Abstract
Development and evolution of animal behaviour and morphology are frequently addressed independently, as reflected in the dichotomy of disciplines dedicated to their study distinguishing object of study (morphology versus behaviour) and perspective (ultimate versus proximate). Although traits are known to develop and evolve semi-independently, they are matched together in development and evolution to produce a unique functional phenotype. Here I highlight similarities shared by both traits, such as the decisive role played by the environment for their ontogeny. Considering the widespread developmental and functional entanglement between both traits, many cases of adaptive evolution are better understood when proximate and ultimate explanations are integrated. A field integrating these perspectives is evolutionary developmental biology (evo-devo), which studies the developmental basis of phenotypic diversity. Ultimate aspects in evo-devo studies—which have mostly focused on morphological traits—could become more apparent when behaviour, ‘the integrator of form and function’, is integrated into the same framework of analysis. Integrating a trait such as behaviour at a different level in the biological hierarchy will help to better understand not only how behavioural diversity is produced, but also how levels are connected to produce functional phenotypes and how these evolve. A possible framework to accommodate and compare form and function at different levels of the biological hierarchy is outlined. At the end, some methodological issues are discussed.
Keywords: evo-devo, evolutionary developmental biology, evolution of behaviour, emergence, levels of analysis, biological hierarchy
1. Introduction
Behaviour has been studied for decades from both proximate (i.e. ontogenetic mechanisms and causes of actual behaviour) as well as ultimate perspectives (i.e. survival value and evolution) [1]. Is there a need for a new perspective? In recent years, fostered by unprecedented technical developments, we have noticed a new way to address (classic) questions that makes use of a more integrative approach and focuses on the system in addition to its component parts (e.g. [2,3]). On the other hand, different disciplines join forces to answer common questions. A clear trend is the integration of proximate and ultimate perspectives for a comprehensive understanding of phenotypes. But why combine development with evolution? A quick, off-the-shelf answer is: because ‘nothing in biology makes sense except in the light of evolution’ [4]. The fact is that, here, this quotation is appropriate. In fact, explaining phenotype and its diversity ‘is fundamentally a problem of explaining the evolution of phenotype development’ [5]. This goes beyond evolutionary biology. Medicine, to name a glossy subject, has begun to realize the importance of evolution, not only in its large praxis [6,7] but also for addressing specific and burning issues such as cancer [8].
A discipline that is successfully integrating proximate with ultimate aspects of phenotype is ‘evolutionary developmental biology’, or evo-devo in short. Evo-devo, as defined by one of its early exponents, is ‘the study of how developmental processes evolve to produce new patterns of development, new developmental gene regulation, new morphologies, new life histories and new behavioural capabilities’ [9]. Evo-devo has recorded many important achievements [10] and has now a diversified research programme that ranges from comparative embryology and morphology, developmental genetics to theoretical approaches [11].
Although evo-devo has successfully succeeded in identifying developmental mechanisms underlying phenotype diversity (e.g. [12,13]), a more evident integration of proximate and ultimate explanations seems to be required [14,15]. The objective could be not less than a ‘mechanistic theory’ for explaining phenotype development and evolution [16]. Since behaviour, considered frequently as ‘integrator’ of form and function [16], plays an important role in adaptive evolution, it would be ‘simplistic, and indeed potentially misleading, to consider the evolution of form without taking into account its interactions with behaviour in the arena of selection’ [10]. Although notable efforts to consider development and evolution of organisms within their natural context—and highlight so the role of behaviour—are being made (e.g. [17,18]), evo-devo studies have mostly focused on morphological aspects of the phenotype, leaving behaviour largely unaddressed [19].
The present issue is a first, more direct attempt to address behaviour within an evo-devo framework. The first obvious benefit we can expect is to understand more about the developmental mechanisms underlying behaviour diversity. But there is another, complementary interest peculiar to addressing traits at different levels of biological organization, such are morphology and behaviour. Although organisms develop and evolve as functional unitary entities, they are composed of a hierarchy of levels (i.e. proteins, cells, organs and so on) [20]. To achieve the fitness of the whole, the individual parts are matched. To understand how the levels are matched to each other and how variation at the genetic level translates into a functional phenotype (up to behaviour), it is crucial to understand the kind of relationships that connect levels. A hypothesis could be that different levels of biological organization may share principles of development and evolution and that, by looking at one level, one may discover principles that are relevant for others. Conversely, principles may be level-specific and focusing on only one level or even a priori assuming that principles obtained by studying one level can be applied tout court to other levels may be misleading. It is hence important to extend the evo-devo paradigm to other levels of the biological hierarchy and include the whole functional phenotype [10].
In this introductory article, I am mainly concerned with showing that morphology and behaviour share many aspects, beginning with the importance that the environment plays for their development. Behaviour and morphology constitute in fact different qualities of the same unique phenotype, tightly linked in development and evolution. Since these qualities appear to be present at each level of the biological hierarchy, a unique framework of analysis encompassing levels seems already possible. Based on that assumption, I provide an example of comparison across levels and discuss some implications. I conclude with some critical considerations on methods used for discovering the genetic basis underlying behavioural diversity. At the end of the article, summaries of the contributions presented in this issue can be found.
2. Different traits, one phenotype
In order to facilitate analyses, the phenotype is often fragmented in distinct traits and every trait analysed separately. This is reflected also in the disciplines dedicated to the study of the different traits, such as developmental biology (i.e. morphology) and ethology (behaviour), as well as their perspectives, such as proximate (e.g. developmental genetics) and ultimate (e.g. behavioural ecology). However, although the independence of traits in development and evolution is a known phenomenon [5], organisms develop and evolve as one entity. Keeping an eye on the whole functional phenotype while analysing individual traits may help to better understand aspects of their development and evolution, as I discuss here.
(a). Developmental aspects
(i). When does development of behaviour begin?
While a distinction may be made between a morphological and a behavioural trait, a clear separation between development of morphology and behaviour does not exist. When does behaviour, and so its development, begin during the lifetime of an animal?
Development of behaviour may be considered as beginning when the organism interacts with the environment. But what is the environment? Is this the ambience external to the developing organism? In many classical examples, behavioural development starts already before a mature behaviour is ‘used’ to interact with the external world [21]. For instance, highly specific responsiveness to the mallard maternal call at the species-typical repetition rate develops in the Peking duck embryo in advance of auditory experience with its own or sib vocalizations [22]. Similarly, correct wiring of the visual system in higher mammals occurs in utero long before vision, when waves of spontaneous neuronal activity are generated by ganglion cells in the retina, even before photoreceptors are present, and propagated across central synapses to target neurons in the lateral geniculate nucleus [23]. In these cases, development of behaviour starts during embryogenesis. Basically, neuronal circuits underlying behaviour develop long before the behaviour they support, and the stimulation of many of them is crucial for their development [24].
Thus, the distinction between the internal and the external environment in order to define when development of behaviour begins is problematic. Further, if influences internal to the developing organism can be considered part of ‘behaviour development’, which of these should be viewed as behaviour development and which not? The development of many neuronal circuits underlying behaviour does not require action potentials and synaptic transmission but is regulated by molecular cues as found on cell surfaces or diffusing in tissues, the same class of signals that regulate morphological development [25]. How should the reaction of a neuron (growth; changing neuronal activity) later involved in behaviour to an impinging stimulus be categorized? Morphological or behavioural development? In an extreme view, behaviour can be simply considered as a series of neuronal impulses within a specified neuronal circuit. In this case, development of behaviour would start when the first neuron is formed. From the perspective of a developing neuronal circuit and neuronal impulses (and hence for behaviour), it does not matter whether a stimulus comes from outside or inside the developing organism or whether it has a chemical or neuronal origin: it will cause a change in developmental events. Thus, development of behaviour and morphology merges at a certain point into each other, but where?
(ii). Development of both morphology and behaviour is sensitive to environmental influences
Changing perspective on what to consider ‘environment’ helps us to recognize more similarities shared by morphology and behaviour. Plasticity is ‘the ability of an organism to react to an internal or external environmental input with a change in form, state, movement, or rate of activity’ [5]. Behaviour is commonly viewed as ‘the’ plastic phenotype, ‘one organismal property that displays high degrees of phenotypic plasticity’ [16]. Certainly, behaviour is ‘highly, and reversibly, combinatorial and recombinatorial during a single life time’ [5], but this is a quality of a trait as it appears in a specific moment. And yet, also its development is considered as being particularly plastic, in which the continuous interaction with the environment is an intrinsic property. Experience and learning are concepts commonly associated with that. Morphological development, in contrast, is mostly believed to be less influenced by environmental perturbations. But which environment are we talking about?
If we shift the focus away from the whole organism and define ‘environment’ as everything different from the genome [5], we realize that the development of morphology is highly plastic and does not require fewer interactions with the environment than behaviour does. Morphological development relies on continuous interactions between developing parts of the body. For instance, shear stress produced during flow is necessary for the correct development of haematopoietic cells [26]. A classical example in morphogenesis is neural induction, when the mesoderm contacts and induces the overlying ectoderm to develop into neural tissue. Manipulations of developmental events reveal how crucial the environment is for a ‘normal’ morphological development: if a small piece of tissue from around the dorsal rim of the blastopore of a developing amphibian embryo is transplanted to the ventral side of another embryo, the host responds by forming an additional embryonic axis containing a virtually complete central nervous system [27]. Interactions among developing tissues can certainly be viewed as a process of ‘experience and learning’, not less than what is known for development of behaviour. Not surprisingly, sensitive periods—phases in development during which a specific trait of an organism can be permanently altered by a particular experience or treatment, preceded and followed by a period of lower sensitivity to the same treatment—are readily described in behavioural [28] as well as morphological development [29].
