Abstract
Virtually all species have developed cellular oscillations and mechanisms that synchronize these cellular oscillations to environmental cycles. Such environmental cycles in biotic (e.g. food availability and predation risk) or abiotic (e.g. temperature and light) factors may occur on a daily, annual or tidal time scale. Internal timing mechanisms may facilitate behavioural or physiological adaptation to such changes in environmental conditions. These timing mechanisms commonly involve an internal molecular oscillator (a ‘clock’) that is synchronized (‘entrained’) to the environmental cycle by receptor mechanisms responding to relevant environmental signals (‘Zeitgeber’, i.e. German for time-giver). To understand the evolution of such timing mechanisms, we have to understand the mechanisms leading to selective advantage. Although major advances have been made in our understanding of the physiological and molecular mechanisms driving internal cycles (proximate questions), studies identifying mechanisms of natural selection on clock systems (ultimate questions) are rather limited. Here, we discuss the selective advantage of a circadian system and how its adaptation to day length variation may have a functional role in optimizing seasonal timing. We discuss various cases where selective advantages of circadian timing mechanisms have been shown and cases where temporarily loss of circadian timing may cause selective advantage. We suggest an explanation for why a circadian timing system has emerged in primitive life forms like cyanobacteria and we evaluate a possible molecular mechanism that enabled these bacteria to adapt to seasonal variation in day length. We further discuss how the role of the circadian system in photoperiodic time measurement may explain differential selection pressures on circadian period when species are exposed to changing climatic conditions (e.g. global warming) or when they expand their geographical range to different latitudes or altitudes.
Keywords: circadian system, photoperiodism, cyanobacteria, seasonal adaptation, suprachiasmatic nucleus, chronobiology
1. Empirical evidence for a selective advantage of circadian rhythms around 24 h
As a result of combined evolutionary pressures, almost all species have developed circadian timing systems that show persistent oscillations in molecular processes, even in constant conditions. Commonly, the observed periods are close to 24 h, hence the term ‘circadian’. Among the species that developed circadian rhythms are cyanobacteria (Synechococcus elongatus), one of the earliest and most primitive organisms known. In itself, this observation suggests that during the early development of life on Earth, rhythms with periods close to the light–dark cycle already generated an evolutionary advantage. The hypothesis of optimization of circadian period was tested in competition experiments under light–dark cycles of different periods with several S. elongatus mutants, each with different intrinsic circadian periods [1,2]. In these experiments, the reproductive success has been quantified of the various strains under different experimental circumstances. It was found that the strain that had a period of its timing system closest to the applied light–dark cycle survived best. Under rhythmic conditions, rhythmic strains outcompeted arrhythmic strains. Under constant light conditions, there were no systematic differences in reproductive success, neither between rhythmic and arrhythmic strains nor between rhythmic strains with different periods. So there is clear adaptive value to the period of the circadian timing system. This may have led to the selection of the KaiA, KaiB and KaiC molecules that are known to play a prominent role in the circadian system of S. elongatus.
In a world with large daily fluctuation in energy supply, it is likely that all cells, including individual cells in complex organisms, have to perform a similar task of harvesting and storing energy when it is available and using up their stores when external energy supplies are insufficient. Yet, in complex organisms, the pathways of energy supply may be many, and the supply may come from various sources and at different times, depending on the specific tasks of the cells and their embedding in the organism. Therefore, in other species, the molecular processes on which the oscillations are based may be very different from cyanobacteria, and may even have evolved independently [3]. Yet, each cell will separately sense fluctuations in energy supply and will adjust its own temporal pattern in response to those fluctuations. In this way, complex organisms might be composed of cells that are rhythmic with a 24 h period but with different timing between the cells, each depending on their specific environments. This is indeed what is observed: many cell types of complex organisms, like mammals, show intrinsic circadian oscillation, but the phases of these oscillations differ between cell types [4]. Yet, in most complex organisms investigated (except plants), it has been observed that a central pacemaker has evolved; a structure that apparently has the specific task to keep track of environmental time and provide that time information to the rest of the body. Other cells use that time information as a reference to influence their own behaviour, albeit in different ways for different cells.
In mammals, this central pacemaker is located in the suprachiasmatic nucleus (SCN) of the hypothalamus [5,6]. Transplantations of SCN tissue between mutant hamsters with different periods of their locomotor activity in constant conditions have proved the circadian pacemaker function of the SCN beyond any doubt [7]. The SCN sets the pace of downstream processes. Since circadian pacemakers have evolved, it seems obvious that they provide fitness value to the species. Yet, experiments to demonstrate the fitness value of having a circadian pacemaker are almost absent. One important exception is an experiment in chipmunks by DeCoursey et al. [8]. She compared individuals having an intact timing system with individuals in which the timing system was shut down by lesioning of the SCN. SCN-lesioned animals and sham-operated animals were reintroduced in nature and their survival was monitored over time. It turned out that SCN-lesioned as well as sham-lesioned animals initially had a hard time, possibly owing to the fact that their territories were taken over by other animals during the few days that were needed for the surgery. After two weeks the survival curves stabilized, but the SCN-lesioned animals persisted to show higher mortality. This suggests specific fitness value of having an intact circadian pacemaker. It is true that this study focused on survival and not on pure fitness consequences [9]. Yet, it is clear that most animals of the SCN-lesioned group did not survive long enough to be able to produce and raise a litter, and therefore it seems to be justified for this specific study to extrapolate the results on survival to fitness.
Several other studies have been performed to test whether a species is optimally adjusted to an environment with 24 h cyclicity against other periods. Fruitflies [10] and blowflies [11] live longer under a 24 h light–dark cycle than under various other periods or under continuous light conditions. Tomatoes grow faster under a 24 h light–dark cycle than under shorter or longer cycles or even in continuous light [12–14]. But this is not universal. Arabidopsis grows faster in continuous light [15]. Yet, even if a species grows faster in a different environment, this does not exclude the possibility that it is optimally adjusted to the environmental circumstances where it lives. Furthermore, growth rate may not be related to fitness and it remains therefore important to test directly the selective advantage of circadian rhythms in other species through competition experiments [9].
2. Early evolution of circadian timing mechanisms: ‘escape from light’ or energy storage?
In the early development of life on our planet, the emerging photosynthesizing organisms that were exposed to sunlight faced several major problems owing to the alternation between day and night. They had to have access to energy throughout the day and night, and they had to protect themselves against damage by ultraviolet (UV) light [16], which was not filtered by the Earth's atmosphere around 3–4 billion years ago when early life emerged [17]. In those cases where the usable form of energy (e.g. sunlight) was not constantly available, organisms had to harvest energy when available and store it for later use. For organisms that relied on photosynthesis, the solutions to these problems must have led almost automatically to rhythmic behaviour at the molecular level. During the day, light had to be absorbed for the formation of ATP through photosynthesis. Part of this ATP is generally used for primary cell functions such as division and growth, whereas another part somehow had to be stored for usage during the subsequent night when the energy supply through photosynthesis was lacking. Simultaneously, the organism's DNA had to be protected against UV radiation during the day. If this protection required mechanisms that interfered with transcription, those mechanisms should have been reversed during the night. Thus, night and day required different fundamental molecular processes.
For cyanobacteria, which are among the most ancient photosynthesizing organisms on Earth, it has recently been proposed that their circadian timing system might have evolved to protect DNA from UV radiation [18,19]. This conjecture explains that the KaiC protein, as a key player in the evolution of timing systems, appeared about 3.5 billion years ago [17]. In these early organisms, KaiC forms hexamers in the presence of ATP during the light phase of the day. These hexamers seem to be involved in the compaction process of the DNA. Simons argues that this compaction protects against UV damage. During the night the hexamers fall apart, thereby allowing unfolding of the DNA. This conjecture is largely a specification of the ‘escape from light hypothesis’, put forward by Pittendrigh [20] and later studied by Hastings and co-workers [21,22]; for a review see [16]. Simons proposes that KaiB and KaiA, the other key players in the molecular circadian oscillator of the cyanobacterium S. elongatus, emerged much later in evolution, KaiB for the purpose of signalling the end of the day, and KaiA to turn the system into a self-sustained oscillation, enabling cyanobacteria to anticipate the day–night cycle rather than just responding to the presence of light.
A recent major breakthrough in the development of our knowledge on circadian regulation is the observation by Kondo's group that DNA transcription is not required for obtaining molecular circadian oscillations in the Kai-system. They have demonstrated that it is sufficient for circadian oscillations in KaiC phosphorylation level to emerge in a test tube, when ATP is added to the proteins KaiA, KaiB and KaiC [23]. The resulting mixture shows oscillations in concentration of the various molecular complexes with large amplitudes and with a period close to 24 h. The resulting period is only marginally dependent on environmental temperature. In addition, measured circadian periods in vitro depended on specific mutations in the Kai proteins, which paralleled periods found in vivo. The apparent post-translational oscillation in the test tube suggests a major role for a similar oscillation in the cells, albeit possibly modified and shaped by transcriptional regulation [19,24].
Mechanistically, the idea is that KaiC occurs in its monomeric form at the beginning of the day. Simultaneously, ATP is produced through photosynthesis. Six KaiC monomers form a hexamer for which they use 12 ATP molecules, not as energy supply but as building blocks [25]. Apart from using ATP as structural elements in the hexamer complex, additional ATP is also used to phosphorylate KaiC. Each KaiC molecule has two phosphorylation sites and KaiA promotes their subsequent phosphorylation in the course of the light phase. Phosphorylation creates additional hydrogen bonds between the six monomers and tightens the hexamer [26,27]. With increasing phosphorylation of the hexamers at the end of the day, KaiB is more likely to attach to the molecular complex. The KaiABC complex is likely to undergo a conformational change, after which phosphorylation is more difficult, and dephosphorylation occurs during the night [28]. Upon dephosphorylation, the hexamers more easily disintegrate. During the night, the amount of KaiC decreases [29]. Upon complete dephosphorylation, the conformational state changes back to the original form, and the remaining KaiC molecules will be phosphorylated again during the next day.
