Abstract
Understanding taste processing in the nervous system is a fundamental challenge of modern neuroscience. Recent research on the neural bases of taste coding in invertebrates and vertebrates allows discussion of whether labelled-line or across-fibre pattern encoding applies to taste perception. While the former posits that each gustatory receptor responds to one stimulus or a very limited range of stimuli and sends a direct ‘line’ to the central nervous system to communicate taste information, the latter postulates that each gustatory receptor responds to a wider range of stimuli so that the entire population of taste-responsive neurons participates in the taste code. Tastes are represented in the brain of the fruitfly and of the rat by spatial patterns of neural activity containing both distinct and overlapping regions, which are in accord with both labelled-line and across-fibre pattern processing of taste, respectively. In both animal models, taste representations seem to relate to the hedonic value of the tastant (e.g. palatable versus non-palatable). Thus, although the labelled-line hypothesis can account for peripheral taste processing, central processing remains either unknown or differs from a pure labelled-line coding. The essential task for a neuroscience of taste is, therefore, to determine the connectivity of taste-processing circuits in central nervous systems. Such connectivity may determine coding strategies that differ significantly from both the labelled-line and the across-fibre pattern models.
Keywords: taste, gustation, labelled-line, across-fibre pattern, invertebrates, vertebrates
1. Introduction: basic models of taste encoding
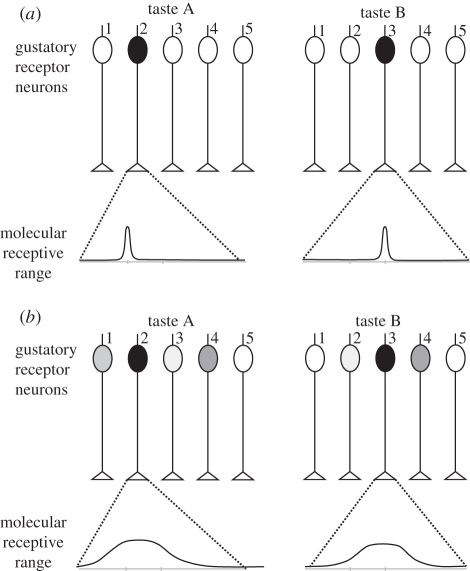
A concept that has guided much taste research is the notion that taste is organized in basic categories that correspond to neuron types narrowly tuned to a single stimulus quality [1,2]. This idea is the basis of the labelled-line theory, which posits that each gustatory receptor neuron is highly specific, responds to one stimulus or a very limited range of stimuli and sends a direct ‘line’ to the central nervous system to communicate information about this (or those) particular taste(s) (figure 1). An alternative view is proposed by the across-fibre pattern theory [3,4] in which individual gustatory receptor neurons are not exclusively labelled for a particular sensation but cooperate with other gustatory receptor neurons in the ensemble to provide a ‘fingerprint’ or neural pattern for the taste. In this case, each gustatory receptor neuron is less specific and responds to a wider range of stimuli; the entire population of taste-responsive neurons participates in the taste code (figure 1).
Figure 1.
Schematic of two theories of taste coding. A simplified gustatory system (without lateral connections) is presented, with five different gustatory receptor neurons. (a) Labelled-line: each molecular receptor has a very limited molecular receptive range, i.e. it is activated by a single (or very few) taste(s). Two different tastes, A and B, are each detected by only one molecular receptor, which activates only one gustatory receptor neuron (in black). Differentiation between A and B does not need further processing, but only five different tastes can be thus coded. (b) Across-fibre pattern: each molecular receptor has a much broader molecular receptive range, i.e. it can be activated by a range of different tastes. The five different molecular receptors have different—but broad—receptive ranges. In our example, taste A will activate several gustatory receptor neurons, although with different intensities depending on the receptor neuron. Receptor neuron 2 will be highly activated by taste A, but only slightly by taste B. Receptor neuron 3 shows the opposite response profile. Among the other gustatory receptor neurons, some will be equally activated by the two tastes (receptor 4), others will show a contrasted response (i.e. responding to A but not to B; gustatory receptor neuron 1), while others will not be activated at all by either taste (gustatory receptor neuron 5). This system allows the fine coding of many tastes, but differentiation among tastes needs additional downstream processing as the representation of each taste is contained in the combination of activations of the different neuronal units.
Labelled-line processing has the advantage of providing very precise knowledge about a limited number of tastes because each separate channel is dedicated to one taste. On the other hand, it cannot code, given the natural constraints of neural systems (e.g. number of neurons), all possible tastes in the environment. Labelled-line processing is therefore a good system for detecting and recognizing a given stimulus with a crucial biological value for the animal, but not for general taste coding. Conversely, the combinatorial across-fibre processing can code a much higher number of tastes with the same number of gustatory receptor neurons. Individual receptor neurons have broad, overlapping response patterns (i.e. they are broadly tuned) so that an individual fibre is non-specific, but collectively, the pattern of activity across multiple receptor neurons is unique for a given stimulus. These two opposing views are well represented by Scott [5] and Yarmolinsky et al. [6], who defend labelled-line coding, and by Smith & St John [7], who defend across-fibre pattern coding. It seems to us that considering important novel findings on neural processing of taste both in vertebrates and invertebrates can enlighten this debate.
In these considerations, a critical question is how perceptual taste sensations arise in the central nervous system [8], or what are the neural mechanisms allowing a given label to be assigned to a taste. In colour vision, for instance, explaining colour sensations on the pure basis of photoreceptor excitations is obviously senseless, as we know that the basis for colour sensations resides in the existence of colour opponent neurons [9–11], which impose a subtractive interaction upon photoreceptor inputs and which are present both in vertebrates and invertebrates capable of colour vision [11,12]. Thus, it is not the receptor signal which is relevant, but the kind of interaction that is imposed upon such receptor input in the neural networks that process tastes upstream of the receptor level. The critical question is, therefore, which kind of processing (i.e. which kind of receptor interaction) is imposed to information coming from taste receptors at the central level. Answering this question may allow deciding whether or not the labelled line or the across-fibre pattern hypothesis makes sense in the case of taste perception. In this framework, we will focus on peripheral (gustatory receptor neuron-level) and central (central nervous system-level) taste encoding to analyse the strategies of taste encoding across various insect and mammalian species.
2. Peripheral taste encoding
(a). The case of insects
The fruitfly Drosophila melanogaster is one of the organisms for which much information has been gained in the last years concerning the neural basis of taste [13,14]. For this insect, the notion of basic tastes prevails, based on the characterization of molecular gustatory receptors. Sixty-eight gustatory receptors (DmGrs, where DM stands for Drosophila melanogaster and Grs for the molecular taste receptors) encoded by 60 genes through alternative splicing have been identified in the fruitfly [15–17]. These encode putative heptahelical 7-transmembrane proteins but it is not clear whether the resulting gustatory receptors signal through G-protein-dependent second-messenger cascades or operate as ligand-gated ion channels. Recently, DmX, a gustatory receptor of the fruitfly tuned to detect a natural toxic molecule, l-canavanine, has been explicitly identified as a G-protein-coupled receptor [18]. Interestingly, this DmX receptor has partially diverged in its ligand-binding pocket from the metabotropic glutamate receptor family and is not related to the Gr family. The expression of the DmX receptor is required in bitter-sensitive gustatory receptor neurons, where it triggers the premature retraction of the proboscis, thus leading to the end of food searching and food aversion.
