Abstract
Management of zoonotic disease is necessary if countryside users are to gain benefit rather than suffer harm from their activities, and to avoid disproportionate reaction to novel threats. We introduce a conceptual framework based on the pressure–state–response model with five broad responses to disease incidence. Influencing public behaviour is one response and requires risk communication based on an integration of knowledge about the disease with an understanding of how publics respond to precautionary advice. A second framework emphasizes how risk communication involves more than information provision and should address dimensions including points-of-intervention over time, place and audience. The frameworks are developed by reference to tick-borne Lyme borreliosis (also known as Lyme disease), for which informed precautionary behaviour is particularly relevant. Interventions to influence behaviour can be directed by knowledge of spatial and temporal variation of tick abundance, what constitutes risky behaviour, how people respond to information of varying content, and an understanding of the social practices related to countryside use. The frameworks clarify the response options and help identify who is responsible for risk communication. These aspects are not consistently understood, and may result in an underestimation of the role of land-based organizations in facilitating appropriate precautionary behaviour.
Keywords: outdoor recreation, influencing behaviour, risk perception, ticks, zoonosis, Lyme borreliosis
1. Introduction
The countryside of the UK is not only a place of work and a source of food and fibre, but is also a set of valued locations for a variety of leisure and recreation pursuits, and an important source of cultural identity [1]. Mechanization and other labour-saving devices have led to a decline in outdoor labour, but there are still substantial workforces; for example, over half a million employed in agriculture, forestry and related industries [2,3]. Each year a substantial proportion of the British population makes a visit to the countryside: for example, in 2001/2002 some 62 per cent made an estimated 1.26 billion trips [4]. Woodland visits were reported by 67 per cent of UK respondents in 2003 and by 77 per cent in 2009 [5].
Countryside and health organizations are increasingly encouraging people to experience and take exercise in the rural environment and urban greenspaces. A proactive approach is being developed to engage more socially and ethnically diverse groups to benefit from outdoor nature [6]. This involves tackling both physical and structural barriers to access and accessibility, and also cultural and perceptual barriers concerned with confidence, knowledge of where to go, permission to use such spaces and concerns about the safety of doing so [7].
There is a range of current high-profile public health concerns around issues such as obesity, diabetes, lack of physical activity, mental health and health inequalities. The costs of these are large; for example, health problems associated with physical inactivity have been estimated to cost £8.2 billion a year in England [8]. The World Health Organization predicts that mental ill health will be the second biggest cause of disease burden globally by 2020 [9]. An increasing body of evidence has shown that visiting, and exercising in, natural environments provide a range of health benefits, such as reductions in blood pressure and heart rate, improvements in physical fitness [10], reductions in stress, and improved mood, well-being and self esteem [11].
Accordingly, a number of initiatives have been targeted at improving access to and use of nature, both in urban areas and in the countryside. These include the ‘Be active, be healthy’ strategy identifying nature as an important setting for physical activity, and campaigns such as the Green Gym (exercise through conservation activities), Blue Gym (exercising in the sea, rivers and waterways), Muckin4life (environmental volunteering as a means to get fit) and Active Woods (use of woodlands for exercise). Forest Schools (and Forest Kindergarten) have been developed to broaden educational opportunities by incorporating physical activities in outdoor settings.
The countryside and urban greenspaces are commonly represented as a benign risk-free environment (‘naturally good for you’), and a place of freedom in contrast to the built environment [12]. Nevertheless, there are hazards associated with activities out of doors. These include physical (e.g. steep slopes, rock fall, avalanche and deep water), activity based (e.g. mountain biking, orienteering and tree top trails), climatic (e.g. hypothermia, sun stroke and sun burn), biological hazards (e.g. stings, bites and allergic reactions to pollen) and abuse of spaces (e.g. anti-social behaviour, fly-tipping, drug use and dog-fouling). Additional hazards are associated with the post-productivist countryside being an environment for a range of amenity and leisure activities and simultaneously a site of production [13]. Of course, the public is protected from many countryside operations (e.g. harvesting sites and chemical treatments), but the working environment still poses dangers (e.g. fallen trees and getting lost in unmarked places).