When stressing the role of the environment in development and evolution [16], it is important to realize that the environment internal to a developing organism plays a role as important as the environment external to it. But basically, external/internal are relative concepts. What is external to the cell is internal to a tissue, which is internal to an organ and the organism. Even stimuli impinging on an organism may still be considered internal to a social group, a colony and so on. The best focus for an integral evo-devo is simply the whole functional phenotype, which may be defined, according to one definition, as ‘all traits of an organism other than its genome’ [5].
(iii). Genes and environment
Morphology and behaviour influence each other's development. Development of the withdrawal reflex system in mice, for instance, requires sensory feedback from muscle contractions to adjust correct synaptic organization [30]. An extreme example illustrating this mutual influence is provided by the so-called two-legged goat and discussed by West-Eberhard [5]. The goat, born without forelegs, adopted a semi-upright posture and bipedal locomotion from the time of its birth. This behaviour caused morphological changes in bones, pelvic skeleton and musculature and thoracic skeleton not found in normal goats of the same age. Besides demonstrating the interplay between morphology and behaviour in development, this example reveals something else: development is contingent and accommodating. That is, if there is a ‘developmental programme’, this is highly sensitive to feedbacks from the environment. If these change, then developmental events can, to a certain degree, adapt. Changes in the environment can have a genetic origin (e.g. altered expression of a transcription factor during limb development) but also a non-genetic basis (e.g. paralysis of forelimbs owing, for example, to polio). It is clear that there was no ‘programme’ telling a priori that bones and muscles should have developed in a certain way if some components had changed. There were no genes telling the limbs to develop differently or telling the animal to behave differently. The ability to adapt to changing developmental conditions—regardless of the cause of variation, whether genetic or environmental—is known as phenotypic accommodation [5]. But a changing environment, we have seen, is a constant of all development, not only of a pathological state. Indeed, many aspects of phenotype development simply emerge from interactions in time and space of underlying component parts and do not probably map one-to-one onto a genetic ‘programme’. For instance, if shear stress is a key player for the development of haematopoietic cells, how is this information encoded in the genome? Is there a gene or genetic programme ‘dedicated’ to ‘shear stress’? For this reason, it has been argued that organisms are not reducible to the component parts [31,32] and several authors [5,33–35] warn against seeing a strictly defined ‘programme’ in the genome and considering genes as the sole source of developmental information.
This realization has been made earlier in behavioural sciences. Probabilistic epigenesis for instance ‘emphasizes the reciprocity of influences within and between levels of an organism's developmental manifold’ (like genetic activity, neural activity as well as influences of the environment external to the developing organism) ‘and the ubiquity of gene–environment interaction in the realization of all phenotypes’ [36,37]. Similarly, in neuroconstructivism, ‘cognitive development is explained as emerging from the experience-dependent development of neural structures supporting mental representations’ [38].
Attempts to include the environment into a theory of development and evolution are being made in ‘developmental systems theory’ (DST), which—not surprisingly—is rooted primarily in developmental and behavioural psychology. For DST ‘developmental information resides neither in the genes nor in the environment, but rather emerges from the interactions of disparate, dispersed developmental resources’ [39]. While genes play clearly an important role in development, they do not seem to be alone. But who plays which role is still debated [40]. An important question is thus understanding which the other developmental resources are, the role they play, and whether and how they could be integrated into the evo-devo framework of analysis.
(b). Evolutionary considerations
(i). Reciprocal influences in evolution
Evolutionary studies have preferred to focus either on morphology or on behaviour. However, if the phenotype develops as one entity, reciprocal influences in evolution are expected, as described in several studies on adaptive evolution.
McPeek [41] compared Damselfly larvae of species inhabiting lakes in which predation was exerted by fishes and of species from fish-less lakes, in which dragonflies were the main predators. The latter are morphologically very similar to one another and differ greatly from fish-lake species. They are large and are active swimmers. All larvae of species from lakes with fishes move very slowly and infrequently but species are morphologically very diverse. Since species in fish-less lakes are more closely related to species in lakes with fishes, McPeek concluded that, following habitat shifts, selection pressures exerted by dragonfly predation apparently favoured swimming as an escape tactic, which mediated selection pressures onto morphologies used in swimming to increase swimming performance. Similar considerations may apply in other cases. Adaptive differences in the jaw morphology in cichlid fishes are related to their feeding habits [42], and the same seems true for variations in beak morphology in Darwin finches [43]. In more extreme cases, such as the evolution of secondary male sexual ornaments, which in some species are driven by female choice [44], the function of morphological structures can only be understood in conjunction with behaviour. These are just a few examples but the ‘potential complexity of morphological and behavioural interactions in the evolution of new adaptive types’ could be ‘much greater than previously considered’ [45].
All elements of the phenotype influence, directly (e.g. neurons) or indirectly (e.g. muscle size), patterns of behavioural adaptation. Katz [46] argues that the functional organization of the nervous system constrains but also promotes the evolvability of behaviour, and Chiel & Beer [47] remind that ‘adaptive behaviour also depends on interactions among the nervous system, body and environment: sensory preprocessing and motor post-processing filter inputs to and outputs from the nervous system; coevolution and co-development of nervous system and periphery create matching and complementarity between them; body structure creates constraints and opportunities for neural control; and continuous feedback between nervous system, body and environment are essential for normal behaviour’.
(ii). Integration of proximate and ultimate explanations
Purely functional considerations of behaviour seem sometimes to forget the historical dimension of the phenotype. Behavioural ecology has thrived on verifying theoretical predictions in natural populations [48]: which is the best behaviour to survive in a particular ecological context? But where does behavioural diversity in developmental terms come from? ‘How comes this diversity to be there in evolutionary terms?’ [5]. As exemplified by the two-legged goat, new behaviour may be the result of phenotypic accommodation or of ancestral inheritance instead of selective pressure alone [49,50]. Moreover, the past evolutionary history constrains future evolutionary trajectories [51,52]: under the same selection pressure, different genomes will respond in different ways, whereas similar traits can be the result of different developmental paths [53,54]. Thus, functional considerations may be used to predict successful behavioural strategies such as particular cognitive abilities [55], but probably not to predict their genetic and developmental basis [56–58]. Behaviour observed in an animal is the result of the interplay between genetic and developmental constraints as well as selection pressures [59].
A topical example to illustrate the above are behavioural syndromes, ‘a suite of correlated behaviours reflecting between-individual consistency in behaviour across multiple situations’ [60]. Their existence appears in conflict with the expectation that organisms are adapted to every situation in an optimal fashion. Without knowing the genetic and developmental basis of behaviour, it is not possible to fully explain why behavioural syndromes are present in some species but not in others, or why behavioural syndromes display in some species correlations across domains (e.g. aggression in feeding as well as mating domain) whereas in others they show domain-specific correlations (e.g. in the mating domain, correlation between aggression towards females and males; no correlation between aggression in the mating versus feeding domain) [61].
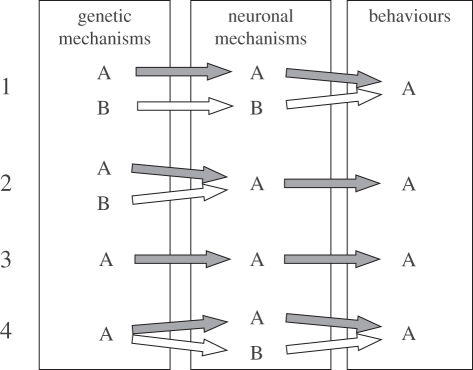
Another conundrum is explaining the adaptive evolution of similar (or homologous) behaviours in different taxa (figure 1). Not only are common behaviours like aggression, sex, or learning and memory present across taxa [63], but also more specific ones, such as tool use [64] or web-spinning [65]. A key developmental question is: can similar behaviours in different taxa rest upon completely different neuronal and/or genetic mechanisms or do similar behaviours (independently of the taxon) rely (or even have to rely) on conserved neuronal and/or genetic mechanisms (figure 1)? Answering this question correctly also depends on clarifying what ‘homologous’ traits are. Homology in biology refers to an historical continuity of traits. As aptly formulated by Wagner [66], ‘intuitively, one would expect that the historical continuity of morphological characters is underpinned by the continuity of the genes that govern the development of these characters. However, things are not that simple: one of the most important results of the past 15 years of molecular developmental genetics is the realization that homologous characters can have different genetic and developmental bases’ [53,54,67]. These findings have occasionally caused misunderstandings as to the correct interpretation of homologous characters [68–70] and new ways to define them are being continuously discussed [66,71–73]. The same kind of difficulties are expected when discussing homology in behavioural traits and, especially, their underlying genetic and developmental bases [63].
Figure 1.
Evolution of ‘homologous’ behaviours. The evolution of similar behaviours across taxa, such as sex or aggression, represents a key developmental and evolutionary phenomenon for understanding genetic and developmental paths through which specific behaviours can be realized. Here, the question is whether similar behaviours can have a different neuronal basis (1), whether particular behaviours can only rest upon conserved neuronal (2) and genetic mechanisms (3) or whether—as in the construction of functionally similar morphological structures [62]—similar behaviours can have the same genetic but different neuronal (i.e. developmental) basis (4).