Another interesting class of receptors has been recently discovered in the fruitfly, the ionotropic receptors (IRs) [19], which are expressed in appendages where gustatory receptor neurons, but also olfactory receptor neurons, are located. These receptors constitute a family of ionotropic glutamate receptors (iGluRs) which do not belong to the well-described kainate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) or N-methyl-d-aspartate (NMDA) receptor classes of iGluRs, and which have divergent ligand-binding domains that lack their characteristic glutamate-interacting residues. IRs are expressed in a combinatorial fashion in sensory neurons that respond to many distinct odours but do not express either insect odorant receptors or gustatory receptors. It has been proposed that IRs constitute a novel family of chemosensory receptors [19], which would be involved in fast-odour signalling [20]. However, their role in gustation cannot be excluded, in particular, because iGluRs are involved in peripheral chemosensing of amino acids in bacteria [21].
Some of the fruitfly's gustatory receptors (Grs) have been linked to specific gustatory stimuli. For instance, Gr5a has been associated with sweet taste as it responds specifically to trehalose and is expressed in most sugar-responsive gustatory receptor neurons [22–25]. Similarly, Gr64a is involved in the detection of other sugars including sucrose, glucose and maltose [26,27]. Gr66a, on the other hand, has been associated with bitter taste as it responds to caffeine and its mutation eliminates caffeine-avoidance behaviour [25,28]. Similar results (inability to respond to caffeine and to theophyline) were obtained upon mutations in Gr93a, which is co-expressed with Gr66a [29]. Flies also possess a taste for carbonated water. A population of neurons was identified which detects CO2 in water and mediates taste-acceptance behaviour [30].
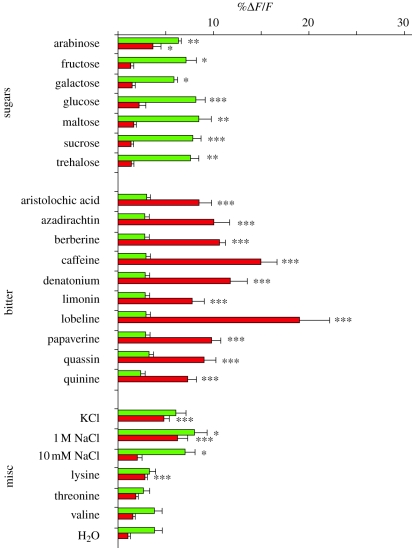
Using neurogenetic methods available in Drosophila, it has been possible to determine that gustatory receptor neurons expressing Gr5a respond to a broad spectrum of sweet substances, while gustatory receptor neurons expressing Gr66a respond to a broad spectrum of bitter substances [25]. A genetically encoded calcium sensor, G-CaMP (see figure 2 legend), was expressed in gustatory receptor neurons of the fruitfly, whose activity was then measured upon stimulation of the proboscis by application of a wide-pore pipette filled with taste solutions. Thus, taste-induced activation of gustatory receptor neurons, which results in calcium increase, could be visualized in terms of fluorescence changes. Besides trehalose, receptor neurons expressing Gr5a also respond to arabinose, fructose, galactose, glucose, maltose and sucrose (figure 2, green bars) while receptor neurons expressing Gr66a responds to caffeine but also to aristolochic acid, berberine, azadirachtin, limonin, lobeline, papaverine, quinine, quassin and denatonium benzoate (figure 2, red bars). Furthermore, other gustatory receptor neurons expressing different Grs (Gr32a and Gr47a) exhibit practically the same profile of responses to a variety of bitter substances as Gr66a receptor neurons. Given the structural differences between these compounds and the fact that receptor neurons with different Grs exhibit similar response profiles to a broad spectrum of bitter substances, the idea that taste receptor neurons in the fly are specifically tuned to single molecules is doubtful. This may be owing to the fact that Gr5a cells and Gr66a receptor neurons coexpress other molecular receptors whose tuning may differ from Gr5a and Gr66a molecular receptors. For instance, the Gr5a molecular receptor, reported as a trehalose receptor [22,24], is coexpressed with another molecular receptor, Gr64f, which is broadly required for the detection of most sugars. Gr64f may also be coexpressed with Gr64a, which appears to be tuned to detect other sugars, such as sucrose, glucose and maltose [27]. Thus, combinations of Gr5a/Gr64f and Gr64a/Gr64f may enhance the spectrum of responsiveness to sugars of a single gustatory receptor neuron [31].
Figure 2.
Gr5a cells respond to a broad spectrum of sugars and Gr66a cells respond to a broad spectrum of bitter substances in the fruitfly Drosophila melanogaster. G-CaMP, a circularly permutated green-fluorescent protein (GFP) linked to calmodulin (CaM) and a CaM-binding peptide, was expressed in taste neurons. Upon neural activation, CaM binds the peptide in the presence of calcium and promotes GFP fluorescence, with fluorescence increasing as a function of calcium concentration. Fluorescence changes (%ΔF/F), which indicate changes in neural activity, are shown for Gr5a cells (green) and Gr66a cells (red) to 23 substances. Gr5a responses were compared with the Gr5a water response, and Gr66a with the Gr66a water response (Student's t-test, ***p < 0.005, **p < 0.01, *p < 0.05). Ten brains were monitored for each stimulus/genotype. Error bars are s.e.m. Adapted from Marella et al. [25].
In speaking about labelled lines, what counts is not the specificity of a molecular receptor but the specificity of the message conveyed by a gustatory receptor neuron to the higher order gustatory centres in the central nervous system. In the periphery of the fruitfly, therefore, tastant detection seems to be organized according to labelled lines segregated, not in terms of single molecule specialization, but in terms of the hedonic value of the tastants (e.g. aversive versus appetitive). Gustatory receptor neurons tuned to respond to bitter substances mediate aversive behaviours, while gustatory receptor neurons tuned to respond to sweet substances mediate appetitive behaviours [25]. If the mammalian molecular receptor for capsaicin, TrpV1, is expressed in Gr66a receptor neurons, flies exhibit aversion towards capsaicin, while expressing it in Gr5a receptor neurons results in preference for capsaicin. Similar tendencies are found if an olfactory molecular receptor is expressed in these cells: the odorant becomes attractive if its molecular receptor is expressed in Gr5a receptor neurons, while it is rejected if its molecular receptor is expressed in Gr66a receptor neurons [32].