Such hazards may be less apparent to those unfamiliar with these environments, who may therefore be ill-prepared to mitigate the risk and consequently reap harm rather than health benefits from visiting the countryside. Here, we regard risk as ‘the probability of a particular adverse event occurring during a stated period of time’ [14], incorporating two key elements: probability and consequence. The distinction between risks taken voluntarily and those that are not is an important one, and public concern is generally greater around risks experienced but not chosen [15]. Organizations encouraging use of the countryside may consider that the combination of naive users and novel hazards indicates the need for communication of appropriate behaviour. Raising awareness of the hazard and associated precautionary actions, however, could heighten concern [16] and may lead to withdrawal from countryside pursuits. This is the essence of the health conundrum that leads to two key challenges for the communication of risk. The first is how to encourage precaution without alarm. The second is how to encourage participation in, and engagement with, the countryside rather than avoidance of the countryside.
Zoonotic diseases present particular hazards to health and may well provoke avoidance of locations of likely infection. The World Health Organization defines zoonoses as ‘diseases and infections, which are transmitted naturally between vertebrate animals and man’. The simplicity of the definition belies the variety of such diseases [17] and their sources (host/reservoir), agents of disease, and modes of transmission. The source of infection may be domestic or wild animals; the infection agent may be a virus, bacterium, protozoon, etc. Transmission may be through direct contact with an infected animal, or by means of a vector, usually an arthropod such as an insect or tick, or by contact with contaminated water, soil, etc. Commonly, while the reservoir host may suffer no ill health, infection of any organ system may be life-threatening to different degrees for humans. There is concern that the global increase in interaction between humans and wildlife, through population expansion and activities such as forest clearance, will fuel an increased transfer of disease and the emergence of new diseases [18]. The hazards result from humans entering an environment within which these diseases are circulating, and the fear-inducing nature of some of the diseases means that there is potential for social amplification of the risk [19,20]. Such concern may lead to calls for action but raises the questions of what action and by whom?
Partial views of zoonotic diseases as an epidemiological or public health issue can be misleading. The management of diseases that impact upon society requires a broader and more integrated approach than has been undertaken in the past. For example, foot and mouth disease was portrayed as a problem for farmers, but the subsequent control practices impacted upon a large cross section of rural enterprises and countryside users [21,22]. The management of plant and animal diseases is increasingly framed as a problem for both government and industry; the former having a particular role in prevention of exotic (and emerging) diseases, the latter in applying known measures to control the incidence and the spread of endemic diseases, with cost-sharing models proposed to join both in resourcing. The appropriate management of zoonotic diseases would appear to be even more challenging with potentially more actors in the system, and the issue not entirely reducible to either ‘human health’ or ‘animal health’.
The value of an integrated view is exemplified by recent analyses of the rise in hantavirus and Lyme borreliosis (also known as Lyme disease) in Belgium [23], and in tick-borne encephalitis in central and eastern Europe. In the latter case, it proved inadequate to see the problem as resulting from climate change enhancing enzootic cycles. Instead, changes to the economy and related human activities were found to play a major part in the increased incidence of the disease among groups from across the social spectrum following the collapse of Communism [24]. Furthermore, spikes in disease incidence in a particular year (2006), appeared to be related to changes in human behaviour as a consequence of the weather, rather than in tick abundance [25].
In such complex circumstances, what actions can be taken by the various protagonists, and where do the responsibilities lie? We suggest that a broad range of responses to the threat to society from zoonotic diseases should arise from a broad view of the whole system. Therefore, in this paper we:
— develop a framework that identifies the range of possible responses to the threat of zoonotic disease;
— provide a second framework that elaborates the response of influencing behaviour and identifies the place of risk communication within this; and
— develop the application of these with respect to Lyme borreliosis.