Simons [18] suggested that a major function of the circadian oscillation is to regulate compaction and decompaction of the DNA, in order to avoid UV-induced DNA damage [24]. Records of DNA compaction and decompaction [29] clearly show that in modern cyanobacteria compaction occurs at night, not during the day. Of course, the current timing of the DNA compaction cycle may be due to a more recent adaptation to the altered atmosphere. The phase of the cycle may have been different 3.5 billion years ago. Apart from DNA protection, however, we suggest that another evolutionary function of the phosphorylation–dephosphorylation cycle of KaiC in cyanobacteria was to store ATP during the day and supply ATP at night. We suggest that ATP storage was the primary reason to develop circadian oscillations.
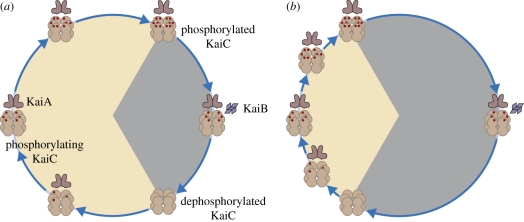
In short (figure 1), we propose that hexamerization of KaiC during the photoperiod served to lock in ATP at a time during evolution when we think that other mechanisms for ATP storage (starch and sugar) had not yet evolved.
Figure 1.
Postulated temporal patterns of phosphorylation and dephosphorylation of KaiC in (a) summer and (b) winter. The phosphorylation phase is hypothesized to be stimulated by KaiA, starting with dephosphorylated KaiC hexamers at dawn and reaching fully phosphorylated hexamers at dusk.
Since ATP was generated by light exposure, the storage should occur in daytime. At night, in the absence of ATP production, the cells released ATP from the store. This release had to be controlled in order to provide the cells with an even flow of ATP. Phosphorylation has that effect. At the beginning of the night, phosphorylation of KaiC is maximal leading to a tight locking in of ATP. As a consequence, a relatively small fraction of the large number of available hexamers dissociates at the beginning of the night. At the end of the night, a relatively larger fraction of the smaller number of remaining hexamers dissociates. This results in a more or less even flow of ATP. Obviously, if the function of this mechanism indeed is to provide ATP at night, the ATP should become available for, say, housekeeping mechanisms, not for rephosphorylating the just dephosphorylated sites of the KaiC molecules. This may be the reason that the two phosphorylations and dephosphorylation per day per KaiC molecule occur in a specific order. Owing to this, rephosphorylation can only occur after complete dephosphorylation [30].
(a). KaiB: a signal of the night phase?
KaiB probably emerged between 2.3 and 3.5 billion years ago [17], much earlier than KaiA. In modern S. elongatus, KaiB binds to hexamers in a specific phosphorylation status, which seems to occur roughly half way through the dark phase (figure 1; [31]). This behaviour is very suitable to signal a specific phase of the cycle in order to trigger relevant processes at that time [18,19]. This signal might ultimately be used to block cell division at night when energy supplies might be limiting to support new cells. Blocking of cell division in S. elongatus was indeed found to be under circadian control. Exposure to constant light revealed a steady increase in population size, except for short intervals of about 3 h that appeared at about 24 h intervals [32–34]. The data show that the 3 h intervals without cell division occur at the very end of the subjective day. Under normal 12 h light : 12 h dark conditions, this leads to the situation that cell division stops 3 h before dusk. During the night, cells do not divide either, but this might be a direct response to the absence of light. At dawn, cell division does not immediately reappear. Apparently, the relevant processes need some time to resume. It might be that KaiB evolved to (indirectly) suppress cell division at the end of the day, at a time when no direct environmental signals are available to trigger this response. This signalling pathway can be considered a clock function: the mechanism is supposed to tell time to the organism through an endogenous mechanism, at a time when direct environmental signals are not available.
(b). KaiA: an adaptation to photoperiod variation?
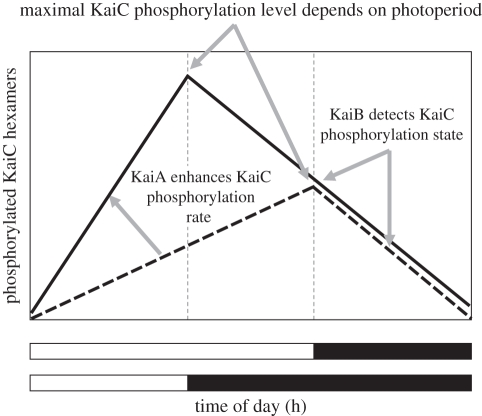
About 1 billion years ago, KaiA appeared [17]. KaiA is known to accelerate KaiC phosphorylation (figure 2; [26,27]). While gene transcription of KaiB and KaiC depends on the same promoter, activation of the KaiA gene is controlled by a different promoter [35], reviewed in Williams [29]. We suggest several possible evolutionary advantages for the development of KaiA. In vitro, KaiA has been demonstrated to increase phosphorylation by a factor of approximately 2.5 in comparison with phosphorylation in the absence of KaiA [26,27]. If this also applies to the in vivo situation, more hexamers may reach the fully phosphorylated state in the presence of KaiA, which will change the timing of the KaiB signal to block cell division. This leads to a functional interpretation of the evolution of KaiA. We propose that KaiA serves to modulate the rate of phosphorylation of KaiC in order to meet the fluctuating demands of changing photoperiod across the seasons (figure 2). Hence, the development of KaiA might have provided S. elongatus and similar cyanobacteria with a selective advantage by generating the capacity to invade areas at different latitudes. This hypothesis can be tested experimentally, by competition experiments in which different KaiA mutants compete with each other under different photoperiods.
Figure 2.
Proposed role of the Kai proteins in evolutionary adaptation to photoperiod. Both in short and in long days, the number of phosphorylated KaiC hexamers decreases at about the same constant low rate. In short days this process lasts longer, so more hexamers must be formed in the day, requiring more KaiC production per hour than in long days and more phosphorylation. Since phosphorylation of KaiC must be complete at the end of the shorter day, KaiA is expected to be present at an even higher concentration in short days. If KaiB signals the dephosphorylation status of the hexamer complexes, this will happen during the night, both in short and in long days.
3. Adjustment to changing day length as a selection force for the circadian system
Although it is likely that circadian timing systems originally evolved on the basis of daily changes in the environment, there is another important function of the circadian timing system, and that is the regulation of annual rhythms in physiology and behaviour [36]. The duration of photoperiod can indicate the time of year, which relates to environmental changes in temperature and food availability. Timing of migration, moult, hibernation and, most importantly, reproduction are behaviours that are adapted to annual changes in the environment to maximize survival and offspring production at temperate climatic zones. Because these behaviours directly relate to fitness, it can be expected that selection pressures for optimal annual timing are strong. Therefore, evolution will have shaped the readouts from the circadian clock to optimize annual timing.
Theoretically, photoperiod can be assessed without a circadian oscillator being present. Organisms that are exposed to the full light phase of the day could have developed systems by which they directly measure photoperiod. However, the evidence so far indicates that all organisms (plants, insects, mammals and birds) that respond to photoperiod changes use a circadian timing mechanism to do so (see [37] for detailed overview). Circadian influence in photoperiodism does not exclude mechanisms of direct responses to day length. This is best exemplified by melatonin secretion in the mammalian pineal. The duration of melatonin secretion during the night is critical in driving downstream photoperiod responses, but melatonin secretion itself is both driven by the circadian system and directly suppressed by light. The most parsimonious explanation for the ubiquitous involvement of a circadian oscillator in photoperiodic responses is that such timing mechanisms were present early in evolution and started to form an internal representation of the day–night cycle. This internal representation now formed an adequate buffering against day-to-day fluctuations in light conditions, yielding a much more reliable seasonal signalling.
(a). Latitudinal clines in circadian parameters
Seasonal modulation of the environment increases at latitudes closer to the poles. The most reliable signal to indicate the time of year is photoperiod, but the relationship between photoperiod and time of year depends on latitude [38]. The amplitude of the annual environmental temperature rhythm also increases with latitude [39]. Species that occur along a wide range of latitudes, therefore, have to adapt to the combined annual changes of temperature and photoperiod in order to optimize annual timing of reproduction. In some species, this has apparently led to latitudinal differences in characteristics of the circadian timing system. Together, these correlative observations between latitude and circadian characteristics form a strong indication that photoperiodic response mechanisms generate a major selection pressure for the circadian system, which, in itself, indicates also a crucial role for the circadian system in annual timing.
One example was found in Arabidopsis. This model plant shows a latitudinal cline in free-running period of its circadian timing system [40], with the period being longer at higher latitudes. A similar positive correlation between circadian period and latitude was found in Drosophila auraria [41], and Drosophila ananassae [42], while Lankinen & Forsman [43] found a negative correlation between free-running period and latitude in Drosophila littoralis.
Other examples of latitudinal clines can be found in the molecular makeup of the circadian system such as polymorphisms in clock genes. Latitudinal clines in circadian clock gene expression levels or clock gene polymorphisms were found in birds [44,45], fish [46] and insects [47–50].
Extreme situations occur in polar regions, where light levels are more or less constant for long periods of time. It has been demonstrated that reindeer (Rangifer tarandus) show daily modulation of behaviour only in spring and in autumn. During continuous darkness in winter and continuous light in summer, they show no 24 h modulation of their rest–activity pattern [51,52] or plasma melatonin levels [53,54]. Apparently, the lack of 24 h rhythmicity in the environment at some parts of the year leads this species to a complete or partial loss of the circadian system at the molecular level [54]. The selective advantage of losing circadian organization under these conditions might be to optimize food intake and ruminant digestion efficiency by a more frequent ultradian organization of feeding [52]. Similar temporal loss of daily organization in an arctic species has also been found in the Svalbard ptarmigan (Lagopus mutus hyperboreus; [55]), but the results in both species do not mean that all species in arctic regions lose circadian organization during the arctic summer or winter. Arctic ground squirrels (Spermophilus parryi, a hind gut fermenter), for instance, maintain a 24 h activity–rest cycle during arctic mid-summer under continuous light conditions well above the polar circle [56,57].
