Abstract
Bees provide an excellent model with which to study the neuronal and molecular modifications associated with the evolution of sociality because relatively closely related species differ profoundly in social behaviour, from solitary to highly social. The recent development of powerful genomic tools and resources has set the stage for studying the social behaviour of bees in molecular terms. We review ‘ground plan’ and ‘genetic toolkit’ models which hypothesize that discrete pathways or sets of genes that regulate fundamental behavioural and physiological processes in solitary species have been co-opted to regulate complex social behaviours in social species. We further develop these models and propose that these conserved pathways and genes may be incorporated into ‘social pathways’, which consist of relatively independent modules involved in social signal detection, integration and processing within the nervous and endocrine systems, and subsequent behavioural outputs. Modifications within modules or in their connections result in the evolution of novel behavioural patterns. We describe how the evolution of pheromonal regulation of social pathways may lead to the expression of behaviour under new social contexts, and review plasticity in circadian rhythms as an example for a social pathway with a modular structure.
Keywords: social behaviour, evolution, gene network, bee, communication, circadian rhythm
1. Introduction
Insect societies represent one of the major transitions in evolution [1] and have attracted the attention of biologists for centuries. Darwin [2] noted that insect societies provide some of the most difficult challenges to the theory of natural selection operating on individuals. In his words: ‘Can we consider the sting of the wasp or of the bee as perfect, which, when used against many attacking animals, cannot be withdrawn, owing to the backward serratures, and so inevitably causes the death of the insect by tearing out its viscera?’ ([2], ch. VI, ‘Difficulties to the theory’, p. 202). He addressed this and other difficulties posed by the biology of social insects by suggesting that natural selection acts also at the group (colony) level: ‘We can see how useful their production may have been to a social community of insects, on the same principle that the division of labour is useful to civilised man’ (p. 242). Since Darwin's time, there has been much debate about the ultimate causes underlying the evolution of social behaviour, resulting in the development of insightful theories such as kin and multi-level selection (e.g. [1,3–5]). Because phylogenetic studies indicate that the solitary lifestyle is the ancestral state, it is assumed that social animals evolved from solitary ancestors [6]. In order to fully understand how natural selection may have been operating to produce this transition, it is necessary to elucidate the proximate mechanisms by which the behaviours of a solitary animal were modified to produce complex social behaviours, such as those underlying division of labour, colony defence and nest construction.
With the development of new genomic tools and resources, it is now possible to employ a comparative approach for understanding the molecular basis of social behaviour within species, and how these behaviours evolved from solitary ancestors [7,8]. A similar approach has been particularly powerful in the field of developmental biology, in which it was shown that reorganization of a core set of regulatory genes has led to a stunning diversity of structural forms [9]. The ‘sociobiological evo-devo approach’ attempts to characterize suites of genes and molecular pathways and test their association with social behaviour. Thus far, most comparative genomics studies have focused only on species with well-developed genomic resources (such as the honeybee and Drosophila), but with the increasing accessibility of genomic information, more relevant comparisons between taxonomically related solitary and social species will become feasible.
While it is possible that novel social behaviours evolved de novo as the result of the appearance of new genes, it seems more likely that discrete pathways that regulate specific behaviours in solitary species have been co-opted to regulate complex social behaviours in social species; this theory underlies the development of various ground plan and genetic toolkit models for social evolution, which have received support from several empirical studies (box 1, see below for further discussion). We further propose that in order to fully understand the evolution of sociality, it is necessary to characterize complete pathways that are involved in social behaviour (hereafter abbreviated as social pathways). We hypothesize that social pathways are modular, and consist of the detection of social signals, the processing of the information conveyed by these signals in the peripheral and the central nervous system and subsequent behavioural outputs (see below). Modifications within modules or in the way modules interact with each other can lead to changes in behaviour and in the context under which it is expressed, and thus can give rise to the evolution of new forms of social behaviour. These models will not only help structure future studies of the evolution of social behaviour in bees, but may also serve as a framework for other behavioural systems in which animals respond to environmental stimuli or signals from other individuals (e.g. courtship and mating, aggression, predator–prey interactions).
Box 1. Terms used in the manuscript (in alphabetical order).
Alloethism Task specialization by different members of a colony of social insects that is related to variation in body size.
Caste A group of individuals displaying a distinct morphological, physiological and behavioural phenotype that specializes on a particular task or set of tasks in a polymorphic social species.
Circadian rhythm A repeating cycle with a period of approximately 24 h. Circadian rhythms are generated by endogenous processes but can be entrained/ adjusted by external cues.
Developmental modules Modules that are involved in the developmental processes generating the form and function of the structures and networks necessary for the execution of a phenotype, including a social behaviour.
Division of labour Specialization of individuals within a social group on specific tasks (see also polyethism and alloethism).
Eusociality A form of social organization in which groups of conspecifics display cooperative care of young, overlapping generations and reproductive division of labour.
Functional modules Modules associated with defined and different functions. For example, in a social pathway, the detection of social signals, the processing of the information conveyed by the social signals or the regulation of exocrine functions.
Genetic toolkit Set of genes with specialized and conserved molecular function (e.g. transcription factors) that is involved in the regulation of similar phenotypes across a wide variety of species and can acquire new or additional functions during evolution to regulate novel physiological or behavioural phenotypes.
Gonadotropic Acting on or stimulating the reproductive organs; in social insects, this refers primarily to ovary activation.
Ground plan Molecular pathways regulating fundamental physiological processes that acquire new or additional functions during evolution to regulate novel physiological or behavioural phenotypes.
Microarray Genomic technology in which thousands of probes, corresponding to DNA with specific nucleotide sequences, are spatially distributed in an organized array. DNA, RNA or cDNA from a sample can be hybridized to the microarray to measure the relative abundance of these nucleotide sequences in the sample. Expression microarrays thus allow analysis of expression levels of thousands, potentially all, of genes in the genome.
Module A set of components (e.g. genes, proteins, cells) that are strongly connected and have relatively weak connectivity to other components or modules. Modules could be subject to divergent evolutionary forces and therefore evolve relatively independently (see also physiological modules, functional modules and developmental modules).
Pheromone Chemical signal that stimulates a stereotyped response in another individual of the same species. Responses are typically instinctive and not learned.
Physiological modules Modules that are involved in the regulation of cellular, neuronal and endocrine processes necessary for the execution of a phenotype, including a social behaviour.
Polyethism Task specialization by different members of a colony of social insects; tasks can be segregated among individuals by age (age polyethism) or morphological differences (caste polyethism).
Polyphenism The ability of a single genome to produce two or more alternative phenotypes (e.g. morphologies) within a single population in response to environmental cues such as photoperiod, temperature, nutrition or social signals.
Primer pheromone Chemical signal that elicits a long-term change in physiology and/or behaviour.
Quantitative trait locus (QTL) A DNA region (which may contain several genes) that is statistically associated with a specific quantitative phenotypic trait.
Releaser pheromone Chemical signal that triggers a rapid behavioural response (of the order of seconds or minutes).
Social pathway The molecular, physiological and cellular components involved in detecting a social signal, processing the information and orchestrating an appropriate behavioural output. Each step of the process may be regulated by a relatively independent ‘module’. At the molecular level, the social pathway may encompass genetic toolkits or ground plans.
2. Insect societies
Social insects, and bees in particular, provide an excellent model system with which to study the evolution of sociality because there is a broad range of taxonomically related species that encompass all levels of sociality, from species with essentially solitary lives all the way to eusocial species with colonies of thousands of highly specialized individuals (figure 1). Furthermore, there are clear effects of genes and social environment on both the social behaviour of individuals and the social organization of the group. Therefore, insect societies provide an excellent model system with which to dissect the effects of nature and nurture on complex behaviour [10–15].
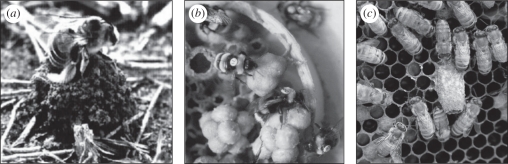
Figure 1.
Bees show diverse levels of social organization, from solitary to highly eusocial. (a) A solitary bee (Eucera palestina) at the entrance to her nest (photo: Abraham Hefetz). (b) Bumble-bee (B. terrestris) colony with a size-based division of labour. The photo shows several relatively small bees that are caring for the brood (photo: Guy Bloch). (c) A honeybee colony with an open queen cell (centre of photo). The rearing of new gynes is regulated by a pheromone blend secreted from the mandibular glands of the colony's queen (photo: Bernardo Niño).
One of the hallmarks of insect societies is a profound bias in reproduction. Some individuals in the colony (typically the queens) are extremely fecund, whereas others (the workers) are sterile or have relatively low reproductive potential. In primitively social species, adult females compete for reproductive dominance; subordinate females typically do not reproduce but rather perform brood care, nest maintenance or foraging activities. In more advanced social systems, females can develop into either queens or workers via alternative developmental processes (known as caste differentiation) that are initiated during pre-adult stages. In species with relatively small-sized colonies, regulation appears to occur mainly through physical aggression, while in larger colonies, regulation is commonly via pheromones. However, differential nutrition, genetic variation and environmental factors have been also implicated in caste determination, mostly in studies with ants [6,16].
A second organization principle is a division of labour among workers specializing in different activities (e.g. brood care, guarding the nest entrance and foraging for food). In bees, division of labour is most commonly related to age (age polyethism) or size (alloethism). Age-related division of labour has been best studied in honeybees (reviewed in [17,18]). In this system, young workers typically specialize in brood care (‘nursing’) and other in-hive activities, while older bees perform activities outside the nest such as guarding and foraging. Size-based division of labour is common in bumble-bees, and is characterized by profound morphological polymorphism with up to ninefold differences in body size [16]. Large individuals are more likely to perform foraging activities, and may start foraging as early as at their first day as adults, while small bees tend to perform in-nest activities. Recent studies demonstrated that large workers are better suited for foraging activities because they have more sensitive sensory detection systems and stronger circadian rhythms [19–21]. These functional differences between small nurses and large foragers are probably determined by pre-adult developmental processes, which are reminiscent of the caste polymorphism division of labour found in many species of ants [22].
Both reproductive and worker division of labour require the development of elaborated communication systems between the individuals in a colony. Information can be conveyed by distinct chemical, vibratory, visual or auditory signals, and can involve the simultaneous use of several modalities [6]. Social bees, particularly those with larger colony sizes such as honeybees, use a broad array of pheromones to organize the activities of the individuals in a colony. Pheromones are chemical signals that convey information between individuals within a species, and can trigger immediate short-term behavioural changes (releaser pheromones) or long-term changes in physiology followed by behavioural changes (primer pheromones). Pheromones are used to regulate both reproductive and worker division of labour in many systems, as well as other colony activities such as nest defence.
3. Ground plans and toolkits
Genes associated with social behaviour in bees have been identified by three main complementary approaches, which have been applied most broadly in honeybees. Candidate gene approaches (using orthologues of genes found in more genetically tractable systems, such as Drosophila melanogaster) have resulted in the characterization and manipulation of key genes, such as the foraging (for) and vitellogenin (Vg) genes that are relatively conserved across species. Genomic approaches, such as quantitative trait locus (QTL) mapping, microarrays, differential display and proteomics, have identified large sets of genes that are associated with social behaviour, but there is typically little information on their molecular function in bees. Finally, genetic and molecular analyses of honeybee populations differing in their social behaviour—either owing to natural variation or artificial selection—have identified genes or genetic loci associated with reproduction and foraging preference [23–25].