2. Methods
The frameworks have emerged from work within a research project ‘Assessing and communicating animal disease risks for countryside users’, and are illustrated with particular reference to Lyme borreliosis, the UK, and temperate climates. Three case study sites ranging from peri-urban to remote upland settings were used for field sampling (Richmond Park on the fringe of Greater London, the New Forest in southern England and Exmoor in southwest England) and in scenario exercises that explored organizational responses to zoonoses. Risk analysis was informed by new survey work of vector tick populations and a population model for the tick Ixodes ricinus, which quantified the relative seasonal abundance of questing ticks in a range of habitats. Risk perception and risk communication were explored through individual interviews, questionnaires and focus groups with land managers, land-based workers, recreational visitors, residents in the case study areas and those diagnosed with Lyme borreliosis. The content of precautionary information currently provided by organizations to employees and visitors was analysed. Organizational responses to existing and plausible future threats were identified through discussions with a Practitioner Panel of representatives from public, charitable and private bodies that employ, encourage or control countryside activities. An Advisory Board provided expert opinion across a range of topics including public health protection, wildlife health, visitor management and land management. Response frameworks evolved from interactions with the Practitioner Panel and Advisory Board, review of international literature, and discussions within the project team based on the natural and social science findings of the project.
3. Elaborating a response framework for zoonotic disease management
A number of frameworks have been developed to facilitate an integrated view of the interaction between human societies and the natural environment. Derivatives of the drivers–pressure–state–impacts–response model have gained widespread support when seeking appropriate societal responses to environmental change [26] and considering causal links between the environment and human health. The OECD and the European Environment Agency have developed a simpler framework of pressure–state–response (P–S–R). Pressures are regarded as drivers of change within a system of interest, such that the state of the system alters, and the changes precipitate some form of response. The response can take the form of interventions to address one or more of the pressures, or to change the state directly.
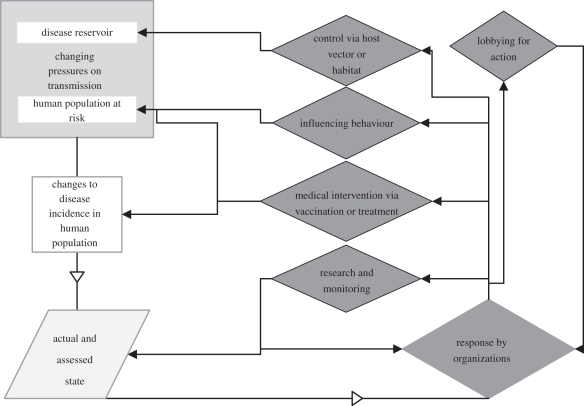
Discussions with the Advisory Board and Practitioner Panel and consideration of literature on disease control informed the development of a framework for zoonotic disease management based on the P–S–R model (figure 1). The framework is intended to support the identification of organizational actions that can be taken to manage the risk and the delineation of responsibilities between and within organizations.
Figure 1.
Zoonotic disease management—framework of pressure, state and responses.
(a). Pressures: changing potential for transmission of a zoonotic disease
In this case, pressures arise from occurrence of disease in animal hosts in the environment, and changes in the susceptibility of human population consequent upon their activities and their behaviour in undertaking them. Numerous factors will govern the disease occurrence in the natural environment, including vertebrate (and vector) population dynamics, climatic effects, habitat quality and land management. Similarly, there are many socio-economic factors that govern the exposure of the human population in the environment, including employment, leisure time and recreational activities.
(b). State: the incidence of infection and disease
Health authorities monitor and report upon zoonoses, and changes in incidence of disease will attract attention and may trigger review of need for a response. The actual state of incidence may be sampled by a number of assessment methods; changes in diagnostic method may also lead to an apparent change in incidence and thus need for action. Even if there is no formal surveillance or reporting, the assessed state of incidence may be sufficient to prompt concern.
(c). Response: five broad responses
The incidence and consequences of the disease may be sufficient to prompt consideration of the need for response to reduce disease incidence.
— Targeted control of hazard. There may be options to control the disease by targeting the reservoir host, targeting the vector or changing the habitat to reduce the prevalence of host or vector at a range of spatial scales.
— Medical intervention. Medical intervention may be seen as the solution to disease incidence. Most advantageous is where a disease can be prevented by the treatment to increase resistance (vaccination) but development of cures to tackle infection is also desirable. Vaccines and treatments are available for relatively few zoonotic diseases.
