Abstract
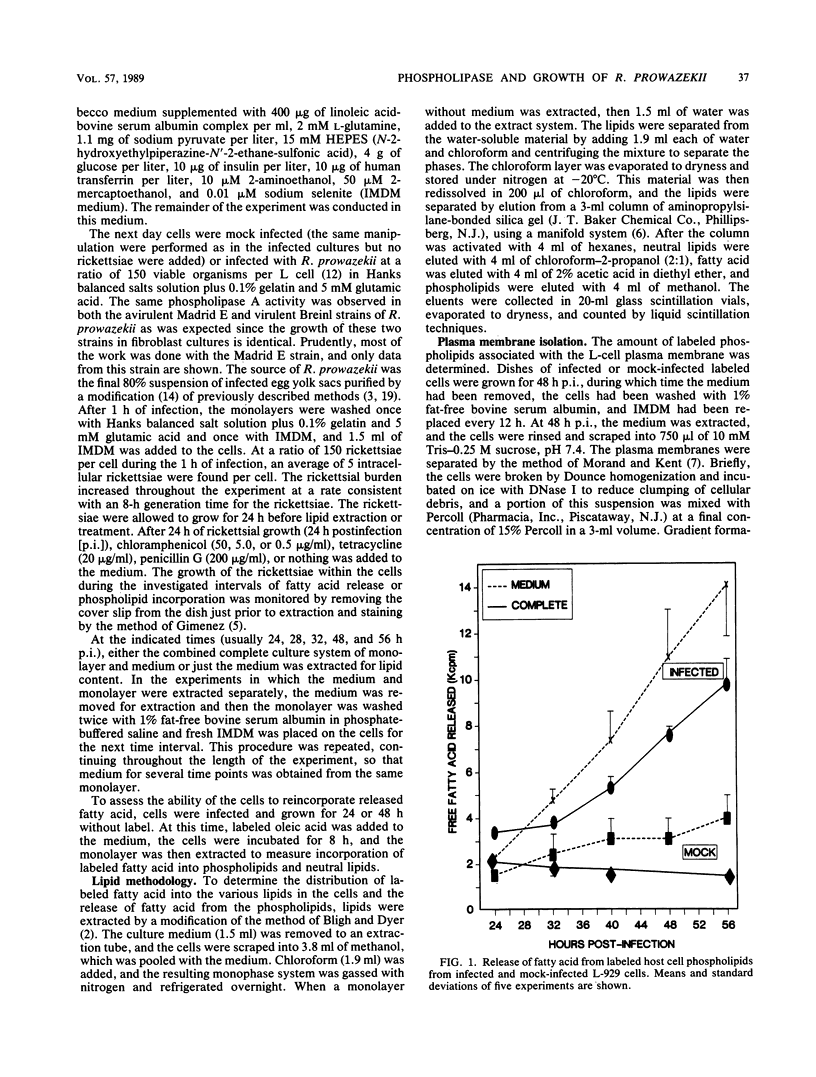
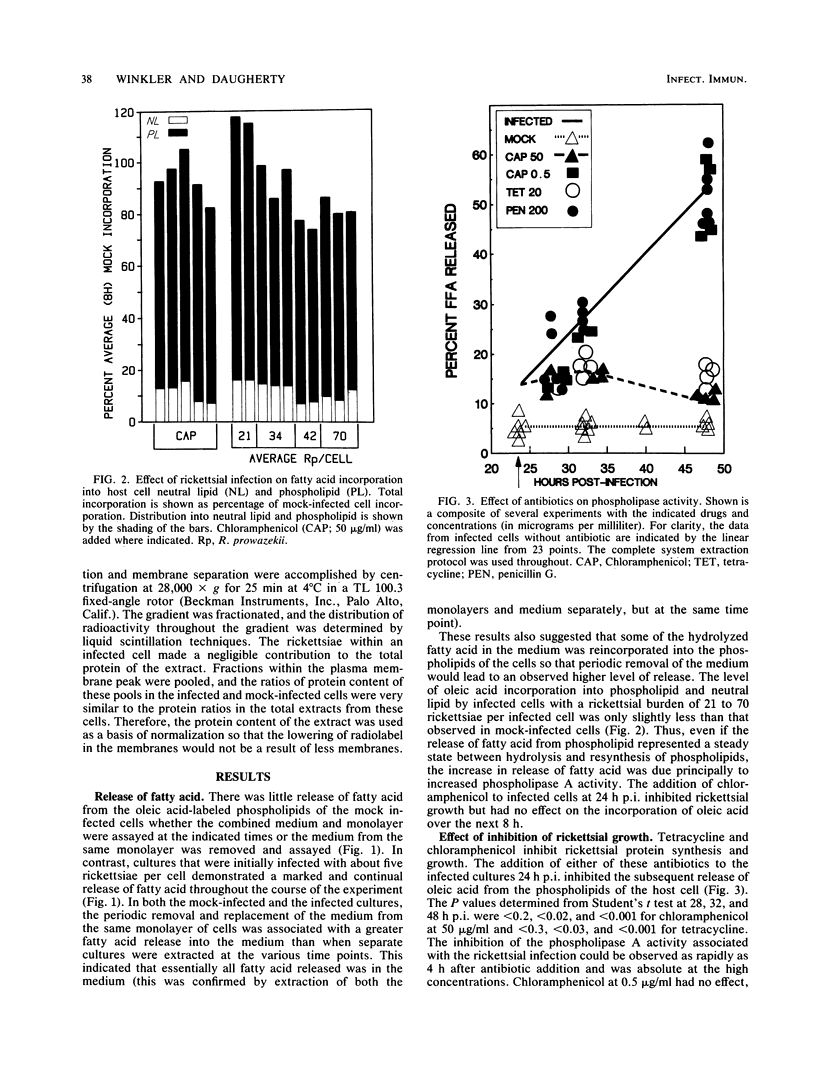
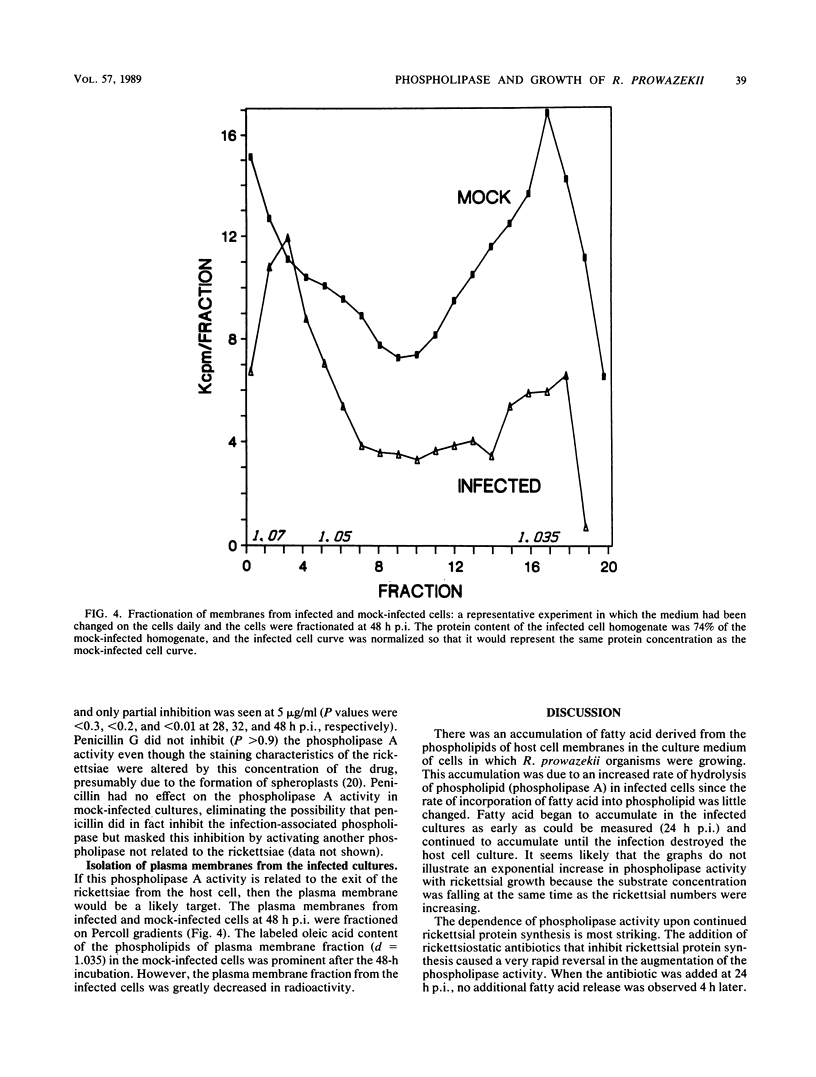
Cultured L929 cells infected with Rickettsia prowazekii had a greatly increased rate of hydrolysis of fatty acid from the oleic acid-radiolabeled phospholipids of the host cell membranes. The incorporation of fatty acid into phospholipid in an infected cell was only moderately inhibited relative to a mock-infected cell. Thus, even if the release of fatty acid from phospholipid represented a steady state between hydrolysis and resynthesis of phospholipids, the increase in release of fatty acid was due principally to increased phospholipase A activity. The increased rate of hydrolysis did not occur only late in the rickettsial infection; this activity began early in infection and continued throughout the course of infection. The addition of tetracycline or chloramphenicol (antibiotics which inhibit rickettsial protein synthesis) to the infected cells caused a rapid and total abatement of this increased rate of phospholipid hydrolysis. In contrast, high concentrations of penicillin affected the morphology of the intracellular rickettsiae, but did not inhibit the phospholipase activity. This phospholipase A activity clearly damages the host cell during the rickettsial infection and may represent the activity by which R. prowazekii escapes from the host cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., BOZEMAN F. M., CAMPBELL J. M., HUMPHRIES J. W., SAWYER T. K. Study on growth of Rickettsia. V. Penetration of Rickettsia tsutsugamushi into mammalian cells in vitro. J Exp Med. 1959 Mar 1;109(3):271–292. doi: 10.1084/jem.109.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Kaluzny M. A., Duncan L. A., Merritt M. V., Epps D. E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985 Jan;26(1):135–140. [PubMed] [Google Scholar]