Taken together, the reindeer and ptarmigan data seem to suggest that a circadian system is only advantageous when the environment has a strong (continuous) 24 h rhythm. These conclusions are fully in line with the selection experiments in cyanobacteria [1,2], but do not explain why arctic ground squirrels maintain circadian organization during the arctic summer. One possible explanation may reside in the strong circannual organization of obligate seasonal hibernation in ground squirrels. Correct timing of hibernation onset and offset is essential to ground squirrels, and a continuous functionality of the circadian (and melatonin) system throughout the summer may therefore be essential to this species. No matter the specific evolutionary advantages of the disappearance of circadian rhythmicity in locomotor activity of reindeer in summer and winter, the data do demonstrate an influence of photoperiod on melatonin [53,54], indicating that the crucial role for melatonin in photoperiodism is preserved. While much of the physiology underlying annual changes in reproduction or moult in mammals are regulated in other areas (like the pituitary and its pars tuberalis [58]), it is commonly assumed that the SCN (at least in mammals outside the arctic region) is involved in the detection of annual changes in photoperiod, and that this serves as a trigger to induce these behaviours. This notion is based on lesion studies in hamsters, showing that photoperiod-induced gonadal regression requires an intact pathway from SCN to the pineal gland [58–65]. The duration of melatonin production in the pineal gland is the signal that regulates gonadal growth or regression [58,66–68], and this melatonin signal is shaped by the SCN through the detection of photoperiod [69].
(b). The evolutionary importance of annual timing and the role of the suprachiasmatic nucleus
Many important events that may have direct fitness consequences are regulated on an annual basis, including reproduction, migration and hibernation. Deviations from optimal seasonal timing will have strong repercussions for survival and reproductive output. Thus, an accurate annual timing mechanism must have been heavily selected for through evolution. In seasonal organisms living in temperate zones, the adaptive significance of the circadian system may lie in its day length signalling capacity rather than in its daily timing function.
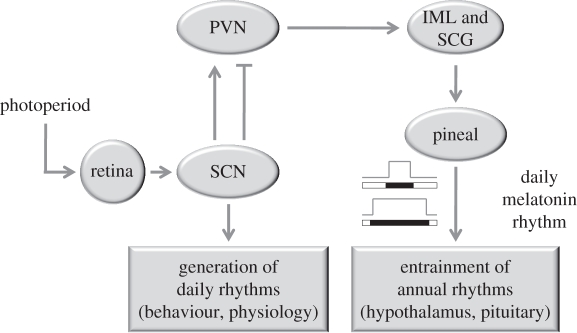
Most body cells show circadian rhythms, which in mammals are coordinated by a central hypothalamic pacemaker, the SCN [4]. To synchronize the body's internal physiology and behaviour to the external daily environmental light cycle, the SCN is facing two tasks: daily synchronization to the light–dark cycle, and adaptation to changing day length over the year (figure 3). The first task drives daily timing (e.g. sleep and activity), the second drives a nocturnal pineal melatonin signal that acts as an input signal to annual timing systems driving seasonal responses in physiology and behaviour (figure 3; [70]).
Figure 3.
The dual role of the SCN in daily and annual rhythms as illustrated by its input and output pathways. Arrow connection ends indicate stimulatory pathways, flat connection ends indicate inhibitory pathways. SCN, suprachiasmatic nucleus; PVN, paraventricular nucleus; IML, intermediolateral column of the spinal cord; SCG, superior cervical ganglion.
Interactions between both functions are illustrated by annual cycles in daily rhythms under (semi-) natural conditions [38,51,52,71–73]. The heterogeneous structure of the SCN and its differentiation between species and sexes [74–80] offers the fascinating possibility that the SCN carries differentiated neuroanatomical substrates for daily and annual timing.
Annual variation in melatonin signal duration has been shown to be a critical input signal for the annual timing mechanism [81,82] that involves the pituitary and its pars tuburalis (PT), which is densely packed with melatonin receptors [83–89]. Pineal melatonin release is driven by the SCN via the paraventricular nucleus (PVN), superior cervical ganglion (SCG) and intermediolateral column of the spinal cord (IML) pathway [58,69]. Pharmacological infusions at the PVN have shown the involvement of nocturnal glutamate excitatory signalling and diurnal GABA (γ-aminobutyric acid) inhibitory signalling to PVN neurons that both shape the melatonin signal to signal the length of the night [61–65].
Selection on annual timing can take place at different levels in the melatonin signalling cascade (figure 3), but most importantly also in the melatonin recipient areas like the PT. Good advances are currently being made in elucidating the molecular mechanism of melatonin signalling in the PT. Although these mechanisms also involve clock genes like Cry1 and Per1 [83,90,91], there are other genes involved that might be subject to selection on annual timing. For this paper, we will limit ourselves to discussing selection on day length measurement on the level of the circadian system.
Recent findings about the characteristics of pacemaker cells within the SCN of several mammalian species may lead to a better understanding of how the SCN might be capable of measuring photoperiod. The issue here is that cells within the SCN appear to show substantial differences with respect to the timing of their activity within the day [92]. While most cells are electrically active during the day, some cells (as deduced from electrical discharge and period gene expression rhythms) seem to be linked to dawn and others are linked to dusk [93]. The phase distribution of SCN cells seems to be organized along the rostro-caudal gradient, where morning cells are dominating the caudal region of the SCN and evening cells are dominating the mid-rostral region of the SCN [94–97]. A compression of photoperiod brings those cell groups closer together in time, while an increase in photoperiod moves them apart [93,98–100]. In this way, the duration of SCN activity becomes related to photoperiod, but so far it is unknown whether those SCN neurons that spread their internal phase relationship under long photoperiods are also involved in shaping the melatonin signal. Furthermore, it is tempting to match this rostro-caudal phase gradient in the SCN to the previously proposed morning–evening model of photoperiod adaptation as proposed by Pittendrigh & Daan [101], but it remains to be established whether the circadian properties of rostral and caudal SCN cells follow the predictions specified for the morning and evening oscillator [101,102].
(c). Selection pressures on intrinsic circadian period: arguments from annual timing
In virtually all circadian systems studied so far, environmental light–dark cycles tune the intrinsic period of the system (τ) to the external period of 24 h. In other words, the organism is synchronized to the outside world owing to the interaction of a response to external light and the organism's intrinsic period. Organisms with τ close to 24 h have a selective advantage over conspecifics that deviate considerably from 24 h (see §1). However, τ measured under continuous darkness may vary between individuals of the same species, and even more so between species. In addition, intrinsic τ within a species was found to vary with latitude (see §3(a)). These arguments indicate that τ was subject to species and environment-specific selection pressures, not tuning τ simply to 24 h, but values slightly away from 24 h to match to specific needs of the organism and maximize fitness through higher survival or reproductive output. There has been surprisingly little debate on what those selection pressures on intrinsic circadian period could be and how these selection mechanisms might work. Here, we will make an attempt to predict qualitative changes in selection pressures with varying environmental conditions, by considering two distinct hypotheses of the role of the circadian system in annual timing: the internal and external coincidence timing models.
(d). Leading and lagging circadian systems: consequences for timing
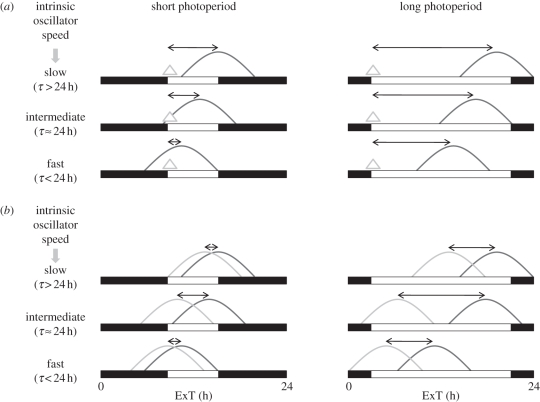
Before zooming in on relationships between circadian and circannual timing, it is important to understand the consequences of differences between the period, T, of the external Zeitgeber, and the endogenous period, τ, of the organism. If T > τ, the overt period of the organism has to lengthen in order to become equal to T. Since the external signal has the effect of slowing down the internal oscillation, the phase of the internal oscillation will be earlier than the phase of the entraining signal. In other words, the internal oscillation will lead relative to the Zeitgeber. By contrast, if T < τ, the internal oscillation will lag relative to the Zeitgeber (figure 4a). As a consequence, under real-life 24 h conditions, the intrinsic period of the oscillator determines to some extent the time of day at which certain events of the internal cycle occur [103]. Correlations between circadian period deviations from 24 h and stability of the circadian rhythm [104] may be important for the organism. Additionally, deviation between the external period and the internal circadian period reduces fitness in bacterial competition experiments [2], and reduces longevity in mammals [105], both confirming the circadian resonance hypothesis. Such properties of circadian systems may have direct fitness consequences that eventually optimize circadian period, but evidence discussed below suggests that circadian period may also be shaped through its effect on day length measurement and subsequent annual responses in physiology, behaviour and reproduction.
Figure 4.
Oscillator speed affects photoperiodic time measurement: differences between external (a) and internal (b) coincidence timings. (a) In an external coincidence timing model, longer circadian periods (slower speed) will generate larger phase angle differences with morning light (grey triangle) owing to a lagging phase angle of the circadian oscillator relative to the light–dark cycle. Short circadian periods (faster speed) will generate smaller phase angle differences with morning light owing to the leading phase angle of the circadian oscillator relative to the light–dark cycle. Hence, internal representation of day length will be longer in slower pacemakers (the reverse being true when evening light induces the photoperiodic response). (b) In an internal coincidence timing model, the circadian oscillator is composed of (at least) two oscillators, a morning (light grey curve) and an evening (dark grey curve) oscillator. The phase angle difference of these two oscillators determines the internal photoperiod representation. Intrinsic period of the combined system will generate leading and lagging properties of both oscillators causing a compression of the phase angle difference when intrinsic period deviates from 24 h. Hence, internal representation of day length will be longer when pacemaker period approaches 24 h.
