Abstract
Here, we bring together and contrast lay (accessible primarily through social science methodologies) and technical (via risk assessment and epidemiological techniques) views of the risk associated with the Escherichia coli O157 pathogen using two case study areas in the Grampian region of Scotland, and North Wales. Epidemiological risk factors of contact with farm animals, visiting farms or farm fields and having a private water supply were associated with postcode districts of higher than average disease incidence in the human population. However, this was not the case for the epidemiological risk factor of consumption of beef burgers, which was independent of disease incidence in the postcode district of residence. The proportion of the population expressing a high knowledge of E. coli O157 was greatest in high-incidence disease districts compared with low-incidence areas (17% cf. 7%). This supports the hypothesis that in high-disease-incidence areas, residents are regularly exposed to information about the disease through local cases, the media, local social networks, etc. or perhaps that individuals are more likely to be motivated to find out about it. However, no statistically significant difference was found between high- and low-incidence postcode districts in terms of the proportion of the population expressing a high likelihood of personal risk of infection (10% cf. 14%), giving a counterintuitive difference between the technical (epidemiological and quantitative microbiological risk assessment (QMRA)) and the lay assessment of E. coli O157 risk. This suggests that lay evaluations of E. coli O157 risk reflect intuitive and experience-based estimates of the risk rather than probabilistic estimates. A generally strong correspondence was found in terms of the rank order given to potential infection pathways, with environment and foodborne infection routes dominating when comparing public understanding with technical modelling results. Two general conclusions follow from the work. First, that integrative research incorporating both lay and technical views of risk is required in order that informed decisions can be made to handle or treat the risk by the groups concerned (e.g. the public, policy makers/risk managers, etc.). Second, when communicating risk, for example, through education programmes, it is important that this process is two-way with risk managers (e.g. including Food Standards Agency officials and communications team, public health infection control and environmental health officers) both sharing information with the public and stakeholder groups, as well as incorporating public knowledge, values and context (e.g. geographical location) into risk-management decisions.
Keywords: E. coli O157, lay understanding, epidemiology, risk perception, zoonoses
1. Introduction
In everyday conversation, a ‘risk’ is something that may cause harm, but the technical conceptualization of risk focuses on the probability of the event as well as on the magnitude of the consequence, frequently being defined as the product of the two [1]. Two technical perspectives are provided by probabilistic (quantitative) risk assessment and epidemiological approaches. The latter ‘just denotes the statistical likelihood of being ill if one is exposed to some factor—it says nothing about whether this factor really causes the disease’ [2]. These factors are termed ‘risk factors’ and one of the main aims in epidemiology is to identify them and also to assess their importance in causality of the disease. Both of these technical perspectives require sufficient data to be available to make predictions regarding risk. This technical or ‘technocratic’ view of risk has received criticism from the social sciences [3]. In particular, how a risk is perceived by individuals depends upon social and cultural context, and personal values and experiences. Thus, technical risk assessment cannot capture all of the complexity of the situation being studied, and combining probability of a risk and consequence by simple multiplication is often difficult to achieve and unlikely to reflect both institutional and lay views on risk.
The study of risk now incorporates insights from several disciplines, including psychology, anthropology, sociology and human geography, as well as embracing the more traditional probabilistic paradigm that underpins epidemiological work on understanding disease risk. Indeed, the concept of risk is complex and contested; a wholly subjective human construct to some, and a verifiable objective truth, independent of human interpretation, to others [4]. What seems clear is that lay perceptions of risk (put simply, people's judgements of a particular hazard) may vary substantially between individuals and groups, and often do not agree with technical measures or predictions of risk [5,6]. This does not mean that one viewpoint is more relevant than the other, but rather that lay and technical assessments both need to be considered when trying to understand and manage risk.
The call for cross-disciplinary cooperation in understanding risk, including the use of a mixed methods empirical approach [7], is therefore growing, driven, in part, by a recognition of the need for greater stakeholder participation in risk management by policy-making organizations such as the UK Food Standards Agency and the US Environmental Protection Agency [8]. Indeed, the need for good risk governance is well established and relies on the three pillars of knowledge, legal procedures/instruments and social values [3]. This paper has as its core an epidemiological examination of risk factors and probabilistic risk assessment for one specific human health hazard: Escherichia coli O157 which is one of a number of gastrointestinal pathogens that causes disease in humans. However, and for the first time, we also attempt to incorporate a lay perspective on aspects of E. coli O157 disease risk from key stakeholder groups, contrasting technical and lay understandings wherever appropriate.
The paper begins with a brief review of current knowledge of E. coli O157 disease and associated risk factors, and the role of different approaches to understanding risk, before presenting specific research hypotheses for the work reported here. Following an explanation of research methods, findings derived for two case study areas in the Grampian region of Scotland and North Wales are presented and discussed, including preliminary implications for future research and policy-making.
(a). Escherichia coli O157 disease risk
Escherichia coli O157 is the most commonly isolated verocytotoxin-producing E. coli O-group in the UK, USA, Canada and Japan [9,10]. The disease is relatively rare with the highest incidence worldwide being reported in Scotland (e.g. 4.7 cases per 100 000 in 2008 [11]). Disease symptoms include bloody diarrhoea in approximately 90 per cent of cases, with 10–15% of cases progressing to haemolytic ureamic syndrome (HUS) a serious condition leading to renal damage and occasionally death [12]. The disease is most common in young children under the age of 5 years and the incidence is highest in rural areas [10].
Escherichia coli O157 is a zoonotic pathogen that can be transmitted from livestock to humans, although it is not pathogenic in farm animals. The main reservoirs for E. coli O157 have been identified as cattle and sheep [13], but it can also be found in a range of other animals including goats, deer, flies, etc. Epidemiological case control studies and analysis of outbreaks from several countries have demonstrated that humans contract the disease by three primary and one secondary transmission pathways with associated risk factors, which vary in relative importance depending on both national and regional contexts. Epidemiology differentiates primary and secondary pathways from a model of transmission that assumes implicitly the reservoir of E. coli O157 is non-human and hence the pathway from non-human source to human is the primary pathway. The secondary pathway is person to person and is particularly prevalent in nurseries and care homes [14]. The three primary transmission pathways, with examples of major outbreaks, are:
— foodborne infection via contaminated food including beef burgers, fermented sausages, dairy products and other produce (e.g. vegetables that have been irrigated or rinsed with contaminated water) [15], for example, the Central Scotland outbreak in 1995, where 512 people were infected and 17 died through consuming contaminated meat products [16];
— waterborne infection primarily through untreated, contaminated drinking-water supplies [17,18], for example, the Walkerton outbreak in Canada in 2000, where 2300 people were infected from public water supply wells contaminated with ruminant faeces [18]; and
— environmental infection either via ingestion of faecal material or by direct contact with animals, their faeces and contaminated soil [19], for example, the millennium boy scout camp in New Deer, Scotland, where 20 fell ill after camping on pasture recently grazed by sheep.