- Morand J. N., Kent C. A one-step technique for the subcellular fractionation of total cell homogenates. Anal Biochem. 1986 Nov 15;159(1):157–162. doi: 10.1016/0003-2697(86)90321-0. [DOI] [PubMed] [Google Scholar]
- Silverman D. J. Rickettsia rickettsii-induced cellular injury of human vascular endothelium in vitro. Infect Immun. 1984 Jun;44(3):545–553. doi: 10.1128/iai.44.3.545-553.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. In vitro studies of Rickettsia-host cell interactions: ultrastructural study of Rickettsia prowazekii-infected chicken embryo fibroblasts. Infect Immun. 1980 Aug;29(2):778–790. doi: 10.1128/iai.29.2.778-790.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, JACKSON E. B., HAHN F. E., LEY A. C., SMADEL J. E. Metabolic studies of rickettsiae. I. The effects of antimicrobial substances and enzyme inhibitors on the oxidation of glutamate by purified rickettsiae. J Immunol. 1951 Aug;67(2):123–136. [PubMed] [Google Scholar]
- Walker T. S. Rickettsial interactions with human endothelial cells in vitro: adherence and entry. Infect Immun. 1984 May;44(2):205–210. doi: 10.1128/iai.44.2.205-210.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. doi: 10.1128/iai.22.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Rickettsial hemolysis: rapid method for enumeration of metabolically active typhus rickettsiae. J Clin Microbiol. 1979 May;9(5):645–647. doi: 10.1128/jcm.9.5.645-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Dobson M. E., Dasch G. A. Biochemistry of rickettsiae: recent advances. Acta Virol. 1987 May;31(3):271–286. [PubMed] [Google Scholar]