One of the important insights emerging from these molecular studies is that honeybee social behaviour may have evolved from modifications in conserved molecular pathways regulating the physiology and behaviour of solitary species. These conserved pathways are commonly referred to as ‘ground plans’ [26–28] or ‘genetic toolkits’ [8]. There are excellent reviews on the social behaviours that are associated with molecular modifications in conserved gene networks regulating reproduction, longevity, food gathering and diapause in solitary insects [8,14,24,29,30]. We therefore review these lines of research briefly below.
The molecular basis for ‘social foraging’ behaviour has been well-characterized, and has led to important insights into the evolution of this pathway. This system is also a good example of a ‘social pathway’ (§4), in that it appears that similar behaviours (foraging) are triggered by different cues in solitary versus social species. In solitary species that do not engage in brood care, foraging is required for personal consumption and triggered by hunger signals. In solitary species that engage in brood care, foraging is also used for offspring provisioning and presumably stimulated by cues from the brood. In honeybees, social foraging is regulated by the colony's nutrition needs (which includes signalling via pheromones produced by the brood [31]), and is carried out by forager bees who do not engage in brood care or reproduction. The ontogenic transition from nest activities to foraging is associated with modifications in key physiological processes including changes in haemolymph titres of juvenile hormone (JH), brain levels of the neuromodulator octopamine and metabolic traits such as lipid levels [17,32,33]. Similarly, there are genetic and physiological differences between foragers that specialize on nectar versus pollen, including differences in levels of JH and ovariole size [24,29]. One of the key questions in the evolution of division of labour in honeybees is whether the molecular factors that regulate social foraging are associated with food-gathering behaviour in solitary species.
Candidate gene studies have revealed that some of the genes that are differentially expressed between nurse and forager honeybees are functionally conserved in solitary insects. For example, the foraging (for) gene, which encodes protein kinase G and is upregulated in foragers, is associated with foraging behaviour in Drosophila, Caenorhabditis elegans, ants and mammals (reviewed in [34]). Additional genes involved in feeding-related pathways in solitary insects have been linked to social foraging, including malvolio [35], insulin-signalling genes [36] and carbohydrate metabolism genes [37]. Peptides that are involved in regulating food intake in solitary insects are also involved in social foraging in honeybees, but interestingly are not linked to ingestion [38]. In addition to these candidate gene studies, microarray comparisons of the brain expression patterns of nurses and foragers revealed significant differences in expression levels of more than 3000 genes, many of which are associated with cell signalling, neuronal development and metabolism [39]. Importantly, large subsets of these genes are regulated by factors known to regulate the transition from nest activities to foraging, such as pheromones or hormone levels (e.g. [40,41]). Thus, these genes may be involved in regulating the transition to foraging—rather than being differentially expressed as a result of foraging behaviour—but their function in food-seeking behaviour in solitary species is largely uncharacterized.
Pathways regulating reproductive physiology appear to be another target for social evolution [27,28,42]. Some of the first data leading to this idea were obtained in studies on the endocrine regulation of division of labour in honeybees. These studies showed that JH, which functions as a major gonadotrophic signal in solitary insects, regulates worker behavioural maturation but not oogenesis in the adult honeybee (reviewed in [32,43,44]). In solitary insects, JH typically upregulates the fat body biosynthesis and accumulation in developing oocytes of the conserved yolk protein vitellogenin (Vg) [45,46]. JH activates Vg biosynthesis in honeybee pupae [47,48], but in adult bees, JH suppresses Vg gene activity [49] and Vg suppresses JH titres [29]. RNA interference-mediated downregulation of Vg caused bees to forage earlier in life [50]. These findings suggest that a network involving JH and Vg was co-opted to regulate worker task (division of labour) in the adult honeybee. Since JH also appears to regulate adult oogenesis in other bee species, including bumble-bees, the modification in this network in this bee lineage may have occurred after the divergence of bumble-bees and honeybees at about 60–70 Ma and may not be a general mechanism for regulating division of labour in bees [32]. It is not yet known whether JH is more strongly associated with reproduction or division of labour in stingless bees, the other major group of highly eusocial bees [51]. However, in ants and wasps, there is also a similar variability in which JH is associated with reproduction in some species and with age polyethism in others [32]. Recent evidence suggests that JH regulatory pathways are involved in other forms of morphological polyphenism in solitary insects [52] and thus JH-regulated molecular pathways may represent a common target for the evolution of polyphenisms in insects.
In addition to their role in regulating age polyethism in honeybees, reproductive pathways may have also been co-opted to regulate foraging preference in honeybees. Artificial selection of lines of honeybees for high versus low levels of pollen hoarding revealed that high pollen-hoarding lines also had higher proportions of pollen foragers and an earlier transition to foraging, indicating that the processes regulating the transition to foraging and forager specialization are linked [53]. There is also a suite of correlated differences in sensory physiology and locomotion, which is also seen in typical unselected (‘wild-type’) colonies in which pollen foragers are similar to bees from the selected high pollen-hoarding line, and nectar foragers are similar to bees from colonies selected for low pollen hoarding (reviewed in [29,30]). Both workers from high pollen-hoarding colonies and pollen foragers from unselected colonies have ovaries with more ovarioles and higher titres of Vg [54]. This association of foraging behaviour/preference and reproductive physiology is consistent with the ‘reproductive ground plan’ hypothesis, which states that task specialization evolved through modification in molecular pathways regulating the reproductive cycle of solitary insects [26,42]. However, it will be important to study additional social species and to demonstrate that these traits are indeed correlated in solitary bee species.
QTL mapping of the high and low selected pollen-hoarding lines identified four major QTLs that have complex pleiotropic and epistatic interactions, and account for much of the observed behavioural and physiological variation. Mapping these QTLs to the honeybee genome revealed that they encompassed a disproportionately high number of orthologues to genes involved in reproduction and insulin signalling in Drosophila, as well as the for gene and the receptor for tyramine, a biogenic amine that may play a role in regulating sensory responsiveness (reviewed in [24,30]). Two candidate genes, PDK1 and HR46, have subsequently been associated to ovarian development [55]. In solitary insects, the insulin/insulin-like signalling (IIS) pathway acts upstream of JH and ecdysteroid-mediated regulation of sensory tuning and reproductive physiology [56], and thus, the IIS pathway may play an important role in this system. Additional studies are necessary to test the expression pattern and function of each of the genes in these QTLs in relation to division of labour and reproduction. The hypothesis that ovarian physiology regulates division of labour was recently challenged, since no differences in task specialization were found between strains of honeybees that vary in reproduction capacity [57]. These findings suggest that the links between ovariole number and foraging behaviour are not always detected or present. Moreover, in solitary bees that provision their brood, the networks regulating foraging and reproduction may need to operate simultaneously (and not separately at different phases of a reproductive cycle) because actively nesting females are foraging. Thus, further investigations on the generality of this model in highly social bees, as well as additional information on the physiology and molecular biology of reproduction in solitary bees, are needed to better understand the relationship between ovarian physiology and foraging behaviour.
An additional hallmark of the division of labour in insect societies is the care of siblings (not offspring) by workers, which led to the theory that sib-care behaviour evolved from maternal behaviour [27,42], and thus these two processes may be regulated by similar pathways [28,58]. Studies with the primitively social paper wasp Polistes metricus support this hypothesis. Female paper wasps show several natural behavioural states that differ in the combination of brood care and reproductive behaviours. Foundresses and queens are reproductively active whereas gynes and workers are not. The pattern of brain gene expression in workers was more similar to that in foundresses, which show maternal care, than to that in queens and gynes, which do not. Genes of the IIS pathway were among the differentially regulated genes, consistent with the hypothesis that this pathway played a critical role in the evolution of eusociality in the hymenoptera [59]. Similarly, in honeybees, microarray analysis of the brains of queens, sterile workers and workers with activated ovaries revealed that genes associated with caste/reproductive differences significantly overlapped with genes associated with nursing behaviour [60].
In many social insects, queens are not only highly fecund but also long lived. Workers, on the other hand, are typically sterile and relatively short lived. This relationship differs from most animals in which there is a trade-off between reproduction and longevity [61,62]. The IIS pathway is involved in the integration of ageing and fertility in vertebrates and invertebrates [63]. Recent studies support the notion that Vg and insulin signalling are also involved in the regulation of longevity in the honeybee [64,65], and queens and workers differ significantly in the expression of genes associated with metabolism, oxidative pathways and immunity [60]. Corona et al. [64] hypothesized that insulin signalling plays a prominent role in the regulation of longevity in honeybees as in other insects, but the traditional relationship between nutrition and IIS is reversed in that high nutritional status inhibits the secretion of insulin-like peptide. Thus, well-fed queens have lower IIS signalling compared with workers.
4. Social molecular pathways
The findings reviewed above revealed a surprising degree of conservation between the molecular pathways regulating behaviour in solitary insects and social behaviour in social insects. But how are these pathways re-organized to produce social versus solitary behaviour? Social behaviour is elicited in response to stimuli from other individuals, and thus these conserved ground plan pathways must be in some way connected to the detection of social signals. We suggest that these ancient molecular pathways are encompassed by social pathways, in which there is a directional flow of information. The pathway starts with the detection of a social signal, subsequent processing and integration of the information conveyed by the signal, and ultimately resulting in a behavioural output (or the inhibition of a behaviour normally performed in a solitary species under certain contexts). We suggest that social pathways commonly include several relatively independent modules, which display strong connectivity within each module and relatively weak connectivity among modules, thereby enabling functional flexibility. These modules could be under different selection pressures and thus serve as the building blocks for evolution [66–68].
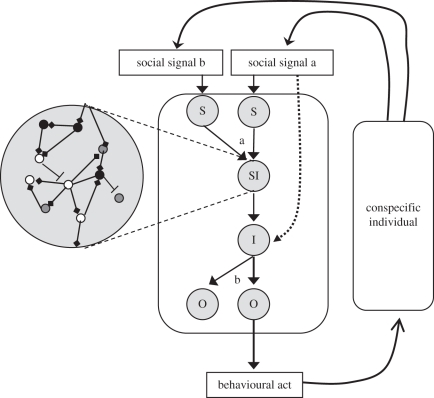
How are the modules of a social pathway organized? At the higher, functional level, a social pathway would consist of a sensory module (e.g. pheromone receptor neurons) that detects the relevant social signal(s), sensory integration modules that process the information conveyed by the social signal (e.g. glomeruli in the antennal lobes), higher integration modules such as neuronal circuits and endocrine systems (e.g. a neuronal network in the mushroom bodies) and motor-controlling modules that regulate the execution of a socially relevant behaviour (e.g. brood care). These high-level functional modules contain nested lower level modules, including physiological and developmental modules [66–68]. Physiological modules (such as JH signalling pathways) regulate cellular, neuronal and endocrine processes, while developmental modules (such as pathways regulating worker body size or caste differentiation) regulate the form and function of the structures and networks necessary for the execution of social behaviour. Importantly, low-level modules are those in which genes (or proteins) interact to form gene networks and signalling pathways. These genes and gene networks may be conserved, and thus can be compared between species or individuals that differ in their social behaviour. It should be noted that regulatory gene networks and signalling pathways can be involved in several functional modules in the same way that hox genes and Delta–Notch pathways are repeatedly used for the development of diverse morphological structures [66]. The term social pathway does not imply a single unidirectional route. Rather it may include bifurcations or points in which several pathways converge. For example, several sensory modules can converge into a single sensory-processing module, and a high-integration module can affect several downstream motor-controlling modules (figure 2). A similar complexity in connectivity can also occur among protein and gene network modules.