Outbreak investigations reveal the range of pathways and risk factors. Hence, assessing human exposure to particular risk factors during outbreaks can help elucidate the transmission of disease in well-defined populations. However, the exposure to these risk factors across the whole population is unknown.
Two approaches that have made major contributions to technical estimation and understanding of human disease risk are spatial epidemiology and risk assessment. The first is the study of the spatial distribution of disease: for E. coli O157 spatially correlated risk factors include densities of livestock and private water supplies [20]. The second, probabilistic risk assessment (more specifically termed QMRA) as developed in food and environmental microbiology, has been applied to E. coli O157, for foodborne (beef burgers) [21], environment (visiting pasture recently grazed by farm animals) [22] and waterborne (drinking from a private water supply) [23] pathways. The risk assessment process consists of the following steps as listed by the World Health Organization (www.who.int/foodsafety/micro/riskassessment/en/index.html): hazard identification, hazard characterization (incorporating dose-response), exposure assessment and risk characterization (estimating the effects on the population under study). Quantitative risk assessment establishes the probability of disease and its consequences (morbidity). Frequently these are presented separately so that risk managers/policy-makers can determine how they should be combined. Thus, both risk assessment and epidemiology can be used as tools to investigate the relative importance of the infection pathways, but how might this relate to lay perceptions of E. coli O157 disease risk and how it can be avoided?
Quantitative risk assessment can evaluate (in terms of a mathematical model) the efficacy of potential interventions to reduce the incidence of a human infectious disease such as E. coli O157 (e.g. vaccination, improving public health education, etc.) [10]. However, success is only likely to be achieved when there is an active engagement of the susceptible population with these interventions [8]. Their motivation to participate is likely to be driven at least in part by whether they have even heard of the disease, the importance that they place on reducing the risk of contracting the disease (i.e. its salience in their lives) as well as their trust in the regulatory bodies involved. It may be hypothesized that in areas of high disease incidence, there will be greater awareness (self-reported knowledge) of the pathogen, including technical (epidemiological) risk factors, because information about it is more readily available (e.g. via local disease incidents, press coverage, public health information campaigns or through job-related or other networks). However, whether this is actually the case is currently unknown.
It is by no means clear whether any increased awareness of E. coli O157 hazard in areas of high disease incidence may influence judgement of the nature of personal risk of infection or disease. Furthermore, it may be suggested that in areas of high disease incidence, there will be a greater expectation of contracting the disease, but, again, whether this is actually the case is unknown.
While an individual's response to a hazard may lead to behaviours that are labelled inappropriate by technical experts (e.g. failure to take protective action when directed), this behaviour may well be in accordance with the personal values and beliefs of that person and the social group to which they belong [1]. Our ability to understand and explain the public perception of, and response to, E. coli O157 risk and risk mitigation is currently very limited. However, it is clear that considering both lay and technical views of risk is a requirement of appropriate policy-making. In the words of the Royal Society Study Group on Risk Analysis, Perception and Management [24], ‘the public viewpoint should not be considered as error but as datum’. The Royal Society Report also acknowledged, however, that uniting academic research on risk across social and natural scientific disciplines had proved elusive. Attempts have been made since (e.g [25]) and as a step in this process addressing the gaps in knowledge identified above, the work reported here incorporates a lay perspective on E. coli O157 disease risk. In particular, research sought to determine whether there exists a correspondence between:
— exposure to risk factors and the incidence of disease;
— awareness of E. coli O157 and incidence of disease (i.e. will those in high incidence disease areas have greater awareness?);
— perceived likelihood of personally catching disease and incidence of disease (i.e. will those in high-incidence areas perceive disease risk to be higher?); and
— epidemiological and QMRA with lay understanding of sources of infection/transmission pathways.
2. Material and methods
(a). Study areas and disease incidence maps
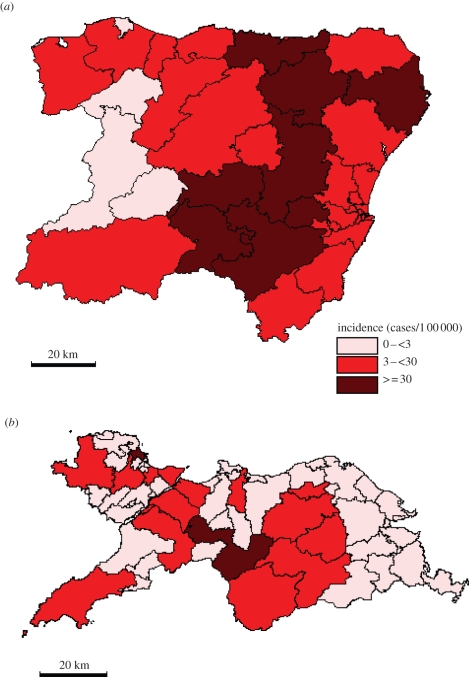
The study areas of Grampian and North Wales were chosen because both have rural farming and urbanized areas and so offer a rural–urban contrast in E. coli O157 disease incidence. Also Grampian has the highest human incidence of E. coli O157 infections in the UK (10.8 cases per 100 000 during 1997–2008) while North Wales has a substantially lower disease burden (2.5 cases per 100 000 during 1999–2007). A case was defined as a symptomatic person, with either microbiological confirmation of E. coli O157 infection or with links to a confirmed case within an outbreak. Human case data were obtained for North Wales (comprising the counties of Anglesey, Conwy, Denbighshire and Gwynedd with a population of 630 152) for the years 1999–2007, totalling 250 postcoded cases; and for the Grampian region of northeast Scotland (comprising the counties of Aberdeenshire and Moray with a population of 519 979) for the years 1997–2008, totalling 667 postcoded cases. The information—from the National Public Health Service of Wales, Communicable Disease Surveillance Center, Cardiff, and from Foresterhill Hospital, Aberdeen—on date of birth, postal district and date of reporting of each individual case was used in this study. Rates of human E. coli O157 infection per 100 000 of population for all postcode districts (figure 1) were calculated by dividing the number of cases with the 2001 population census (there are 38 postcode districts in Grampian and 58 in North Wales). Human disease incidence was split into three categories (high: greater than 30, medium: 3–30 and low: less than three cases per 100 000 yr−1).