This organization may not be shared by all insects as different lifestyles may lead to dramatic modifications of the gustatory repertoire. In the case of the honeybee, for instance, experiments performed with harnessed bees in the laboratory could not find clear evidence for bitter taste perception until now [33]. Electrophysiological as well as behavioural analyses performed on several gustatory appendages have shown that bees in the laboratory are quite insensitive to bitter substances [34] and even consume important quantities of them despite their high concentration, toxicity and resulting mortality [35]. However, when tested in free-flight conditions, bees exhibit avoidance of highly concentrated bitter substances thus suggesting that gustatory thresholds may dramatically vary with the experimental situation and the possibility of expressing avoidance in an overt way [36].
Interestingly, the honeybee presents only 10 gustatory receptor genes, a finding that has been interpreted as an indication of a limited gustatory world [37]. None of these receptors share homologies with the bitter-tuned gustatory receptor gene Gr66a of the fruitfly. Although the ligands of the honeybee gustatory receptor neurons have not yet been identified, the dramatic difference existing between bees and flies in gustatory receptor genes underlines the necessity of comparative studies on insect gustation. Clearly, focusing on a single species, even if it allows using state-of-the art techniques for molecular receptor characterization at different levels, will not allow understanding per se the logic of taste perception.
What happens if one follows the projections of gustatory receptor neurons to central taste-processing organs in the fruitfly? Marella et al. [25] traced the projections of gustatory receptor neurons to the suboesophageal ganglion (SOG), the first relay in the central nervous system for the processing of taste information in insects. They found that different gustatory receptor neurons show segregated projections in the SOG, with neurons expressing Gr5a projecting lateral and anterior to projections of neurons expressing Gr66a. Marella et al. [25] concluded that there is a spatial activity map of different taste modalities in the fly brain that corresponds to the anatomical projections of Gr5a and Gr66a receptor neurons. Imaging taste responses in the SOG by expression of an exogenous ligand-gated ion channel showed that activation of Gr5a receptor neurons drives acceptance, while that of Gr66a receptor neurons drive rejection behaviour [25]. Although the spatial segregation of projections of receptor neurons seems to support the labelled-line hypothesis, this is not necessarily the case: such a spatial segregation refers to the receptor neuron level but not to second-order neurons.
(b). The case of mammals
Although insects and mammals diverged from a common ancestor in the Cambrian, 550 Ma, common principles can be identified between these phyla in the organization of their gustatory system [6]. Studies on taste detection in rats have yielded the notion that tastant quality is mediated by labelled lines defined by distinct and strictly segregated populations of taste receptor cells. Such cells are endowed with either heteromeric G-coupled receptors assembled by combinatorial arrangement of T1R1, T1R2 and T1R3 subunits or T2R receptors [6]. T1R3 combines with T1R2 (T1R2 + 3) to form a sweet taste receptor that responds to all classes of sweet tastants. Thus, cells expressing T1R2 + 3 are the sweet-sensing receptor cells [38]. On the other hand, T1R1 and T1R3 molecular receptors combine to form a broadly tuned amino acid taste receptor at the basis of umami sensations. Thus, cells expressing T1R1 + 3 are umami-sensing cells. Bitter taste is mediated by T2R molecular receptors [38]. T2R genes are selectively expressed in subsets of gustatory receptor cells distinct from those containing sweet and umami receptors. A large number of T2Rs have been shown to function as bitter taste receptors in heterologous expression assays [38] and several have distinctive polymorphisms that are associated with significant variations in sensitivity to selective bitter tastants in mice [39], chimpanzees [40] and humans [41].
Salt is detected by various mechanisms, one of which is mediated by the sodium channel ENaC [42], while the membrane-tethered carbonic anhydrase CA IV is required for carbonated taste [43]. Sour tastes are mediated by a member of the transient receptor potential ion-channel family, PKD2L1 [44]. This molecular receptor is selectively expressed in a population of gustatory receptor cells distinct from those mediating sweet, umami and bitter tastes. Genetic ablation of PKD2L1-expressing cells produced animals that lost sour taste [44]. These results indicate that the PKD2L1 ion channel is the candidate mechanism involved in the sour taste detection.
Variations in the peripheral coding of these basic tastes—sweet, umami, bitter, salty and sour—are present in some mammal species. Cats, for instance, carry a naturally occurring deletion in their T1R2 gene, which explains why Felidae do not respond to sweets [45]. In fact, the specificity of the sweet receptors differs among carnivore species that vary substantially in dietary habits. In the case of cats and dogs, for instance, difference in dietary habits is reflected in the different functional states of the T1R2 molecular receptor for sweet taste. Dogs are omnivores and have a functional T1R2 receptor gene, whereas cats are obligate carnivores and do not have such a gene [45].
As in Drosophila, gustatory receptor neurons tuned to respond to bitter substances mediate aversive behaviours, while gustatory receptor neurons tuned to respond to sweet substances mediate appetitive behaviours. For instance, spiradoline is a synthetic opiate that is tasteless to mice; however, expressing a spiradoline molecular receptor in gustatory receptor neurons responding to sweet substances results in attraction to spiradoline [46] while expressing the same molecular receptor in gustatory receptor neurons responding to bitter substances results in aversion towards spiradoline [47].
3. Central taste encoding: hedonic value of tastants as basic encoding criterion?
In general, what remains unclear, except in a few cases (see below), is the kind of neural interaction that is imposed upon taste receptor input at the central level. Whether or not taste neurons are embedded in interactive ensembles (i.e. whether the information conveyed by taste neurons is processed by networks of interconnected neurons in which a single neuron rarely encodes taste characteristics) remains a discussed question in most animals apart from a few exceptions discussed below.