Figure 2.
A schematic of a social pathway. From a functional perspective, a social pathway includes sensory modules (S), sensory integration modules (SI), higher integration modules (I) and modules regulating effecter mechanisms such as motor control or exocrine glands (O). Each of these functional modules consisted of nested molecular modules (insert, note that specific genes can be involved in more than a single module). The molecular modules can be developmental or physiological (see text and box 1 for details). Light grey circles outline functional modules, arrows depict the flow of information, small circles within the insert depict individual genes (or proteins) and lines connecting the small circles depict activation (diamond end) or inhibition (line end) of gene (or protein) activity. The dotted line depicts a social signal directly activating integration modules, bypassing sensory integration (see text for details). a, a point in which two pathways converge; b, a point of bifurcation.
What are the molecular mechanisms by which the connections between modules in a social pathway may be reorganized? New genes may have evolved (e.g. by gene duplication) that regulate or connect conserved modules; this seems likely in the case of sensory modules for olfactory signals, since there can be considerable diversity of olfactory receptor sequences even among related species [69]. Alternatively, expression patterns of existing genes may be altered by changes in protein structure/function or cis-regulatory regions, which allow these genes to be expressed in novel contexts allowing for the reorganization of connections between modules [70–72]. Epigenetic regulation, which occurs without any changes in DNA sequence, can also alter expression patterns of a gene (or suite of genes) involved in regulating a particular module or forming connections between modules. Epigenetic regulation may be caused by modifications to DNA or chromatin (the DNA–protein complex in chromosomes) structure, which alter the transcriptional potential of certain genes or chromosomal regions [73]. There is evidence that epigenetic regulation associated with DNA methylation is involved in caste determination, one of the key processes in the evolution of advanced insect societies [74,75]. DNA methylation has been shown to be involved in integrating social signals and result in long-term behavioural changes in rodents [76], but its function in regulating behaviour in social insects remains to be fully characterized [77].
The social pathway may be a useful conceptual framework for studying how an ‘ancient’ ground plan pathway was modified to give rise to diverse forms of social behaviours. Comparative analyses of social pathways can be used for identifying parts of the pathways that diverge between species differing in their social behaviour. An effective study of social pathways therefore requires the availability of molecular and genomics resources for related insects differing in their social behaviour. The evolution of a certain social behaviour can be associated with modifications in the interactions among modules or by changes within modules that change their function. One of the ways in which such a change can occur is by expressing a behaviour in a new (social) context [27,42]. From a social pathway perspective, this means that a sensory module that detects a social signal evolved to be linked to an ancient ground plan pathway. For example, social brood care could have evolved because a sensory module that detects brood signals has been connected to the ancient modular pathway that is associated with food seeking or processing in solitary insects, but the remainder of the pathways may remain mostly intact. Indeed, studies in honeybees and Drosophila have suggested that the for gene underlies food-related locomotion in both species, but it is regulated by factors associated with the division of labour in honeybees [78]. Similarly, the biogenic amine octopamine is implicated in feeding and food-related activity in several solitary insects [79,80] and in social foraging in honeybees [81].
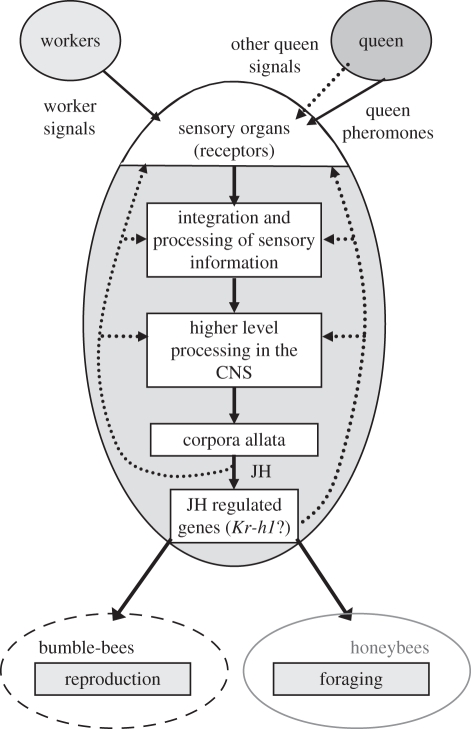
Social pathways can be also modified such that a similar social signal evolved to regulate different social behaviours. We recently used the social pathway framework in a comparative study on the social behaviour of bumble-bees (Bombus terrestris) and honeybees (Apis mellifera). In honeybees, queen pheromone inhibits worker reproduction and slows down behavioural maturation of the worker bees; this latter process is probably modulated by pheromone-mediated regulation of JH levels [82,83]. In the bumble-bees, the presence of the queen—which is communicated by behavioural and possibly chemical signals [84,85]—inhibits worker reproduction via decreased JH levels, but was suggested to have no effect on division of labour [86,87]. In both species, the presence of the queen is associated with a decrease in JH biosynthesis rates [88,89], and therefore requires a pathway linking the sensory system detecting the queen signal with the regulation of the corpora allata, the endocrine glands that synthesize and release JH (figure 3). JH has pleiotropic effects and the pathways downstream of JH are predicted to at least partially diverge in order to accommodate the differential influences on division of labour and reproduction in adult workers of the two species. We hypothesized that by comparing gene expression patterns across the two species, it should be possible to identify genes that are sensitive to the social environment versus genes that are directly linked to the downstream behavioural changes. We examined the expression patterns of a transcription factor, Krüppel-homologue 1 (Kr-h1), to test this model. Kr-h1 expression is downregulated in honeybee workers upon exposure to queen pheromone, and upregulated in foragers [40,90]. In the bumble-bee, Kr-h1 levels were similarly downregulated by the presence of the queen or the dominant worker. Kr-h1 levels were also associated with JH-mediated changes in reproductive state, but elevated levels were not associated with foraging behaviour [91]. Thus, it is possible that Kr-h1 is a part of a JH signalling pathway that in social bees is linked to social regulation, but was differentially co-opted during social evolution of bumble-bees and honeybees (figure 3). However, owing to the large pleiotropic effects of JH on insect physiology, further studies are necessary to determine whether the mechanisms by which JH and Kr-h1 interact are similar in the two species.
Figure 3.
A simplified scheme for a social pathway associated with juvenile hormone (JH)-mediated regulation of social behaviour. The social inhibition of JH biosynthesis by the corpora allata is similar in bumble-bees and honeybees, but the influence of JH on worker behaviour differs between the two species. Krüppel homologue 1 (Kr-h1) is a transcription factor that appears to be involved in the conserved part of the pathway. The arrows depict the flow of information, dotted arrows are hypothetical and the white boxes depict modules in which gene expression or activity can be studied.
5. Pheromone-regulated social pathways
The studies reviewed above suggest that the molecular pathways involved in key physiological processes in solitary insects have been modified in social insects, such that they are activated by social signals and therefore expressed in a novel social context. In order for a molecular pathway to be expressed in a social context, it needs to be linked to sensory systems associated with social communication. Communication can occur via multiple modalities, but pheromonal communication is particularly widespread and sophisticated in insects (reviewed in [92,93]). The evolution of social pathways regulated by pheromonal communication requires the development of multiple pheromone (exocrine) glands, a sensory system that can decode and process multiple pheromonal signals, and the linkage of this sensory system, via processing modules, to downstream behavioural modules that regulate reproduction, brood care, nest construction, defence and/or foraging. Pheromone detection can lead to neurophysiological changes that result in the production of a specific behaviour, or changes in sensory thresholds that result in altered behaviour under different contexts. Below we discuss the putative modules of pheromonally regulated social pathways, and highlight examples in honeybees in which the modules or the connections between the modules of these pathways appear to have been altered to produce a different behavioural response.
There can be considerable variation in the production of pheromonal signals, which can affect the meaning of the chemical signal. For example, the reproductive state of the honeybee queen is associated with variation in the pheromones she produces; queens that have a higher reproductive potential appear to signal this to the workers [94,95]. Modulation of pheromone biosynthetic pathways is also associated with caste differentiation in honeybees. Queens produce a specific pheromone blend in their mandibular glands (five components of which are termed ‘queen mandibular pheromone’, or QMP). Modifications in these biosynthetic pathways, presumably via modulation of gene expression, result in the production of a different blend in workers. Furthermore, queenless and reproductive workers can produce queen-like pheromone blends, demonstrating that these changes in biosynthetic pathways are plastic and regulated by the social environment [96–98].
A pheromone-regulated social pathway requires the detection of the chemical signal by receptors in the peripheral chemosensory systems. Binding of the pheromones to specific odorant receptors results in the activation of receptor neurons, which subsequently modulates downstream neural networks. These neurophysiological changes in the central nervous system are thought to result in downstream behavioural or physiological changes (reviewed in [69,99]). Sequencing of the honeybee genome has identified numerous olfactory receptors [100], most of which do not have any known function, though one (AmOR11) was recently demonstrated to detect 9-ODA, the major component of queen pheromone [101].
There is also some evidence that pheromones can interact directly with neuronal networks in the central brain (dotted line in figure 2). In Drosophila, male accessory gland proteins that are transferred to the female during copulation pass from the reproductive tract into the circulation system and affect female behaviour, perhaps by interacting with receptors in the central nervous system (reviewed in [102]). In honeybees, one of the components of QMP, homovanillyl alcohol (HVA), has a similar structure to the biogenic amine dopamine and appears to interact directly with and activate dopamine receptors in the worker bee brain [103–105]. Since worker bees do lick and consume queen pheromone as part of a behavioural response to the pheromone, it appears that this component may be directly altering activity of a neuronal network rather than acting via receptors and processing in the olfactory system. Thus, based on the social pathways model, HVA may have evolved to bypass the sensory detection system and act directly on neuroprocessing pathways (figure 2, see also [106]).
In order to understand how pheromone-regulated social pathways may be modulated, it is necessary to first characterize the molecular responses to the pheromonal signal. Pheromones may cause changes in endocrine or neuroendocrine signalling, which affect downstream physiological processes regulating behaviour. Several studies have demonstrated that exposure of honeybee workers to pheromones causes changes in brain gene expression that are associated with downstream changes in behaviour [40,107,108]. For example, exposure of young honeybee workers to QMP causes significant changes in global brain gene expression patterns. These changes are behaviourally relevant: upregulated expression of genes that are upregulated in the brains of nurse bees (relative to foragers), and downregulated expression of genes that are upregulated in the brains of forager bees. These expression changes are consistent with behavioural and physiological data demonstrating that QMP delays the transition from nursing to foraging activities [83].