If the above discussion reminds us of the importance to correctly [74] integrate proximate and ultimate aspects for an accurate understanding of behaviour [75], the latter example raises another, related problem: how are the levels in the biological hierarchy connected?
3. Towards formulating a framework of analysis across levels of organization
(a). Broadening the perspective
Upto here, I have tried to highlight the importance of keeping an eye on the whole phenotype while studying individual traits. This helps us to recognize qualities shared by morphology and behaviour (e.g. the importance of the environment) and reciprocal influences in development and evolution. Here I change perspective. I use broader definitions of ‘form’ and ‘behaviour’ to develop a possible framework to describe and compare traits at different levels of the biological hierarchy.
(i). The form of behaviour
One apparent quality of morphology is to have a defined structure, a form. As can be found in any English dictionary, form is defined as ‘the spatial arrangement of something as distinct from its substance’. In that respect, also behaviour has, like morphology, a form. Different from morphology, behaviour may be viewed as a dynamic (i.e. involving movement) ‘spatial arrangement of’ e.g. body limbs, but this is nevertheless ‘form’. In this sense and in analogy with a morphological trait, the form of a particular species-specific behavioural pattern (e.g. the courtship displays) remains within certain limits (i.e. is repeatable) and can be recognized as such despite some intra- and inter-individual variation in members of the same species. Additionally, this characteristic allows a particular behaviour to achieve a determined function (i.e. grooming behaviour, or a communication display). Frequently, a specific motoric pattern with specific parts of the body produces a reaction but not when the same motoric pattern is produced with other parts of the body (if possible at all) or if another motoric pattern is produced using the same body parts. For instance, a hand movement in a certain fashion signals ‘hello!’ Another movement with the same hand may signify completely opposite intentions. This specificity is more evident in elementary behaviours involving motoric patterns such as feeding, swallowing, excreting (urinating and defecating), displacing in space (walking, flying), yawning, vomiting, grooming and so on. But also more complex behaviours, such as aggression, sex, fear, to even more elaborate functions commonly defined under the term ‘cognition’, such as decision making, may still be characterized by having a determined ‘form’. As known for morphological traits, motoric patterns in a species are so constant that they can be used to classify species and build phylogenies (e.g. [76,77]). The idea of form in behaviour is not new. Lorenz, for instance, when referring to ‘Instinkthandlungen’ (instinctive acts), insisted on viewing and studying species-specific behaviours as organs [78].
(ii). The behaviour of form and the link to function
Up to here, behaviour has been considered from its ethological perspective (i.e. as ‘the behaviour of an animal’). But behaviour has, of course, a broader definition, as found for example in Wikipedia: ‘the actions or reactions of an object or organism, usually in relation to the environment’. Behaviour so defined is not restricted to any specific level of the biological hierarchy. Protozoa, for instance, display complex actions and reactions, from escaping from a threat, mating and replicating to moving towards a food source and feeding [79]. These movements are not coordinated by a nervous system but are nevertheless classified as animal ‘behaviour’. Basically, morphological structures at every level of the hierarchy are regularly engaged in behaviour: organs, cells and enzymes ‘act or react’. This ‘acting or reacting’ is strictly associated with function, meant here as the way in which a morphological trait operates to achieve function. The heart behaves in a defined way in order to function as a pump. Enzymes ‘behave’ (i.e. function) in a very specific way in order to operate (e.g. catalyze reactions).
A second element in the definition is that behaviour may or may not involve movement. Even structures that appear static may owe their form to passive behaviour, like for instance the structure of a bone, which is meant to maximize resilience when involved in a specific motoric behaviour, or colour patterns on the exoskeleton of an insect, meant to provoke a specific behaviour, like sexual attraction in a potential partner or warning to competitors or other insects, or even to avoid being seen by predators.
Generally, morphological structures have a determined form in view of a specific way of operating. The way in which they operate, involving or not movement, because it is an ‘action or reaction’, can be considered ‘behaviour’. Put differently, the mean of functioning (the behaviour) of a morphological structure reveals its (form and) function. This is why behaviour is frequently seen as an integrator of function [16]. This is not to say that morphology does not have function on its own, but a more correct view is that function is achieved through morphology (i.e. structure) behaving (or used) in a specific way.
(iii). A framework for comparing levels of organization
Considering the similarities between morphology and behaviour highlighted above and, especially, the form characterizing morphology as well as behavioural displays, a unique evo-devo programme may now be more within reach. A common programme would for instance try to understand developmental mechanisms underlying ‘form’ at each level of the biological hierarchy and how these levels relate to each other. In order to integrate proximate and ultimate perspectives, the ‘mechanistic theory’ for evo-devo envisioned by Laubichler [16] should hence also integrate levels of biological organization. With respect to ‘mechanistic explanations’, evo-devo has been mainly considering gene regulatory networks acting in development and changing through evolution (e.g. [80]). Yet, we have seen above that many features of the phenotype have emergent properties that would not map necessarily one-to-one with the genotype. To the reductionistic criticism Hamilton replies ‘by noticing that mechanistic explanations are reductionistic’—in the sense that a ‘mechanistic explanation works by decomposing systems (…) into their component parts and operations’—‘but they need not lead to gene-centrism’. In particular, reductionistic explanations ‘need not lead anyone to ignore the overall system in favour of the actions of one “fundamental” part of it [81]. That is, the properties of a system depend as much on the component parts as on their organization.
By reuniting the qualities of form and behaviour outlined above, one may recognize that ‘behaviour’—in its broader definition—is in fact the way in which parts composing a specific level of the hierarchy function, their organization. The parts so organized (i.e. behaving in a certain way) acquire and have a specific ‘form’ and so a function. This perspective applies to any level of the biological hierarchy (figure 2). Parts at the ‘behavioural level’ may be body parts that, organized (i.e. moving, behaving) in a specific way, achieve a certain function; parts at a lower level may be amino acids in a protein that, organized in a determined way (i.e. having a specific three-dimensional structure) achieve a specific function.
Figure 2.
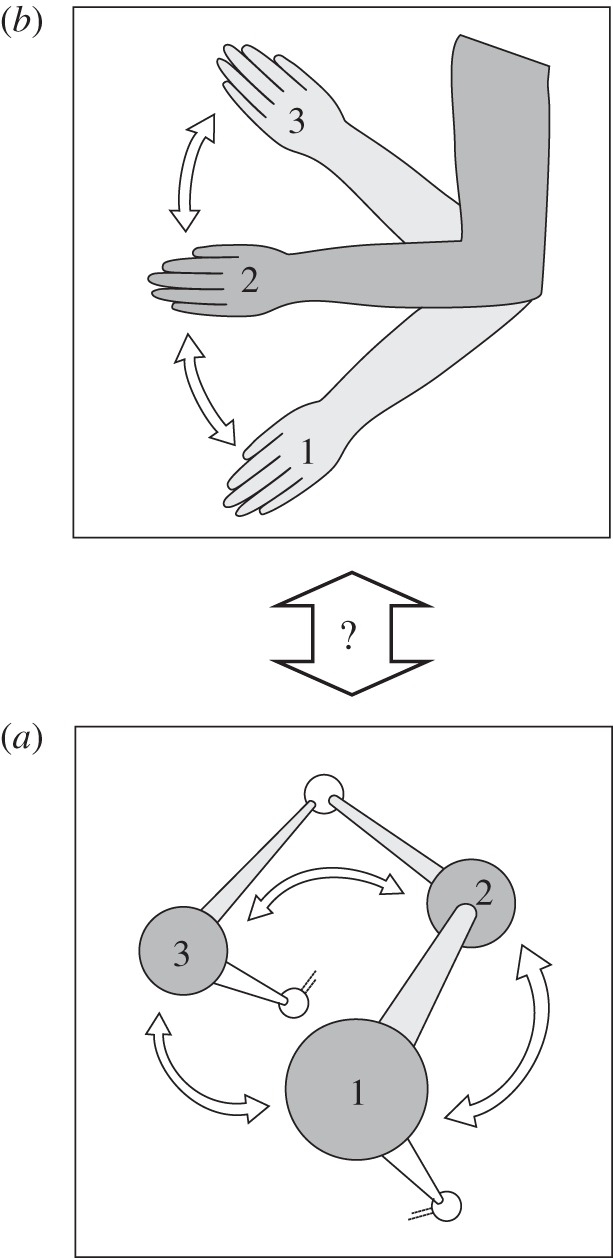
Organization of levels in the biological hierarchy. The organization of elements at each level may be similar across the biological hierarchy. Because elements or parts (indicated by the numbers) behave or interact (indicated by arrows connecting the parts) in a specific way, they acquire (in the case of dynamic interactions between the parts) or have (in the case of static interactions) a form with a defined function. (a) A level may be a protein, composed of amino acids having a specific spatial organization or, (b) in a behavioural display with an arm, a determined coordination in time and space of parts of the arm (hand, forearm). A key question is understanding how the levels are connected (vertical double arrow with question mark).
In this way, some of the difficulties encountered above, as for example establishing when, during development, to consider the beginning of behaviour development, would disappear. Because biological systems are inherently functional, they are always composed of behaviour and form at the same time. Form and behaviour describe together the specific way in which parts at a certain level of the biological hierarchy are organized and function. As soon as this organization changes in time or space, development begins.
A ‘mechanistic theory’ for development and evolution would thus have to understand (i) which are the parts composing each level in the biological hierarchy, (ii) how these parts are organized in order to produce each level, (iii) how the levels are connected, and (iv) how the organization in levels evolves and affects evolutionary trajectories in order to produce phenotypic diversity.