Figure 1.
Escherichia coli O157 disease incidence (cases per 100 000) in (a) Grampian and (b) North Wales.
(b). Exposure assessment survey
A telephone-based exposure assessment questionnaire based on a sub-sample (n = 580) of the Grampian population stratified by age, population density and socio-economic status was carried out during the period September 2008 to June 2009. The questionnaire asked specific questions with regard to an individual's potential exposure via the three primary pathways:
— foodborne (‘How often do you eat a beef burger?’);
— waterborne (‘Are you on a public or private water supply?’); and
— environmental (‘How often do you visit farms or cross fields with cattle, sheep or goats in them?’ and ‘How many occasions in a year do you think you handle or touch or stroke cattle, sheep or goats?’).
The postcode of the interviewee was used to link to postcode district area and hence disease incidence. The answers to the environmental and food questions were classed as high (combining ‘weekly’ and ‘every day’) or low (combining ‘never’, ‘annually’ or ‘monthly’). The proportion of highly exposed respondents for each of the food and environment questions as well as the proportion on a private water supply was calculated for postal district areas of high, medium and low disease incidence.
Percentiles (2.5% and 97.5%) for the proportion of highly exposed respondents to each of the factors were obtained by bootstrapping using the PopTools (available from http://www.cse.csiro.au/poptools). Randomization tests [26] were applied independently to each of the exposures to establish statistical significance between postcode district areas of high, medium and low disease incidence. The randomization test used the Monte Carlo method and was based on 10 000 simulations.
(c). Attitude and awareness survey
Awareness of, and attitudes towards, E. coli O157 risk in three equally represented groups—farmers (who live on a farm or smallholding), rural visitors (who do not live in the countryside but were surveyed in it) and non-farming rural residents (who lived in the countryside but were not designated as farmers)—in the two study areas, comprising 900 respondents in total, was assessed using a paper-based, self-complete questionnaire. These groups were chosen because of their likely exposure to the environmental pathway. Completed questionnaires were acquired through: a mailing administered by the National Farmers Union Scotland for Grampian farmers; dissemination at agricultural shows and farmers' meetings, assisted by the National Farmers Union Cymru and the Farmers Union of Wales, for North Wales farmers (both Welsh and English language versions of the questionnaire were made available); dissemination at popular countryside locations for rural residents and visitors in both study areas; and attendance at meetings of local groups, including Community Councils and primary schools, for additional responses from non-farming residents in rural Grampian. Participants were drawn widely from across the two study areas, with questionnaire returns received between April 2008 and January 2009. The questionnaire included mostly closed response, Likert-type questions covering self-reported knowledge, harmfulness, symptoms, seriousness of illness, likelihood of infection and infection pathways for E. coli O157, and also open-ended questions on any risk avoidance behaviours undertaken and how participants had heard about E. coli O157. Key responses presented here relate to self-reported knowledge (awareness) of E. coli O157, perceived personal likelihood of acquiring E. coli O157 disease and recognizing sources of E. coli O157 infection. The questionnaire also collected basic socio-demographic information on each respondent including postcode of address, date of birth, employment status and gender.
Self-reported knowledge of respondents was categorized into two levels (high and low). Those respondents who had not heard of E. coli O157, as asked by an initial routing question, were put into the low group as were those respondents who responded to the question, ‘How much would you say you know about E. coli O157?’ by ticking the options ‘just the name’ or ‘a little’. The others who responded with the options ‘quite a bit’, ‘a lot’ or ‘expert’ were put into the high-knowledge group. Overall 87 per cent of respondents were categorized as having low self-reported knowledge, with 13 per cent categorized as having high self-reported knowledge. Respondents were grouped into postcode district areas of high, medium or low E. coli O157 disease incidence using their postcode address information. Percentiles and randomization tests for statistical significance were applied to the proportion of respondents claiming high levels of knowledge to establish statistical significance between areas of high, medium and low disease incidence.
Perceived likelihood of personal illness was determined in response to the question, ‘Do you think it is likely that you will suffer from E. coli O157 in your lifetime?’ Responses were grouped into two categories representing perceived risk: high (those responding with ‘likely’ and ‘very likely’) and low (those responding with ‘very unlikely’, ‘unlikely’ and ‘neither likely nor unlikely’). Eighty-eight per cent of respondents were categorized as having low perceived risk, and 12 per cent as having high perceived risk. The proportion of respondents with high perceived risk was calculated for postcode district areas of high, medium and low disease incidence and bootstrapped percentiles and statistical significance between the areas calculated as above.
Respondents were also asked to indicate the likelihood that where they live, ‘people in general’ might acquire E. coli O157 infection from a list of 12 possible transmission sources. Responses to these risk factors (possible sources) were also combined into two groups for each source to indicate two levels of perceived likelihood: unlikely (including ‘very unlikely’, ‘unlikely’ or ‘neither likely nor unlikely’ responses) and likely (including ‘likely’ or ‘very likely’ responses). The proportions of respondents in each area perceiving a general likely risk of infection were compared for each of the 12 sources to construct contingency tables and were tested for significance using χ2 (using confidence intervals of 95%).
(d). Spatial epidemiology risk factor model
A multivariate linear regression model was developed to link together proxy risk factors, calculated as follows in 2 × 2 km tetrads (using human and farm animal population density data as well as number of individuals on a private water supply) for each of the three primary transmission pathways [27]:
— foodborne—proportional to the number of people in the human population under consideration;
— waterborne—proportional to the number of properties on a private water supply multiplied by the total number of E. coli O157 excreted by cattle and sheep; and
— environmental—proportional to the human population in the area multiplied by the total number of E. coli O157 excreted by cattle and sheep.
Person to person transmission was not included as it was assumed in the first instance to be similar for each pathway.
The three proxy risk variables were then integrated up to postal districts and regressed against the number of observed cases. This was performed independently for Grampian and North Wales using PASW Statistics 17 (www.spss.com). The number of predicted cases attributed to each transmission pathway for each postal district was determined by calculating the appropriate regression coefficient multiplied by the risk factor proxy. This was then summed for all the postal districts in both study areas. The percentage attribution for each transmission pathway was then determined.