There is evidence that the organization of pheromone-regulated social pathways in honeybees may be modified by social context and behavioural/physiological state. For example, unlike nurse bees, forager bees are not behaviourally or molecularly responsive to QMP, despite the fact that antennal responses to QMP are similar [90,109,110]. Worker bees in social groups will respond behaviourally and physiologically to alarm pheromone, but isolated bees do not, though antennal responses remain intact [111,112]. Thus, the sensory module of the social pathway is functional, but the modules downstream that are involved in the processing or behavioural output are disabled or disconnected.
Studies with brood pheromone (BP) suggest that the connections between the modules of this social pathway may be labile. BP is produced by the developing larvae, and has different effects on nurses and foragers. BP delays the transition from nursing to foraging in young nurse bees [113], but it stimulates pollen-foraging behaviour in forager bees [31,114]. Exposure of young bees to BP shifts brain gene expression patterns to be more ‘nurse-like’, while exposure of older bees produces a more ‘forager-like’ brain expression pattern [107]. Thus, BP has age- or task-dependent effects on both behaviour and gene expression. These results suggest that the sensory module is intact, while the connections to the downstream processing or behavioural modules have been modified.
The above studies highlight the fact that pheromone-regulated social pathways can be modified by both social context and behavioural state. However, social pathways may also be the target of selective pressures, which result in population- or even species-level differences in the molecular response to social cues. For example, exposure of young honeybees to alarm pheromone (which stimulates defensive behaviour) has large-scale effects on brain gene expression that largely overlap with expression differences between Africanized and European bees, two races that are characterized by differences in aggression and response to alarm pheromone [108]. Thus, genotypic differences in behaviour may be associated with changes in expression patterns of modules associated with pheromone response.
In fire ants, interactions between queens and workers differ between two genetic morphs, and this may also be related to changes in social pathways. Fire ants exhibit a natural social polymorphism with monogynous (single queen) or polygynous (multiple queen) colonies. This polymorphism in social organization is associated with allelic variation in the gene encoding putative olfactory binding protein general protein-9 (Gp-9). Homozygous workers with the BB allele will only accept a single BB queen, while heterozygous workers will accept multiple Bb queens. This strong association between a putative odorant binding protein and a social phenotype is consistent with the hypothesis that changes in a pheromonal communication system affect social organization (reviewed in [115]), perhaps at the level of the sensory detection module. However, Gp-9 allelic variation is also associated with a suite of queen-related behavioural and morphological traits, and thus may result from the combined effect of several genes linked to the Gp-9 locus, and not from the Gp-9 gene alone [106].
In summary, the organization of pheromone communication systems is inherently modular, encompassing sensory detection, neural processing and downstream endocrine and behavioural changes. Within honeybees, there is evidence for plasticity in pheromone production and responses to pheromones. Responses to pheromones can be modulated by physiological, genotypic and social factors, and, in the examples highlighted above, appear to be due to changes in the processing or behavioural output modules. With the identification of genes associated with pheromone-mediated behavioural changes in honeybees, it now becomes feasible to bridge into other systems and determine whether these pathways are conserved. Queen regulation of worker reproduction is common across social insect species, making this an ideal system for studying the conservation or divergence of different modules in social pathways.
6. Task-related plasticity in the circadian clockwork
The circadian clock is an internal system that organizes the temporal physiology and behaviour of animals [116]. Socially regulated plasticity in circadian rhythms provides a good model for a social pathway because the circadian clock is one of the systems in which the relationships between genes and behaviour are best understood, and the molecular clockwork is a well-defined module. In addition, the circadian clock appears to play important roles in the temporal organization of insect societies, and thus was probably influenced by the evolution of social behaviour. For example, the circadian clock is involved in social synchronization of the behaviour of individual bees, in plasticity in circadian rhythms of queens that is associated with their reproductive status and in task related plasticity in circadian rhythms of workers that appears to contribute to the division of labour and overall colony efficiency (reviewed in [117]).
Task-related plasticity in circadian rhythms was first discovered in the honeybee, where division of labour relates to age. Young bees (less than two weeks of age) typically perform activities inside the constantly dark and homeostatically regulated hive with no circadian rhythms. Foragers, which are typically older (more than three weeks of age), have strong circadian rhythms with elevated activity during the day in which they forage for nectar and pollen, while at night they sleep inside the hive [118–121]. Differences in the exposure of nest bees and foragers to environmental factors such as light and temperature cannot account for this plasticity in circadian rhythms; the rhythms are strongly linked to the behavioural state of the bee. Nurses are active around-the-clock even when they experience a light–dark illumination regime that is similar to that of the foragers [121,122]. In addition, an ontogeny of circadian rhythms similar to that seen in the hive is also evident in young bees that are housed individually or in small broodless groups in constant laboratory environment, consistent with the premise that the ontogeny of circadian rhythms is not regulated by changes in ambient illumination or temperature [123,124]. The social context has profound influence on circadian rhythmicity in bees. Foragers switch back to activity with no circadian rhythms when induced to revert to brood care activity [125,126]; nurse bees switch to activity with robust circadian rhythms shortly after removal from the hive [122,127]. Given the association between brood care and plasticity in circadian rhythms, it is not surprising that the brood is the most potent social factor influencing plasticity in the clockwork. Young honeybees that are confined to a broodless piece of comb or develop on empty combs in small cages do show robust circadian rhythms in clock gene expression, whereas full-sister bees of a similar age developing on a comb containing brood are active around the clock with attenuated molecular oscillations [127].
The molecular module generating circadian rhythms in animals is well characterized; it consists of interlocked autoregulatory transcriptional/translational feedback loops with positive and negative elements [128]. This organizational principle of the circadian clock is manifested in an approximately 24 h cycle in both mRNA and protein abundance of a group of canonical ‘clock genes’, and provides the means to assess the molecular clockwork by measuring clock gene expression over the course of a day. The molecular clockwork in the honeybee appears to be in some characteristics more similar to that of mammals than to Drosophila. The honeybee genome does not encode orthologues for two of the canonical clock genes in Drosophila, Timeless (Tim1), and the Drosophila-type Cryptochrome (Cry-d, also known as Cry1), but it does encode the mammalian-type Cryptochrome (Cry-m, also known as Cry2), which is not encoded by the Drosophila genome [122,129]. Task-related plasticity in circadian rhythms is associated with plasticity in the molecular clockwork. Brain mRNA abundance of Per, Cry-m, Tim2 and Cycle show robust oscillations in foragers but not in nurses. Social manipulations uncoupling age and task indicated the temporal pattern of clock gene expression is linked more strongly to task than to age [122,126,130]. Taken together these studies suggest that the circadian system in social insects was co-opted to organize individual behaviour such that it fits with the social environment.
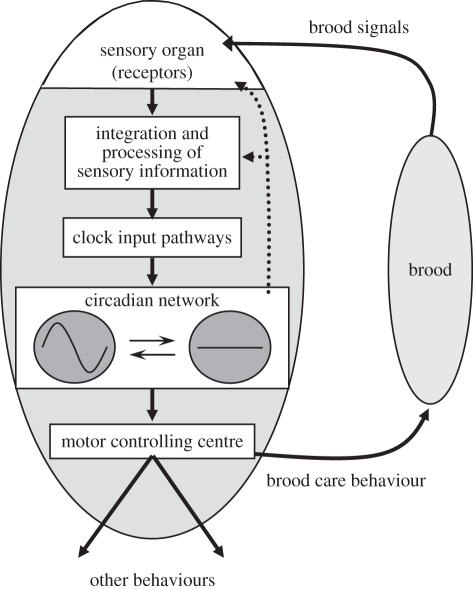
Comparative studies support this hypothesis: there is a similar task-related plasticity in behavioural and molecular rhythms in the bumble-bee B. terrestris in which division of labour is based primarily on size rather than age as in honeybees [21,131], and in ants in which age-related division of labour evolved independently of that in honeybees [132,133]. In the bumble-bees, large workers, that are more likely to perform foraging activity, emerge from the pupae with stronger circadian rhythms [21], and more cells expressing the putative clock neuropeptide pigment-dispersing factor (PDF) [134]. This latter finding is different from the honeybee in which there are a similar number of PDF immunoreactive cells in nurses and foragers [135]. Thus, in bumble-bees with a size-based division of labour, developmental factors that control body size also appear to influence the structure of the circadian system. The plasticity in circadian rhythms is hypothesized to improve the division of labour and colony efficiency because around-the-clock activity by nurses facilitates continuous brood care, whereas foraging behaviour relies on the circadian clock for timing visits to flowers and for time-compensated sun-compass navigation. This suggests that the evolution of sociality was associated with an increased plasticity in an integrative module, the circadian system, and with linking this module to sensory modules detecting signals from the brood. Little is known about the molecular pathways linking the clock to sensory modules and to downstream output pathways (figure 4).
Figure 4.
A simplified scheme for a social pathway associated with task-related plasticity in circadian rhythms. Nurse bees that interact with the brood are active around the clock with no circadian rhythms in behaviour or brain clock gene expression. Nurse bees that do not interact with the brood switch to activity with strong behavioural and molecular circadian rhythms. The brood signal and the sensory system detecting and conveying this information to the circadian clock network are yet unknown. It is not known whether social signals influence the circadian system via dedicated clock input pathways or indirectly by changing motor activity or arousal state which then act on the circadian system. Other details as in figure 3.
But how did the clock of the worker evolve for this plasticity? In animals, including humans, imposing around-the-clock activity is typically associated with increased pathologies and deterioration in performance [116]. As reviewed above, it is commonly assumed that nursing behaviour in workers evolved from maternal behaviour in the ancestors of social insects. This hypothesis predicts an association between reproductive state and circadian rhythms in bees with developed maternal care. There is no information on the activity rhythms of solitary mother bees, but there is evidence suggesting that female cockroaches with active ovaries show attenuated circadian rhythms [136], suggesting an interaction between reproductive physiology and the circadian system in this species. The association of maternal behaviour, reproductive state and circadian rhythms was investigated in the bumble-bee B. terrestris in which the mother queen cares for the first developing batch of brood. This study shows that queens with brood are active around the clock, but queens of similar age for which the brood was removed switched to activity with strong circadian rhythms [137]. Queens with only eggs were also active with no circadian rhythms, making it difficult to determine whether around-the-clock activity is associated with brood care behaviour, reproductive physiology or both. In ants [138–140], and honeybees [141,142], egg-laying queens that do little, if any, maternal care are also active around the clock, suggesting an association of circadian rhythmicity with reproductive physiology, but not with brood care in these species. Thus, pathways that regulate reproductive physiology, maternal behaviour or both may be involved in regulating plasticity in circadian rhythms.
Little is known about pathways linking reproductive physiology and the circadian system. Manipulations of JH levels in honeybee workers or cockroaches did not affect their behavioural rhythms or the pattern of brain clock gene expression [136,143,144]. The involvement of insulin signalling has not yet been explored in bees but there is some evidence that these two systems interact. In Drosophila, elevated insulin signalling and FOXO mutations increase the susceptibility of the circadian clock to oxidative stress. FOXO mutations also advanced ageing-related attenuation of circadian rhythms [145]. In addition, nutrition and metabolism, which are regulated by insulin signalling, have diverse influences on circadian rhythms (reviewed in [146]). The IIS pathway is also a good candidate to influence size-related variation in the circadian system of bumble-bees because it plays a major role in the regulation of cell number and body size [147,148].