(b). Evo-devo on behaviour: comparing levels
If developmental mechanisms are similarly organized through the levels of the biological hierarchy, then conserved principles may be expected across them. An approach to understand this is applying the available evo-devo framework to what we already know about development and evolution of behaviour (see e.g. [82]). Here, I briefly illustrate an example of how this approach could look like and generate new specific questions that can be experimentally addressed.
(i). The conservation of developmental elements across species
An ongoing discovery in evo-devo studies is that many genes and gene regulatory networks underlying morphology are evolutionarily conserved and inform the evolutionary process [83]. Some are used for similar tasks in distant taxa, such as genetic cascades underlying eye [62] or heart [84] formation, or for the specification of proximal–distal limb patterning [85]. Others are deployed multiple times during development for the construction of completely different structures [86,87]. Is there conservation in the elements (genes, neurons) underlying behaviour? Some behaviours, like aggression [88] or learning [89], seem to rely on molecular mechanisms that are conserved between vertebrates and invertebrates [63] (see also [63] for a broader discussion). This might be explained by the fact that a centralized nervous system was already present in the common ancestor of bilateria, as supported by genetic and developmental data [90,91]. These data suggest that even more molecular mechanisms underlying ‘homologous’ behaviours could be shared by vertebrates and invertebrates. It may hence not be surprising to find conservation within more related groups. FoxP2, for instance, even though it is also expressed in non-vocal learner species, is necessary for the elaboration of sensory-motor information underlying vocalization from crocodiles to humans [92,93].
Conservation seems also to be widespread in the neuronal mechanisms underlying behaviour. Neuronal structures responsible for acquisition and processing of odorant and taste information display a striking degree of similarity between invertebrates and vertebrates [59,94]. Since specific neuronal circuits can support different behaviours [95], conservation across taxa may not be surprising and could also explain the observation that entire circuits may be retained even across taxa that display different behaviours [96].
Even if limited, these data suggest that conservation might not be specific to a level of the biological hierarchy (e.g. genes, proteins) but might be common also at other levels (e.g. neuronal circuits). Still, many questions remain open. If elements are conserved, how is context-specificity during development realized? That is, how are conserved elements used to produce different developmental outcomes? Do conserved gene regulatory networks underlie particular behaviours as they do for particular morphological structures [83]? If elements (i.e. proteins, neuronal circuits) are conserved, how is behaviour diversity produced?
(ii). Developmental mechanisms underlying phenotypic novelty
The fact that many proteins and protein cascades are conserved across taxa but are deployed for the construction of different morphologies suggested that their differential use (rather than their composition) could lie at the basis of the observed diversity. Since differential gene use is obtained through changes in regulatory regions, this came to be considered by some as a major mechanism behind the evolution of phenotypic novelty [97,98]. Although still debated [99,100], this conclusion is supported by studies on the evolution of morphological and physiological traits [101–104]. Genes contributing to behaviour are probably involved in other, non-behavioural functions [105]. The genes needed for the construction of the central nervous system (e.g. Hox, Otx, Pax, Wnt), for instance, are used for the construction of other organs as well [106]. This applies also for many genes (e.g. Pax6) involved in the development of central pattern generators (CPGs), neuronal circuits underlying many rhythmic behaviours like breathing and walking [107]. This could suggest that also diversity in behaviour may be produced mainly through changes in the use rather than the coding sequence of genes, a hypothesis supported already by few data. For example, an important species-specific difference in affiliative behaviour in voles is caused by changes in the regulatory region of the vasopressin V1a receptor [108]. The fact that in humans, retained non-coding sequences underwent accelerated evolution compared with other primates [109], and genes specifically expressed in the brain display a lower degree of amino acid divergence than other genes [110], lent further support for such a mechanism. Finally, since FoxP2 is expressed in vocal learner and non-vocal learner species, its involvement in vocal learning has also been attributed to differences in expression [92].
With regard to neuronal mechanisms, since homologous neurons and neuronal circuits can be found in species that appear and act very differently, it has been suggested that functional differences could arise by re-specification of common circuits [19,96]. Different authors have observed that rather subtle changes in the nervous system (e.g. change in neuromodulation) can cause important changes in behaviour [56,96]. For instance, the evolution of language in humans entailed modifications of pre-existing neuronal circuits [111] and the switch between aggression and courtship in Drosophila relies on the neuromodulatory action of octopamine on male-specific neuronal circuits [112].
The first impression when considering these data is that both at the genetic as well as the neuronal level the way for producing phenotypic diversity may be similar. That is, changes in the use (rather than the nature) of conserved elements at one phenotypic level (genes, neuronal circuits) seem responsible for changes at higher levels (morphology, behaviour). The data presented above are certainly not enough to make a conclusive assessment, but the idea may still serve as a working hypothesis. Basic questions related to this are: How do changes at the higher levels needed for producing new behaviour correlate with changes at the genetic level? Is it all about ‘teaching old genes (or neuronal circuits) new ‘tricks’’—to use an expression familiar to evo-devo practitioners [113]—or are other changes (i.e. the coding sequence of genes) also or even more important for producing new behaviour? Are the genetic and developmental changes responsible for behaviour variation within a species the same as those causing behaviour differences between species?
These few considerations already raise excitement: the conservation of principles governing development and evolution of phenotypes across levels of phenotypic complexity (i.e. morphology, behaviour) could be more than a hypothesis.
4. The search for genetic changes underlying behavioural diversity: some considerations
(a). The limits of comparative genomics
How to find changes in the genome that are relevant for behavioural diversity? Comparisons between the genomes of distinct species may reveal associations between new genes and novel phenotypes [114]. In general, however, as already known for new morphological structures, new behaviours are probably the product of conserved genes rather than new, species-specific ones. For instance, the development of CPGs relies on genes involved in the development of many other structures [107]. For the same reason, it will be difficult to infer function from the evolutionary conservation of the DNA sequence [115], both regulatory and coding, unless the compared species are closely related and the involvement of some genes in behaviour is known at least in one species. In the case of new morphology, relevant genes can be inferred from their expression in the new structure they build (e.g. [43]). As to behaviour, it will be difficult to associate genes with a particular behaviour without knowing which specific neuronal elements the behaviour needs, which is rarely the case. Additionally, the fact that the same neuronal structures can support many behaviours [95] renders the analysis even more daunting.
If new behaviour is the result of small changes in regulatory regions of conserved genes, it will be difficult to spot the relevant ones: small changes in the genome are frequently the product of drift and non-selective mutations. Between humans and mice, for instance, many regulatory elements are not conserved, and many conserved elements in regulatory regions appear not to have a regulatory function [116]. Ideally, changes in behaviour should be unambiguously associated with changes at the genetic level. This is only possible for experiments carried out in the same genetic background, as in mutagenesis screens and selection experiments.
(b). Mutagenesis screens and selection experiments
If diversity in behaviour would be caused by a change in the regulatory region of a gene, a small but relevant mutation in a regulatory element should affect only one or few functions (e.g. in behaviour) of that gene. Mutagenesis screens for behaviour have been crucial to identify genes underlying important behaviours in Drosophila [117], but this method seems nowadays not very popular. This may be partly due to the belief that behaviour is genetically more complex than morphology, because of, for example, more widespread pleiotropy. However, there are no reasons for assuming that genes affecting behaviour would display more pleiotropy than genes underlying complex morphology (compare for instance [118,119]). Another reason could be that we are possibly not used to reducing complex behaviours into defined and quantifiable behavioural patterns as we easily do with complex morphology. New technologies can be combined with mutagenesis screens to overcome these problems, as for instance the use of software for screening behavioural mutants in high-throughput format [120,121], or microarrays and next-generation sequencing methods to spot changes in gene expression as a consequence of the induced mutation (see below).
However, understanding the primary function of a wild-type allele by looking at the effects of its mutated version could be misleading. In fact, if phenotypic accommodation is extensive, the mutant phenotype we observe would be caused primarily by the reaction of the developing system to the mutation, which has less to do with the primary function of the gene. For instance, if the two-legged goat phenotype was caused by a mutation, what is the primary function of the mutated gene? Causing the goat to walk normally? Or should we not better say that we have discovered the gene (i.e. the mutant allele) responsible for bipedal posture?
Heritable behavioural variation in a population can be screened directly or used to select lines that behave differently. Differences in behaviour can then be correlated with genetic changes through microarrays [118], QTL analyses [122] or, nowadays, next-generation sequencing methods [123]. Although selection is applied on behavioural differences observed within and not between species, selection experiments can identify genes associated with a particular behaviour [118] and reveal genetic correlations underlying a particular behaviour, but also—since development and evolution of behaviour and morphology influence each other—underlying particular associations between behaviour and morphology in adaptive evolution.
5. Conclusions
I have argued for the importance of keeping an eye on the whole phenotype while analysing individual traits. This helps for instance to recognize that key developmental elements are shared by morphology and behaviour. Behaviour is not more plastic than morphology. It is only dynamic and can be repeatedly used. Environment is a key element of development of both traits and should find a place in the framework of analysis of evo-devo. The tight entanglement of morphology and behaviour in development and evolution suggests that an integration of proximate and ultimate perspectives could offer a better understanding of present phenotypes. The functional phenotype, made of morphology and behaviour alike, develops and evolves as one entity. Therefore, there is only one evo-devo that includes the whole functional phenotype [10].
