical clinics. J Neurosci Nurs. 2006;38:447–52. doi: 10.1097/01376517-200612000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Levin HS, Gary HE, Eisenberg HM, Ruff RM, Barth JT, Kreutzer J, et al. Neurobehavioral outcome 1 year after severe head injury: Experience of the Traumatic Coma Data Bank. J Neurosurg. 1990;73:699–709. doi: 10.3171/jns.1990.73.5.0699. [DOI] [PubMed] [Google Scholar]
- 14.Litvan I, Saposnik G, Maurino J, Gonzales L, Saizar R, Sica RE. Pupillary diameter assessment: Need for a graded scale. Neurology. 2000;54:530–1. doi: 10.1212/wnl.54.2.530. [DOI] [PubMed] [Google Scholar]
- 15.Loewenfeld IE. 1st ed. Detroit: Wayne State University Press; 1993. The pupil: Anatomy, Physiology, and Clinical Applications. [Google Scholar]
- 16.Manley GT, Larson MD. Infrared pupillometry during uncal herniation. J Neurosurg Anesthesiol. 2002;14:223–8. doi: 10.1097/00008506-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Marmarou A, Anderson RL, Ward JD, Choi SC, Young HF. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J Neurosurg. 1991;75:S59–66. [Google Scholar]
- 18.Marmarou A, Lu J, Butcher I, McHugh GS, Murray GD, Steyerberg EW, et al. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrolment: An IMPACT analysis. J Neurotrauma. 2007;24:270–80. doi: 10.1089/neu.2006.0029. [DOI] [PubMed] [Google Scholar]
- 19.Marshall LF, Barba D, Toole BM, Bowers SA. The oval pupil: Clinical significance and relationship to intracranial hypertension. J Neurosurg. 1983;58:566–8. doi: 10.3171/jns.1983.58.4.0566. [DOI] [PubMed] [Google Scholar]
- 20.Marshall LF, Gautille T, Klauber M, Eisenberg HM, Jane JA, Luerssen TG, et al. The outcome of severe closed head injury. J Neurosurg. 1991;75:S28–36. [Google Scholar]
- 21.Marshall LF, Smith RW, Shapiro HM. The outcome with aggressive treatment in severe head injuries.Part I: The significance of intracranial pressure monitoring. J Neurosurg. 1979;50:20–5. doi: 10.3171/jns.1979.50.1.0020. [DOI] [PubMed] [Google Scholar]
- 22.Meeker M, Du R, Bacchetti P, Privitera CM, Larson MD, Holland MC, et al. Pupil examination: Validity and clinical utility of an automated pupillometer. J Neurosci Nurs. 2005;37:34–40. [PubMed] [Google Scholar]
- 23.Narayan RK, Greenberg RP, Miller JD, Enas GG, Choi SC, Kishore PR, et al. Improved confidence of outcome prediction in severe head injury: A comparative analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J Neurosurg. 1981;54:751–62. doi: 10.3171/jns.1981.54.6.0751. [DOI] [PubMed] [Google Scholar]
- 24.Narayan RK, Kishore PR, Becker DP, Ward JD, Enas GG, Greenberg RP, et al. Intracranial pressure: To monitor of not to monitor? J Neurosurg. 1982;56:650–9. doi: 10.3171/jns.1982.56.5.0650. [DOI] [PubMed] [Google Scholar]
- 25.Nijboer JM, van der Naalt J, ten Duis HJ. Patients beyond salvation? Various categories of trauma patients with a minimal Glasgow Coma Score. Injury. 2010;41:52–7. doi: 10.1016/j.injury.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 26.Olivecrona M, Rodling-Wahlström M, Naredi S, Koskinen LO. Effective ICP reduction by decompressive craniectomy in patients with severe traumatic brain injury treated by an ICP-targeted therapy. J Neurotrauma. 2007;24:927–35. doi: 10.1089/neu.2005.356E. [DOI] [PubMed] [Google Scholar]