(e). Quantitative microbiological risk assessment
QMRAs were developed for foodborne, waterborne and environmental pathways [27]. Briefly, the average probability of infection and consequently disease was determined for consumption of a beef burger, a glass of water drunk from a private water supply and a visit or camp on a field grazed by cattle or sheep. The beef burger QMRA was an extension of one developed in Canada [21], and the environment QMRA was an updated version of one previously described by Strachan et al. [22]. All the QMRAs were parametrized with data, where available, from the Grampian region of Scotland (e.g. farm animal prevalence and E. coli O157 shed). To predict the number of cases and also the relative importance of the pathways, it was possible to integrate up the risk with the number of exposures, using data from the exposure assessment survey (i.e. each year in Grampian there are reportedly 3.45 million beef burgers consumed, 34.7 million glasses of water drunk from a private water supply and 2.58 million person visits to pasture).
3. Results and discussion
(a). Exposure assessment and disease incidence
In areas of Grampian with a high incidence of E. coli O157 disease, there was a significantly higher proportion of people reporting more frequent contact with farm animals, visitation of farms/fields and reliance on private water supplies (table 1). This is in accord with previous spatial epidemiological studies which have identified that these risk factors for E. coli O157 are more common in rural areas where disease incidence is highest [10,20,28]. However, table 1 indicates that a lower proportion of people reported high frequency consumption of beef burgers in areas with highest reported disease incidence. The explanation for this finding may be that high-incidence areas are typically rural and as such the rural risk factor (e.g. contact with farm animals and their faeces) may dominate E. coli O157 risk in those communities. Epidemiological investigations have shown burgers to be a risk factor in the USA [15] and Wales [29], although such evidence is not apparent in Scotland [19]. This may be due to cultural differences (e.g. in the USA, there are particular communities that consume their burgers ‘rare’—it is not known whether this is the case in Wales and Scotland), or that beef burgers are less of a risk per se in Scotland.
Table 1.
Population proportions reporting frequent exposure to risk factors, high likelihood of suffering personal illness during lifetime from E. coli O157 and a high level of knowledge of E. coli O157, differentiated according to area-based E. coli O157 disease incidence.
| % population (2.5 and 97.5 percentiles) |
|||
|---|---|---|---|
| low incidence area | medium incidence area | high incidence area | |
| proportion reporting frequent direct contact with cattle, sheep and goats (Grampian)a | 2.27 (0.53–4.71) | 1.62 (0–3.64) | 7.76c (4.33–11.54) |
| proportion reporting frequent visits to farms or grazed fields (Grampian)a | 10.64 (7.10–14.61) | 12.57 (8.16–17.39) | 26.81d (18.29–35.85) |
| proportion reporting frequent consumption of burgers (Grampian)a | 8.52 (12.81–4.57) | 9.74 (14.12–5.67) | 4.36e (7.39–1.85) |
| proportion on a private water supply (Grampian)a | 5.89 (8.81–3.27) | 6.03 (9.52–3.02) | 17.48f (25.53–10.10) |
| proportion perceiving high likelihood of personal illness from E. coli O157 within lifetime (Grampian and Wales combined)b | 13.75 (10.35–17.32) | 11.46 (7.82–15.15) | 10.46 (6.51–15.05) |
| proportion claiming high level of knowledge of E. coli O157 (Grampian and Wales combined)b | 7.02g (4.58–9.57) | 17.30 (13.37–21.43) | 16.63 (22.16–11.30) |
aExposure assessment survey.
bAttitude and awareness survey.
cA higher proportion reported frequent direct contact with farm animals in high disease incidence areas compared with medium incidence (p = 0.0006) and low incidence (p = 0.0034) areas.
dA higher proportion reported frequent farm or grazed field visitation in high disease incidence areas compared with medium incidence (p = 0.002) and low incidence (p = 0.004) areas.
eA lower proportion reported high frequency consumption of burgers in high disease incidence areas compared with medium incidence areas (p = 0.0238) (no significant difference comparing high and low incidence areas (p = 0.0635)).
fA higher proportion reported use of a private water supply in high disease incidence areas compared with medium incidence areas (p = 0.0047) (no significant difference comparing high and low incidence areas (p = 0.0981)).
gA lower proportion reported a high level of knowledge in low disease incidence areas compared with medium (p < 0.0001) and high (p = 0.0003) incidence areas.
(b). Awareness, perceived likelihood of illness and disease incidence
In general, high levels of self-reported knowledge (awareness) of E. coli O157 tended to be reported more by people living in medium to high disease incidence areas (17% cf. 7%; table 1). This may reflect the ready availability of information through newspaper and TV reports, educational initiatives by public authorities, local social networks, etc. and/or the greater motivation to seek out information for those living in higher incidence areas. Most respondents had first heard of E. coli or E. coli O157 via the ‘media’. In Grampian, the Press and Journal, a regional paper for northeast Scotland, was often mentioned by name. People described particular outbreaks or events related to food poisoning, food scares, death, infection, illness, kidney failure, butchers, children, school lunches and old people. Grampian respondents were more likely to describe Scottish outbreak examples such as Wishaw and Lanarkshire and respondents in North Wales cited the South Wales outbreak or the Anglesey petting farm. Thus, it is likely that in areas of high disease incidence, there will be examples of outbreaks or knowledge of someone (or someone who knows of someone) who has contracted the disease that will raise public awareness [1].
Claimed knowledge level may also be important in interpreting findings relating to perceived likelihood of disease and disease incidence. However, no association was found between perceived likelihood of personal lifetime illness from E. coli O157 and relative levels of disease incidence (table 1). While this might appear at first to be counterintuitive, particularly as our findings suggest a positive association between level of awareness and disease incidence, it is possible to offer explanation based on an understanding of risk perception. For example, risk information seeking and processing models [30] predict that as perceived risk in response to an unfamiliar and dreaded event or hazard is heightened, individuals will be more likely to actively seek out and process available information about that risk in forming their attitudes and associated behaviours.
In this study, visitors to rural Grampian more often described intentional behaviour to reduce risk than resident farmers (data presented in Jones et al. [31]), but these behaviours were directed at personal and food-related hygiene indoors (e.g. the kitchen) rather than at environmental risks outdoors, and only a very small proportion of respondents overall claimed to undertake risk reduction behaviour in the outdoor environment [31]. Greater seeking and processing of information then closes the gap between what an individual knows about a risk and what they feel they need to know in order to take appropriate action. As this gap closes and the hazard therefore becomes more familiar and subject to personal control [32], the perception of the risk as ‘bad’ may lessen. This corresponds with other research suggesting that hazards that are dreaded (e.g. through lack of control) or less familiar (e.g. new risks) may be perceived as posing greater risk than those that become more familiar through increased awareness and knowledge acquisition [33,34].