Since behaviour can describe ‘the way of interacting’ of elements at any level of the biological hierarchy and form is the specific way—dynamic or static—in which these elements are organized, morphology and animal behaviour could be reunited within the same framework of analysis. This framework may be applied beyond the organism level, such as to social groups, to understand whether principles valid within organisms can be used to describe development and evolution beyond the organism level [81,124]. Behaviour hence is truly the missing partner: not only animal behaviour at the organism level, needed for a better interpretation of adaptive evolution but, more generally, behaviour as the way in which elements at any level of the hierarchy need to be organized in order to obtain a specific functional form. The question now is understanding which are the key players at every level, how they interact and how levels are connected. Perhaps, once having clarified this, understanding the role of genes—i.e. whether they are the major players in development and evolution—will emerge as a natural consequence.
6. What the articles say
While studies on behaviour development and evolution have been carried out for many years, the treatment of behaviour within an evo-devo paradigm is only beginning, as reflected by the contributions presented in the present issue. The first articles [10,46,63,74] set in motion (or refresh) important discussions on general aspects of development and evolution pertaining to behaviour. These are then more specifically addressed in the studies discussed in the second series of articles [58,93,94,124,125].
For all those not familiar with evo-devo, the article by Paul Brakefield [10] is a very useful introduction. He reviews some major themes and achievements of evo-devo, illustrating the scope of the field to reveal ‘how the processes of development can contribute to explaining patterns of evolutionary diversification in animal form’. In addition to stressing the exciting potential of extending the approach of evo-devo to patterns of behavioural diversification, Brakefield [10] reasons that ‘to consider the evolution of form without taking into account its interactions with behaviour’ (…) ‘is simplistic and potentially misleading’. The author concludes by agreeing with some other workers in the field that understanding more about evolvability—‘the capacity of a developmental system to evolve’—should be a key step towards answering many of the open questions in evo-devo.
Studies of the evolution and the development of behaviour take place at the ultimate and proximate levels of analyses, respectively. There has been much debate in past decades about how to (or how not to) integrate studies across these levels. Scott MacDougall-Shackleton [74] reviews different uses of the term ‘levels of analysis’ and highlights how studies of function and mechanism can be integrated, an approach epitomized by evolutionary developmental biology.
Although genetic differences can affect behaviour, genes ultimately act through the nervous system. Paul Katz [46] maintains that the functional organization of the nervous system constrains but also promotes the evolvability of behaviour. He reviews evidence showing how neural mechanisms such as activity-dependent plasticity and neuromodulation allow basal neural circuits to be employed for different behaviours, as observed in sensory processing, motor output and even in complex social behaviour. Katz concludes that the qualities of the nervous system that enable it to produce complex behaviour may also play a role in the evolvability of species-typical behaviour.
Christopher Reaume and Marla Sokolowski [63] discuss how gene function, as a hierarchical biological phenomenon, relates to behavioural homology across species. They suggest that gene function homology in behaviour can be addressed independently using different levels of investigation, including the DNA sequence, the gene's position in a genetic pathway, spatial–temporal tissue expression and neuronal circuit. Several examples are used to illustrate these points, including circadian rhythms, learning and memory, food-related behaviours and sleep. They also discuss how qualitative and quantitative comparisons of the behavioural phenotype, its function and the importance of the environmental and social context should be used in cross-species comparisons.
In mammals, the statistics of allometric variation in brains within species (swine, mink, and multiple strains of laboratory mice) closely resemble phylogenetic brain variation over all mammals. Barbara Finlay, Flora Hinz and Richard Darlington [58] argue that, first, this pattern reveals the selection of developmental parameters for brain organization robust to environmental and niche variation at the individual level, and, second, that only a restricted set of computational models of the brain can be consistent with an adaptive function for conserved patterns of variation at within- and between-species scales.
Language in animals evolved via modifications of existing molecular and morphological hardware. Connstance Scharff & Jana Petri [93] focus on one protein, FoxP2, which is required for language in humans, for song learning in birds, and for motor skill learning in mice. They argue that it is a good candidate to study deep homologies of molecular toolkits and neural circuits relevant for the emergence of language. They also critically examine the (lack of) evidence for some of the claims about the uniqueness of human language.
Circadian systems are ubiquitous, implying an essential role for daily timing in evolution. Roleof Hut & Domien Beersma [125] suggest that energy storage was crucial for the earliest evolution of circadian clock systems in primitive photosynthesizing life forms. The modification of ATP storage/release capacity of KaiC proteins through KaiA suggests a daylength adaptation mechanism that may explain the global expansion of cyanobacteria. In general, different models for photoperiodic adaptation predict different selection pressures on the circadian period. Hut & Beersma [125] therefore conclude that establishing latitudinal clines in circadian tau for various species is crucial to reveal circadian photoperiod adaptation mechanisms and how these may constrain responses to latitudinal expansion and global warming.
Bee societies have fascinated humans for centuries because of their complexity, and led to many theories about the evolution of cooperative and altruistic behaviours. Guy Bloch & Christina Grozinger [124] review recent genomic studies on the development and evolution of social behaviour in bees and propose a model of ‘social pathways’, modules consisting in the detection, elaboration and output of signals involved in social behaviour. The authors then use the model to illustrate how the hormonal system and plasticity in the circadian clockwork have been modified during and contributed to the evolution of bee societies.
Taste, the sense that distinguishes between chemical compounds and the sensations they produce based on contact with chemoreceptors, allows discriminating edible from non-edible items and is, therefore, crucial for survival. Gabriela De Brito Sanchez & Martin Giurfa [94] discuss the principles of taste coding in the animal brain, from insects to mammals. They argue that an essential task for a neuroscience of taste is to determine the connectivity of taste-processing circuits in the central nervous system, and suggest that, beyond labelled-line or across-fibre pattern coding, taste representations are conserved across species and seem to relate to the hedonic value of the tastant (e.g. palatable versus non-palatable).
Acknowledgements
I thank D. Beersma, L. Beukeboom, P.-H. Clergeot, A. Kamping, S. Mitchell, M. Olmedo, S. Paolucci, T. Schwander, S. Verhulst, C. Vermeulen, L. van de Zande and three anonymous reviewers for useful discussions and comments on previous versions of the manuscript. I thank all contributors in this Special Issue for their tenaciousness in coping with my and reviewers' comments and apologize with all those whose contributions could not be included in this issue. I wish to thank the following people at Philosophical Transactions of the Royal Society: Joseph James for his invitation to guest-edit the present issue; Claire Rawlinson and Joanna Bolesworth for their patience with many changes along the way. R.C.B. received support with a grant from the Swiss National Science Foundation (SNF) for prospective researchers and an ALW grant (nr. 817.02.020) from the Netherlands Organization for Scientific Research (NWO).
Footnotes
One contribution of 10 to a Theme Issue ‘Evolutionary developmental biology (evo-devo) and behaviour’.