— Influencing behaviour. The complexity of the zoonotic system may mean that direct control and medical intervention are not feasible and that precaution is a highly desirable alternative to be encouraged, informed by biological knowledge of the zoonoses. This may include maintenance of some existing behaviours but also introduction or modification of others.
— Research and further surveillance. Trends in the state of the disease may prompt responsible authorities to continue or develop enhanced surveillance of the human population or of the reservoir or vector, or to refine other control methods and interventions.
— Lobbying for action. Whether or not there is formal surveillance, the assessed state of incidence may trigger divergent views of the need for and nature of response. Action groups may form to demand response (e.g. increased research), or oppose particular actions (e.g. widespread culling of host animals) based upon a variety of personal experiences (e.g. knowledge within social groups and delays in obtaining treatment). A variety of outcomes may result, including increased communication around the disease and the promotion of unconventional treatments.
Criteria governing the chosen response are likely to include the specifics of the disease cycle, the geographical extent of the problem and the nature of the threat to human health. The framework does not identify who should initiate or undertake particular responses, but it is possible to identify implicated candidate groups (table 1). This highlights the range of potential organizations, and the complexity of achieving a coordinated response, particularly when responsibility for initiation is unclear. For example, behavioural responses may be promoted by concerned groups, those with responsibility for land or for those visiting land, or by public bodies concerned to minimize burden on health services.
Table 1.
Candidate organizations who may be involved in responses to changing state of zoonotic disease incidence.
| type of response | candidate organizations |
|---|---|
| control of agent or host | government—public health bodies and animal health bodies |
| government—landowners and land managers | |
| business—landowners and land managers | |
| community-based—landowners and land managers | |
| non-governmental organizations—landowners and land managers | |
| medical intervention | government—public health bodies |
| business—pharmaceutical developers and suppliers | |
| business—healthcare providers | |
| influencing behaviour | government—public health bodies |
| government—countryside access bodies | |
| government—landowners and land managers | |
| business—landowners and land managers | |
| business—recreation and accommodation providers | |
| community-based—landowners and land managers | |
| non-governmental organizations—landowners and land managers | |
| non-governmental organizations—special interest groups | |
| research and monitoring | international—world health and disease bodies |
| government—public health services | |
| research institutions | |
| lobbying for action | non-governmental organizations—special interest groups, staff groups |
4. Elaborating a risk communication framework for intentional influencing of behaviour
A belief that providing information will be sufficient to change behaviour is prevalent among many organizations and together with education is certainly a key type of policy instrument [27]. However, the idea (often referred to as the information deficit model) that provision of information, either about the risk or recommended responses to the risk, will lead to appropriate behaviour change has proved to be ill-conceived in relation to a whole range of issues [28,29]. People may consider that they have sufficient information, they may consider themselves not at risk and exhibit ‘optimistic bias’ (e.g. [30]), they may not trust the source of the information, and the target behaviour may be habitual or enjoyable [31,32]. Considerable attention has, therefore, been paid towards understanding the change and maintenance of behaviour and how policy interventions can facilitate desired changes, for example, in promoting health and sustainable living [33,34]. Rather than focus on information provision, ways of influencing public behaviour should take account, and ‘go with the grain’, of the contexts in which people act and the networks within which they interact. This underlines the importance of developing a more holistic approach, bringing together a whole suite of influence options. The Diamond model is one way of capturing these insights and highlights that attention should be paid to developing behaviour change initiatives that encourage (give the right signals), exemplify (lead by example), engage (get people involved) and enable (make it easier) [28].
Arguably there are some distinctive elements in seeking to influence behaviour in contexts where risk is a key focus of communication and where, for example, there may be increased anxiety or concern and also considerable uncertainty [35]. The way in which people make sense of risk takes account of many more factors than are contained within the probability/consequence algorithm used by experts to characterize risk. People may scrutinize communications and communicators for signals of trustworthiness and be influenced by the ‘personality profile’ of the hazard—is it familiar, dreaded, new and so on [36,37]. The benefits of stopping and starting behaviours may be quite different when located within the broad context of people's everyday experiences and practices. Furthermore, communication in other response areas (such as publicizing a vaccine or lack of one, or sanctioning or disapproving certain types of land management) will provide statements from which risk can be read and which may communicate risk even if this is not the intention of the communicator.