Photoperiodic responses are characterized by a sharp transition between short- and long-day responses, indicating strong selection for optimal annual timing. For instance, photoperiodic control of testes growth in hamsters can distinguish between a 1 h change in photoperiod: regressed testes are switched to developing testes in a 12.5 h photoperiod but not in an 11.5 h photoperiod [106]. The involvement of the circadian system in this photoperiodic regulation of testis size is indicated by the observation that hamsters with a short circadian period (owing to the tau mutation) show regressed testes at dark durations of 9 h or longer, whereas their wild-type counterparts need at least 11 h darkness per day to regress their testes [107,108]. In addition, selection experiments in Peromyscus leucopus for short-day response of the reproductive system showed a difference in circadian period between responsive and non-responsive mice [109], although subsequent experiments in continuous darkness might indicate that photoresponsiveness in these strains is also determined by other factors.
Two different models have been postulated to describe the role of the circadian system in photoperiodic time measurement: external coincidence timing [110,111] and internal coincidence timing [101,102,112]. External coincidence timing proposes that the circadian system acts as a single oscillator with a specific phase angle to the onset (or offset) of the light phase of the day. When day length increases, the additional light will eventually coincide with a certain sensitive phase of the oscillator and thereby trigger a long-day response (figure 4a). Internal coincidence timing proposes the existence of (at least) two oscillators, one entraining to dawn and the other one to dusk. When day length increases, the phase angle difference between these two oscillators will also increase, which will eventually trigger a long-day response (figure 4b).
Both external and internal coincidence timing models assume an ‘internal photoperiod representation’, generated by the circadian system in response to the external day length. The two photoperiodic models appear to predict very different effects of external photoperiod on intrinsic photoperiod representation when variation in circadian period, and subsequent leading or lagging phase angles, is considered.
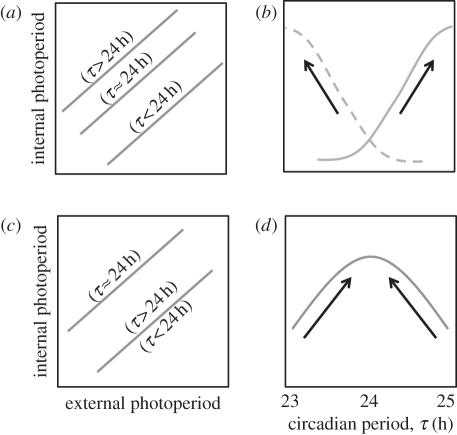
In the external coincidence timing model, consider the case of a sensitive phase in the morning: if light hits the system at this phase, the long-day photoperiod response is triggered. If an individual has a longer intrinsic circadian period (a more lagging phase angle), the sensitive phase will occur at a later time of the day (figure 4a, the reverse being true for external coincidence with evening light). Hence, light will hit the sensitive phase at an earlier day in the season. Phrased differently, long-day photoperiod responses will occur at shorter external photoperiods in animals with longer τ (figure 5a), given that all other things are being equal. Longer circadian periods will, therefore, lengthen the internal photoperiod representation (figure 5b, grey line). Conversely, when evening light induces a photoperiod response, lengthening of circadian period will shorten internal photoperiod (figure 5b, dashed line).
Figure 5.
External (a,b) and internal (c,d) coincidence timing models lead to different predictions for directional selection pressure on circadian period. As follows from figure 4, the relationship between internal and external photoperiods will be differentially affected by circadian period when external (a) or internal (c) coincidence timing is assumed. Plotting internal photoperiod (for a given external photoperiod) against circadian period (τ) yields different curves for external (b) and internal (d) coincidence timing. For external coincidence timing, morning light would generate an inverse relationship between τ and internal photoperiod (b, continuous curve) compare with evening light (b, dashed curve). The curves in (b) and (d) enable predictions on how selection pressure would act on τ when an earlier long-day response is favoured during spring (e.g. at lower latitudes or warmer climatic conditions) for external (b) coincidence and internal (d) coincidence timing. For this example, the directional selection pressures on τ are indicated for both models (black arrows).
In the internal coincidence timing model, both the morning and evening oscillators will tend to lead when intrinsic period shortens and both oscillators will tend to lag when intrinsic period lengthens. Only when intrinsic period approaches 24 h, both oscillators will have no tendency to lead or lag and as a result their phase angle difference will be maximal when τ approaches 24 h (figures 4b and 5c). When internal coincidence timing is assumed, the internal representation of any given external photoperiod is expected to be maximal when τ = 24 h (figure 5d).
Once the influence of intrinsic circadian period on the internal representation of photoperiod is known (figure 5b,d), we can try to establish how selection would act upon intrinsic circadian period when, for instance, a changing selection pressure for earlier reproduction is present. There are at least two environmental conditions where it is relatively easy to see how environmental conditions would select for earlier reproduction in spring.
First, we can expect a latitudinal cline in the relationship between spring temperatures and photoperiod. In general, a certain long-day photoperiod (longer than 12 h) corresponds to lower temperatures at higher latitudes for two reasons: (i) because it occurs earlier in spring closer to the poles, and (ii) because the Sun projects less heat on the Earth surface because it radiates from a position closer to the horizon at higher latitudes. For plants, invertebrates and poikilothermic vertebrates, development depends on ambient temperature and at higher latitudes reproduction and growth will commence later in spring. These species, but also the predators depending on them, will face a selection pressure favouring later breeding than their conspecifics living closer to the equator. As a result, latitude-dependent selection pressure on timing of breeding may result in latitudinal clines in circadian clock gene polymorphism frequencies or expression levels, as indicated by studies in birds [44,45], fish [46] and insects [47–50].
Second, global warming generates a new relationship between photoperiod and ambient temperature. Without debating the cause of global warming, climate data show that a certain latitudinal location faces increasing ambient temperatures during the last 50–100 years, while the annual cycle in photoperiod duration remains constant. This yields a changing relationship between photoperiod and temperature [113,114]. Warm spring temperatures that allow for developmental growth or reproduction will occur at shorter photoperiods than before. This suggests that animals breeding in temperate zones could breed earlier for energetic reasons, but also because the growth season for plants and invertebrate prey species starts earlier [115–117]. Indeed, selection pressure for earlier reproduction attributed to global warming was found in long-term population records for temperate zone birds [118,119].
When selection pressure for earlier reproduction increases, chronobiology would predict an effect on circadian period, given the importance of the circadian system in photoperiodic time measurement. Both models for photoperiodic time measurement (external versus internal coincidence) yield different predictions when earlier reproduction in spring is favoured (figure 5b,d). In such situations, external coincidence timing would select for circadian periods that may deviate from 24 h (increasing τ when morning light induces the photoperiod response; decreasing τ when evening light induces the photoperiod response), while internal coincidence timing would specifically select for τ closer to 24 h. Inherent to this line of reasoning is that the external coincidence timing model would, in principle, allow for a continuous increase or decrease of critical day length by changing circadian period. Internal coincidence timing would have a clear limitation in that maximal internal photoperiod representation is obtained when circadian period equals 24 h. Deviations from 24 h, in any direction, would always yield shortening of internal photoperiod representation. When selection pressures would call for even longer internal photoperiod representation, additional modifying response parameters would have to be recruited. Internal coincidence timing, therefore, seems to be the more complex solution, because it inherently carries its limitations in regulatory possibilities. For this reasoning, it seems likely that natural selection may have favoured external coincidence timing over internal coincidence timing, but we cannot exclude that a combination of both mechanisms can exist, even within a species. Neither can we exclude that in certain species, selection pressure acted on mechanisms downstream from the melatonin signal, leaving the circadian system untouched but yielding similar effects on annual timing.
(e). Data from latitudinal clines fit external, but not internal coincidence timing
When a population moves up towards higher latitudes, selection pressure would change to later reproduction. As a consequence, τ can be predicted to move away from 24 h under the assumption of internal coincidence timing (figure 5d), but under the assumption of external coincidence timing τ will continuously decline (photo induction phase in the subjective morning), or increase (photo induction phase in the subjective evening; figure 5b). Hence, both models will predict a latitudinal cline in circadian τ, but only the external coincidence timing model predicts that this cline will cross the 24 h limit if the measured range of latitudes is large enough. This difference was tested using published data in insects (table 1).
Table 1.
Properties of latitudinal clines in circadian period. Latitudinal clines in circadian period are summarized for Arabidopsis thaliana and several Drosophila species. The latitudinal range indicates the minimal and maximal latitudes from which the samples were taken; τ range indicates the outcome of the regression of τ against latitude over the latitudinal range that was sampled. The slope and significance of the regression of τ against latitude is given, as well as whether the regression crossed the 24 h limit over the latitudinal range sampled. No dataset was corrected for other geographical parameters like altitude or distance from large open water areas. Genus name abbreviation: A, Arabidopsis; D, Drosophila; asterisk (*) indicates European data only.
| species | development state | latitude range | τ range | regression slope |
τ range crosses 24 h? | reference | |
|---|---|---|---|---|---|---|---|
| A. thaliana | seedling | 5–65 | 23.7–24.9 | + | n.s. | yes | [40] |
| D. subobscura | pupae | 28.2–62.9 | 24.7–25.9 | − | significant | no | [120] |
| D. littoralis | pupae | 41.6–69 | 20.7–23.4 | − | significant | no | [43] |
| D. ananassae | adult | 6–36 | 22.2–25.4 | + | significant | yes | [42] |
| D. auraria | adult | 34–43 | 22.4–24.4 | + | significant | yes | [41] |
| D. melanogaster | adult | 33.6–52.2 | 23.6–23.8 | + | n.s. | no | [121] |
| D. melanogaster | adult | 39.5–52.2 | 23.9–23.6 | − | significant | no | [121]* |
The data in table 1 indicate that, both in Arabidopsis and Drosophila, latitude can cause selection pressure that changes circadian period from above to below 24 h or vice versa, as predicted by an external coincidence model (figure 5b). In these data, there is no indication that selection on circadian period pushes against a limit around 24 h, as can be expected when the system follows an internal coincidence model (figure 5d). It would be very valuable to obtain similar datasets in mammals and birds, but such data are presently not known to us.