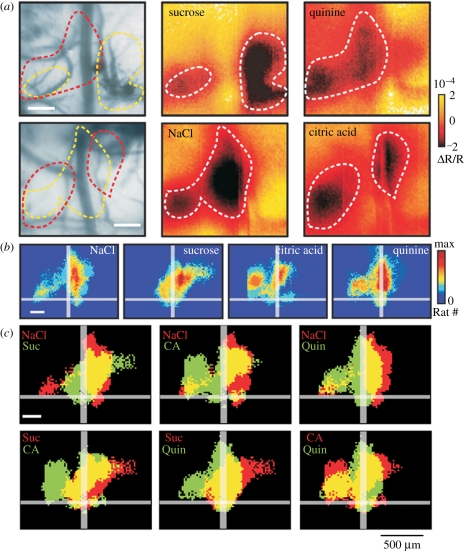
Do neuronal responses to taste follow the same organizational principle as gustatory receptor neurons in the central nervous system? In mammals, many central gustatory neurons exhibit a strong response to a specific tastant but are also able to respond to other tastants. Both narrowly and broadly tuned gustatory neurons can be found at every stage of the mammalian central gustatory pathways, which suggests that labelled-line and across-fibre pattern coexist as coding mechanisms [48]. Recent studies performed on central processing of taste in the rat have provided a clearer picture in which an organized form of across-fibre pattern seems to be present [49]. In mammals, the process of encoding chemical information into taste perception extends from the receptor level to the primary gustatory cortex and to other multimodal areas [17,50]. Imaging the gustatory cortex upon gustatory stimulation in rats showed that the four different tastes tested (salty, sour, sweet and bitter) are represented by specific spatial patterns containing both distinct and overlapping regions, which can account for both labelled-line and across-fibre pattern processing of taste, respectively (figure 3). Quantifying the overlap between different taste representations allowed the emergence of two groups of stimuli, related to what can be defined as the appetitive (or hedonic) value of the stimulus itself. Higher overlap values were found between NaCl and sucrose, associated with good nutrients, or between quinine and citric acid, associated with noxious substances. This may suggest a possible additional level of spatial organization representing a taste's hedonic value: a pattern for attractive and a pattern for aversive stimuli [49]. In associated behavioural tests, it was shown that it was possible to associate the positive or negative hedonic value of a given taste with the cortical patterns elicited by each taste in the imaging experiments [49]. These imaging results suggest that the hedonic value of the tastes (palatable versus non-palatable) corresponds to a neural classification principle, far beyond a pure labelled-line strategy. Thus, these imaging studies [49] indicate that an across-fibre pattern, evinced through overlapping of activation areas between tastes (see yellow areas in figure 3c), coexists with specific maps for each taste category in the gustatory cortex. Thus, while taste-specific maps would allow differentiation of tastes within a hedonic category, their grouping in a common region would label them with a hedonic value. Again, the kind of neural interaction leading to the emergence of such hedonic maps remains unclear.
Figure 3.
Different tastants induce different activation patterns in the rat gustatory cortex. In vivo intrinsic optical imaging of the rat gustatory cortex. Following gustatory stimulation of the rat's oral cavity, intrinsic signals from the blood vessel pattern of the gustatory cortex are recorded by illuminating it with a 546 nm filter. These intrinsic signals are associated with an initial increase in the concentration of deoxyhaemoglobin. (a) Different examples of activation maps induced by sucrose and quinine stimulation (upper row) and NaCl and citric acid (lower row). While sucrose (dashed yellow line) and quinine (dashed red line) induce patterns with low overlapping (upper left panel), NaCl and citric acid exhibited more overlapping (lower left panel). Twenty-eight presentations of each stimulus are averaged. (b) Population maps for the four basic taste modalities, NaCl (n = 18), sucrose (n = 15), citric acid (n = 8) and quinine (n = 8). Imaging was done on a total of 27 animals, testing at least two tastants chosen randomly among the four. Colour scale ‘max’ represents 60% of total number of animals. (c) Comparison of activated areas between tastants (Suc, sucrose; CA, citric acid; Quin, quinine). The yellow areas represent overlapping regions. Single stimulus surfaces are derived from (b) considering pixels responding with a consistency index of 20%. Adapted from Accolla et al. [49], by generous courtesy of A. Carleton.
Even more unclear are the neural interactions underlying the experience-dependent reorganization of such taste maps in the rat gustatory cortex. Associating a sweet taste with a visceral malaise leads to a plastic rearrangement of its cortical representation, becoming more similar to a bitter and unpleasant taste representation [51]. Thus, an internal state of malaise induces a plastic reshaping in the gustatory cortex, which is translated into a behavioural shift of the stimulus hedonic value [51]. This cortical reshaping considerably challenges the labelled-line hypothesis as it is difficult to reconcile with a rigid linear transmission of taste information from the periphery to the central nervous system. It rather supports the notion that taste information is subjected to considerable processing that reshapes the original message in its way to higher order centres.
Are taste-encoding principles similar in insects and mammals at the central level? In the larva of Drosophila, neurons expressing the gene hugin, which arborize in the SOG, are closely related with gustatory receptor neurons expressing Gr66a [52] and may act as second-order neurons for these receptor neurons [53]. While silencing Gr66a results in flies accepting bitter substances (see above), eliminating hugin lowers the threshold for initiating feeding. In other words, blocking hugin neurons would not influence the choice of food but rather the decision to begin feeding on a certain food. Which kind of interaction exists between projection of neurons expressing Gr66a and hugin neurons in the SOG remains to be determined. Thus, characterizing the computation processes performed by second-order neurons on receptor input is a priority that needs to be addressed to determine whether beyond peripheral taste detection, an across-fibre pattern model or a spike timing model (in which the precise pattern of action potentials that communicate taste quality) accounts for central taste coding in insects.
In that sense, electrophysiological studies performed in the desert locus Schistocerca migratoria [54,55] provide fundamental information as they report how tastes detected by gustatory receptor neurons on the hind legs are encoded by a population of interneurons of the methathoracic ganglion (MG). Previous studies on fleshflies Sarcophaga bullata [56] and the locust Locusta migratoria [57] have reported single-neuron recordings performed either at the level of the SOG (fleshflies) or the MG (locust) but they did not perform a systematic testing of a broad spectrum of tastants so that the gustatory response profiles of these neurons was unclear. On the contrary, Rogers & Newland [55] focused on spiking interneurons located in the midline of the MG and analysed their responses upon stimulation of gustatory receptor neurons of the locust hind leg with various tastants. These gustatory receptor neurons send their afferents to the MG and contact the spiking interneurons of the midline of the MG [54]. These interneurons responded differently to various tastants such as NaCl, water, sucrose and nicotine hydrogen tartrate (NHT), thus showing that there is convergence of a large number of taste qualities onto the same interneurons [55]. Furthermore, the response durations of these interneurons are a function of chemical identity and concentration. Thus, at first sight, spiking interneurons of the MG are broadly tuned to different chemical stimuli in a manner consistent with across-fibre pattern coding. However, contrary to assumptions of the across-fibre pattern theory, spiking interneurons of the locust MG showed all the same response profile instead of exhibiting broad but different, overlapping response profiles (figure 1). Indeed, the seven interneurons recorded responded highly to the deterrent substances NHT and NaCl at a high concentration (250 mM), while showing a low response to attractive sucrose and water. Rogers & Newland [55] affirm that this response profile is inconsistent with a system that is concerned with establishing chemical identity because, for instance, water could not be distinguished from sucrose or NHT from NaCl. They propose instead that the duration of response to different chemicals provides a direct measure of aversiveness because the relative size of the neuronal response of spiking local interneurons and motor neurons correlates strongly with behavioural withdrawal responses. One could, in fact, push the argument further and state that what spiking interneurons in the MG of the locus encode is the hedonic value of the tastants perceived. Firstly, although all neurons recorded in the midline of the MG showed the same response profile, the number of neurons recorded upon stimulation with NaCl, water, sucrose and NHT was low so that further recordings could show different profiles. Secondly, neurons in other regions of the MG may also show coincident response profiles but different from those of the MG midline, responding more, for instance, to appetitive rather than to aversive substances. In any case, the idea of having a central hedonic coding is present in the work of Rogers & Newland [55] who stated that local circuits in the MG ‘mediate motor responses that differentiate between acceptable and unacceptable, and a neural representation of this appears fully apparent at an early synaptic stage of chemosensory integration’.