A better characterization of the environmental and neuroendocrine regulation of the circadian system in social and solitary bees is necessary for understanding the social pathways underlying plasticity in circadian rhythms and their relationships with pathways regulating reproduction and nutrition.
7. Concluding remarks
The recent development of genomic and molecular tools has revolutionized the study of insect sociobiology. Thus far, many of the studies that we reviewed suggest that modifications in core pathways, such as insulin signalling, the circadian system, reproductive physiology and hormone signalling, play key roles in the evolution of social behaviour. However, how these conserved molecular pathways are incorporated into a social pathway—and especially how they have been linked to social communication systems—remains to be elucidated. One obvious limitation of our current state of knowledge is that most of the characterized modifications were inferred from comparisons between the highly social honeybee and the solitary fruitfly D. melanogaster. Thus, it is difficult to separate changes that are genuinely associated with social evolution from those related to phylogenetic variation between bees and flies, given that these lineages are estimated to have diverged over 300 Ma [149]. With the anticipated sequencing of the genomes of several bee species in the near future (G. Robinson 2010, personal communication), and the increasing accessibility of genomic technologies, it will become possible to conduct more informative comparative genomics analyses involving closely related solitary and eusocial species. Key bee species for socio-comparative genomics include halictid bees, a family of bees showing all levels of sociality, and Euglossine (orchid) bees, bumble-bees and stingless bees, which are closely related to honeybees.
Another key challenge is to determine whether there are specific elements or modules of social pathways that serve as the main targets of social evolution. For example, it is possible that core pathways remain intact and highly conserved from solitary to eusocial species, while sensory modules regulating detection of a social signal are modified such that the behaviour is expressed in a social context. Are there specific ‘hot spots’ that are a common target for the modifications associated with social evolution? It is also important to determine whether social pathways are indeed modular, and whether these modules are subject to different selection pressures. Alternatively, selection may operate by targeting highly pleiotropic genes that may act as ‘prime movers’ for evolutionary changes. For example, many of the studies that we reviewed above suggest that modifications in insulin-signalling pathways were important in the evolution of social behaviour in bees (e.g. longevity, reproduction and feeding behaviour). Does this emphasis of IIS pathways represent the current state of knowledge and research focus, or did the evolution of sociality in bees indeed involve only a few modifications in key pathways, such as insulin signalling, which act upstream of diverse processes involved in social behaviours? In order to answer these questions, it will be necessary to develop genomic resources in a range of species, from solitary to social, and to characterize the genes and pathways regulating similar behavioural processes. With the recent genomic sequencing of Nasonia [150], ants [151], transcriptome sequencing of P. metricus [59] and Polistes dominulus (Toth & Grozinger 2011, unpublished data) and ongoing sequencing projects for a number of other bee species, it will soon be possible to address these questions.
Acknowledgements
We would like to thank Abraham Hefetz and members of the Bloch and Grozinger laboratories for helpful discussions and critical reading of the manuscript. We thank two anonymous reviewers for their useful comments on an earlier version of this manuscript. The Bloch laboratory has been supported by grants for the Israel Science Foundation (ISF), the US-Israel Bi-National Science Foundation (BSF), the National Institute for Psychobiology in Israel (NIPI) and the German Israeli Foundation (GIF). The Grozinger laboratory has been supported by funding from the National Science Foundation (NSF), US Department of Agriculture (USDA) and BSF.
Footnotes
One contribution of 10 to a Theme Issue ‘Evolutionary developmental biology (evo-devo) and behaviour’.
References
- 1.Maynard Smith J., Szathmáry E. 1998. The major transitions in evolution. Oxford, London: Oxford University Press [Google Scholar]
- 2.Darwin C. 1859. On the origin of species by means of natural selection, 1st edn, 1st issue. London: John Murray. [Google Scholar]
- 3.Hamilton W. D. 1964. The genetical evolution of social behaviour. J. Theor. Biol. 7, 1–16 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 4.Okasha S. 2006. Evolution and the levels of selection. Oxford, London: Oxford University Press [Google Scholar]
- 5.Sober E., Wilson D. S. 1998. Unto others: the evolution and psychology of unselfish behavior. Cambridge, MA: Harvard University Press [Google Scholar]
- 6.Wilson E. O. 1971. The insect societies. Cambridge, MA: The Belkap Press of Harvard University Press [Google Scholar]
- 7.Robinson G. E., Grozinger C. M., Whitfield C. W. 2005. Sociogenomics: social life in molecular terms. Nat. Rev. Genet. 6, 257–270 10.1038/nrg1575 (doi:10.1038/nrg1575) [DOI] [PubMed] [Google Scholar]
- 8.Toth A. L., Robinson G. E. 2007. Evo-devo and the evolution of social behavior. Trends Genet. 23, 334–341 10.1016/j.tig.2007.05.001 (doi:10.1016/j.tig.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 9.Carroll S. B., Grenier J. K., Weatherbee S. D. 2005. From DNA to diversity: molecular genetics and the evolution of animal design. Malden, MA: Blackwell Publishing [Google Scholar]
- 10.Grozinger C. M., Robinson G. E. 2010. Sociogenomics. In Encyclopedia of animal behavior (eds Breed M. D., Moore J.), pp. 2672 Oxford, UK: Elsevier Press [Google Scholar]
- 11.Nijhout H. F. 2003. Development and evolution of adaptive polyphenisms. Evol. Develop. 5, 9–18 10.1046/j.1525-142X.2003.03003.x (doi:10.1046/j.1525-142X.2003.03003.x) [DOI] [PubMed] [Google Scholar]
- 12.Oldroyd B. P., Fewell J. H. 2007. Genetic diversity promotes homeostasis in insect colonies. Trends Ecol. Evol. 22, 408–413 10.1016/j.tree.2007.06.001 (doi:10.1016/j.tree.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 13.Robinson G. E., Fernald R. D., Clayton D. F. 2008. Genes and social behavior. Science 322, 896–900 10.1126/science.1159277 (doi:10.1126/science.1159277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith C. R., Toth A. L., Suarez A. V., Robinson G. E. 2008. Genetic and genomic analyses of the division of labour in insect societies. Nat. Rev. Genet. 9, 735–748 10.1038/nrg2429 (doi:10.1038/nrg2429) [DOI] [PubMed] [Google Scholar]
- 15.West-Eberhard M. J. 2003. Developmental plasticity and evolution. New York, NY: Oxford University Press [Google Scholar]
- 16.Michener C. D. 1974. The social behavior of the bees, 2nd edn. Cambridge, MA: The Belknap Press of Harvard University Press [Google Scholar]
- 17.Robinson G. E. 1992. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 37, 637–665 10.1146/annurev.en.37.010192.003225 (doi:10.1146/annurev.en.37.010192.003225) [DOI] [PubMed] [Google Scholar]
- 18.Seeley T. D. 1996. The wisdom of the hive: the social physiology of honey bee colonies. Cambridge, MA: Harvard University Press [Google Scholar]
- 19.Spaethe J., Brockmann A., Halbig C., Tautz J. 2007. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften 94, 733–739 10.1007/s00114-007-0251-1 (doi:10.1007/s00114-007-0251-1) [DOI] [PubMed] [Google Scholar]
- 20.Spaethe J., Chittka L. 2003. Interindividual variation of eye optics and single object resolution in bumblebees. J. Exp. Biol. 206, 3447–3453 10.1242/jeb.00570 (doi:10.1242/jeb.00570) [DOI] [PubMed] [Google Scholar]
- 21.Yerushalmi S., Bodenhaimer S., Bloch G. 2006. Developmentally determined attenuation in circadian rhythms links chronobiology to social organization in bees. J. Exp. Biol. 209, 1044–1051 10.1242/jeb.02125 (doi:10.1242/jeb.02125) [DOI] [PubMed] [Google Scholar]
- 22.Hölldobler B., Wilson E. O. 1990. The Ants. Cambridge, MA: Belknap Press [Google Scholar]
- 23.Barron A. B., Oldroyd B. P., Ratnieks F. L. W. 2001. Worker reproduction in honey-bees (Apis) and the anarchic syndrome. A review. Behav. Ecol. Sociobiol. 50, 199–208 10.1007/s002650100362 (doi:10.1007/s002650100362) [DOI] [Google Scholar]
- 24.Hunt G. J., et al. 2007. Behavioral genomics of honeybee foraging and nest defense. Naturwissenschaften 94, 247–267 10.1007/s00114-006-0183-1 (doi:10.1007/s00114-006-0183-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lattorff H. M., Moritz R. F., Crewe R. M., Solignac M. 2007. Control of reproductive dominance by the thelytoky gene in honeybees. Biol. Lett. 3, 292–295 10.1098/rsbl.2007.0083 (doi:10.1098/rsbl.2007.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amdam G. V., Norberg K., Fondrk M. K., Page R. E., Jr 2004. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc. Natl Acad. Sci. USA 101, 11 350–11 355 10.1073/pnas.0403073101 (doi:10.1073/pnas.0403073101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West-Eberhard M. J. 1987. The epigenetical origins of insect sociality. In Tenth Int. Congress of the Int. Union Study of Social Insects (eds Eder J., Rembold J.), pp. 369–372 Munich, Germany: Verlag J. Peperny [Google Scholar]
- 28.West-Eberhard M. J. 1996. Wasp societies as microcosms for the study of development and evolution. In Natural history and evolution of paper wasps (eds Turillazzi S., West-Eberhard M. J.), pp. 290–317 Oxford, London: Oxford University Press [Google Scholar]
- 29.Page R. E., Linksvayer T. A., Amdam G. V. 2009. Social life from solitary regulatory networks: a paradigm for insect sociality. In Organization of insect societies, from genome to sociocomplexity (eds Gadau J., Fewell J.), pp. 357–367 Cambridge, MA: Harvard University Press [Google Scholar]