- 27.Privitera CM, Stark LW. A binocular pupil model for simulation of relative afferent pupil defects and the swinging flashlight test. Biol Cybern. 2006;94:215–24. doi: 10.1007/s00422-005-0042-8. [DOI] [PubMed] [Google Scholar]
- 28.Ritter AM, Muizelaar JP, Barnes T, Choi S, Fatouros P, Ward J, et al. Brain stem blood flow, pupillary response, and outcome in patients with severe head injuries. Neurosurgery. 1999;44:941–8. doi: 10.1097/00006123-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Roukoz CB, Robertson CS, Gopinath SP. Outcome in patients with blunt head trauma and a Glasgow Coma Scale score of 3 at presentation. J Neurosurg. 2009;111:683–7. doi: 10.3171/2009.2.JNS08817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakas DE, Bullock MR, Teasdale GM. One-year outcome following craniotomy for traumatic hematoma in patients with fixed dilated pupils. J Neurosurg. 1995;82:961–5. doi: 10.3171/jns.1995.82.6.0961. [DOI] [PubMed] [Google Scholar]
- 31.Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J Neurosurg. 1982;56:498–503. doi: 10.3171/jns.1982.56.4.0498. [DOI] [PubMed] [Google Scholar]
- 32.Shallenberg M, Bangre V, Steuhl K, Kremmer S, Selbach M. Comparison of the Colvard, Procyon, and Neuroptics pupillometers for measuring pupil diameter under low ambient illumination. J Refract Surg. 2010;26:134–43. doi: 10.3928/1081597X-20100121-09. [DOI] [PubMed] [Google Scholar]
- 33.Taylor WR, Chen JW, Meltzer H, Gennarelli TA, Kelbch C, Knowlton S. Quantitative pupillometry, a new technology: Normative data and preliminary observations in patients with acute head injury. J Neurosurg. 2003;98:205–13. doi: 10.3171/jns.2003.98.1.0205. [DOI] [PubMed] [Google Scholar]
- 34.Tien HC, Cunha JR, Wu SN, Chughtai T, Tremblay LN, Brenneman FD, et al. Do trauma patients with a Glasgow Coma Scale score of 3 and bilateral fixed and dilated pupils have any chance of survival? J Trauma. 2006;60:274–8. doi: 10.1097/01.ta.0000197177.13379.f4. [DOI] [PubMed] [Google Scholar]
- 35.Usui S, Stark LW. Sensory and motor mechanisms interact to control amplitude of pupil noise. Vision Res. 1978;18:505–7. doi: 10.1016/0042-6989(78)90065-2. [DOI] [PubMed] [Google Scholar]
- 36.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomized controlled trials. Lancet Neurol. 2007;6:215–22. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 37.Venes J. Intracranial pressure monitoring in perspective. Childs Brain. 1980;7:236–51. doi: 10.1159/000119951. [DOI] [PubMed] [Google Scholar]
- 38.Williams RF, Magnotti LJ, Croce MA, Hargraves BB, Fischer PE, Schroeppel TJ, et al. Impact of decompressive craniectomy on functional outcome after severe traumatic brain injury. J Trauma. 2009;66:1570–4. doi: 10.1097/TA.0b013e3181a594c4. [DOI] [PubMed] [Google Scholar]
- 39.Wilson SF, Amling JK, Floyd SD, McNair ND. Determining interrater reliability of nurses’ assessments of pupillary size and reaction. J Neurosci Nurs. 1988;20:189–92. doi: 10.1097/01376517-198806000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Worthley LI. The pupillary light reflex in the critically ill patient. Crit Care Resusc. 2000;2:34–7. [PubMed] [Google Scholar]
- 41.Young GB. Coma. Ann N Y Acad Sci. 2009;1157:32–47. doi: 10.1111/j.1749-6632.2009.04471.x. [DOI] [PubMed] [Google Scholar]