Given the characteristics of a hazard that tend to determine perceived risk (e.g. controllability, familiarity), it should not be a surprise that those living in higher disease incidence areas did not, in general, perceive themselves at greater risk from E. coli O157 disease. There is also the possibility that in areas of higher disease incidence, individuals who have frequent exposure, such as farmers, may have (or perceive themselves to have) immunity to the disease [35,36] and as such perceive the likelihood of symptomatic infection to be relatively low. Attitude and awareness may be influenced by a complexity of demographic factors including age, sex, working in farming and place of residence [31]. More qualitative research generally is also required to determine how knowledge influences protective behaviour. What does seem clear at this stage is that lay evaluations of E. coli O157 risk reflect a risk as feelings-based approach as opposed to risk as analysis [37], meaning their evaluations are not based on differing likelihood of exposure, but rather intuitive and experience-based evaluations of the risk. This finding offers important insights into drivers of E. coli O157 and gastrointestinal disease risk perception and, ultimately, decisions about appropriate risk mitigation behaviour among individuals in the communities studied. An important intermediary step here is to try to understand how self-reported knowledge relates to technical knowledge, which brings us to a comparison of technical and popular understandings of E. coli O157 transmission pathways (risk factors).
(c). Transmission pathways
Estimates of risk factors using spatial epidemiology indicated that environmental and food sources were responsible for a higher proportion of cases than water from private water supplies (table 2). There were regional differences in that the environment appears to be the most important source of E. coli O157 infection in Grampian, with food the primary source in North Wales. Epidemiological studies [38,39] also show that person to person transmission is an important pathway accounting for between 4 and 20 per cent of cases, although it is unknown whether person to person transmission occurs more frequently in cases associated with food, water or the environment. However, both studies demonstrated that secondary transmission is more likely when young children are involved.
Table 2.
A comparison of the relative importance of different E. coli O157 infection transmission pathways in Grampian and North Wales derived from predictive epidemiological risk factor modelling of cases, quantitative microbiological risk assessment (QMRA) and a questionnaire survey of attitudes and awareness.
| relative importance of infection source by approach |
|||||
|---|---|---|---|---|---|
| epidemiological risk factor model | QMRA | attitude and awareness survey |
|||
| source: | % cases (95% CI) | % cases (95% CI) | source: | % likely (95% CI) | |
| Grampian | environment | 65.8 (49.6–82.0) | 56.1 (52.2–60.4) | contact with animal faeces | 62.1 (56–68.3) |
| handling farm animals | 35.8 (30.3–41.3) | ||||
| contact with soil and mud | 28.6 (23.5–33.8) | ||||
| streams, rivers, ponds, lakes | 23.7 (19–28.4) | ||||
| contact with household pets | 13.8 (10.1–17.4) | ||||
| breathing outside air | 2.5 (1–4.1) | ||||
| food | 26.9 (11–42.8) | 34.0 (28.7–39.4) | eating undercooked meat | 55.9 (49.7–62.1) | |
| eating raw vegetables | 12.3 (8.9–15.8) | ||||
| water | 7.3 (0–16) | 9.9 (0–11.1) | using private water supplies | 24.6 (19.9–29.4) | |
| using mains water | 3.5 (1.7–5.3) | ||||
| person to persona | toilets and wash hand basins | 28.3 (23.3–33.3) | |||
| contact with other people | 10.1 (6.9–13.3) | ||||
| source: | % cases (95% CI) | source: | |||
| North Wales | environment | 21.9 (9.3–34.5) | contact with animal faeces | 56.4 (49.8–62.9) | |
| handling farm animals | 33.9 (28.2–39.6) | ||||
| contact with soil and mud | 27.3 (21.9–32.8) | ||||
| streams, rivers, ponds, lakes | 25.3 (20.1–30.5) | ||||
| contact with household pets | 17.9 (13.4–22.3) | ||||
| breathing outside air | 2.6 (0.9–4.2) | ||||
| food | 62.6 (48–77.2) | eating undercooked meat | 66.7 (60.3–73) | ||
| eating raw vegetables | 17.0 (12.8–21.3) | ||||
| water | 15.5 (9.7–21.3) | using private water supplies | 18.6 (14.1–23.2) | ||
| using mains water | 8.0 (5–10.9) | ||||
| person to persona | toilets and wash hand basins | 37.2 (31.3–43) | |||
| contact with other people | 17.9 (13.6–22.3) | ||||
The Grampian QMRAs produced the same rank order (environment, food and then water) as for the spatial epidemiological modelling risk factors. However, caution is required when assessing the absolute level of risk rather than relative risk estimate in these results, as both types of model have underlying assumptions associated with them. Further, the QMRAs actually over-predict by a factor of 30 the number of cases associated with each of the three pathways [27]—a sizeable discrepancy that may be explained by immunity, non-symptomatic infection and the physiological status of the pathogen at the point of ingestion. All of these factors would affect the dose–response relationship which is not possible to test in humans for obvious ethical reasons. It should also be noted that the foodborne QMRA considered only beef burgers, but E. coli O157 may come from other foodstuffs which would increase the overestimate in the number of human cases further.
Lay attitude findings (table 2) yielded a similar rank order of transmission pathways deemed to be a probable cause of infection between the two study areas. Contact with animal faeces and eating undercooked meat were perceived to be the most likely sources in both areas. Interestingly, animal contact ranked more highly in Grampian, though not significantly so, while eating undercooked meat appeared a more important perceived infection source in North Wales (p < 0.05); in effect, giving the same rank ordering as for the quantitative modelling approaches. Similarly, ‘breathing outside air’ was identified as having the lowest risk by questionnaire respondents in both study areas, and this is not included as a risk factor for E. coli O157 infection in epidemiological models (although there may be some risk associated with muck spreading and composting activities). It is also worth noting that in North Wales, person to person transmission (toilets/wash-hand basins and contact with other people) appeared to be considered more important than in Grampian. The questionnaire did not specifically ask whether children were considered to be a higher risk (particularly for secondary transmission), though the answer to this question would be helpful since the epidemiological information indicates that this group are an important source of secondary infection in both sporadic and outbreak situations.