Intracellular recordings from central gustatory neurons in the SOG of the moth Heliothis virescens combined with fluorescent staining have recently revealed a large diversity of neurons responding with varying tuning breadth to sucrose, quinine, water and mechanosensory stimuli applied to the antennae, proboscis and right tarsus [58]. The integration of information across stimuli and appendages contradicts a simple labelled-line mechanism in the central nervous system for coding identity and location of taste stimuli. Instead, responses recorded suggest a population coding mechanism in which information is represented by distinct activity patterns in partly overlapping populations of SOG neurons [58]. With just one appetitive (sucrose 1 M) and one aversive stimulus (quinine hydrochloride 0.1 M) tested, it is difficult to determine whether or not a spatial form of hedonic coding can be found in the SOG of the moth H. virescens [58]. Further studies should analyse whether the moth's SOG presents a hedonic spatial, organization.
4. A comparative analysis of taste perception
The fact that hedonics seems to be a guiding principle in the organization of the gustatory system raises the question of whether neural architectures allowing such encoding originate from a common ancestor or whether they represent cases of parallel evolution. So far, answering these questions is difficult given the lack of enough data allowing across-species comparisons. In that sense, even if model organisms such as the fruitfly and the rat paved the way of gustatory research, we should keep in mind that they offer a rather narrow view on the multiplicity of neural solutions that may underlie the neural processing of tastes. In that sense, focusing, either for commodity reasons or for pressures imposed by current scientific policies, on a couple of model species may be misleading as it might lead to loss of the evolutionary perspective necessary for interpreting the logics of a sensory system.
In insects, for instance, the honeybee seems to differ dramatically from the fruitfly in its gustatory repertoire [33,37]. As mentioned above, the bee possesses fewer gustatory receptors (10 versus 68 in the fruitfly; [37]). Is it because bees have a lifestyle in which sucrose-associated taste of nectars is over-dimensioned so that other taste modalities have been lost? This hypothesis seems too simplistic: bees collect pollen and vegetal resins and these tasks could provide an adaptive framework for perceiving bitter substances and amino acids.
Thus, the study of gustatory coding and perception should be enlarged to other species in order to answer questions on particularities and commonalities of functional principles and underlying neural architectures. What is nevertheless interesting is that, despite our limited dataset, insects and mammals seem to use a common principle for coding tastes at the central level: the segregation of tastes in terms of their hedonic value. This may be associated with the fact that organisms with different evolutionary histories are nevertheless confronted with the same problem: distinguishing palatable from non-palatable food items. This distinction is crucial for individual survival as non-palatable items are usually associated with toxicity and mortality. In that sense, taste encoding would follow a basic principle of the nervous system that is present across phyla, which is the capacity to encode in an unambiguous way the positive (appetitive) and negative (aversive) experiences. Dedicated neural systems exist to this end both in insects and mammals. In insects, for instance, appetitive reinforcements are signalled through the activity of octopaminergic neurons [59–62] while aversive reinforcements are signalled through dopaminergic neurons [61–66]. In mammals, dopaminergic activity underlies appetitive reward systems and dopamine neurons are said to encode the prediction error of rewarding outcomes [67–71] but no specific punishment neurotransmitter system is known. A critical question is if and how these reinforcement systems, positive and negative, interact—when present—with gustatory pathways to instruct gustatory processing circuits about the hedonic value of tastes. Such interaction, if any, would support the idea that the hedonics could grow out (at least in part) of the message conveyed by reinforcement neurons upon taste perception. In other words, a concomitant activation of a negative reinforcement neuron upon taste perception would result in a negative labelling of that taste. In the olfactory system of honeybees, this labelling has been clearly demonstrated. Any odour paired with the artificial activation (through intracellular current injection via an electrode) of an octopaminergic, reinforcement neuron called VUMmx1 (ventral unpaired median neuron of the maxillar neuromere 1) acquires a positive value and is therefore learned as an appetitive stimulus ([72], see [73] for review). In the fruitfly, similar results have been found for aversive learning: optophysiological [65] or temperature-dependent [66] activation of a specific subset of dopaminergic neurons upon odour stimulation leads to the formation of odour-aversive memories and therefore to the avoidance of the odour that was learned as an aversive stimulus.
Interactions between the gustatory pathways and reinforcement pathways have not been explored so far. Do these two pathways converge already at the peripheral level to facilitate fast hedonic classification of taste, which would be important for survival? Are they confused so that neurons tuned to respond to aversive tastes release a negative-reinforcement neurotransmitter to further signal the negative experience? Do these two systems share similar topologies at the central level? If yes, are there several classes of reinforcement (e.g. dopaminergic) neurons, among which particular classes would be dedicated to the gustatory system? Answering these and other questions, i.e. understanding how and where in the brain interactions between gustatory and reinforcement processing do occur, is therefore a research programme in which across-species comparisons should play a fundamental role.
5. Conclusion
The labelled-line design of the gustatory periphery remains uncontested, both for insects and mammals, as convincingly illustrated by the recent genetic and physiological data. Whether the labelled-line principle is carried on or not to higher taste centres remains unknown. On the one hand, it can be argued that a labelled-line organization of higher pathways cannot be ruled out a priori, given that basic taste qualities such as sweet, sour, bitter or salty are not only recognized by different receptor neurons, but represent clearly distinct perceptual entities, at least in humans. On the other hand, the facts that taste is encoded in broad hedonic categories and that even the hedonic value of a taste may change as a result of experience argue against the validity of the labelled-line hypothesis when it comes to the central processing of taste.
The study of interactive population coding in central areas related to taste processing has still a long way to go [74]. Characterizing such interactions will provide the necessary framework for a complete understanding of the functioning of taste networks. Gustatory responses in the taste cortex of rats are dynamic. Indeed, chronic recordings in active, tasting rats uncovered slower dynamics but also oscillations [75] in the gustatory cortex, a region rife with inhibitory cross-talk based on GABAergic transmission [76]. These oscillations, however, are apparent only when rats are not engaged in taste processing. These and other results show that the emergence of taste sensations at the central level is complex and rather distant from a labelled-line coding strategy. In the case of insects, no study has determined so far how taste is encoded by higher order neurons at the level of the SOG. Single-cell recording combined with multi-electrode and imaging recording methods may allow progress in our understanding of central taste processing.
We conclude that the labelled-line hypothesis can eventually account for peripheral processing of taste as sensory neurons can be tuned to basic tastes, but we argue that it is critical to unravel the neural processing of tastes occurring at the central level focusing on interactive neural population coding.