- 30.Page R. E., Jr, Scheiner R., Erber J., Amdam G. V. 2006. The development and evolution of division of labor and foraging specialization in a social insect (Apis mellifera L.). Curr. Top. Dev. Biol. 74, 253–286 10.1016/S0070-2153(06)74008-X (doi:10.1016/S0070-2153(06)74008-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pankiw T. 2004. Brood pheromone regulates foraging activity of honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 97, 748–751 10.1603/0022-0493(2004)097[0748:BPRFAO]2.0.CO;2 (doi:10.1603/0022-0493(2004)097[0748:BPRFAO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 32.Bloch G., Shpingler H., Wheeler D. E., Robinson G. E. 2009. Endocrine influences on the organization of insect societies. In Hormones, brain and behavior (eds Pfaff D., Arnold A., Etgen A., Fahrbach S. E., Rubin R.). New York, NY: Academic Press [Google Scholar]
- 33.Toth A. L., Kantarovich S., Meisel A. F., Robinson G. E. 2005. Nutritional status influences socially regulated foraging ontogeny in honey bees. J. Exp. Biol. 208, 4641–4649 10.1242/jeb.01956 (doi:10.1242/jeb.01956) [DOI] [PubMed] [Google Scholar]
- 34.Kaun K. R., Sokolowski M. B. 2009. cGMP-dependent protein kinase: linking foraging to energy homeostasis. Genome 52, 1–7 10.1139/g08-090 (doi:10.1139/g08-090) [DOI] [PubMed] [Google Scholar]
- 35.Ben-Shahar Y., Dudek N. L., Robinson G. E. 2004. Phenotypic deconstruction reveals involvement of manganese transporter malvolio in honey bee division of labor. J. Exp. Biol. 207, 3281–3288 10.1242/jeb.01151 (doi:10.1242/jeb.01151) [DOI] [PubMed] [Google Scholar]
- 36.Ament S. A., Corona M., Pollock H. S., Robinson G. E. 2008. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc. Natl Acad. Sci. USA 105, 4226–4231 10.1073/pnas.0800630105 (doi:10.1073/pnas.0800630105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunieda T., et al. 2006. Carbohydrate metabolism genes and pathways in insects: insights from the honey bee genome. Insect Mol. Biol. 15, 563–576 10.1111/j.1365-2583.2006.00677.x (doi:10.1111/j.1365-2583.2006.00677.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brockmann A., Annangudi S. P., Richmond T. A., Ament S. A., Xie F., Southey B. R., Rodriguez-Zas S. R., Robinson G. E., Sweedler J. V. 2009. Quantitative peptidomics reveal brain peptide signatures of behavior. Proc. Natl Acad. Sci. USA 106, 2383–2388 10.1073/pnas.0813021106 (doi:10.1073/pnas.0813021106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitfield C. W., Cziko A. M., Robinson G. E. 2003. Gene expression profiles in the brain predict behavior in individual honey bees. Science 302, 296–299 10.1126/science.1086807 (doi:10.1126/science.1086807) [DOI] [PubMed] [Google Scholar]
- 40.Grozinger C. M., Sharabash N. M., Whitfield C. W., Robinson G. E. 2003. Pheromone-mediated gene expression in the honey bee brain. Proc. Natl Acad. Sci. USA 100(Suppl. 2), 14 519–14 525 10.1073/pnas.2335884100 (doi:10.1073/pnas.2335884100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitfield C. W., Ben-Shahar Y., Brillet C., Leoncini I., Crauser D., Leconte Y., Rodriguez-Zas S., Robinson G. E. 2006. Genomic dissection of behavioral maturation in the honey bee. Proc. Natl Acad. Sci. USA 103, 16 068–16 075 10.1073/pnas.0606909103 (doi:10.1073/pnas.0606909103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West-Eberhard M. J. 1987. Flexible strategy and social evolution. In Animal societies: theories and facts (eds Ito Y., Brown J. L., Kikkawa J.), pp. 35–51 Tokyo, Japan: Japan Scientific Societies Press [Google Scholar]
- 43.Hartfelder K., Engels W. 1998. Social insect polymorphism: hormonal regulation of plasticity in development and reproduction in the honeybee. Curr. Top. Dev. Biol. 40, 45–77 10.1016/S0070-2153(08)60364-6 (doi:10.1016/S0070-2153(08)60364-6) [DOI] [PubMed] [Google Scholar]
- 44.Robinson G. E., Vargo E. L. 1997. Juvenile hormone in adult eusocial Hymenoptera: gonadotropin and behavioral pacemaker. Arch. Insect Biochem. Physiol. 35, 559–583 (doi:10.1002/(SICI)1520-6327(1997)35:4<559::AID-ARCH13>3.0.CO;2-9) [DOI] [PubMed] [Google Scholar]
- 45.Goodman W. G., Granger N. A. 2005. The juvenile hormones. In Comprehensive molecular insect science (ed. Lawrence I. G. E. A.), pp. 319–408 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 46.Wyatt G. R., Davey K. G. 1996. Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormone in adult insects. Adv. Insect Physiol. 26, 2–155 [Google Scholar]
- 47.Barchuk A. R., Bitondi M. M., Simoes Z. L. 2002. Effects of juvenile hormone and ecdysone on the timing of vitellogenin appearance in hemolymph of queen and worker pupae of Apis mellifera. J. Insect. Sci. 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinto L. Z., Bitondi M. M., Simoes Z. L. 2000. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J. Insect Physiol. 46, 153–160 [DOI] [PubMed] [Google Scholar]
- 49.Guidugli K. R., Nascimento A. M., Amdam G. V., Barchuk A. R., Omholt S., Simoes Z. L., Hartfelder K. 2005. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 579, 4961–4965 10.1016/j.febslet.2005.07.085 (doi:10.1016/j.febslet.2005.07.085) [DOI] [PubMed] [Google Scholar]
- 50.Nelson C. M., Ihle K. E., Fondrk M. K., Page R. E., Amdam G. V. 2007. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 5, e62. 10.1371/journal.pbio.0050062 (doi:10.1371/journal.pbio.0050062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartfelder K., Makert G. R., Judice C. C., Pereira G. A. G., Santana W. C., Dallacqua R., Bitondi M. M. G. 2006. Physiological and genetic mechanisms underlying caste development, reproduction and division of labor in stingless bees. Apidologie 37, 144–163 10.1051/apido:2006013 (doi:10.1051/apido:2006013) [DOI] [Google Scholar]
- 52.Suzuki Y., Nijhout H. F. 2006. Evolution of a polyphenism by genetic accommodation. Science 311, 650–652 10.1126/science.1118888 (doi:10.1126/science.1118888) [DOI] [PubMed] [Google Scholar]
- 53.Page R. E., Fondrk M. K. 1995. The effects of colony level selection on the social-organization of honey-bee (Apis mellifera L) colonies—colony level components of pollen hoarding. Behav. Ecol. Sociobiol. 36, 135–144 10.1007/BF00170718 (doi:10.1007/BF00170718) [DOI] [Google Scholar]
- 54.Amdam G. V., Csondes A., Fondrk M. K., Page R. E., Jr 2006. Complex social behaviour derived from maternal reproductive traits. Nature 439, 76–78 10.1038/nature04340 (doi:10.1038/nature04340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y., Amdam G. V., Rueppell O., Wallrichs M. A., Fondrk M. K., Kaftanoglu O., Page R. E., Jr 2009. PDK1 and HR46 gene homologs tie social behavior to ovary signals. PLoS ONE 4, e4899. 10.1371/journal.pone.0004899 (doi:10.1371/journal.pone.0004899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flatt T., Tu M. P., Tatar M. 2005. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays 27, 999–1010 10.1002/bies.20290 (doi:10.1002/bies.20290) [DOI] [PubMed] [Google Scholar]
- 57.Oldroyd B. P., Beekman M. 2008. Effects of selection for honey bee worker reproduction on foraging traits. PLoS Biol. 6, e56. 10.1371/journal.pbio.0060056 (doi:10.1371/journal.pbio.0060056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linksvayer T. A., Wade M. J. 2005. The evolutionary origin and elaboration of sociality in the aculeate Hymenoptera: maternal effects, sib-social effects, and heterochrony. Q. Rev. Biol. 80, 317–336 10.1086/432266 (doi:10.1086/432266) [DOI] [PubMed] [Google Scholar]
- 59.Toth A. L., et al. 2007. Wasp gene expression supports an evolutionary link between maternal behavior and eusociality. Science 19, 441–444 10.1126/science.1146647 (doi:10.1126/science.1146647) [DOI] [PubMed] [Google Scholar]
- 60.Grozinger C. M., Fan Y., Hoover S. E., Winston M. L. 2007. Genome-wide analysis reveals differences in brain gene expression patterns associated with caste and reproductive status in honey bees (Apis mellifera). Mol. Ecol. 16, 4837–4848 10.1111/j.1365-294X.2007.03545.x (doi:10.1111/j.1365-294X.2007.03545.x) [DOI] [PubMed] [Google Scholar]
- 61.Keller L., Jemielity S. 2006. Social insects as a model to study the molecular basis of ageing. Exp. Gerontol. 41, 553–556 10.1016/j.exger.2006.04.002 (doi:10.1016/j.exger.2006.04.002) [DOI] [PubMed] [Google Scholar]
- 62.Page R. E., Jr, Peng C. Y. 2001. Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp. Gerontol. 36, 695–711 10.1016/S0531-5565(00)00236-9 (doi:10.1016/S0531-5565(00)00236-9) [DOI] [PubMed] [Google Scholar]
- 63.Finch C. E., Ruvkun G. 2001. The genetics of aging. Annu. Rev. Genomics Hum. Genet. 2, 435–462 10.1146/annurev.genom.2.1.435 (doi:10.1146/annurev.genom.2.1.435) [DOI] [PubMed] [Google Scholar]
- 64.Corona M., Velarde R. A., Remolina S., Moran-Lauter A., Wang Y., Hughes K. A., Robinson G. E. 2007. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl Acad. Sci. USA 104, 7128–7133 10.1073/pnas.0701909104 (doi:10.1073/pnas.0701909104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seehuus S. C., Norberg K., Gimsa U., Krekling T., Amdam G. V. 2006. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl Acad. Sci. USA 103, 962–967 10.1073/pnas.0502681103 (doi:10.1073/pnas.0502681103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlosser G. 2002. Modularity and the units of evolution. Theory Biosci. 121, 1–80 10.1078/1431-7613-00049 (doi:10.1078/1431-7613-00049) [DOI] [Google Scholar]
- 67.Wagner G. P., Pavlicev M., Cheverud J. M. 2007. The road to modularity. Nat. Rev. Genet. 8, 921–931 10.1038/nrg2267 (doi:10.1038/nrg2267) [DOI] [PubMed] [Google Scholar]
- 68.Winther R. G. 2001. Varieties of modules: kinds, levels, origins, and behaviors. J. Exp. Zool. 291, 116–129 10.1002/jez.1064 (doi:10.1002/jez.1064) [DOI] [PubMed] [Google Scholar]
- 69.Hallem E. A., Dahanukar A., Carlson J. R. 2006. Insect odor and taste receptors. Annu. Rev. Entomol. 51, 113–135 10.1146/annurev.ento.51.051705.113646 (doi:10.1146/annurev.ento.51.051705.113646) [DOI] [PubMed] [Google Scholar]
- 70.Hoekstra H. E., Coyne J. A. 2007. The locus of evolution: evo devo and the genetics of adaptation. Evolution 61, 995–1016 10.1111/j.1558-5646.2007.00105.x (doi:10.1111/j.1558-5646.2007.00105.x) [DOI] [PubMed] [Google Scholar]