References
- 1.Bolhuis J. J., Giraldeau L.-A. (eds) 2005. The behavior of animals. Oxford, UK: Blackwell Publishing [Google Scholar]
- 2.Geschwind D. H., Konopka G. 2009. Neuroscience in the era of functional genomics and systems biology. Nature 461, 908–915 10.1038/nature08537 (doi:10.1038/nature08537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayroles J. F., et al. 2009. Systems genetics of complex traits in Drosophila melanogaster. Nat. Genet. 41, 299–307 10.1038/ng.332 (doi:10.1038/ng.332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobzhansky T. 1964. Biology, molecular and organismic. Am. Zool. 4, 443–452 [DOI] [PubMed] [Google Scholar]
- 5.West-Eberhard M. J. 2003. Developmental plasticity and evolution. Oxford, NY: Oxford University Press [Google Scholar]
- 6.Gluckman P. D., Beedle A. S., Hanson M. 2009. Principles of evolutionary medicine. 1st edn. Oxford, NY: Oxford University Press [Google Scholar]
- 7.Stearns S. C., Nesse R. M., Govindaraju D. R., Ellison P. T. 2010. Evolutionary perspectives on health and medicine. Proc. Natl Acad. Sci. USA 107, 1691–1695 10.1073/pnas.0914475107 (doi:10.1073/pnas.0914475107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson R. D. 2009. Evo-Devo and the evolution of cancer: a hypothesis for metamorphic therapies for the cancers of prolactin-influenced tumourigenesis: with special reference to glioblastoma multiforme (GBM). Med. Hypotheses 72, 629–630 10.1016/j.mehy.2009.01.014 (doi:10.1016/j.mehy.2009.01.014) [DOI] [PubMed] [Google Scholar]
- 9.Raff R. A. 2007. Written in stone: fossils, genes and evo-devo. Nat. Rev. Genet. 8, 911–920 10.1038/nrg2225 (doi:10.1038/nrg2225) [DOI] [PubMed] [Google Scholar]
- 10.Brakefield P. M. 2011. Evo-devo and accounting for Darwin's endless forms. Phil. Trans. R. Soc. B 366, 2069–2075 10.1098/rstb.2011.0007 (doi:10.1098/rstb.2011.0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller G. B. 2007. Evo-devo: extending the evolutionary synthesis. Nat. Rev. Genet. 8, 943–949 10.1038/nrg2219 (doi:10.1038/nrg2219) [DOI] [PubMed] [Google Scholar]
- 12.Salazar-Ciudad I., Jernvall J. 2010. A computational model of teeth and the developmental origins of morphological variation. Nature 464, 583–586 10.1038/nature08838 (doi:10.1038/nature08838) [DOI] [PubMed] [Google Scholar]
- 13.Jeong S., Rebeiz M., Andolfatto P., Werner T., True J., Carroll S. B. 2008. The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell 132, 783–793 10.1016/j.cell.2008.01.014 (doi:10.1016/j.cell.2008.01.014) [DOI] [PubMed] [Google Scholar]
- 14.Breuker C. J., Debat V., Klingenberg C. P. 2006. Functional evo-devo. Trends Ecol. Evol. 21, 488–492 10.1016/j.tree.2006.06.003 (doi:10.1016/j.tree.2006.06.003) [DOI] [PubMed] [Google Scholar]
- 15.Laubichler M. D., Maienschein J. (eds) 2009. Form and function in developmental evolution. Cambridge, NY: Cambridge University Press [Google Scholar]
- 16.Laubichler M. D. 2009. Form and function in evo devo: historical and conceptual reflections. In Form and function in developmental evolution. (eds Laubichler M. D., Maienschein J.), pp. 10–46, 1st edn. Cambridge, NY: Cambridge University Press [Google Scholar]
- 17.Gilbert S. F. 2001. Ecological developmental biology: developmental biology meets the real world. Dev. Biol. 233, 1–12 10.1006/dbio.2001.0210 (doi:10.1006/dbio.2001.0210) [DOI] [PubMed] [Google Scholar]
- 18.Gilbert S. F., Epel D. 2009. Ecological developmental biology: integrating epigenetics, medicine, and evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- 19.Katz P. S. 2006. Evolution and development of neural circuits in invertebrates. Curr. Opin. Neurobiol. 17, 59–64 10.1016/j.conb.2006.12.003 (doi:10.1016/j.conb.2006.12.003) [DOI] [PubMed] [Google Scholar]
- 20.Morowitz H. J. 2002. The emergence of everything: how the world became complex. New York, NY: Oxford University Press [Google Scholar]
- 21.Fentress J. C., McLeod P. J. 1986. Motor patterns in development (excerpt). In The development of animal behavior: a reader. (eds Bolhuis J. J., Hogan J. A.), pp. 219–244 Oxford, UK: Blackwell Publishers [Google Scholar]
- 22.Gottlieb G. 1980. Development of species identification in ducklings. VI: specific embryonic experience required to maintain species-typical perception in Peking ducklings. In The Development of animal behavior: a reader. (eds Bolhuis J. J., Hogan J. A.), pp. 161–169 Oxford, UK: Blackwell Publishers; [DOI] [PubMed] [Google Scholar]
- 23.Shatz C. J. 1996. Emergence of order in visual system development. Proc. Natl Acad. Sci. USA 93, 602–608 10.1073/pnas.93.2.602 (doi:10.1073/pnas.93.2.602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marder E., Rehm K. J. 2005. Development of central pattern generating circuits. Curr. Opin. Neurobiol. 15, 86–93 10.1016/j.conb.2005.01.011 (doi:10.1016/j.conb.2005.01.011) [DOI] [PubMed] [Google Scholar]
- 25.Goodman C. S., Shatz C. J. 1993. Developmental mechanisms that generate precise patterns of neuronal connectivity (Review). Cell 72, 77–98 10.1016/S0092-8674(05)80030-3 (doi:10.1016/S0092-8674(05)80030-3) [DOI] [PubMed] [Google Scholar]
- 26.Adamo L., et al. 2009. Biomechanical forces promote embryonic haematopoiesis. Nature 459, 1131–1135 10.1038/nature08073 (doi:10.1038/nature08073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown M. C., Keynes R. J., Lumsden A. 2001. The developing brain. Oxford, NY: Oxford University Press [Google Scholar]
- 28.Bateson P., Hinde R. A. 1987. developmental changes in sensitivity to experience. In The development of animal behavior: a reader (eds Bolhuis J. J., Hogan J. A.), pp. 66–75 Oxford, UK: Blackwell Publishers [Google Scholar]
- 29.Waddington C. H. 1966. Principles of development and differentiation. New York, NY: Macmillan [Google Scholar]
- 30.Schouenborg J. 2002. Modular organisation and spinal somatosensory imprinting. Brain Res. Rev. 40, 80–91 10.1016/S0165-0173(02)00191-1 (doi:10.1016/S0165-0173(02)00191-1) [DOI] [PubMed] [Google Scholar]
- 31.Polanyi M. 1968. Life's irreducible structure. Live mechanisms and information in DNA are boundary conditions with a sequence of boundaries above them. Science 160, 1308–1312 10.1126/science.160.3834.1308 (doi:10.1126/science.160.3834.1308) [DOI] [PubMed] [Google Scholar]
- 32.Elsasser W. M. 1998. Reflections on a theory of organisms: holism in biology. Baltimore, MD: The Johns Hopkins University Press [Google Scholar]
- 33.Nijhout H. F. 1990. Metaphors and the role of genes in development. Bioessays 12, 441–446 10.1002/bies.950120908 (doi:10.1002/bies.950120908) [DOI] [PubMed] [Google Scholar]
- 34.Goodwin B. C. 1994. How the leopard changed its spots: the evolution of complexity. New York, NY: C. Scribner's Sons [Google Scholar]
- 35.Pigliucci M. 2010. Genotype–phenotype mapping and the end of the ‘genes as blueprint' metaphor. Phil. Trans. R. Soc. B 365, 557–566 10.1098/rstb.2009.0241 (doi:10.1098/rstb.2009.0241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottlieb G. 1992. Individual development and evolution: the genesis of novel behavior. New York, NY: Oxford University Press [Google Scholar]
- 37.Gottlieb G. 2007. Probabilistic epigenesis. Dev. Sci. 10, 1–11 10.1111/j.1467-7687.2007.00556.x (doi:10.1111/j.1467-7687.2007.00556.x) [DOI] [PubMed] [Google Scholar]
- 38.Westermann G., Mareschal D., Johnson M. H., Sirois S., Spratling M. W., Thomas M. S. C. 2007. Neuroconstructivism. Dev. Sci. 10, 75–83 10.1111/j.1467-7687.2007.00567.x (doi:10.1111/j.1467-7687.2007.00567.x) [DOI] [PubMed] [Google Scholar]
- 39.Robert J. S., Hall B. K., Olson W. M. 2001. Bridging the gap between developmental systems theory and evolutionary developmental biology. Bioessays 23, 954–962 10.1002/bies.1136 (doi:10.1002/bies.1136) [DOI] [PubMed] [Google Scholar]
- 40.Oyama S., Griffiths P. E., Russell D. G. (eds) 2001. Cycles of contingency: developmental systems and evolution. Cambridge, MA: The MIT Press [Google Scholar]
- 41.McPeek M. A. 1995. Morphological evolution mediated by behavior in the damselflies of 2 communities. Evolution 49, 749–769 10.2307/2410328 (doi:10.2307/2410328) [DOI] [PubMed] [Google Scholar]
- 42.Albertson R. C., Streelman J. T., Kocher T. D., Yelick P. C. 2005. From the cover: integration and evolution of the cichlid mandible: the molecular basis of alternate feeding strategies. Proc. Natl Acad. Sci. USA 102, 16 287–16 292 10.1073/pnas.0506649102 (doi:10.1073/pnas.0506649102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abzhanov A., Protas M., Grant B. R., Grant P. R., Tabin C. J. 2004. Bmp4 and morphological variation of beaks in Darwin's finches. Science 305, 1462–1465 10.1126/science.1098095 (doi:10.1126/science.1098095) [DOI] [PubMed] [Google Scholar]