Acknowledgements
We thank four anonymous reviewers and Frederic Marion-Poll for helpful comments on previous versions of the manuscript, and Rinaldo Bertossa for careful editing of our work. We thank Alan Carleton for kindly providing figure 3 and the French National Research Agency (ANR; Project INSAVEL), the University Paul Sabatier (Project APIGENE) and the CNRS for support.
Footnotes
One contribution of 10 to a Theme Issue ‘Evolutionary developmental biology (evo-devo) and behaviour’.
References
- 1.Pfaffmann C. 1959. The afferent code for sensory quality. Am. Psychol. 14, 226–232 10.1037/h0049324 (doi:10.1037/h0049324) [DOI] [Google Scholar]
- 2.Scott T. R., Plata-Salaman C. R. 1991. Coding of taste quality. In Smell and taste in health and disease (eds Getchell T. V., Bartoshuk L. M., Doty R. L., Snow J. B.), pp. 345–369 New York, NY: Raven Press [Google Scholar]
- 3.Erickson R. P. 1968. Stimulus coding in topographic and non-topographic sensory modalities: on the significance of the activity of individual sensory neurons. Psychol. Rev. 75, 447–465 10.1037/h0026752 (doi:10.1037/h0026752) [DOI] [PubMed] [Google Scholar]
- 4.Erickson R. P. 2007. A study of the science of taste: on the origins and influence of core ideas. Behav. Brain Sci. 31, 59–74 10.1017/S0140525X08003348 (doi:10.1017/S0140525X08003348) [DOI] [PubMed] [Google Scholar]
- 5.Scott K. 2004. The sweet and the bitter of mammalian taste. Curr. Opin. Neurobiol. 14, 423–427 10.1016/j.conb.2004.06.003 (doi:10.1016/j.conb.2004.06.003) [DOI] [PubMed] [Google Scholar]
- 6.Yarmolinsky D. A., Zuker C. S., Ryba N. J. P. 2009. Common sense about taste: from mammals to insects. Cell 139, 234–244 10.1016/j.cell.2009.10.001 (doi:10.1016/j.cell.2009.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith D. V., St John S. J. 1999. Neural coding of gustatory information. Curr. Opin. Neurobiol. 9, 427–435 10.1016/S0959-4388(99)80064-6 (doi:10.1016/S0959-4388(99)80064-6) [DOI] [PubMed] [Google Scholar]
- 8.Schiffman S. S. 2000. Taste quality and neural coding: implications from psychophysics and neurophysiology. Physiol. Behav. 69, 147–159 10.1016/S0031-9384(00)00198-0 (doi:10.1016/S0031-9384(00)00198-0) [DOI] [PubMed] [Google Scholar]
- 9.Derrington A. M., Krauskopf J., Lennie P. 1984. Chromatic mechanisms in lateral geniculate nucleus of macaque. J. Physiol. 357, 241–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid R. C., Shapley R. M. 1992. Spatial structure of cone inputs to receptive fields in primate lateral geniculate nucleus. Nature 356, 716–718 10.1038/356716a0 (doi:10.1038/356716a0) [DOI] [PubMed] [Google Scholar]
- 11.Schluppeck D., Engel S. A. 2002. Colour opponent neurons in V1: a review and model reconciling results from imaging and single-unit recording. J. Vis. 2, 480–492 10.1167/2.6.5 (doi:10.1167/2.6.5) [DOI] [PubMed] [Google Scholar]
- 12.Kien J., Menzel R. 1977. Chromatic properties of interneurons in the optic lobes of the bee. II. Narrow band and colour opponent neurons. J. Comp. Physiol. 113, 35–53 10.1007/BF00610452 (doi:10.1007/BF00610452) [DOI] [Google Scholar]
- 13.Amrein H., Thorne N. 2005. Gustatory perception and behavior in Drosophila melanogaster. Curr. Biol. 15, R673–R684 10.1016/j.cub.2005.08.021 (doi:10.1016/j.cub.2005.08.021) [DOI] [PubMed] [Google Scholar]
- 14.Hallem E. A., Dahanukar A., Carlson J. R. 2005. Insect odor and taste receptors. Annu. Rev. Entomol. 51, 113–135 10.1146/annurev.ento.51.051705.113646 (doi:10.1146/annurev.ento.51.051705.113646) [DOI] [PubMed] [Google Scholar]
- 15.Dunipace L., Meister S., McNealy C., Amrein H. 2001. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 11, 822–835 10.1016/S0960-9822(01)00258-5 (doi:10.1016/S0960-9822(01)00258-5) [DOI] [PubMed] [Google Scholar]
- 16.Scott K., Brady R., Jr, Cravchik A., Morozov P., Rzhetsky A., Zuker C. S., Axel R. 2001. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 104, 661–673 10.1016/S0092-8674(01)00263-X (doi:10.1016/S0092-8674(01)00263-X) [DOI] [PubMed] [Google Scholar]
- 17.Scott K. 2005. Taste recognition: food for thought. Neuron 48, 455–464 10.1016/j.neuron.2005.10.015 (doi:10.1016/j.neuron.2005.10.015) [DOI] [PubMed] [Google Scholar]
- 18.Mitri C., Soustelle L., Framery B., Bockaert J., Parmentier M.-L., Grau Y. 2009. Plant insecticide L-canavanine repels Drosophila via the insect orphan GPCR DmX. PLoS Biol 7, e1000147. 10.1371/journal.pbio.1000147 (doi:10.1371/journal.pbio.1000147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benton R., Vannice K. S., Gomez-Diaz C., Vosshall L. B. 2009. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162 10.1016/j.cell.2008.12.001 (doi:10.1016/j.cell.2008.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silbering A. F., Benton R. 2010. Ionotropic and metabotropic mechanisms in chemoreception: ‘chance or design?’ EMBO Rep. 11, 173–179 10.1038/embor.2010.8 (doi:10.1038/embor.2010.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen G. Q., Cui C., Mayer M. L., Gouaux E. 1999. Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature 402, 817–821 10.1038/990080 (doi:10.1038/990080) [DOI] [PubMed] [Google Scholar]
- 22.Dahanukar A., Foster K., van der Goes van Naters W. M., Carlson J. R. 2001. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 4, 1182–1186 10.1038/nn765 (doi:10.1038/nn765) [DOI] [PubMed] [Google Scholar]
- 23.Ueno K., Ohta M., Morita H., Mikuni Y., Nakajima S., Yamamoto K., Isono K. 2001. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr. Biol. 11, 1451–1455 10.1016/S0960-9822(01)00450-X (doi:10.1016/S0960-9822(01)00450-X) [DOI] [PubMed] [Google Scholar]
- 24.Chyb S., Dahanukar A., Wickens A., Carlson J. R. 2003. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc. Natl Acad. Sci. USA 100(Suppl. 2), 14 526–14 530 10.1073/pnas.2135339100 (doi:10.1073/pnas.2135339100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marella S., Fischler W., Kong P., Asgarian S., Rueckert E., Scott K. 2006. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron 49, 285–295 10.1016/j.neuron.2005.11.037 (doi:10.1016/j.neuron.2005.11.037) [DOI] [PubMed] [Google Scholar]