The complexity of zoonotic disease transmission, the unavailability or cost of other responses and in some cases the dynamic nature of the threat, may mean that influencing public behaviours is the most viable response. We therefore propose a further framework that situates communication of risk in relation to a broader appreciation of mechanisms for influencing behaviour and firmly based on biological understanding of a hazard. We focus on strategies to maximize the likelihood of particular behaviours, or at least that allow people to make informed choices knowing the likely links between particular behaviours and particular consequences. There are five dimensions to the proposed risk communication framework:
— WHO? Do actions need to be tailored to particular audiences and their activities?
— WHERE? Is the risk or the underlying hazards place/site-specific?
— WHEN? Is the risk specific to time of day or season, and should actions be taken before, during and after a visit?
— WHAT? Are there behaviours that can minimize the risk of acquiring the disease?
— HOW? Can behaviours be influenced by measures that encourage, enable, exemplify and/or engage?
5. Framework analysis of the management of lyme borreliosis
We drew together biological and social understanding to explore the frameworks developed above, with specific reference to Lyme borreliosis. The disease is caused by bacteria (Borrelia burgdorferi s.l.) transmitted between the reservoir hosts (a wide range of birds and mammals) and humans by an arthropod vector (ticks, especially I. ricinus in UK). Lyme borreliosis was first recognized in the USA in the late 1970s [38], but there is evidence of early occurrence in a number of European countries [39]. Early symptoms include a bull-eye rash and flu-like symptoms, and at this stage, the disease is readily treated with antibiotics. Without treatment, there can be late stage complications involving many tissues, especially the nervous, musculoskeletal and cardiovascular systems [40].
(a). The biological and social pressures on Lyme borreliosis transmission
A number of pressures, both biological and sociological, can influence disease incidence:
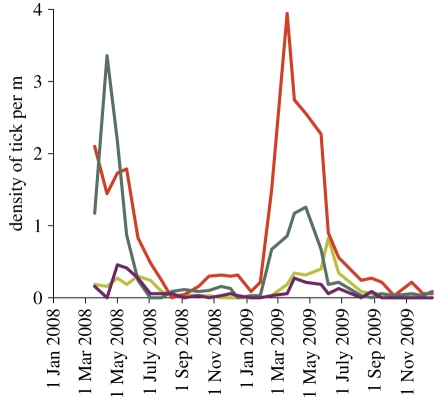
Tick populations and the proportion that carry the bacteria. Ticks hatch from eggs as larvae, and develop into nymphs, then adults, with a single, large blood meal taken by each stage. All stages of I. ricinus will feed on an extremely wide range of hosts, and their abundance varies with micro-climate (and hence habitat), host abundance, and time of year (figure 2). Nymphal tick density, which may vary by up to two orders of magnitude, is the main determinant of the density of infected nymphs as this is more variable than the prevalence of B. burgdorferi s.l. infection in ticks. The latter is affected by the composition of the host assemblage, since some hosts are non-competent to act as reservoirs for infection. Results from our study sites indicated that 3–9% of nymphal ticks and 6–33% of adults were found to carry B. burgdorferi s.l., broadly in line with the few other surveys undertaken in the UK; much higher incidence has been found in particular circumstances elsewhere in Europe [41] and typically in the USA [39]. Several strands of evidence suggest that there has been an increase in tick numbers and range expansion in the UK (e.g. [42]), though a lack of suitable long-term monitoring precludes definitive conclusions.
Figure 2.
The influence of seasonality and vegetation on abundance of I. ricinus nymphs at Exmoor, as sampled at three week intervals by standardized blanket dragging. Light green line, short grass; red line, mixed ericaceous shrub; dark green line, bracken; purple line, heather.