4. Conclusions
The everlasting alternation between light and darkness and the concomitant oscillation in environmental temperature provides systematic variation in the environment to many organisms on Earth. Those organisms have adjusted their physiology to those environmental changes to profit optimally from the daily changes. The underlying machinery is, however, very different between cyanobacteria and complex animals. In animals, the rhythms of individual cells and tissues are controlled by a master pacemaker that evolved because of the apparent benefit of a conductor that regulates the phasing of the activities of all other cells in the organism. This line of reasoning seems rather straightforward: small evolutionary steps are required to reach more complex circadian adaptation known today and each step has fitness advantage of its own. Available data seem to support quite a few of those steps. Yet, the proof of fitness advantages of having a pacemaker is meagre and mostly indirect.
The advantage for early cyanobacteria to be able to anticipate daily fluctuations of the environment through oscillations in KaiC hexamers might also have created a new problem: adaptation to a specific light–dark cycle would have caused limitations on expanding to geographical regions at different latitudes. We suggest that KaiA might have emerged to enable this system to adjust to different durations of day and night, thereby allowing cyanobacteria to expand their geographical range to different latitudes. A testable prediction that follows from this hypothesis is that KaiA would be upregulated under short photoperiods and downregulated under long photoperiods (figure 2). Specific experiments of Kai expression under different photoperiods would not only teach us more about the function of circadian regulation in photoperiodic adaptation in cyanobacteria, but it would also shed light from a different angle on the evolution of the earliest circadian clock system known.
The proposed early evolutionary force of photoperiod adjustment in the earliest organisms that were equipped with a circadian system indicates that circadian clocks and photoperiodic adjustment must have been intricately connected from the start. Indeed, in higher plants, insects and mammals, the involvement of the circadian system in photoperiodic responses has been confirmed by various experiments, albeit that its precise mechanism remains to be elucidated. Another line of reasoning originates from accumulating evidence that latitudinal clines were found in circadian period in various species. This suggests that photoperiod-dependent selection pressures on circadian period exist, and that circadian period plays a critical role in the involvement of the circadian system in photoperiodic responses. We suggest that these latitudinal clines can actually be used to distinguish between two leading hypotheses in circadian photoperiod regulation, of internal and external coincidence timings. Both models yield different predictions on the effect of changing selection pressures on circadian period when photoperiodic responses change with different latitudes. Using this line of reasoning enables us to determine which photoperiodic mechanism may be present in a specific species, when latitudinal clines of its circadian period are established. Data obtained in Drosophila seem to indicate that this species uses an external coincidence model since the latitudinal cline of circadian period crosses 24 h, while internal coincidence would predict a limit around 24 h. To obtain insight in mechanisms of photoperiodism in other taxa, it will be valuable to obtain information on latitudinal clines of circadian period in, for instance, mammals, birds and fish. The qualitative model presented here using latitudinal clines of circadian period will be equally relevant to predict what the directional selection pressures on circadian period may be when temperate zone species are exposed to changing climatic conditions (e.g. global warming) that shift their optimal timing of breeding and other annual events in physiology and behaviour.
Acknowledgements
We thank B. Helm, M. Menaker, S. Daan, M. Merrow, M. Maas, M. Simons, M. Sturre and the referees for helpful discussions and comments on the manuscript. Part of this work was developed during the 2008 Lorenz Center workshop ‘Keeping track of the Seasons’ organized by M. Visser, A. Dawson and B. Helm. This work was supported by the European Commission 6th Framework Project EUCLOCK (No. 018741).
Footnotes
One contribution of 10 to a Theme Issue ‘Evolutionary developmental biology (evo-devo) and behaviour’.
References
- 1.Ouyang Y., Andersson C. R., Kondo T., Golden S. S., Johnson C. H. 1998. Resonating circadian clocks enhance fitness in cyanobacteria. Proc. Natl Acad. Sci. USA 95, 8660–8664 10.1073/pnas.95.15.8660 (doi:10.1073/pnas.95.15.8660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woelfle M. A., Ouyang Y., Phanvijhitsiri K., Johnson C. H. 2004. The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr. Biol. 14, 1481–1486 10.1016/j.cub.2004.08.023 (doi:10.1016/j.cub.2004.08.023) [DOI] [PubMed] [Google Scholar]
- 3.Rosbash M. 2009. The implications of multiple circadian clock origins. PLoS Biol. 7, e62. 10.1371/journal.pbio.1000062 (doi:10.1371/journal.pbio.1000062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herzog E. D., Tosini G. 2001. The mammalian circadian clock shop. Semin. Cell Dev. Biol. 12, 295–303 10.1006/scdb.2001.0257 (doi:10.1006/scdb.2001.0257) [DOI] [PubMed] [Google Scholar]
- 5.Stephan F. K., Zucker I. 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl Acad. Sci. USA 69, 1583–1586 10.1073/pnas.69.6.1583 (doi:10.1073/pnas.69.6.1583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore R. Y., Eichler V. B. 1972. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42, 201–206 10.1016/0006-8993(72)90054-6 (doi:10.1016/0006-8993(72)90054-6) [DOI] [PubMed] [Google Scholar]
- 7.Ralph M. R., Foster R. G., Davis F. C., Menaker M. 1990. Transplanted suprachiasmatic nucleus determines circadian period. Science 247, 975–978 10.1126/science.2305266 (doi:10.1126/science.2305266) [DOI] [PubMed] [Google Scholar]
- 8.DeCoursey P. J., Walker J. K., Smith S. A. 2000. A circadian pacemaker in free-living chipmunks: essential for survival? J. Comp. Physiol. A 186, 169–180 10.1007/s003590050017 (doi:10.1007/s003590050017) [DOI] [PubMed] [Google Scholar]
- 9.Johnson C. H. 2005. Testing the adaptive value of circadian systems. Methods Enzymol. 393, 818–837 10.1016/S0076-6879(05)93043-7 (doi:10.1016/S0076-6879(05)93043-7) [DOI] [PubMed] [Google Scholar]
- 10.Pittendrigh C. S., Minis D. H. 1972. Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 69, 1537–1539 10.1073/pnas.69.6.1537 (doi:10.1073/pnas.69.6.1537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Saint Paul U., Aschoff J. 1978. Longevity among blowflies Phormia terraenovae R.D. kept in non-24-hour light–dark cycles. J. Comp. Physiol. 127, 191–195 10.1007/BF01350109 (doi:10.1007/BF01350109) [DOI] [Google Scholar]
- 12.Highkin H. R., Hanson J. B. 1954. Possible interaction between light–dark cycles and endogenous daily rhythms on the growth of tomato plants. Plant Physiol. 29, 301–302 10.1104/pp.29.3.301 (doi:10.1104/pp.29.3.301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillman W. S. 1956. Injury of tomato plants by continuous light and unfavorable photoperiodic cycles. Am. J. Bot. 43, 89–96 10.2307/2438816 (doi:10.2307/2438816) [DOI] [Google Scholar]
- 14.Withrow A. P., Withrow R. B. 1949. Photoperiodic chlorosis in tomato. Plant Physiol. 24, 657–663 10.1104/pp.24.4.657 (doi:10.1104/pp.24.4.657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green R. M., Tingay S., Wang Z. Y., Tobin E. M. 2002. Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol. 129, 576–584 10.1104/pp.004374 (doi:10.1104/pp.004374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pittendrigh C. S. 1993. Temporal organisation: reflections of a Darwinian clock-watcher. Annu. Rev. Physiol. 55, 17–54 10.1146/annurev.ph.55.030193.000313 (doi:10.1146/annurev.ph.55.030193.000313) [DOI] [PubMed] [Google Scholar]
- 17.Dvornyk V., Vinogradova O., Nevo E. 2003. Origin and evolution of circadian clock genes in prokaryotes. Proc. Natl Acad. Sci. USA 100, 2495–2500 10.1073/pnas.0130099100 (doi:10.1073/pnas.0130099100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simons M. J. 2009. The evolution of the cyanobacterial posttranslational clock from a primitive ‘phoscillator’. J. Biol. Rhythms 24, 175–182 10.1177/0748730409333953 (doi:10.1177/0748730409333953) [DOI] [PubMed] [Google Scholar]