- 71.Wagner G. P., Lynch V. J. 2008. The gene regulatory logic of transcription factor evolution. Trends Ecol. Evol. 23, 377–385 10.1016/j.tree.2008.03.006 (doi:10.1016/j.tree.2008.03.006) [DOI] [PubMed] [Google Scholar]
- 72.Wray G. A. 2007. The evolutionary significance of cis-regulatory mutations. Nat. Rev. Genet. 8, 206–216 10.1038/nrg2063 (doi:10.1038/nrg2063) [DOI] [PubMed] [Google Scholar]
- 73.Martin C., Zhang Y. 2007. Mechanisms of epigenetic inheritance. Curr. Opin. Cell Biol. 19, 266–272 10.1016/j.ceb.2007.04.002 (doi:10.1016/j.ceb.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 74.Elango N., Hunt B. G., Goodisman M. A., Yi S. V. 2009. DNA methylation is widespread and associated with differential gene expression in castes of the honeybee, Apis mellifera. Proc. Natl Acad. Sci. USA 106, 11 206–11 211 10.1073/pnas.0900301106 (doi:10.1073/pnas.0900301106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kucharski R., Maleszka J., Foret S., Maleszka R. 2008. Nutritional control of reproductive status in honeybees via DNA methylation. Science 319, 1827–1830 10.1126/science.1153069 (doi:10.1126/science.1153069) [DOI] [PubMed] [Google Scholar]
- 76.Szyf M., McGowan P., Meaney M. J. 2008. The social environment and the epigenome. Environ. Mol. Mutagen. 49, 46–60 10.1002/em.20357 (doi:10.1002/em.20357) [DOI] [PubMed] [Google Scholar]
- 77.Foret S., Kucharski R., Pittelkow Y., Lockett G. A., Maleszka R. 2009. Epigenetic regulation of the honey bee transcriptome: unravelling the nature of methylated genes. BMC Genomics 10, 472. 10.1186/1471-2164-10-472 (doi:10.1186/1471-2164-10-472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ben-Shahar Y., Leung H. T., Pak W. L., Sokolowski M. B., Robinson G. E. 2003. cGMP-dependent changes in phototaxis: a possible role for the foraging gene in honey bee division of labor. J. Exp. Biol. 206, 2507–2515 10.1242/jeb.00442 (doi:10.1242/jeb.00442) [DOI] [PubMed] [Google Scholar]
- 79.Long T. F., Murdock L. L. 1983. Stimulation of blowfly feeding behavior by octopaminergic drugs. Proc. Natl Acad. Sci. USA 80, 4159–4163 10.1073/pnas.80.13.4159 (doi:10.1073/pnas.80.13.4159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., Heisenberg M. 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 23, 10 495–10 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schulz D. J., Barron A. B., Robinson G. E. 2002. A role for octopamine in honey bee division of labor. Brain Behav. Evol. 60, 350–359 10.1159/000067788 (doi:10.1159/000067788) [DOI] [PubMed] [Google Scholar]
- 82.Hoover S. E., Keeling C. I., Winston M. L., Slessor K. N. 2003. The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften 90, 477–480 10.1007/s00114-003-0462-z (doi:10.1007/s00114-003-0462-z) [DOI] [PubMed] [Google Scholar]
- 83.Pankiw T., Huang Z., Winston M. L., Robinson G. E. 1998. Queen mandibular gland pheromone influences worker honey bee (Apis mellifera L.) foraging ontogeny and juvenile hormone titers. J. Insect Physiol. 44, 685–692 10.1016/S0022-1910(98)00040-7 (doi:10.1016/S0022-1910(98)00040-7) [DOI] [PubMed] [Google Scholar]
- 84.Bloch G., Hefetz A. 1999. Reevaluation of the role of mandibular glands in regulation of reproduction in bumblebee colonies. J. Chem. Ecol. 25, 881–896 10.1023/A:1020805103379 (doi:10.1023/A:1020805103379) [DOI] [Google Scholar]
- 85.Bloch G., Hefetz A. 1999. Regulation of reproduction by dominant workers in bumblebee (Bombus terrestris) queenright colonies. Behav. Ecol. Sociobiol. 45, 125–135 10.1007/s002650050546 (doi:10.1007/s002650050546) [DOI] [Google Scholar]
- 86.Bloch G., Borst D. W., Huang Z., Robinson G. E., Cnaani J., Hefetz A. 2000. Juvenile hormone titers, juvenile hormone biosynthesis, ovarian development and social environment in Bombus terrestris. J. Insect Physiol. 46, 47–57 10.1016/S0022-1910(99)00101-8 (doi:10.1016/S0022-1910(99)00101-8) [DOI] [PubMed] [Google Scholar]
- 87.Cameron S. A., Robinson G. E. 1990. Juvenile hormone does not affect division of labor in bumble bee colonies (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 83, 626–631 [Google Scholar]
- 88.Bloch G., Borst D. W., Huang Z.-Y., Robinson G. E., Hefetz A. 1996. Effects of social conditions on juvenile hormone-mediated reproductive development in Bombus terrestris workers. Physiol. Entomol. 21, 257–267 10.1111/j.1365-3032.1996.tb00863.x (doi:10.1111/j.1365-3032.1996.tb00863.x) [DOI] [Google Scholar]
- 89.Huang Z. Y., Robinson G. E., Tobe S. S., Yagi K. J., Strambi C., Strambi A., Stay B. 1991. Hormonal regulation of behavioural development in the honey bee is based on changes in the rate of juvenile hormone biosynthesis. J. Insect Physiol. 37, 733–742 10.1016/0022-1910(91)90107-B (doi:10.1016/0022-1910(91)90107-B) [DOI] [Google Scholar]
- 90.Grozinger C. M., Robinson G. E. 2007. Endocrine modulation of a pheromone-responsive gene in the honey bee brain. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 193, 461–470 10.1007/s00359-006-0202-x (doi:10.1007/s00359-006-0202-x) [DOI] [PubMed] [Google Scholar]
- 91.Shpigler H., Patch H. M., Cohen M., Fan Y., Grozinger C. M., Bloch G. 2010. The transcription factor Kruppel homolog 1 is linked to hormone mediated social organization in bees. BMC Evol. Biol. 10, 120. 10.1186/1471-2148-10-120 (doi:10.1186/1471-2148-10-120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le Conte Y., Hefetz A. 2008. Primer pheromones in social hymenoptera. Annu. Rev. Entomol. 53, 523–542 10.1146/annurev.ento.52.110405.091434 (doi:10.1146/annurev.ento.52.110405.091434) [DOI] [PubMed] [Google Scholar]
- 93.Wyatt T. D. 2003. Pheromones and animal behavior: communication by taste and smell. Cambridge, UK: Cambridge University Press [Google Scholar]
- 94.Kocher S. D., Richard F. J., Tarpy D. R., Grozinger C. M. 2009. Queen reproductive state modulates queen pheromone production and queen–worker interactions in honey bees. Behav. Ecol. 20, 1007–1014 10.1093/beheco/arp090 (doi:10.1093/beheco/arp090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Richard F. J., Tarpy D. R., Grozinger C. M. 2007. Effects of insemination quantity on honey bee queen physiology. PLoS ONE 2, e980. 10.1371/journal.pone.0000980 (doi:10.1371/journal.pone.0000980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hasegawa M., Asanuma S., Fujiyuki T., Kiya T., Sasaki T., Endo D., Morioka M., Kubo T. 2009. Differential gene expression in the mandibular glands of queen and worker honeybees, Apis mellifera L.: implications for caste-selective aldehyde and fatty acid metabolism. Insect Biochem. Mol. Biol. 39, 661–667 10.1016/j.ibmb.2009.08.001 (doi:10.1016/j.ibmb.2009.08.001) [DOI] [PubMed] [Google Scholar]
- 97.Malka O., Karunker I., Yeheskel A., Morin S., Hefetz A. 2009. The gene road to royalty—differential expression of hydroxylating genes in the mandibular glands of the honeybee. FEBS J. 276, 5481–5490 10.1111/j.1742-4658.2009.07232.x (doi:10.1111/j.1742-4658.2009.07232.x) [DOI] [PubMed] [Google Scholar]
- 98.Plettner E., Slessor K. N., Winston M. L., Oliver J. E. 1996. Caste-selective pheromone biosynthesis in honeybees. Science 271, 1851–1853 10.1126/science.271.5257.1851 (doi:10.1126/science.271.5257.1851) [DOI] [Google Scholar]
- 99.Dulac C., Torello A. T. 2003. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat. Rev. Neurosci. 4, 551–562 10.1038/nrn1140 (doi:10.1038/nrn1140) [DOI] [PubMed] [Google Scholar]
- 100.Robertson H. M., Wanner K. W. 2006. The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res. 16, 1395–1403 10.1101/gr.5057506 (doi:10.1101/gr.5057506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wanner K. W., Nichols A. S., Walden K. K., Brockmann A., Luetje C. W., Robertson H. M. 2007. A honey bee odorant receptor for the queen substance 9-oxo-2-decenoic acid. Proc. Natl Acad. Sci. USA 104, 14 383–14 388 10.1073/pnas.0705459104 (doi:10.1073/pnas.0705459104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Avila F. W., Sirot L. K., Laflamme B. A., Rubinstein C. D., Wolfner M. F. 2010. Insect seminal fluid proteins: identification and function. Annu. Rev. Entomol. 56, 21–40 10.1146/annurev-ento-120709-144823 (doi:10.1146/annurev-ento-120709-144823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beggs K. T., Glendining K. A., Marechal N. M., Vergoz V., Nakamura I., Slessor K. N., Mercer A. R. 2007. Queen pheromone modulates brain dopamine function in worker honey bees. Proc. Natl Acad. Sci. USA 104, 2460–2464 10.1073/pnas.0608224104 (doi:10.1073/pnas.0608224104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beggs K. T., Mercer A. R. 2009. Dopamine receptor activation by honey bee queen pheromone. Curr. Biol. 19, 1206–1209 10.1016/j.cub.2009.05.051 (doi:10.1016/j.cub.2009.05.051) [DOI] [PubMed] [Google Scholar]
- 105.Vergoz V., Schreurs H. A., Mercer A. R. 2007. Queen pheromone blocks aversive learning in young worker bees. Science 317, 384–386 10.1126/science.1142448 (doi:10.1126/science.1142448) [DOI] [PubMed] [Google Scholar]
- 106.Keller L. 2009. Adaptation and the genetics of social behaviour. Phil. Trans. R. Soc. B 364, 3209–3216 10.1098/rstb.2009.0108 (doi:10.1098/rstb.2009.0108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alaux C., Le Conte Y., Adams H. A., Rodriguez-Zas S., Grozinger C. M., Sinha S., Robinson G. E. 2009. Regulation of brain gene expression in honey bees by brood pheromone. Genes Brain Behav. 8, 309–319 10.1111/j.1601-183X.2009.00480.x (doi:10.1111/j.1601-183X.2009.00480.x) [DOI] [PubMed] [Google Scholar]
- 108.Alaux C., et al. 2009. Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc. Natl Acad. Sci. USA 106, 15 400–15 405 10.1073/pnas.0907043106 (doi:10.1073/pnas.0907043106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pham-Delegue M. H., Trouiller J., Caillaud C. M., Roger B., Masson C. 1993. Effect of queen pheromone on worker bees of different ages: behavioural and electrophysiological responses. Apidologie 24, 267–281 10.1051/apido:19930307 (doi:10.1051/apido:19930307) [DOI] [Google Scholar]
- 110.Vergoz V., McQuillan H. J., Geddes L. H., Pullar K., Nicholson B. J., Paulin M. G., Mercer A. R. 2009. Peripheral modulation of worker bee responses to queen mandibular pheromone. Proc. Natl Acad. Sci. USA 106, 20 930–20 935 10.1073/pnas.0907563106 (doi:10.1073/pnas.0907563106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moritz R. F. A., Burgin H. 1987. Group response to alarm pheromones in social wasps and the honeybee. Ethology 76, 15–26 10.1111/j.1439-0310.1987.tb00668.x (doi:10.1111/j.1439-0310.1987.tb00668.x) [DOI] [Google Scholar]