Broadly speaking, a good degree of agreement was found between lay and technical understandings of disease risk pathways. Relevant research in the literature is very sparse, but a recent review of case studies involving non-scientific stakeholders in risk (exposure) assessment relating to food chain hazards concluded that these stakeholders were generally ‘fully aware of alternative pathways contributing to exposures’ [8]. Our results are further evidence of a disconnect between knowledge and perceived risk, individuals may be well aware of the factors determining exposure, but may still over- or underestimate their own personal likelihood of exposure through other psychological factors (e.g. level of dread, control, familiarity, etc.).
4. Conclusions
This study has been a first step at bringing together technical and lay understandings of E. coli O157 disease risk. Data from the exposure survey were used in the QRMA to predict the number of cases by infection pathway, and, perhaps surprisingly given the diversity of disciplinary tradition and technique involved, there was considerable agreement between findings derived from technical (epidemiological and QMRA) and lay perspectives. To some extent, for example, findings from the exposure assessment questionnaire survey support spatial epidemiological work on E. coli O157 disease incidence with regard to exposure to environmental and water-related risk factors. Lay and technical views of the relative importance of different infection pathways showed a strong degree of consistency, although this was not the case between perceived personal likelihood of risk and living in an area of high risk. This exposes important and alternative views of concern/risk between scientific and non-scientific viewpoints. Indeed, even the interpretation of technical data by scientists can result in a range of views on risk (e.g. the recent petting farm outbreak at the Godstone farm in England led to calls by some scientists to ban access to children less than 5 year old [40,41]). Knowledge, expertise and technical understanding are distributed across both scientific and non-scientific constituencies and this together with individual values, concerns and perceptions must be considered when dealing with E. coli O157 risk. One area where this broad and combined perspective would prove valuable in future research would be in refining our understanding of the role of person to person transmission of E. coli O157.
A lay understanding of risk also serves to highlight the limitations inherent in an approach based solely on a technocratic (e.g. epidemiological and probabilistic risk assessment) view of risk involving the traditions of positivism and claimed objectivity, with its allied reliance on mathematical/probabilistic constructs to understand disease risk. Human perception of risk is a multi-dimensional construct that comprises the processing, assimilation and evaluation of both experiential (i.e. feelings-based) and analytic (i.e. knowledge-based) responses to a hazard. Hence, a social science perspective on risk can aid our understanding of why individuals and groups may not feel themselves to be at greater risk of a disease, including E. coli O157, even when statistical evidence strongly suggests the contrary. This type of apparent mismatch between technical and lay understandings of risk underpins the now widely held belief that formulating appropriate policy to reduce disease risk requires collaboration between the natural and social sciences, although such collaboration is still in its infancy [8]. Further, public understandings of risk can be used to inform the technical risk models. For example, if it is known that the behaviours of particular population groups vary (e.g. consuming burgers rare or well done or likelihood of washing hands after contact with farm animals), then this can be included into the models so that revised estimates of risk can be calculated and this information can then be communicated to demonstrate the positive/negative effect of that behaviour.
With specific reference to potential implications for policy-making to reduce E. coli O157 risk in particular, our findings indicate a general lack of claimed knowledge (only a relatively small proportion—16.6 per cent in high-incidence areas—of respondents expressed a high level of knowledge of E. coli O157), but a generally sound understanding of potential disease transmission pathways, gained, perhaps, through an appreciation of E. coli O157 as some form of ‘stomach bug’ and, therefore, an association with common hygiene concerns. It might be appropriate, therefore, to build risk mitigation strategy (e.g. public education campaigns) on what might broadly be seen already as good hygiene; i.e. raising specific awareness of the risk of E. coli O157 infection, but in the familiar context of ‘normal’ hygiene practice in the outdoor environment and in relation to food storage and preparation. That said, the risk from private water supplies should not be ignored, and clearly more research is needed into how enhanced awareness and knowledge might translate into appropriate risk reduction behaviour within different stakeholder groups, allowing the better tailoring of information to those statistically most at risk. Such education campaigns to communicate that risk must be two-way [42], with risk managers (e.g. public health, Food Standards Agency, etc.) as well as the public/targeted groups both engaging in the social learning process. The aim being that the public can make informed choices about the risk in a relationship of reciprocated trust with the risk managers [3].
Although rare, E. coli O157 is a severe gastrointestinal pathogen, and this has prompted a significant amount of research into its occurrence and health impacts from natural and medical science perspectives. Much that remains to be done in these areas is readily applicable to the other primary human zoonotic pathogens (e.g. Salmonella, Listeria and Cryptosporidium). If we are to more fully understand how appropriate risk reduction behaviours can be effectively designed and implemented, then integrative (interdisciplinary) research effort is required, incorporating a better understanding of how individuals and groups construct perceptions of disease risk/precautionary measures. While the simplest approach for academics would be to revert back to, or remain locked within, specific disciplinary domains in studying E. coli O157 risk and risk mitigation, it is likely that interdisciplinarity offers a more efficient research model, encouraging, for example, an early appreciation of where key gaps in knowledge lie and how different concepts and methodologies can be used to gather and interpret required empirical evidence. An in-depth appreciation of why individuals and groups react to a given hazard in the way they do will require the greater use of both quantitative (e.g. surveys) and qualitative research techniques (e.g. semi-structured interviews and focus groups) [43], as well as mixed-method approaches aimed specifically at understanding the gap between lay and technical assessment of risk (see mental models methodology [44]); techniques not designed to produce information suitable for traditional probabilistic analysis. Integrative research therefore challenges those involved to embrace new investigative traditions and techniques, and we are still at a very early stage in meeting these challenges in the management of E. coli O157 disease risk and that of the other gastrointestinal pathogens.
Acknowledgements
This research was undertaken in association with a project funded under the UK Research Councils Rural Economy and Land Use (RELU) Programme entitled ‘Reducing E. coli O157 Risk in Rural Communities’ (RES-229-31-0003). RELU is funded jointly by the Economic and Social Research Council, the Biotechnology and Biological Sciences Research Council and the Natural Environment Research Council, with additional funding from the Department for Environment, Food and Rural Affairs and the Scottish Government. Dr Tom Reid (Foresterhill hospital, Aberdeen), Dr Roland Salmon and Dr Robert Smith (NPHS Communicable Disease Surveillance Center, Cardiff) are thanked for supplying the clinical E. coli O157 case data from Grampian and North Wales, respectively, Rachel Cartwright for the telephone exposure assessment survey and RELU E. coli O157 project colleagues for comments on the work and article.