- 19.Brunner M., Simons M. J., Merrow M. 2008. Lego clocks: building a clock from parts. Genes Dev. 22, 1422–1426 10.1101/gad.1686608 (doi:10.1101/gad.1686608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pittendrigh C. S. 1965. Biological clocks: the functions, ancient and modern, of circadian oscillations. Science in the Sixties. In Proc. 1965 Cloudcroft Symp. Air Force Office of Scientific Research, pp. 95–111 [Google Scholar]
- 21.Roenneberg T., Hastings J. W. 1991. Are the effects of light on phase and period of the Gonyaulax clock mediated by different pathways? Photochem. Photobiol. 53, 525–533 10.1111/j.1751-1097.1991.tb03665.x (doi:10.1111/j.1751-1097.1991.tb03665.x) [DOI] [PubMed] [Google Scholar]
- 22.Johnson C. H., Kondo T., Hastings J. W. 1991. Action spectrum for resetting the circadian phototaxis rhythm in the CW15 Strain of Chlamydomonas: II. Illuminated cells. Plant Physiol. 97, 1122–1129 10.1104/pp.97.3.1122 (doi:10.1104/pp.97.3.1122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima M., Imai K., Ito H., Nishiwaki T., Murayama Y., Iwasaki H., Oyama T., Kondo T. 2005. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 308, 414–415 10.1126/science.1108451 (doi:10.1126/science.1108451) [DOI] [PubMed] [Google Scholar]
- 24.Kitayama Y., Nishiwaki T., Terauchi K., Kondo T. 2008. Dual KaiC-based oscillations constitute the circadian system of cyanobacteria. Genes Dev. 22, 1513–1521 10.1101/gad.1661808 (doi:10.1101/gad.1661808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi F., et al. 2003. ATP-induced hexameric ring structure of the cyanobacterial circadian clock protein KaiC. Genes Cells 8, 287–296 10.1046/j.1365-2443.2003.00633.x (doi:10.1046/j.1365-2443.2003.00633.x) [DOI] [PubMed] [Google Scholar]
- 26.Williams S. B., Vakonakis I., Golden S. S., LiWang A. C. 2002. Structure and function from the circadian clock protein KaiA of Synechococcus elongatus: a potential clock input mechanism. Proc Natl. Acad Sci USA 99, 15 357–15 362 10.1073/pnas.232517099 (doi:10.1073/pnas.232517099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y., Mori T., Johnson C. H. 2003. Cyanobacterial circadian clockwork: roles of KaiA, KaiB and the kaiBC promoter in regulating KaiC. EMBO J. 22, 2117–2126 10.1093/emboj/cdg168 (doi:10.1093/emboj/cdg168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito H., Kageyama H., Mutsuda M., Nakajima M., Oyama T., Kondo T. 2007. Autonomous synchronization of the circadian KaiC phosphorylation rhythm. Nat. Struct. Mol. Biol. 14, 1084–1088 10.1038/nsmb1312 (doi:10.1038/nsmb1312) [DOI] [PubMed] [Google Scholar]
- 29.Williams S. B. 2007. A circadian timing mechanism in the cyanobacteria. Adv. Microb. Physiol. 52, 229–296 10.1016/S0065-2911(06)52004-1 (doi:10.1016/S0065-2911(06)52004-1) [DOI] [PubMed] [Google Scholar]
- 30.Nishiwaki T., Satomi Y., Kitayama Y., Terauchi K., Kiyohara R., Takao T., Kondo T. 2007. A sequential program of dual phosphorylation of KaiC as a basis for circadian rhythm in cyanobacteria. EMBO J. 26, 4029–4037 10.1038/sj.emboj.7601832 (doi:10.1038/sj.emboj.7601832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pattanayek R., Williams D. R., Pattanayek S., Mori T., Johnson C. H., Stewart P. L., Egli M. 2008. Structural model of the circadian clock KaiB-KaiC complex and mechanism for modulation of KaiC phosphorylation. EMBO J. 27, 1767–1778 10.1038/emboj.2008.104 (doi:10.1038/emboj.2008.104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson C. H., Egli M., Stewart P. L. 2008. Structural insights into a circadian oscillator. Science 322, 697–701 10.1126/science.1150451 (doi:10.1126/science.1150451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson C. H., Mori T., Xu Y. 2008. A cyanobacterial circadian clockwork. Curr. Biol. 18, R816–R825 10.1016/j.cub.2008.07.012 (doi:10.1016/j.cub.2008.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith R. M., Williams S. B. 2006. Circadian rhythms in gene transcription imparted by chromosome compaction in the cyanobacterium Synechococcus elongatus. Proc. Natl Acad. Sci. USA 103, 8564–8569 10.1073/pnas.0508696103 (doi:10.1073/pnas.0508696103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishiura M., Kutsuna S., Aoki S., Iwasaki H., Andersson C. R., Tanabe A., Golden S. S., Johnson C. H., Kondo T. 1998. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science 281, 1519–1523 10.1126/science.281.5382.1519 (doi:10.1126/science.281.5382.1519) [DOI] [PubMed] [Google Scholar]
- 36.Goldman B. D. 2001. Mammalian photoperiodic system: Formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms 16, 283–301 10.1177/074873001129001980 (doi:10.1177/074873001129001980) [DOI] [PubMed] [Google Scholar]
- 37.Foster R. G., Kreitzman L. 2009. Seasons of life. London, UK: Profile books Ltd [Google Scholar]
- 38.Daan S., Aschoff J. 1975. Circadian rhythms of locomotor activity in captive birds and mammals: their variations with season and latitude. Oecologia 18, 269–316 10.1007/BF00345851 (doi:10.1007/BF00345851) [DOI] [PubMed] [Google Scholar]
- 39.Bradshaw W. E., Holzapfel C. M. 2006. Climate change. Evolutionary response to rapid climate change. Science 312, 1477–1478 10.1126/science.1127000 (doi:10.1126/science.1127000) [DOI] [PubMed] [Google Scholar]
- 40.Michael T. P., Salome P. A., Yu H. J., Spencer T. R., Sharp E. L., McPeek M. A., Alonso J. M., Ecker J. R., McClung C. R. 2003. Enhanced fitness conferred by naturally occurring variation in the circadian clock. Science 302, 1049–1053 10.1126/science.1082971 (doi:10.1126/science.1082971) [DOI] [PubMed] [Google Scholar]
- 41.Pittendrigh C. S., Takamura T. 1989. Latitudinal clines in the properties of a circadian pacemaker. J. Biol. Rhythms 4, 217–235 10.1177/074873048900400209 (doi:10.1177/074873048900400209) [DOI] [PubMed] [Google Scholar]
- 42.Joshi D. S. 1999. Latitudinal variation in locomotor activity rhythm in adult Drosophila ananassae. Can. J. Zool. Rev. Can. Zool. 77, 865–870 10.1139/cjz-77-6-865 (doi:10.1139/cjz-77-6-865) [DOI] [Google Scholar]
- 43.Lankinen P., Forsman P. 2006. Independence of genetic geographical variation between photoperiodic diapause, circadian eclosion rhythm, and Thr-Gly repeat region of the period gene in Drosophila littoralis. J. Biol. Rhythms 21, 3–12 10.1177/0748730405283418 (doi:10.1177/0748730405283418) [DOI] [PubMed] [Google Scholar]
- 44.Johnsen A., et al. 2007. Avian Clock gene polymorphism: evidence for a latitudinal cline in allele frequencies. Mol. Ecol. 16, 4867–4880 10.1111/j.1365-294X.2007.03552.x (doi:10.1111/j.1365-294X.2007.03552.x) [DOI] [PubMed] [Google Scholar]
- 45.Liedvogel M., Szulkin M., Knowles S. C., Wood M. J., Sheldon B. C. 2009. Phenotypic correlates of Clock gene variation in a wild blue tit population: evidence for a role in seasonal timing of reproduction. Mol. Ecol. 18, 2444–2456 10.1111/j.1365-294X.2009.04204.x (doi:10.1111/j.1365-294X.2009.04204.x) [DOI] [PubMed] [Google Scholar]
- 46.O'Malley K. G., Banks M. A. 2008. A latitudinal cline in the Chinook salmon (Oncorhynchus tshawytscha) Clock gene: evidence for selection on PolyQ length variants. Proc. Biol. Sci. 275, 2813–2821 10.1098/rspb.2008.0524 (doi:10.1098/rspb.2008.0524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kyriacou C. P., Peixoto A. A., Sandrelli F., Costa R., Tauber E. 2008. Clines in clock genes: fine-tuning circadian rhythms to the environment. Trends Genet. 24, 124–132 10.1016/j.tig.2007.12.003 (doi:10.1016/j.tig.2007.12.003) [DOI] [PubMed] [Google Scholar]
- 48.Mathias D., Jacky L., Bradshaw W. E., Holzapfel C. M. 2005. Geographic and developmental variation in expression of the circadian rhythm gene, timeless, in the pitcher-plant mosquito, Wyeomyia smithii. J. Insect Physiol. 51, 661–667 10.1016/j.jinsphys.2005.03.011 (doi:10.1016/j.jinsphys.2005.03.011) [DOI] [PubMed] [Google Scholar]
- 49.Tauber E., Kyriacou C. P. 2005. Molecular evolution and population genetics of circadian clock genes. Methods Enzymol. 393, 797–817 10.1016/S0076-6879(05)93042-5 (doi:10.1016/S0076-6879(05)93042-5) [DOI] [PubMed] [Google Scholar]
- 50.Mathias D., Jacky L., Bradshaw W. E., Holzapfel C. M. 2007. Quantitative trait loci associated with photoperiodic response and stage of diapause in the pitcher-plant mosquito, Wyeomyia smithii. Genetics 176, 391–402 10.1534/genetics.106.068726 (doi:10.1534/genetics.106.068726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Oort B. E., Tyler N. J., Gerkema M. P., Folkow L., Blix A. S., Stokkan K. A. 2005. Circadian organization in reindeer. Nature 438, 1095–1096 10.1038/4381095a (doi:10.1038/4381095a) [DOI] [PubMed] [Google Scholar]
- 52.van Oort B. E., Tyler N. J., Gerkema M. P., Folkow L., Stokkan K. A. 2007. Where clocks are redundant: weak circadian mechanisms in reindeer living under polar photic conditions. Naturwissenschaften 94, 183–194 10.1007/s00114-006-0174-2 (doi:10.1007/s00114-006-0174-2) [DOI] [PubMed] [Google Scholar]