- Winkler H. H. Early events in the interaction of the obligate intracytoplasmic parasite, Rickettsia prowazekii, with eucaryotic cells: entry and lysis. Ann Inst Pasteur Microbiol. 1986 May-Jun;137A(3):333–336. doi: 10.1016/s0769-2609(86)80044-8. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Miller E. T. Activated complex of L-cells and Rickettsia prowazekii with N-ethylmaleimide-insensitive phospholipase A. Infect Immun. 1984 Sep;45(3):577–581. doi: 10.1128/iai.45.3.577-581.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Miller E. T. Phospholipase A activity in the hemolysis of sheep and human erythrocytes by Rickettsia prowazeki. Infect Immun. 1980 Aug;29(2):316–321. doi: 10.1128/iai.29.2.316-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Miller E. T. Phospholipase A and the interaction of Rickettsia prowazekii and mouse fibroblasts (L-929 cells). Infect Immun. 1982 Oct;38(1):109–113. doi: 10.1128/iai.38.1.109-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Silverman D. J., Waddell A., Brown D. T. Penicillin-induced unstable intracellular formation of spheroplasts by rickettsiae. J Infect Dis. 1982 Aug;146(2):147–158. doi: 10.1093/infdis/146.2.147. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D. In vitro studies on rickettsia-host cell interactions: intracellular growth cycle of virulent and attenuated Rickettsia prowazeki in chicken embryo cells in slide chamber cultures. Infect Immun. 1975 Jun;11(6):1391–1404. doi: 10.1128/iai.11.6.1391-1401.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. D., Silverman D. J. In vitro studies on Rickettsia-host cell interactions: lag phase in intracellular growth cycle as a function of stage of growth of infecting Rickettsia prowazeki, with preliminary observations on inhibition of rickettsial uptake by host cell fragments. Infect Immun. 1976 Jun;13(6):1749–1760. doi: 10.1128/iai.13.6.1749-1760.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