Footnotes
One contribution of 11 to a Theme Issue ‘Interdisciplinary perspectives on the management of infectious animal and plant diseases’.
References
- 1.Kasperson R. E., Renn O., Slovic P., Brown H. S., Emel J., Goble R., Kasperson J. X., Ratick S. 1988. The social amplification of risk: a conceptual framework. Risk Anal. 8, 177–187 10.1111/j.1539-6924.1988.tb01168.x (doi:10.1111/j.1539-6924.1988.tb01168.x) [DOI] [Google Scholar]
- 2.Giesecke J. 2002. Modern infectious disease epidemiology, 2nd edn London, UK: Arnold [Google Scholar]
- 3.Renn O. 2008. Risk governance—coping with uncertainty in a complex world, 1st edn. London, UK: Earthscan [Google Scholar]
- 4.Pidgeon N., Hood C., Jones D., Turner B., Gibson R. 1992. Risk perception. In Risk analysis, perception and management (eds Royal Society Study Group), pp. 89–134 London, UK: The Royal Society [Google Scholar]
- 5.Douglas M. 1992. Risk and blame: essays in cultural theory. London, UK: Routledge [Google Scholar]
- 6.Slovic P. 1999. Trust, emotion, sex, politics, and science: surveying the risk-assessment battlefield. Risk Anal. 19, 689–701 [DOI] [PubMed] [Google Scholar]
- 7.Ben-Ari A., Or-Chen K. 2009. Integrating competing conceptions of risk: a call for future directions of research. J. Risk Res. 12, 865–877 10.1080/13669870902899674 (doi:10.1080/13669870902899674) [DOI] [Google Scholar]
- 8.Barker G. C., Bayley C., Cassidy A., French S., Hart A., Malakar P. K., Maule J., Petkov M., Shepherd R. 2010. Can a participatory approach contribute to food chain risk analysis? Risk Anal. 30, 766–781 (doi:10.1111/j.1539-6924.2010.01385.x) [DOI] [PubMed] [Google Scholar]
- 9.Chase-Topping M., Gally D., Low C., Matthews L., Woolhouse M. 2008. Super-shedding and the link between human infection and livestock carriage of Escherichia coli O157. Nat. Rev. Microbiol. 6, 904–912 10.1038/nrmicro2029 (doi:10.1038/nrmicro2029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strachan N. J., Dunn G. M., Locking M. E., Reid T. M., Ogden I. D. 2006. Escherichia coli O157: burger bug or environmental pathogen? Int. J. Food Microbiol. 112, 129–137 10.1016/j.ijfoodmicro.2006.06.021 (doi:10.1016/j.ijfoodmicro.2006.06.021) [DOI] [PubMed] [Google Scholar]
- 11.Locking M., Cowden J. 2009. Escherichia coli O157. BMJ 339, b4076 10.1136/bmj.b4076 (doi:10.1136/bmj.b4076) [DOI] [PubMed] [Google Scholar]
- 12.Tarr P. I., Gordon C. A., Chandler W. L. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365, 1073–1086 10.1016/S0140-6736(05)7114-2 (doi:10.1016/S0140-6736(05)7114-2) [DOI] [PubMed] [Google Scholar]
- 13.Solecki O., MacRae M., Strachan N., Lindstedt B. A., Ogden I. 2009. E. coli O157 from sheep in northeast Scotland: prevalence, concentration shed, and molecular characterization by multilocus variable tandem repeat analysis. Foodborne Pathogen. Dis. 6, 849–854 10.1089/fpd.2008.0216 (doi:10.1089/fpd.2008.0216) [DOI] [PubMed] [Google Scholar]
- 14.Reiss G., Kunz P., Koin D., Keeffe E. B. 2006. Escherichia coli O157 : H7 infection in nursing homes: review of literature and report of recent outbreak. J. Am. Geriatr. Soc. 54, 680–684 10.1111/j.1532-5415.2006.00682.x (doi:10.1111/j.1532-5415.2006.00682.x) [DOI] [PubMed] [Google Scholar]
- 15.Rangel J. M., Sparling P. H., Crowe C., Griffin P. M., Swerdlow D. L. 2005. Epidemiology of Escherichia coli O157 : H7 outbreaks, United States, 1982–2002. Emerg. Infect. Dis. 11, 603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowden J. M., Ahmed S., Donaghy M., Riley A. 2001. Epidemiological investigation of the central Scotland outbreak of Escherichia coli O157 infection, November to December 1996. Epidemiol. Infect. 126, 335–341 10.1017/S0950268801005520 (doi:10.1017/S0950268801005520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Licence K., Oates K. R., Synge B. A., Reid T. M. 2001. An outbreak of E. coli O157 infection with evidence of spread from animals to man through contamination of a private water supply. Epidemiol. Infect. 126, 135–138 [PMC free article] [PubMed] [Google Scholar]
- 18.Hrudey S. E., Payment P., Huck P. M., Gillham R. W., Hrudey E. J. 2003. A fatal waterborne disease epidemic in Walkerton, Ontario: comparison with other waterborne outbreaks in the developed world. Water Sci. Technol. 47, 7–14 [PubMed] [Google Scholar]
- 19.Locking M. E., O'Brien S. J., Reilly W. J., Wright E. M., Campbell D. M., Coia J. E., Browning L. M., Ramsay C. N. 2001. Risk factors for sporadic cases of Escherichia coli O157 infection: the importance of contact with animal excreta. Epidemiol. Infect. 127, 215–220 10.1017/S0950268801006045 (doi:10.1017/S0950268801006045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innocent G. T., et al. 2005. Spatial and temporal epidemiology of sporadic human cases of Escherichia coli O157 in Scotland, 1996–1999. Epidemiol. Infect. 133, 1033–1041 10.1017/S0950268805003687 (doi:10.1017/S0950268805003687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassin M. H., Lammerding A. M., Todd E. C., Ross W., McColl R. S. 1998. Quantitative risk assessment for Escherichia coli O157 : H7 in ground beef hamburgers. Int. J. Food Microbiol. 41, 21–44 10.1016/S0168-1605(98)00028-2 (doi:10.1016/S0168-1605(98)00028-2) [DOI] [PubMed] [Google Scholar]
- 22.Strachan N. J., Dunn G. M., Ogden I. D. 2002. Quantitative risk assessment of human infection from Escherichia coli O157 associated with recreational use of animal pasture. Int. J. Food Microbiol. 75, 39–51 10.1016/S0168-1605(01)00727-9 (doi:10.1016/S0168-1605(01)00727-9) [DOI] [PubMed] [Google Scholar]