- 53.Stokkan K. A., van Oort B. E., Tyler N. J., Loudon A. S. 2007. Adaptations for life in the Arctic: evidence that melatonin rhythms in reindeer are not driven by a circadian oscillator but remain acutely sensitive to environmental photoperiod. J. Pineal Res. 43, 289–293 10.1111/j.1600-079X.2007.00476.x (doi:10.1111/j.1600-079X.2007.00476.x) [DOI] [PubMed] [Google Scholar]
- 54.Lu W., Meng Q. J., Tyler N. J., Stokkan K. A., Loudon A. S. 2010. A circadian clock is not required in an arctic mammal. Curr. Biol. 20, 533–537 10.1016/j.cub.2010.01.042 (doi:10.1016/j.cub.2010.01.042) [DOI] [PubMed] [Google Scholar]
- 55.Reierth E., Van't Hof T. J., Stokkan K. A. 1999. Seasonal and daily variations in plasma melatonin in the high-arctic Svalbard ptarmigan (Lagopus mutus hyperboreus). J. Biol. Rhythms 14, 314–319 10.1177/074873099129000731 (doi:10.1177/074873099129000731) [DOI] [PubMed] [Google Scholar]
- 56.Long R. A., Martin T. J., Barnes B. M. 2005. Body temperature and activity patterns in free-living arctic ground squirrels. J. Mammal 86, 314–322 10.1644/BRG-224.1 (doi:10.1644/BRG-224.1) [DOI] [Google Scholar]
- 57.Long R. A., Hut R. A., Barnes B. M. 2007. Simultaneous collection of body temperature and activity data in burrowing mammals: a new technique. J. Wildlife Manage. 71, 1375–1379 10.2193/2006-399 (doi:10.2193/2006-399) [DOI] [Google Scholar]
- 58.Lincoln G. A., Clarke I. J., Hut R. A., Hazlerigg D. G. 2006. Characterizing a mammalian circannual pacemaker. Science 314, 1941–1944 10.1126/science.1132009 (doi:10.1126/science.1132009) [DOI] [PubMed] [Google Scholar]
- 59.Rusak B., Morin L. P. 1976. Testicular responses to photoperiod are blocked by lesions of the suprachiasmatic nuclei in golden hamsters. Biol. Reprod. 15, 366–374 10.1095/biolreprod15.3.366 (doi:10.1095/biolreprod15.3.366) [DOI] [PubMed] [Google Scholar]
- 60.Lincoln G. A., Ebling F. J., Almeida O. F. 1985. Generation of melatonin rhythms. Ciba Found Symp. 117, 129–148 [DOI] [PubMed] [Google Scholar]
- 61.Kalsbeek A., Cutrera R. A., van Heerikhuize J. J., Van Der Vliet J., Buijs R. M. 1999. GABA release from suprachiasmatic nucleus terminals is necessary for the light-induced inhibition of nocturnal melatonin release in the rat. Neuroscience 91, 453–461 10.1016/S0306-4522(98)00635-6 (doi:10.1016/S0306-4522(98)00635-6) [DOI] [PubMed] [Google Scholar]
- 62.Kalsbeek A., Garidou M. L., Palm I. F., Van Der Vliet J., Simonneaux V., Pevet P., Buijs R. M. 2000. Melatonin sees the light: blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur. J. Neurosci. 12, 3146–3154 10.1046/j.1460-9568.2000.00202.x (doi:10.1046/j.1460-9568.2000.00202.x) [DOI] [PubMed] [Google Scholar]
- 63.Perreau-Lenz S., Kalsbeek A., Garidou M. L., Wortel J., Van der Vliet J., van H. C., Simonneaux V., Pévet P., Buijs R. M. 2003. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 17, 221–228 10.1046/j.1460-9568.2003.02442.x (doi:10.1046/j.1460-9568.2003.02442.x) [DOI] [PubMed] [Google Scholar]
- 64.Perreau-Lenz S., Kalsbeek A., Pevet P., Buijs R. M. 2004. Glutamatergic clock output stimulates melatonin synthesis at night. Eur. J. Neurosci. 19, 318–324 10.1111/j.0953-816X.2003.03132.x (doi:10.1111/j.0953-816X.2003.03132.x) [DOI] [PubMed] [Google Scholar]
- 65.Perreau-Lenz S., Kalsbeek A., Van der Vliet J., Pevet P., Buijs R. M. 2005. In vivo evidence for a controlled offset of melatonin synthesis at dawn by the suprachiasmatic nucleus in the rat. Neuroscience 130, 797–803 10.1016/j.neuroscience.2004.10.014 (doi:10.1016/j.neuroscience.2004.10.014) [DOI] [PubMed] [Google Scholar]
- 66.Reiter R. J. 1980. The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev. 1, 109–131 10.1210/edrv-1-2-109 (doi:10.1210/edrv-1-2-109) [DOI] [PubMed] [Google Scholar]
- 67.Lincoln G. A., Almeida O. F., Arendt J. 1981. Role of melatonin and circadian rhythms in seasonal reproduction in rams. J. Reprod. Fertil. Suppl. 30, 23–31 [PubMed] [Google Scholar]
- 68.Karsch F. J., Bittman E. L., Foster D. L., Goodman R. L., Legan S. J., Robinson J. E. 1984. Neuroendocrine basis of seasonal reproduction. Recent Prog. Horm. Res. 40, 185–232 [DOI] [PubMed] [Google Scholar]
- 69.Oster H., Maronde E., Albrecht U. 2002. The circadian clock as a molecular calendar. Chronobiol. Int. 19, 507–516 10.1081/CBI-120004210 (doi:10.1081/CBI-120004210) [DOI] [PubMed] [Google Scholar]
- 70.Stehle J. H., Von G. C., Schomerus C., Korf H. W. 2001. Of rodents and ungulates and melatonin: creating a uniform code for darkness by different signaling mechanisms. J. Biol. Rhythms 16, 312–325 10.1177/074873001129002033 (doi:10.1177/074873001129002033) [DOI] [PubMed] [Google Scholar]
- 71.Hut R. A., Van Oort B. E. H., Daan S. 1999. Natural entrainment without dawn and dusk: the case of the European ground squirrel (Spermophilus citellus). J. Biol. Rhythms 14, 290–299 10.1177/074873099129000704 (doi:10.1177/074873099129000704) [DOI] [PubMed] [Google Scholar]
- 72.Hut R. A., Van der Zee E. A., Jansen K., Gerkema M. P., Daan S. 2002. Gradual reappearance of post-hibernation circadian rhythmicity correlates with numbers of vasopressin-containing neurons in the suprachiasmatic nuclei of European ground squirrels. J. Comp. Physiol. B 172, 59–70 [DOI] [PubMed] [Google Scholar]
- 73.Hut R. A., Barnes B. M., Daan S. 2002. Body temperature patterns before, during, and after semi-natural hibernation in the European ground squirrel. J. Comp. Physiol. B 172, 47–58 [DOI] [PubMed] [Google Scholar]
- 74.Swaab D. F., Nijveldt F., Pool C. W. 1975. Distribution of oxytocin and vasopressin in the rat supraoptic and paraventricular nucleus. J. Endocrinol. 67, 461–462 10.1677/joe.0.0670461 (doi:10.1677/joe.0.0670461) [DOI] [PubMed] [Google Scholar]
- 75.Van Leeuwen F. W., Swaab D. F., de R. C. 1978. Immunoelectronmicroscopic localization of vasopressin in the rat suprachiasmatic nucleus. Cell Tissue Res. 193, 1–10 [DOI] [PubMed] [Google Scholar]
- 76.Hofman M. A., Fliers E., Goudsmit E., Swaab D. F. 1988. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: sex differences and age-dependent changes. J. Anat. 160, 127–143 [PMC free article] [PubMed] [Google Scholar]
- 77.Card J. P., Moore R. Y. 1991. The organisation of visual circuits influencing the circadian activity of the suprachiasmatic nucleus. In Suprachiasmatic nucleus; the mind's clock (eds Klein D. C., Moore R. Y., Reppert S. M.), pp. 51–76 Oxford, UK: Oxford University Press [Google Scholar]
- 78.Moore R. Y. 1983. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed. Proc. 42, 2783–2789 [PubMed] [Google Scholar]
- 79.Morin L. P., Shivers K. Y., Blanchard J. H., Muscat L. 2006. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience 137, 1285–1297 10.1016/j.neuroscience.2005.10.030 (doi:10.1016/j.neuroscience.2005.10.030) [DOI] [PubMed] [Google Scholar]
- 80.Silver R., Sookhoo A. I., LeSauter J., Stevens P., Jansen H. T., Lehman M. N. 1999. Multiple regulatory elements result in regional specificity in circadian rhythms of neuropeptide expression in mouse SCN. Neuroreport 10, 3165–3174 10.1097/00001756-199910190-00008 (doi:10.1097/00001756-199910190-00008) [DOI] [PubMed] [Google Scholar]
- 81.Carter D. S., Goldman B. D. 1983. Progonadal role of the pineal in the Djungarian hamster (Phodopus sungorus sungorus): mediation by melatonin. Endocrinology 113, 1268–1273 10.1210/endo-113-4-1268 (doi:10.1210/endo-113-4-1268) [DOI] [PubMed] [Google Scholar]
- 82.Carter D. S., Goldman B. D. 1983. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology 113, 1261–1267 10.1210/endo-113-4-1261 (doi:10.1210/endo-113-4-1261) [DOI] [PubMed] [Google Scholar]
- 83.Dardente H., Menet J. S., Poirel V.-J., Streicher D., Gauer F., Vivien-Roels B., Klosen P., Pévet P., Masson-Pévet M. 2003. Melatonin induces Cry1 expression in the pars tuberalis of the rat. Brain Res Mol. Brain Res. 114, 101–106 10.1016/S0169-328X(03)00134-7 (doi:10.1016/S0169-328X(03)00134-7) [DOI] [PubMed] [Google Scholar]
- 84.Dardente H., Klosen P., Pevet P., Masson-Pevet M. 2003. MT1 melatonin receptor mRNA expressing cells in the pars tuberalis of the European hamster: effect of photoperiod. J. Neuroendocrinol. 15, 778–786 10.1046/j.1365-2826.2003.01060.x (doi:10.1046/j.1365-2826.2003.01060.x) [DOI] [PubMed] [Google Scholar]
- 85.Johnston J. D., Tournier B. B., Andersson H., Masson-Pevet M., Lincoln G. A., Hazlerigg D. G. 2006. Multiple effects of melatonin on rhythmic clock gene expression in the mammalian pars tuberalis. Endocrinology 147, 959–965 10.1210/en.2005-1100 (doi:10.1210/en.2005-1100) [DOI] [PubMed] [Google Scholar]
- 86.Lincoln G. A., Andersson H., Hazlerigg D. 2003. Clock genes and the long-term regulation of prolactin secretion: evidence for a photoperiod/circannual timer in the pars tuberalis. J. Neuroendocrinol. 15, 390–397 10.1046/j.1365-2826.2003.00990.x (doi:10.1046/j.1365-2826.2003.00990.x) [DOI] [PubMed] [Google Scholar]