- 26.Dahanukar A., Lei Y. T., Kwon J. Y., Carlson J. R. 2007. Two Gr genes underlie sugar reception in Drosophila. Neuron 56, 503–516 10.1016/j.neuron.2007.10.024 (doi:10.1016/j.neuron.2007.10.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao Y., Moon S. J., Wang X., Ren Q., Montell C. 2008. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr. Biol. 18, 1797–1801 10.1016/j.cub.2008.10.009 (doi:10.1016/j.cub.2008.10.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon S. J., Kottgen M., Jiao Y., Xu H., Montell C. 2006. A taste receptor required for the caffeine response in vivo. Curr. Biol. 16, 1812–1817 10.1016/j.cub.2006.07.024 (doi:10.1016/j.cub.2006.07.024) [DOI] [PubMed] [Google Scholar]
- 29.Lee Y., Moon S. J., Montell C. 2009. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl Acad. Sci. USA 106, 4495–4500 10.1073/pnas.0811744106 (doi:10.1073/pnas.0811744106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischler W., Kong P., Marella S., Scott K. 2007. The detection of carbonation by the Drosophila gustatory system. Nature 448, 1054–1057 10.1038/nature06101 (doi:10.1038/nature06101) [DOI] [PubMed] [Google Scholar]
- 31.Jiao Y., Moon S. J., Montell C. 2007. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc. Natl Acad. Sci. USA 104, 14 110–14 115 10.1073/pnas.0702421104 (doi:10.1073/pnas.0702421104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiroi M., Tanimura T., Marion-Poll F. 2008. Hedonic taste in Drosophila revealed by olfactory receptors expressed in taste neurons. PloS ONE 3, e2610. 10.1371/journal.pone.0002610 (doi:10.1371/journal.pone.0002610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Brito Sanchez M. G., Ortigao-Farias J. R., Gauthier M., Liu F., Giurfa M. 2007. Taste perception in honeybees: just a taste of honey? Arthropod Plant Interact. 1, 69–76 10.1007/s11829-007-9012-5 (doi:10.1007/s11829-007-9012-5) [DOI] [Google Scholar]
- 34.de Brito Sanchez M. G., Giurfa M., de Paula Mota T. R., Gauthier M. 2005. Electrophysiological and behavioral characterization of gustatory responses to antennal ‘bitter’ taste in honeybees. Eur. J. Neurosci. 22, 3161–3170 10.1111/j.1460-9568.2005.04516.x (doi:10.1111/j.1460-9568.2005.04516.x) [DOI] [PubMed] [Google Scholar]
- 35.Ayestaran A., Giurfa M., de Brito Sanchez M. G. 2010. Toxic but drank: gustatory aversive compounds induce post-ingestional malaise in harnessed honeybees. PLoS ONE. 5, e15000. 10.1371/journal.pone.0015000 (doi:10.1371/journal.pone.0015000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avarguès-Weber A., de Brito Sanchez M. G., Giurfa M., Dyer A. G. 2010. Aversive reinforcement improves visual discrimination learning in free-flying honeybees. PLoS ONE 5, e15370. 10.1371/journal.pone.0015370 (doi:10.1371/journal.pone.0015370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson H. M., Wanner K. W. 2006. The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Gen. Res. 16, 1395–1403 10.1101/gr.5057506 (doi:10.1101/gr.5057506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandrashekar J., Hoon M. A., Ryba N. J., Zuker C. S. 2006. The receptors and cells for mammalian taste. Nature 444, 288–294 10.1038/nature05401 (doi:10.1038/nature05401) [DOI] [PubMed] [Google Scholar]
- 39.Chandrashekar J., Mueller K. L., Hoon M. A., Adler E., Feng L., Guo W., Zuker C. S., Ryba N. J. 2000. T2Rs function as bitter taste receptors. Cell 100, 703–711 10.1016/S0092-8674(00)80706-0 (doi:10.1016/S0092-8674(00)80706-0) [DOI] [PubMed] [Google Scholar]
- 40.Wooding S., et al. 2006. Independent evolution of bitter-taste sensitivity in humans and chimpanzees. Nature 440, 930–934 10.1038/nature04655 (doi:10.1038/nature04655) [DOI] [PubMed] [Google Scholar]
- 41.Kim U. K., Jorgenson E., Coon H., Leppert M., Risch N., Drayna D. 2003. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 299, 1221–1225 10.1126/science.1080190 (doi:10.1126/science.1080190) [DOI] [PubMed] [Google Scholar]
- 42.Heck G. L., Mierson S., DeSimone J. A. 1984. Salt-taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science 223, 403–405 10.1126/science.6691151 (doi:10.1126/science.6691151) [DOI] [PubMed] [Google Scholar]
- 43.Chandrashekar J., Yarmolinsky D., von Buchholtz L., Oka Y., Sly W., Ryba N. J. P., Zuker C. S. 2009. The taste of carbonation. Science 326, 455–458 10.1126/science.1174601 (doi:10.1126/science.1174601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang A. L., Chen X., Hoon M. A., Chandrashekar J., Guo W., Trankner D., Ryba N. J., Zuker C. S. 2006. The cells and logic for mammalian sour taste detection. Nature 442, 934–938 10.1038/nature05084 (doi:10.1038/nature05084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X., et al. 2005. Pseudogenization of a sweet-receptor gene accounts for cats' indifference toward sugar. PLoS Genet. 1, e3. 10.1371/journal.pgen.0010003 (doi:10.1371/journal.pgen.0010003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao G. Q., Zhang Y., Hoon M. A., Chandrashekar J., Erlenbach I., Ryba N. J., Zuker C. S. 2003. The receptors for mammalian sweet and umami taste. Cell 115, 255–256 10.1016/S0092-8674(03)00844-4 (doi:10.1016/S0092-8674(03)00844-4) [DOI] [PubMed] [Google Scholar]
- 47.Mueller K. L., Hoon M. A., Erlenbach I., Chandrashekar J., Zuker C. S. 2005. The receptors and coding logic for bitter taste. Nature 434, 225–229 10.1038/nature03352 (doi:10.1038/nature03352) [DOI] [PubMed] [Google Scholar]
- 48.Spector A. C., Travers S. P. 2005. The representation of taste quality in the mammalian nervous system. Behav. Cognit. Neurosci. Rev. 4, 143–191 10.1177/1534582305280031 (doi:10.1177/1534582305280031) [DOI] [PubMed] [Google Scholar]
- 49.Accolla R., Bathellier B., Petersen C. C., Carleton A. 2007. Differential spatial representation of taste modalities in the rat gustatory cortex. J. Neurosci. 27, 1396–1404 10.1523/JNEUROSCI.5188-06.2007 (doi:10.1523/JNEUROSCI.5188-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolls E. T. 2005. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol. Behav. 85, 45–56 10.1016/j.physbeh.2005.04.012 (doi:10.1016/j.physbeh.2005.04.012) [DOI] [PubMed] [Google Scholar]