- 44.Bakker T. C. M. 1993. Positive genetic correlation between female preference and preferred male ornament in sticklebacks. Nature 363, 255–257 10.1038/363255a0 (doi:10.1038/363255a0) [DOI] [Google Scholar]
- 45.Emerson S. B., Koehl M. A. R. 1990. The interaction of behavioral and morphological change in the evolution of a novel locomotor type—flying frogs. Evolution 44, 1931–1946 10.2307/2409604 (doi:10.2307/2409604) [DOI] [PubMed] [Google Scholar]
- 46.Katz P. S. 2011. Neural mechanisms underlying the evolvability of behaviour. Phil. Trans. R. Soc. B 366, 2086–2099 10.1098/rstb.2010.0336 (doi:10.1098/rstb.2010.0336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiel H. J., Beer R. D. 1997. The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci. 20, 553–557 10.1016/S0166-2236(97)01149-1 (doi:10.1016/S0166-2236(97)01149-1) [DOI] [PubMed] [Google Scholar]
- 48.Parker G. A., Smith J. M. 1990. Optimality theory in evolutionary biology. Nature 348, 27–33 10.1038/348027a0 (doi:10.1038/348027a0) [DOI] [Google Scholar]
- 49.Gittleman J. L. 1989. The comparative approach in ethology: aims and limitations. In Perspectives in ethology: vol. 8, Whither ethology? (eds Bateson P. P. G., Klopfer P. H.), pp. 55–83 New York, NY: Plenum Press [Google Scholar]
- 50.Gould S. J., Vrba E. S. 1982. Exaptation; a missing term in the science of form. Paleobiology 8, 4–15 [Google Scholar]
- 51.Brakefield P. M. 2006. Evo-devo and constraints on selection. Trends Ecol. Evol. 21, 362–368 10.1016/j.tree.2006.05.001 (doi:10.1016/j.tree.2006.05.001) [DOI] [PubMed] [Google Scholar]
- 52.Wilkins A. S. 2007. Colloquium papers: between ‘design’ and ‘bricolage’: genetic networks, levels of selection, and adaptive evolution. Proc. Natl Acad. Sci. USA 104, 8590–8596 10.1073/pnas.0701044104 (doi:10.1073/pnas.0701044104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duboule D., Wilkins A. S. 1998. The evolution of ‘bricolage’. Trends Genet. 14, 54–59 10.1016/S0168-9525(97)01358-9 (doi:10.1016/S0168-9525(97)01358-9) [DOI] [PubMed] [Google Scholar]
- 54.Wray G. A. 2002. Do convergent developmental mechanisms underlie convergent phenotypes? Brain Behav. Evol. 59, 327–336 10.1159/000063566 (doi:10.1159/000063566) [DOI] [PubMed] [Google Scholar]
- 55.Sherry D. 2005. Do ideas about function help in the study of causation? Anim. Biol. (formerly Netherlands J. Zool.) 55, 441–456 [Google Scholar]
- 56.Nishikawa K. C. 2002. Evolutionary convergence in nervous systems: insights from comparative phylogenetic studies. Brain Behav. Evol. 59, 240–249 10.1159/000063561 (doi:10.1159/000063561) [DOI] [PubMed] [Google Scholar]
- 57.Bolhuis J. 2005. Function and mechanism in neuroecology: looking for clues. Anim. Biol. (formerly Netherlands J. Zool.) 55, 457–490 [Google Scholar]
- 58.Finlay B. L., Hinz F., Darlington R. B. 2011. Mapping behavioural evolution onto brain evolution: the strategic roles of conserved organization in individuals and species. Phil. Trans. R. Soc. B 366, 2111–2123 10.1098/rstb.2010.0344 (doi:10.1098/rstb.2010.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisthen H. L. 2002. Why are olfactory systems of different animals so similar? Brain Behav. Evol. 59, 273–293 10.1159/000063564 (doi:10.1159/000063564) [DOI] [PubMed] [Google Scholar]
- 60.Sih A., Bell A., Johnson J. C. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 61.Coleman K., Wilson D. S. 1998. Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim. Behav. 56, 927–936 10.1006/anbe.1998.0852 (doi:10.1006/anbe.1998.0852) [DOI] [PubMed] [Google Scholar]
- 62.Kozmik Z. 2005. Pax genes in eye development and evolution. Curr. Opin. Genet. Dev. 15, 430–438 10.1016/j.gde.2005.05.001 (doi:10.1016/j.gde.2005.05.001) [DOI] [PubMed] [Google Scholar]
- 63.Reaume C. J., Sokolowski M. B. 2011. Conservation of gene function in behaviour. Phil. Trans. R. Soc. B 366, 2100–2110 10.1098/rstb.2011.0028 (doi:10.1098/rstb.2011.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emery N. J., Clayton N. S. 2004. The mentality of crows: convergent evolution of intelligence in corvids and apes. Science 306, 1903–1907 10.1126/science.1098410 (doi:10.1126/science.1098410) [DOI] [PubMed] [Google Scholar]
- 65.Blackledge T. A., Gillespie R. G. 2004. Convergent evolution of behavior in an adaptive radiation of Hawaiian web-building spiders. Proc. Natl Acad. Sci. USA 101, 16 228–16 233 10.1073/pnas.0407395101 (doi:10.1073/pnas.0407395101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner G. P. 2007. The developmental genetics of homology. Nat. Rev. Genet. 8, 473–479 10.1038/nrg2099 (doi:10.1038/nrg2099) [DOI] [PubMed] [Google Scholar]
- 67.Wray G. A., Abouheif E. 1998. When is homology not homology? Curr. Opin. Genet. Dev. 8, 675–680 10.1016/S0959-437X(98)80036-1 (doi:10.1016/S0959-437X(98)80036-1) [DOI] [PubMed] [Google Scholar]
- 68.Abouheif E., Akam M., Dickinson W. J., Holland P. W., Meyer A., Patel N. H., Rudolf A. R., Louise Roth V., Gregory A. W. 1997. Homology and developmental genes. Trends Genet. 13, 432–433 10.1016/S0168-9525(97)01271-7 (doi:10.1016/S0168-9525(97)01271-7) [DOI] [PubMed] [Google Scholar]
- 69.Abouheif E. 1997. Developmental genetics and homology: a hierarchical approach. Trends Ecol. Evol. 12, 405–408 10.1016/S0169-5347(97)01125-7 (doi:10.1016/S0169-5347(97)01125-7) [DOI] [PubMed] [Google Scholar]
- 70.Gehring W. J., Ikeo K. 1999. Pax 6—mastering eye morphogenesis and eye evolution. Trends Genet. 15, 371–377 10.1016/S0168-9525(99)01776-X (doi:10.1016/S0168-9525(99)01776-X) [DOI] [PubMed] [Google Scholar]
- 71.Hall B. K. (ed.) 1994. Homology: the hierarchical basis of comparative biology. 1st edn San Diego, CA: Academic Press [Google Scholar]
- 72.Minelli A. 1998. Molecules, developmental modules, and phenotypes: a combinatorial approach to homology. Mol. Phylogenet. Evol. 9, 340–347 10.1006/mpev.1997.0490 (doi:10.1006/mpev.1997.0490) [DOI] [PubMed] [Google Scholar]
- 73.Hall B. K. 2003. Descent with modification: the unity underlying homology and homoplasy as seen through an analysis of development and evolution. Biol. Rev. Camb. Phil. Soc. 78, 409–433 10.1017/S1464793102006097 (doi:10.1017/S1464793102006097) [DOI] [PubMed] [Google Scholar]
- 74.MacDougall-Shackleton S. A. 2011. The levels of analysis revisited. Phil. Trans. R. Soc. B 366, 2076–2085 10.1098/rstb.2010.0363 (doi:10.1098/rstb.2010.0363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryan M. J. 2005. The evolution of behaviour, and integrating it towards a complete and correct understanding of behavioural biology. Anim. Biol. 55, 419–439 10.1163/157075605774841012 (doi:10.1163/157075605774841012) [DOI] [Google Scholar]
- 76.Cap H., Deleporte P., Joachim J., Reby D. 2008. Male vocal behavior and phylogeny in deer. Cladistics 24, 917–931 10.1111/j.1096-0031.2008.00223.x (doi:10.1111/j.1096-0031.2008.00223.x) [DOI] [PubMed] [Google Scholar]
- 77.Scholes E. 2008. Evolution of the courtship phenotype in the bird of paradise genus Parotia (Aves: Paradisaeidae): homology, phylogeny, and modularity. Biol. J. Linn. Soc. 94, 491–504 10.1111/j.1095-8312.2008.01012.x (doi:10.1111/j.1095-8312.2008.01012.x) [DOI] [Google Scholar]
- 78.Lorenz K. 1937. Über die Bildung des Instinktbegriffes. Naturwiss 25, 324–331 10.1007/BF01774269 (doi:10.1007/BF01774269) [DOI] [Google Scholar]
- 79.Verni F., Gualtieri P. 1997. Feeding behaviour in ciliated protists. Micron 28, 487–504 10.1016/S0968-4328(97)00028-0 (doi:10.1016/S0968-4328(97)00028-0) [DOI] [Google Scholar]
- 80.Erwin D. H., Davidson E. H. 2009. The evolution of hierarchical gene regulatory networks. Nat. Rev. Genet. 10, 141–148 10.1038/nrg2499 (doi:10.1038/nrg2499) [DOI] [PubMed] [Google Scholar]
- 81.Hamilton A. L. 2009. Toward a mechanistic evo devo. In Form and function in developmental evolution (eds Laubichler M. D., Maienschein J.), pp. 213–224 Cambridge, NY: Cambridge University Press [Google Scholar]
- 82.Toth A. L., Robinson G. E. 2007. Evo-devo and the evolution of social behavior. Trends Genet. 23, 334–341 10.1016/j.tig.2007.05.001 (doi:10.1016/j.tig.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 83.Davidson E. H., Erwin D. H. 2006. Gene regulatory networks and the evolution of animal body plans. Science 311, 796–800 10.1126/science.1113832 (doi:10.1126/science.1113832) [DOI] [PubMed] [Google Scholar]
- 84.Olson E. N. 2006. Gene regulatory networks in the evolution and development of the heart. Science 313, 1922–1927 10.1126/science.1132292 (doi:10.1126/science.1132292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pueyo J. I., Couso J. P. 2005. Parallels between the proximal–distal development of vertebrate and arthropod appendages: homology without an ancestor? Curr. Opin. Genet. Dev. 15, 439–446 10.1016/j.gde.2005.06.007 (doi:10.1016/j.gde.2005.06.007) [DOI] [PubMed] [Google Scholar]