- 87.Lincoln G. A., Andersson H., Loudon A. 2003. Clock genes in calendar cells as the basis of annual timekeeping in mammals–a unifying hypothesis. J. Endocrinol. 179, 1–13 10.1677/joe.0.1790001 (doi:10.1677/joe.0.1790001) [DOI] [PubMed] [Google Scholar]
- 88.Masson-Pevet M., Gauer F. 1994. Seasonality and melatonin receptors in the pars tuberalis in some long day breeders. Biol. Signals 3, 63–70 10.1159/000109527 (doi:10.1159/000109527) [DOI] [PubMed] [Google Scholar]
- 89.Von Gall C., Weaver D. R., Moek J., Jilg A., Stehle J. H., Korf H. W. 2005. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann. N Y Acad. Sci. 1040, 508–511 10.1196/annals.1327.105 (doi:10.1196/annals.1327.105) [DOI] [PubMed] [Google Scholar]
- 90.Wagner G. C., Johnston J. D., Tournier B. B., Ebling F. J., Hazlerigg D. G. 2007. Melatonin induces gene-specific effects on rhythmic mRNA expression in the pars tuberalis of the Siberian hamster (Phodopus sungorus). Eur. J. Neurosci. 25, 485–490 10.1111/j.1460-9568.2006.05291.x (doi:10.1111/j.1460-9568.2006.05291.x) [DOI] [PubMed] [Google Scholar]
- 91.Ono H., Nakao N., Yoshimura T. 2009. Identification of the photoperiodic signaling pathway regulating seasonal reproduction using the functional genomics approach. Gen. Comp. Endocrinol. 163, 2–6 10.1016/j.ygcen.2008.11.017 (doi:10.1016/j.ygcen.2008.11.017) [DOI] [PubMed] [Google Scholar]
- 92.Quintero J. E., Kuhlman S. J., McMahon D. G. 2003. The biological clock nucleus: a multiphasic oscillator network regulated by light. J. Neurosci. 23, 8070–8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.VanderLeest H. T., Houben T., Michel S., DeBoer T., Albus H., Vansteensel M. J., Block G. D., Meijer J. H. 2007. Seasonal encoding by the circadian pacemaker of the SCN. Curr. Biol. 17, 468–473 10.1016/j.cub.2007.01.048 (doi:10.1016/j.cub.2007.01.048) [DOI] [PubMed] [Google Scholar]
- 94.Hazlerigg D. G., Ebling F. J. P., Johnston J. D. 2005. Photoperiod differentially regulates gene expression rhythms in the rostral and caudal SCN. Curr. Biol. 15, 1–2 10.1016/j.cub.2005.06.010 (doi:10.1016/j.cub.2005.06.010) [DOI] [PubMed] [Google Scholar]
- 95.Naito E., Watanabe T., Tei H., Yoshimura T., Ebihara S. 2008. Reorganization of the suprachiasmatic nucleus coding for day length. J. Biol. Rhythms 23, 140–149 10.1177/0748730408314572 (doi:10.1177/0748730408314572) [DOI] [PubMed] [Google Scholar]
- 96.Inagaki N., Honma S., Ono D., Tanahashi Y., Honma K. 2007. Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc. Natl Acad. Sci. USA 104, 7664–7669 10.1073/pnas.0607713104 (doi:10.1073/pnas.0607713104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sosniyenko S., Hut R. A., Daan S., Sumova A. 2009. Influence of photoperiod duration and light–dark transitions on entrainment of Per1 and Per2 gene and protein expression in subdivisions of the mouse suprachiasmatic nucleus. Eur. J. Neurosci. 30, 1802–1814 10.1111/j.1460-9568.2009.06945.x (doi:10.1111/j.1460-9568.2009.06945.x) [DOI] [PubMed] [Google Scholar]
- 98.Beersma D. G. M., van Bunnik B. A. D., Hut R. A., Daan S. 2008. Emergence of circadian and photoperiodic system level properties from interactions among pacemaker cells. J. Biol. Rhythms 23, 362–373 10.1177/0748730408317992 (doi:10.1177/0748730408317992) [DOI] [PubMed] [Google Scholar]
- 99.Rohling J., Wolters L., Meijer J. H. 2006. Simulation of day-length encoding in the SCN: from single-cell to tissue-level organization. J. Biol. Rhythms 21, 301–313 10.1177/0748730406290317 (doi:10.1177/0748730406290317) [DOI] [PubMed] [Google Scholar]
- 100.Rohling J., Meijer J. H., VanderLeest H. T., Admiraal J. 2006. Phase differences between SCN neurons and their role in photoperiodic encoding; a simulation of ensemble patterns using recorded single unit electrical activity patterns. J. Physiol. Paris 100, 261–270 10.1016/j.jphysparis.2007.05.005 (doi:10.1016/j.jphysparis.2007.05.005) [DOI] [PubMed] [Google Scholar]
- 101.Pittendrigh C. S., Daan S. 1976. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J. Comp. Physiol. A 106, 333–355 10.1007/BF01417860 (doi:10.1007/BF01417860) [DOI] [Google Scholar]
- 102.Daan S., Albrecht U., van der Horst G. T., Illnerova H., Roenneberg T., Wehr T. A., Schwartz W. J. 2001. Assembling a clock for all seasons: are there M and E oscillators in the genes? J. Biol. Rhythms 16, 105–116 10.1177/074873001129001809 (doi:10.1177/074873001129001809) [DOI] [PubMed] [Google Scholar]
- 103.Johnson C. H., Elliott J. A., Foster R. 2003. Entrainment of circadian programs. Chronobiol. Int. 20, 741–774 10.1081/CBI-120024211 (doi:10.1081/CBI-120024211) [DOI] [PubMed] [Google Scholar]
- 104.Daan S., Beersma D. G. M. 2002. Circadian frequency and its variability. In Biological rhythms. (ed. Kumar V.), pp. 24–37 New Delhi, India: Narosa [Google Scholar]
- 105.Wyse C. A., Coogan A. N., Selman C., Hazlerigg D. G., Speakman J. R. 2010. Association between mammalian lifespan and circadian free-running period: the circadian resonance hypothesis revisited. Biol. Lett. 6, 696–698 10.1098/rsbl.2010.0152 (doi:10.1098/rsbl.2010.0152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elliott J. A. 1976. Circadian rhythms and photoperiodic time measurement in mammals. Fed. Proc. 35, 2339–2346 [PubMed] [Google Scholar]
- 107.Stirland J. A., Mohammad Y. N., Loudon A. S. 1996. A mutation of the circadian timing system (tau gene) in the seasonally breeding Syrian hamster alters the reproductive response to photoperiod change. Proc. Biol. Sci. 263, 345–350 10.1098/rspb.1996.0053 (doi:10.1098/rspb.1996.0053) [DOI] [PubMed] [Google Scholar]
- 108.Stirland J. A., Hastings M. H., Loudon A. S., Maywood E. S. 1996. The tau mutation in the Syrian hamster alters the photoperiodic responsiveness of the gonadal axis to melatonin signal frequency. Endocrinology 137, 2183–2186 10.1210/en.137.5.2183 (doi:10.1210/en.137.5.2183) [DOI] [PubMed] [Google Scholar]
- 109.Majoy S. B., Heideman P. D. 2000. Tau differences between short-day responsive and short-day nonresponsive white-footed mice (Peromyscus leucopus) do not affect reproductive photoresponsiveness. J. Biol. Rhythms 15, 501–513 10.1177/074873000129001611 (doi:10.1177/074873000129001611) [DOI] [PubMed] [Google Scholar]
- 110.Bünning E. 1936. Die endogene Tagesrhythmik als Grundlage der photoperiodischen Reaktion. Ber dtsch bot Ges 54, 590–607 [Google Scholar]
- 111.Bünning E. 1960. Circadian rhythms and time measurement in photoperiodism. Cold Spring Harbor Symp. Quant. Biol. 25, 249–256 [DOI] [PubMed] [Google Scholar]
- 112.Pittendrigh C. S., Bruce V. G. 1959. Daily rhythms as coupled oscillator systems and their relation to thermoperiodism and photoperiodism. In Photoperiodism and related phenomena in plants and animals. (ed. Withrow R. B.), pp. 475–505 Washington, DC: A.A.A.S [Google Scholar]
- 113.Bradshaw W. E., Holzapfel C. M. 2008. Genetic response to rapid climate change: it's seasonal timing that matters. Mol. Ecol. 17, 157–166 10.1111/j.1365-294X.2007.03509.x (doi:10.1111/j.1365-294X.2007.03509.x) [DOI] [PubMed] [Google Scholar]
- 114.Bradshaw W. E., Holzapfel C. M. 2010. Light, time, and the physiology of biotic response to rapid climate change in animals. Annu. Rev. Physiol. 72, 147–166 10.1146/annurev-physiol-021909-135837 (doi:10.1146/annurev-physiol-021909-135837) [DOI] [PubMed] [Google Scholar]
- 115.Visser M. E., Holleman L. J. 2001. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. Biol. Sci. 268, 289–294 10.1098/rspb.2000.1363 (doi:10.1098/rspb.2000.1363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Visser M. E., Holleman L. J., Gienapp P. 2006. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 164–172 10.1007/s00442-005-0299-6 (doi:10.1007/s00442-005-0299-6) [DOI] [PubMed] [Google Scholar]
- 117.Van Asch M., Visser M. E. 2007. Phenology of forest caterpillars and their host trees: the importance of synchrony. Annu. Rev. Entomol. 52, 37–55 10.1146/annurev.ento.52.110405.091418 (doi:10.1146/annurev.ento.52.110405.091418) [DOI] [PubMed] [Google Scholar]
- 118.Nussey D. H., Postma E., Gienapp P., Visser M. E. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 10.1126/science.1117004 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- 119.Both C., et al. 2004. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. Biol. Sci. 271, 1657–1662 10.1098/rspb.2004.2770 (doi:10.1098/rspb.2004.2770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lankinen P. 1993. North south differences in circadian eclosion rhythm in European populations of Drosophila-subobscura. Heredity 71, 210–218 10.1038/hdy.1993.126 (doi:10.1038/hdy.1993.126) [DOI] [Google Scholar]
- 121.Sawyer L. A., Hennessy J. M., Peixoto A. A., Rosato E., Parkinson H., Costa R., Kyriacou C. P. 1997. Natural variation in a Drosophila clock gene and temperature compensation. Science 278, 2117–2120 10.1126/science.278.5346.2117 (doi:10.1126/science.278.5346.2117) [DOI] [PubMed] [Google Scholar]