- 112.Robinson G. E. 1987. Modulation of alarm pheromone perception in the honey bee: evidence for division of labor based on hormonally regulated response thresholds. J. Comp. Physiol. 160, 613–619 10.1007/BF00611934 (doi:10.1007/BF00611934) [DOI] [Google Scholar]
- 113.Le Conte Y., Mohammedi A., Robinson G. E. 2001. Primer effects of a brood pheromone on honeybee behavioural development. Proc. R. Soc. Lond. 268, 163–168 10.1098/rspb.2000.1345 (doi:10.1098/rspb.2000.1345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pankiw T., Roman R., Sagili R. R., Zhu-Salzman K. 2004. Pheromone-modulated behavioral suites influence colony growth in the honey bee (Apis mellifera). Naturwissenschaften 91, 575–578 10.1007/s00114-004-0568-y (doi:10.1007/s00114-004-0568-y) [DOI] [PubMed] [Google Scholar]
- 115.Gotzek D., Ross K. G. 2007. Genetic regulation of colony social organization in fire ants: an integrative overview. Q. Rev. Biol. 82, 201–226 10.1086/519965 (doi:10.1086/519965) [DOI] [PubMed] [Google Scholar]
- 116.Dunlap J. C., Loros J. J., DeCoursey P. J. 2004. Chronobiology: biological timekeeping. Sunderland, MA: Sinauer Associates [Google Scholar]
- 117.Bloch G. 2009. Socially mediated plasticity in the circadian clock of social insects. In Organization of insect societies—from genome to sociocomplexity (eds Gadau J., Fewell J.), pp. 402–431 Cambridge, MA: Harvard University Press [Google Scholar]
- 118.Crailsheim K., Hrassnigg N., Stabentheiner A. 1996. Diurnal behavioural differences in forager and nurse honey bees (Apis mellifera carnica Pollm). Apidologie 27, 235–244 10.1051/apido:19960406 (doi:10.1051/apido:19960406) [DOI] [Google Scholar]
- 119.Kaiser W. 1988. Busy bees need rest, too: behavioral and electromyographical sleep signs in honeybees. J. Comp. Physiol. A 163, 565–584 10.1007/BF00603841 (doi:10.1007/BF00603841) [DOI] [Google Scholar]
- 120.Klein B. A., Olzsowy K. M., Klein A., Saunders K. M., Seeley T. D. 2008. Caste-dependent sleep of worker honey bees. J. Exp. Biol. 211, 3028–3040 10.1242/jeb.017426 (doi:10.1242/jeb.017426) [DOI] [PubMed] [Google Scholar]
- 121.Moore D., Angel J. E., Cheeseman I. M., Fahrbach S. E., Robinson G. E. 1998. Timekeeping in the honey bee colony: integration of circadian rhythms and division of labor. Behav. Ecol. Sociobiol. 43, 147–160 10.1007/s002650050476 (doi:10.1007/s002650050476) [DOI] [Google Scholar]
- 122.Shemesh Y., Cohen M., Bloch G. 2007. Natural plasticity in circadian rhythms is mediated by reorganization in the molecular clockwork in honeybees. FASEB J. 21, 2304–2311 10.1096/fj.06-8032com (doi:10.1096/fj.06-8032com) [DOI] [PubMed] [Google Scholar]
- 123.Meshi A., Bloch G. 2007. Monitoring circadian rhythms of individual honey bees in a social environment reveals social influences on postembryonic ontogeny of activity rhythms. J. Biol. Rhythms 22, 343–355 10.1177/0748730407301989 (doi:10.1177/0748730407301989) [DOI] [PubMed] [Google Scholar]
- 124.Moore A. D. 2001. Honey bee circadian clocks: behavioral control from individual workers to whole-colony rhythms. J. Insect Physiol. 47, 843–857 10.1016/S0022-1910(01)00057-9 (doi:10.1016/S0022-1910(01)00057-9) [DOI] [Google Scholar]
- 125.Bloch G., Robinson G. E. 2001. Chronobiology. Reversal of honeybee behavioural rhythms. Nature 410, 1048. 10.1038/3507418335074183pii (doi:10.1038/3507418335074183pii) [DOI] [PubMed] [Google Scholar]
- 126.Bloch G., Toma D. P., Robinson G. E. 2001. Behavioral rhythmicity, age, division of labor and period expression in the honey bee brain. J. Biol. Rhythms 16, 444–456 10.1177/074873001129002123 (doi:10.1177/074873001129002123) [DOI] [PubMed] [Google Scholar]
- 127.Shemesh Y., Eban-Rothschild A., Cohen M., Bloch G. 2010. Molecular dynamics and social regulation of context-dependent plasticity in the circadian clockwork of the honey bee. J. Neurosci. 30, 12517–12525 10.1523/JNEUROSCI.1490-10.2010 (doi:10.1523/JNEUROSCI.1490-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dunlap J. C. 1999. Molecular bases for circadian clocks. Cell 96, 271–290 [DOI] [PubMed] [Google Scholar]
- 129.Rubin E. B., Shemesh Y., Cohen M., Elgavish S., Robertson H. M., Bloch G. 2006. Molecular and phylogenetic analyses reveal mammalian-like clockwork in the honey bee (Apis mellifera) and shed new light on the molecular evolution of the circadian clock. Genome Res. 16, 1352–1365 10.1101/gr.5094806 (doi:10.1101/gr.5094806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bloch G., Rubinstein C. D., Robinson G. E. 2004. Period expression in the honey bee brain is developmentally regulated and not affected by light, flight experience, or colony type. Insect Biochem. Mol. Biol. 34, 879–891 10.1016/j.ibmb.2004.05.004S0965174804000839pii (doi:10.1016/j.ibmb.2004.05.004S0965174804000839pii) [DOI] [PubMed] [Google Scholar]
- 131.Bloch G. 2008. The functional organization of size-related division of labor in bumblebees—from circadian rhythms and phototaxis to gene expression. In Proc. of the XXIII Int. Congress of Entomology, Durban, South Africa, 6–12 July 2008, p. 462 [Google Scholar]
- 132.Ingram K. K., Krummey S., LeRoux M. 2009. Expression patterns of a circadian clock gene are associated with age-related polyethism in harvester ants, Pogonomyrmex occidentalis. BMC Ecol. 9, 7. 10.1186/1472-6785-9-7 (doi:10.1186/1472-6785-9-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jong J. J., Lee H. J. 2008. Differential expression of circadian locomotor rhythms among castes of the gray-black spiny ant, Polyrhachis dives (Hymenoptera: Formicidae). Sociobiology 52, 167–184 [Google Scholar]
- 134.Weiss R., Dov A., Fahrbach S. E., Bloch G. 2009. Body size-related variation in pigment dispersing factor-immunoreactivity in the brain of the bumblebee Bombus terrestris (Hymenoptera, Apidae). J. Insect Physiol. 55, 479–487 10.1016/j.jinsphys.2009.01.016 (doi:10.1016/j.jinsphys.2009.01.016) [DOI] [PubMed] [Google Scholar]
- 135.Bloch G., Solomon S. M., Robinson G. E., Fahrbach S. E. 2003. Patterns of PERIOD and pigment-dispersing hormone immunoreactivity in the brain of the European honeybee (Apis mellifera): age- and time-related plasticity. J. Comp. Neurol. 464, 269–284 10.1002/cne.10778 (doi:10.1002/cne.10778) [DOI] [PubMed] [Google Scholar]
- 136.Lin T., Lee H. 1998. Parallel control mechanisms underlying locomotor activity and sexual receptivity of the female German cockroach, Blattella germanica (L.). J. Insect Physiol. 44, 1039–1051 [DOI] [PubMed] [Google Scholar]
- 137.Eban-Rothschild A., Belluci S., Bloch G. 2011. Maternity-related plasticity in circadian rhythms of bumble-bee queens. Proc. R. Soc. B (in press) (doi:10.1098/rspb.2011.0579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCluskey E. S. 1967. Circadian rhythms in female ants, and loss after mating flight. Comp. Biochem. Physiol. 23, 665–677 10.1016/0010-406X(67)90418-5 (doi:10.1016/0010-406X(67)90418-5) [DOI] [PubMed] [Google Scholar]
- 139.Sharma V. K., Lone S. R., Goel A. 2004. Clocks for sex: loss of circadian rhythms in ants after mating? Naturwissenschaften 91, 334–337 10.1007/s00114-004-0526-8 (doi:10.1007/s00114-004-0526-8) [DOI] [PubMed] [Google Scholar]
- 140.Sharma V. K., Lone S. R., Goel A., Chandrashekaran M. K. 2004. Circadian consequences of social organization in the ant species Camponotus compressus. Naturwissenschaften 91, 386–390 10.1007/s00114-004-0544-6 (doi:10.1007/s00114-004-0544-6) [DOI] [PubMed] [Google Scholar]
- 141.Free J. B., Ferguson A. W., Simpkins J. R. 1992. The behaviour of queen honeybees and their attendants. Physiol. Entomol. 17, 43–55 10.1111/j.1365-3032.1992.tb00988.x (doi:10.1111/j.1365-3032.1992.tb00988.x) [DOI] [Google Scholar]
- 142.Harano K., Sasaki M., Sasaki K. 2007. Effects of reproductive state on rhythmicity, locomotor activity and body weight in the European honeybee, Apis mellifera queens (Hymenoptera, Apini). Sociobiology 50, 189–200 [Google Scholar]
- 143.Bloch G., Meshi A. 2007. Influences of octopamine and juvenile hormone on locomotor behavior and period gene expression in the honeybee, Apis mellifera. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 193, 181–199 10.1007/s00359-006-0179-5 (doi:10.1007/s00359-006-0179-5) [DOI] [PubMed] [Google Scholar]
- 144.Bloch G., Sullivan J. P., Robinson G. E. 2002. Juvenile hormone and circadian locomotor activity in the honey bee Apis mellifera. J. Insect Physiol. 48, 1123–1131 10.1016/S0022-1910(02)00205-6 (doi:10.1016/S0022-1910(02)00205-6) [DOI] [PubMed] [Google Scholar]
- 145.Zheng X., Yang Z., Yue Z., Alvarez J. D., Sehgal A. 2007. FOXO and insulin signaling regulate sensitivity of the circadian clock to oxidative stress. Proc. Natl Acad. Sci. USA 104, 15 899–15 904 10.1073/pnas.0701599104 (doi:10.1073/pnas.0701599104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Green C. B., Takahashi J. S., Bass J. 2008. The meter of metabolism. Cell 134, 728–742 10.1016/j.cell.2008.08.022 (doi:10.1016/j.cell.2008.08.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ikeya T., Galic M., Belawat P., Nairz K., Hafen E. 2002. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 12, 1293–1300 [DOI] [PubMed] [Google Scholar]
- 148.Oldham S., Hafen E. 2003. Insulin/IGF and target of rapamycin signaling: a TOR de force in growth control. Trends Cell Biol. 13, 79–85 10.1016/S0962-8924(02)00042-9 (doi:10.1016/S0962-8924(02)00042-9) [DOI] [PubMed] [Google Scholar]
- 149.Grimaldi D., Engel M. S. 2005. Evolution of the insects. Cambridge, London: Cambridge University Press [Google Scholar]
- 150.Werren J. H., et al. 2010. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science 327, 343–348 10.1126/science.1178028 (doi:10.1126/science.1178028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bonasio R., et al. 2010. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science 329, 1068–1071 10.1126/science.1192428 (doi:10.1126/science.1192428) [DOI] [PMC free article] [PubMed] [Google Scholar]