The amount of time spent in tick-bearing habitat. Increased leisure time, mobility and variety of outdoor pursuits may lead to increases in the amount of time people spend in tick-infested habitats. Interventions, whether site-specific or as broader campaigns to encourage activities in nature, might also lead to more time spent outside. Provision of particular facilities can lead to rapid increases in visitor numbers; for example, the activities and infrastructure improvements at Bedgebury Forest (as part of the Active England programme) resulted in visit numbers increasing from approximately 51K in 2005/2006 to 273K in 2007/2008, while at Haldon numbers rose from 10K in 2003 to 224K in 2007/2008 [43]. Less site-specific are an increasing range of organized activities targeted at different age and groups, such as the ‘Green Gym’, ‘Walking to Health’, Nordic walking and ‘buggycise’ for mothers with young children.
(b). The changing state of Lyme borreliosis incidence
The incidence of Lyme borreliosis in the UK is monitored by the Health Protection Agency (HPA) and Health Protection Scotland (HPS). It is reportable to the Health and Safety Executive under RIDDOR (The Reporting of Injuries, Diseases and Dangerous Occurrences Regulations 1995) but only in Scotland is Lyme borreliosis a notifiable disease (now following laboratory diagnosis). Published HPA data show an increase in incidence of the disease in England and Wales by 384 per cent over the period 1997–2008 (to 813 cases in England and Wales in 2008) of which approximately one-sixth are reported to have been acquired overseas. Even more dramatically in Scotland, the number of cases has increased by 1500 per cent between 2000 (37 cases) and 2009 (605 cases), with most of that increase occurring since 2005 (when there were 96 cases). It is unclear to what extent the increase is in part attributable to improved diagnosis, but a number of observers have suggested that this infection is still under-reported. Nevertheless, the recorded prevalence is very much lower than in mainland Europe.
(c). Current responses to Lyme borreliosis
Discussions with the Practitioner Panel and in interviews with a range of organizations provided information on the extent to which the range of potential responses is currently being considered.
Direct control of hazard and indirect control of environment. In the UK, there is little attempt to direct control. Some reported targeted vegetation control close to provided footpaths in an attempt to reduce encounters with ticks. Management for some specific land uses, in particular grouse moors, has resulted in localized action to remove ticks through the use of culling (of mountain hares), and acaricides (on sheep flocks) in an attempt to reduce tick burden on young birds and the transmission of louping ill [44,45]. However, change in the mandatory use of sheep dips has reportedly limited the deployment of acaricides. In North America, environmental control to reduce tick burdens includes area-wide application of acaricides, exclusion of deer, treatment of tick hosts and landscape practices (primarily vegetation management) [46,47]. However, such measures are typically applied in regions where large deer populations exist in close proximity to residential areas, a situation with only limited parallels in the UK. Extensive habitat modification and ground-based acaricide application, for example, are unlikely ever to be appropriate in areas used for public recreation, such as National Parks or Forestry Commission woodlands, given that Lyme borreliosis prevention is only one of many varied considerations faced by land managers. The prevalence of ticks and the diversity of reservoir hosts also militate against such action on a widespread scale.
Medical intervention. There is currently no vaccine for Lyme borreliosis, since the one once available in the USA was withdrawn [48], so pre-infection interventions are not available. In the USA, there is only one strain of Lyme disease spirochaetes, B. burgdorferi s.s., while in the UK, there are at least three (the others being Borrelia afzelii and Borrelia garinii), requiring multi-valent vaccines that are particularly challenging, and likely to take 15–20 years to develop. Post-infection treatment is available (either on the basis of clinical signs or following serological tests). It is most effective when made available promptly, which requires an understanding of when to seek medical assistance among countryside users and awareness of the symptoms of Lyme borreliosis among medical professionals.
Influencing behaviour. Some clear preventative measures (also termed personal protective behaviours [46]) involve avoidance of tick bites, and prompt removal of ticks. The former can be achieved in a variety of ways including covering skin with clothing, use of repellents or avoidance of tick-bearing habitat. Prompt removal will minimize the risk of transmission of the bacteria from the tick to humans; transmission is rare during the first 24 h of attachment, but may be more common with some European strains, e.g. B. afzelii [39]. However, both avoidance and removal presuppose awareness and adoption of precautionary behaviour. In Connecticut, northeastern USA, district-wide health education campaigns have been successful in raising awareness and adoption of precautions; individual precautions were adopted more readily than steps to modify the environment, such as vegetation control.