- 23.Vinten A. J. A., Potts J., Avery L., Strachan N. J. C. 2009. Microbial pollution of water by livestock: approaches to risk assessment and mitigation. Animal 3, 744–752 10.1017/S1751731109004005 (doi:10.1017/S1751731109004005) [DOI] [PubMed] [Google Scholar]
- 24.Anonymous 1992. Risk: analysis, perception and management. London, UK: Report of a Royal Society Study Group [Google Scholar]
- 25.Burningham K., Fielding J., Thrush D. 2008. ‘It'll never happen to me’: understanding public awareness of local flood risk. Disasters 32, 216–238 10.1111/j.1467-7717.2007.01036.x (doi:10.1111/j.1467-7717.2007.01036.x) [DOI] [PubMed] [Google Scholar]
- 26.Manly B. F. J. 2007. Randomization, bootstrap and Monte Carlo methods in biology, 3rd edn. Boca Raton, FL: Chapman & Hall/CRC [Google Scholar]
- 27.Rotariu O., Ogden I. D., MacRitchie L., Forbes K. J., Cross P., Williams A. P., Hunter C. J., Teunis P. F. M., Strachan N. J. C. Submitted. Applying risk assessment and spatial epidemiology to elucidate the source of human E. coli O157 infection. Epidemiol. Infect. [DOI] [PubMed] [Google Scholar]
- 28.Valcour J. E., Michel P., McEwen S. A., Wilson J. B. 2002. Associations between indicators of livestock farming intensity and incidence of human Shiga toxin-producing Escherichia coli infection. Emerg. Infect. Dis. 8, 252–257 10.3201/eid0803.010159 (doi:10.3201/eid0803.010159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parry S. M., Salmon R. L., Willshaw G. A., Cheasty T. 1998. Risk factors for and prevention of sporadic infections with vero cytotoxin (shiga toxin) producing Escherichia coli O157. Lancet 351, 1019–1022 10.1016/S0140-6736(97)08376-1 (doi:10.1016/S0140-6736(97)08376-1) [DOI] [PubMed] [Google Scholar]
- 30.Griffin R. J., Dunwoody S., Neuwirth K. 1999. Proposed model of the relationship of risk information seeking and processing to the development of preventive behaviors. Environ. Res. 80, S230–S245 10.1006/enrs.1998.3940 (doi:10.1006/enrs.1998.3940) [DOI] [PubMed] [Google Scholar]
- 31.Jones C. D. R., Hunter C., William A. P., Strachan N. J. C., Cross P. 2011. Escherichia coli O157: comparing disease awareness of rural residents and visitors in livestock farming areas. Epidemiol. Infect. 10.1017/S0950268810002918 (doi:10.1017/S0950268810002918) [DOI] [PubMed] [Google Scholar]
- 32.Frewer L. J., Shepherd R., Sparks P. 1994. The interrelationship between perceived knowledge, control and risk associated with a range of food-related hazards targeted at the individual, other people and society. J. Food Saf. 14, 19–40 10.1111/j.1745-4565.1994.tb00581.x (doi:10.1111/j.1745-4565.1994.tb00581.x) [DOI] [Google Scholar]
- 33.Slovic P. 1988. Perception of risk. Science 236, 280–285 10.1126/science.3563507 (doi:10.1126/science.3563507) [DOI] [PubMed] [Google Scholar]
- 34.Loewenstein G. F., Weber E. U., Hsee C. K., Welch N. 2001. Risk as feelings. Psychol. Bull. 127, 267–286 10.1037/0033-2909.127.2.267 (doi:10.1037/0033-2909.127.2.267) [DOI] [PubMed] [Google Scholar]
- 35.Silvestro L., Caputo M., Blancato S., Decastelli L., Fioravanti A., Tozzoli R., Morabito S., Caprioli A. 2004. Asymptomatic carriage of verocytotoxin-producing Escherichia coli O157 in farm workers in Northern Italy. Epidemiol. Infect. 132, 915–919 10.1017/S0950268804002390 (doi:10.1017/S0950268804002390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quilliam R. S., et al. Submitted Seroprevalence and risk factors associated with Escherichia coli 0157 in a farming population. Zoonoses Public Health. [DOI] [PubMed] [Google Scholar]
- 37.Slovic P., Finucane M. L., Peters E., MacGregor D. G. 2004. Risk as analysis and risk as feelings: some thoughts about affect, reason, risk and rationality. Risk Anal. 24, 311–322 10.1111/j.0272-4332.2004.00433.x (doi:10.1111/j.0272-4332.2004.00433.x) [DOI] [PubMed] [Google Scholar]
- 38.Parry S. M., Salmon R. L. 1998. Sporadic STEC O157 infection: secondary household transmission in Wales. Emerg. Infect. Dis. 4, 657–661 10.3201/eid0404.980419 (doi:10.3201/eid0404.980419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snedeker K. G., Shaw D. J., Locking M. E., Prescott R. J. 2009. Primary and secondary cases in Escherichia coli O157 outbreaks: a statistical analysis. BMC Infect. Dis. 9, 144 10.1186/1471-2334-9-144 (doi:10.1186/1471-2334-9-144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffin G. 2010. Review of the major outbreak of E. coli O157 in Surrey, 2009. Available from the Health Protection Agency: http://www.griffininvestigation.org.uk/default.htm (accessed 28 March 2011). [Google Scholar]
- 41.Nikkah R. 2009. Young children must avoid farm animals says E. coli expert Hugh Pennington. The Daily Telegraph, 19 September 2009
- 42.Leiss W. 1996. Three phases in risk communication practice. In Challenges in risk assessment and risk management (eds Kunreuther H., Slovic P.), pp. 85–94 Thousan Oaks, CA: American Academy of Political and Social Science [Google Scholar]
- 43.Hunter C., Vergunst P., Jones C., Ioris A., Strachan N. J. C., Farrington J. 2009. Private water supplies (Scotland) regulations 2006; understanding engagement of owners and users. See http://www.scotland.gov.uk/Publications/2009/11/06133634/0 (accessed 17 February 2010)
- 44.Morgan M. G., Fischoff B., Bostrom A., Atman C. J. 2002. Risk communication: a mental models approach. Cambridge, UK: Cambridge University Press [Google Scholar]