- 86.Pires-daSilva A., Sommer R. J. 2003. The evolution of signalling pathways in animal development. Nat. Rev. Genet. 4, 39–49 10.1038/nrg977 (doi:10.1038/nrg977) [DOI] [PubMed] [Google Scholar]
- 87.Lemons D., McGinnis W. 2006. Genomic evolution of Hox gene clusters. Science 313, 1918–1922 10.1126/science.1132040 (doi:10.1126/science.1132040) [DOI] [PubMed] [Google Scholar]
- 88.Edwards D. H., Kravitz E. A. 1997. Serotonin, social status and aggression. Curr. Opin. Neurobiol. 7, 812–819 10.1016/S0959-4388(97)80140-7 (doi:10.1016/S0959-4388(97)80140-7) [DOI] [PubMed] [Google Scholar]
- 89.Alberini C. M. 1999. Genes to remember. J. Exp. Biol. 202, 2887–2891 [DOI] [PubMed] [Google Scholar]
- 90.Denes A. S., Jekely G., Steinmetz P. R., Raible F., Snyman H., Prud'homme B., Ferrier D. E. K., Balavoine G., Arendt D. 2007. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell 129, 277–288 10.1016/j.cell.2007.02.040 (doi:10.1016/j.cell.2007.02.040) [DOI] [PubMed] [Google Scholar]
- 91.Arendt D., Denes A. S., Jekely G., Tessmar-Raible K. 2008. The evolution of nervous system centralization. Phil. Trans. R. Soc. B 363, 1523–1528 10.1098/rstb.2007.2242 (doi:10.1098/rstb.2007.2242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scharff C., Haesler S. 2005. An evolutionary perspective on FoxP2: strictly for the birds? Curr. Opin. Neurobiol. 15, 694–703 10.1016/j.conb.2005.10.004 (doi:10.1016/j.conb.2005.10.004) [DOI] [PubMed] [Google Scholar]
- 93.Scharff C., Petri J. 2011. Evo-devo, deep homology and FoxP2: implications for the evolution of speech and language. Phil. Trans. R. Soc. B 366, 2124–2140 10.1098/rstb.2011.0001 (doi:10.1098/rstb.2011.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Brito Sanchez G., Giurfa M. 2011. A comparative analysis of neural taste processing in animals. Phil. Trans. R. Soc. B 366, 2171–2180 10.1098/rstb.2010.0327 (doi:10.1098/rstb.2010.0327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Briggman K. L., Kristan W. B. 2008. Multifunctional pattern-generating circuits. Annu. Rev. Neurosci. 31, 271–294 10.1146/annurev.neuro.31.060407.125552 (doi:10.1146/annurev.neuro.31.060407.125552) [DOI] [PubMed] [Google Scholar]
- 96.Katz P. S., Harris-Warrick R. M. 1999. The evolution of neuronal circuits underlying species-specific behavior. Curr. Opin. Neurobiol. 9, 628–633 10.1016/S0959-4388(99)00012-4 (doi:10.1016/S0959-4388(99)00012-4) [DOI] [PubMed] [Google Scholar]
- 97.Wray G. A. 2007. The evolutionary significance of cis-regulatory mutations. Nat. Rev. Genet. 8, 206–216 10.1038/nrg2063 (doi:10.1038/nrg2063) [DOI] [PubMed] [Google Scholar]
- 98.Carroll S. B. 2008. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134, 25–36 10.1016/j.cell.2008.06.030 (doi:10.1016/j.cell.2008.06.030) [DOI] [PubMed] [Google Scholar]
- 99.Hoekstra H. E., Coyne J. A. 2007. The locus of evolution: evo devo and the genetics of adaptation. Evol. Int. J. Org. Evol. 61, 995–1016 [DOI] [PubMed] [Google Scholar]
- 100.Craig L. R. 2009. Defending evo-devo: a response to Hoekstra and Coyne. Phil. Sci. 76, 335–344 10.1086/649808 (doi:10.1086/649808) [DOI] [Google Scholar]
- 101.Chan Y. F., et al. 2010. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science 327, 302–305 10.1126/science.1182213 (doi:10.1126/science.1182213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shapiro M. D., Marks M. E., Peichel C. L., Blackman B. K., Nereng K. S., Jonsson B., Schluter D., Kingsley D. M. 2004. Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature 428, 717–723 10.1038/nature02415 (doi:10.1038/nature02415) [DOI] [PubMed] [Google Scholar]
- 103.Cretekos C. J., Wang Y., Green E. D., Martin J. F., Rasweiler J. J. T., Behringer R. R. 2008. Regulatory divergence modifies limb length between mammals. Genes Dev. 22, 141–151 10.1101/gad.1620408 (doi:10.1101/gad.1620408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tishkoff S. A., et al. 2007. Convergent adaptation of human lactase persistence in Africa and Europe. Nat. Genet. 39, 31–40 10.1038/ng1946 (doi:10.1038/ng1946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sokolowski M. B. 2001. Drosophila: genetics meets behaviour. Nat. Rev. Genet. 2, 879–890 10.1038/35098592 (doi:10.1038/35098592) [DOI] [PubMed] [Google Scholar]
- 106.Redies C., Puelles L. 2004. Central nervous system development: from embryonic modules to functional modules. In Modularity in development and evolution (eds Schlosser G., Wagner G. P.), pp. 154–182 Chicago, IL: University of Chicago Press [Google Scholar]
- 107.Goulding M. 2009. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 10, 507–518 10.1038/nrn2608 (doi:10.1038/nrn2608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Young L. J., Nilsen R., Waymire K. G., MacGregor G. R., Insel T. R. 1999. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature 400, 766–768 10.1038/23650 (doi:10.1038/23650) [DOI] [PubMed] [Google Scholar]
- 109.Prabhakar S., Noonan J. P., Paabo S., Rubin E. M. 2006. Accelerated evolution of conserved noncoding sequences in humans. Science 314, 786. 10.1126/science.1130738 (doi:10.1126/science.1130738) [DOI] [PubMed] [Google Scholar]
- 110.Fisher S. E., Marcus G. F. 2006. The eloquent ape: genes, brains and the evolution of language. Nat. Rev. Genet. 7, 9–20 10.1038/nrg1747 (doi:10.1038/nrg1747) [DOI] [PubMed] [Google Scholar]
- 111.Rilling J. K., Glasser M. F., Preuss T. M., Ma X., Zhao T., Hu X., Behrens T. E. J. 2008. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat. Neurosci. 11, 426–428 10.1038/nn2072 (doi:10.1038/nn2072) [DOI] [PubMed] [Google Scholar]
- 112.Certel S. J., Savella M. G., Schlegel D. C., Kravitz E. A. 2007. Modulation of Drosophila male behavioral choice. Proc. Natl Acad. Sci. USA 104, 4706–4711 10.1073/pnas.0700328104 (doi:10.1073/pnas.0700328104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carroll S. B. 2005. Endless forms most beautiful: the new science of evo devo and the making of the animal kingdom. New York, NY: Norton [Google Scholar]
- 114.Canestro C., Yokoi H., Postlethwait J. H. 2007. Evolutionary developmental biology and genomics. Nat. Rev. Genet. 8, 932–942 10.1038/nrg2226 (doi:10.1038/nrg2226) [DOI] [PubMed] [Google Scholar]
- 115.Boguski M. S., Jones A. R. 2004. Neurogenomics: at the intersection of neurobiology and genome sciences. Nat. Neurosci. 7, 429–433 10.1038/nn1232 (doi:10.1038/nn1232) [DOI] [PubMed] [Google Scholar]
- 116.Clark A. G. 2006. Genomics of the evolutionary process. Trends Ecol. Evol. 21, 316–321 10.1016/j.tree.2006.04.004 (doi:10.1016/j.tree.2006.04.004) [DOI] [PubMed] [Google Scholar]
- 117.Hall J. C. 2003. A neurogeneticist's manifesto. J. Neurogenet. 17, 1–90 [PubMed] [Google Scholar]
- 118.Toma D. P., White K. P., Hirsch J., Greenspan R. J. 2002. Identification of genes involved in Drosophila melanogaster geotaxis, a complex behavioral trait. Nat. Genet. 31, 349–353 [DOI] [PubMed] [Google Scholar]
- 119.Long A. D., Mullaney S. L., Reid L. A., Fry J. D., Langley C. H., Mackay T. F. C. 1995. High resolution mapping of genetic factors affecting abdominal bristle number in Drosophila melanogaster. Genetics 139, 1273–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chronis N., Zimmer M., Bargmann C. I. 2007. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat. Methods 4, 727–731 10.1038/nmeth1075 (doi:10.1038/nmeth1075) [DOI] [PubMed] [Google Scholar]
- 121.Branson K., Robie A. A., Bender J., Perona P., Dickinson M. H. 2009. High-throughput ethomics in large groups of Drosophila. Nat. Methods 6, 451–477 10.1038/nmeth.1328 (doi:10.1038/nmeth.1328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anholt R. R., Mackay T. F. 2004. Quantitative genetic analyses of complex behaviours in Drosophila. Nat. Rev. Genet. 5, 838–849 10.1038/nrg1472 (doi:10.1038/nrg1472) [DOI] [PubMed] [Google Scholar]
- 123.Stapley J., et al. 2010. Adaptation genomics: the next generation. Trends Ecol. Evol. 25, 705–712 10.1016/j.tree.2010.09.002 (doi:10.1016/j.tree.2010.09.002) [DOI] [PubMed] [Google Scholar]
- 124.Bloch G., Grozinger C. M. 2011. Social molecular pathways and the evolution of bee societies. Phil. Trans. R. Soc. B 366, 2155–2170 10.1098/rstb.2010.0346 (doi:10.1098/rstb.2010.0346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hut R. A., Beersma D. G. M. 2011. Evolution of time-keeping mechanisms: early emergence and adaptation to photoperiod. Phil. Trans. R. Soc. B 366, 2141–2154 10.1098/rstb.2010.0409 (doi:10.1098/rstb.2010.0409) [DOI] [PMC free article] [PubMed] [Google Scholar]