- 51.Accolla R., Carleton A. 2008. Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proc. Natl Acad. Sci. USA 105, 4010–4015 10.1073/pnas.0708927105 (doi:10.1073/pnas.0708927105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bader R., Colomb J., Pankratz B., Schroeck A., Stocker R., Pankratz M. J. 2007. Genetic dissection of neural circuit anatomy underlying feeding behavior in Drosophila: distinct classes of hugin-expressing neurons. J. Comp. Neurol. 502, 848–856 10.1002/cne.21342 (doi:10.1002/cne.21342) [DOI] [PubMed] [Google Scholar]
- 53.Melcher C., Bader R., Pankratz M. J. 2007. Amino acids, taste circuits, and feeding behavior in Drosophila: towards understanding the psychology of feeding in flies and man. J. Endocrinol. 192, 467–472 10.1677/JOE-06-0066 (doi:10.1677/JOE-06-0066) [DOI] [PubMed] [Google Scholar]
- 54.Newland P. L. 1999. Processing of gustatory information by spiking local interneurones in the locust. J. Neurophysiol. 82, 3149–3159 [DOI] [PubMed] [Google Scholar]
- 55.Rogers S. M., Newland P. L. 2002. Gustatory processing in thoracic local circuits of locusts. J. Neurosci 2, 8324–8333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell B. K., Itagaki H. 1992. Physiology and morphology of gustatory interneurons in the subesophageal ganglion of the fleshfly Sarcophaga bullata. J. Comp. Physiol. A 171, 213–230 10.1007/BF00188929 (doi:10.1007/BF00188929) [DOI] [PubMed] [Google Scholar]
- 57.Rogers S. M., Simpson S. J. 1999. Chemodiscriminatory neurones in the sub-oesophageal ganglion of Locusta migratoria L. Entomol. Exper. Appl. 91, 19–28 10.1046/j.1570-7458.1999.00462.x (doi:10.1046/j.1570-7458.1999.00462.x) [DOI] [Google Scholar]
- 58.Kvello P., Jørgensen K., Mustaparta H. 2010. Central gustatory neurons integrate taste quality information from four appendages in the moth Heliothis virescens. J. Neurophysiol. 103, 2965–2981 10.1152/jn.00985.2009 (doi:10.1152/jn.00985.2009) [DOI] [PubMed] [Google Scholar]
- 59.Hammer M., Menzel R. 1998. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn. Mem. 5, 146–156 [PMC free article] [PubMed] [Google Scholar]
- 60.Farooqui T., Robinson K., Vaessin H., Smith B. H. 2003. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J. Neurosci. 23, 5370–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., Heisenberg M. 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10 495–10 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unoki S., Matsumoto Y., Mizunami M. 2005. Participation of octopaminergic reward system and dopaminergic punishment system in insect olfactory learning revealed by pharmacological study. Eur. J. Neurosci. 22, 1409–1416 10.1111/j.1460-9568.2005.04318.x (doi:10.1111/j.1460-9568.2005.04318.x) [DOI] [PubMed] [Google Scholar]
- 63.Vergoz V., Roussel E., Sandoz J. C., Giurfa M. 2007. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PloS ONE 2, e288. 10.1371/journal.pone.0000288 (doi:10.1371/journal.pone.0000288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riemensperger T., Völler T., Stock P., Buchner E., Fiala A. 2005. Punishment prediction by dopaminergic neurons in Drosophila. Curr. Biol. 15, 1953–1960 10.1016/j.cub.2005.09.042 (doi:10.1016/j.cub.2005.09.042) [DOI] [PubMed] [Google Scholar]
- 65.Claridge-Chang A., Roorda R. D., Vrontou E., Sjulson L., Li H., Hirsh J., Miesenböck G. 2009. Writing memories with light-addressable reinforcement circuitry. Cell 139, 405–415 10.1016/j.cell.2009.08.034 (doi:10.1016/j.cell.2009.08.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aso Y., Siwanowicz I., Bräcker L., Ito K., Kitamoto T., Tanimoto H. 2010. Specific dopaminergic neurons for the formation of labile aversive memory. Curr. Biol. 20, 1445–1451 10.1016/j.cub.2010.06.048 (doi:10.1016/j.cub.2010.06.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wise R. A., Rompre P. P. 1989. Brain dopamine and reward. Annu. Rev. Psychol. 40, 191–225 10.1146/annurev.ps.40.020189.001203 (doi:10.1146/annurev.ps.40.020189.001203) [DOI] [PubMed] [Google Scholar]
- 68.Schultz W. 1998. Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27 [DOI] [PubMed] [Google Scholar]
- 69.Schultz W. 1997. Dopamine neurons and their role in reward mechanisms. Curr. Opin. Neurobiol. 7, 191–197 10.1016/S0959-4388(97)80007-4 (doi:10.1016/S0959-4388(97)80007-4) [DOI] [PubMed] [Google Scholar]
- 70.Schultz W., Dickinson A. 2000. Neuronal coding of prediction errors. Annu. Rev. Neurosci. 23, 473–500 10.1146/annurev.neuro.23.1.473 (doi:10.1146/annurev.neuro.23.1.473) [DOI] [PubMed] [Google Scholar]
- 71.Wise R. A. 2004. Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483–494 10.1038/nrn1406 (doi:10.1038/nrn1406) [DOI] [PubMed] [Google Scholar]
- 72.Hammer M. 1993. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 366, 59–63 10.1038/366059a0 (doi:10.1038/366059a0) [DOI] [PubMed] [Google Scholar]
- 73.Giurfa M. 2007. Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J. Comp. Physiol. A 193, 801–824 10.1007/s00359-007-0235-9 (doi:10.1007/s00359-007-0235-9) [DOI] [PubMed] [Google Scholar]
- 74.Fontanini A., Grossman S. A., Revill B. R., Katz D. B. 2008. Cortical ensembles in taste coding. In Handbook of the senses: olfaction and taste (eds Firestein S., Beauchamp G. K.), pp. 329–338 San Diego, CA: Academic Press [Google Scholar]
- 75.Fontanini A., Katz D. B. 2005. 7 to 12 Hz activity in rat gustatory cortex reflects disengagement from a fluid self-administration task. J. Neurophysiol. 93, 2832–2840 10.1152/jn.01035.2004 (doi:10.1152/jn.01035.2004) [DOI] [PubMed] [Google Scholar]
- 76.Ogawa H., Hasegawa K., Otawa S., Ikeda I. 1998. GABAergic inhibition and modifications of taste responses in the cortical taste area in rats. Neurosci. Res. 32, 85–95 10.1016/S0168-0102(98)00071-6 (doi:10.1016/S0168-0102(98)00071-6) [DOI] [PubMed] [Google Scholar]