We found that some countryside user groups organize awareness-raising at periods of higher risk (e.g. Mountaineering Council of Scotland), and many employers provide information to promote precaution as part of Health and Safety at Work commitments (e.g. Health and Safety at Work Act 1974). However, provision of information to different sectors of the public is currently uncoordinated, with some suggestion that organizations are reticent about raising the visibility of Lyme borreliosis. This could be for reasons associated with the conundrum discussed at the outset; alerting people to possible risks may compromise messages promoting the benefits of recreational spaces for health and restoration. Organizations may also be reticent because by assuming a responsibility to inform it may be inferred that they are acknowledging a legal responsibility which they do not accept. In the case of workers, the duty of care and legal liability may be unambiguous, but this may not necessarily be so in relation to visitors to the countryside who may voluntarily put themselves into positions of risk. This is reflected in differences in risk communication with a focused dissemination of information such as risk assessments and briefing notes to staff, but a variety of leaflets and other sources made available for visitors seeking information. Of the large number and wide variety of messages obtained on Lyme borreliosis and precaution, most were types of Health and Safety guidance for staff members. With respect to external communication, many organizations relied upon the provision of leaflets, whose content varied with organizational type and whether they were prepared primarily for employees or for others. Much information was recycled, with many organizations adopting information from others without an appreciation or indication of its origin.
Surveys at our case study sites indicated that understanding of the existence of Lyme borreliosis and precautions was sometimes confused, but generally low and variable both across visitors to a particular site and between sites. For example, at the Exmoor study site, approximately two-thirds of those interviewed were unaware of what precautions to take. Additionally some visitors, and even recent patients, although aware of the risk of Lyme borreliosis, were reluctant to take precautionary measures during their visit to the countryside. There were also disparate views among visitors and members of our Practitioner Panel around who was considered responsible for providing information or managing the disease.
Research and monitoring. The national Health Protection Agencies monitor and disseminate numbers of reported cases of Lyme disease, but the reporting process itself is not standardized, and there is no systematic monitoring of reservoirs, vectors (or vector hosts) or infection prevalence within vectors. Lyme borreliosis is not viewed as a priority for medical research, and the polyvalent nature of the disease (multiple strains) makes vaccine development costly and slow. The lack of impact upon animal health (and domestic animal health) means that it is not a priority for those concerned.
Pressures for action. In the USA, there is substantial controversy over the prevalence, diagnosis and the treatment of Lyme borreliosis [49,50]. In Europe, a number of groups have formed to encourage more action by public bodies, and promote awareness; including Borreliosis and Associated Diseases Awareness (BADA), Lyme Disease Action (LDA) and EuroLyme. Views of the prevalence and nature of clinical disease diverge between some of these groups, scientists and public health bodies. Internet searches for information on Lyme borreliosis often return links to information provided by such groups ahead of those provided by the public health bodies. Some managers reported the occurrence of ‘guerrilla signage’ whereby warning notices were placed on their land anonymously. There have been calls for the disease to be publicized further and made reportable throughout UK, including lobbying of the Scottish and UK Parliaments and petitions to Downing Street. A number of pressure groups have suggested that more attention be given to GP awareness of the diagnosis of the disease to overcome what they see as failures to recognize it and a consequent under-reporting of cases.
(d). Influencing behaviour as part of Lyme borreliosis disease management
The application of the P–S–R framework above, based on an explicit integration of biological and socio-psychological information, suggests that influencing behaviour is the most viable line of response to Lyme borreliosis. This reflects the lack of protective vaccination, the lack of post-exposure protective immunity, and the extent of tick-bearing habitat and the range of potential hosts that together make direct control impractical. Furthermore, while field data show the presence of ticks in most habitats within recreational woodlands (A. D. M. Dobson 2008–2009, unpublished data), behavioural observations indicate that humans already predominantly stick to paths, thereby limiting the degree to which their contact with ticks can be further diminished without severely limiting their enjoyment of the countryside. There is, however, a range of options available for influencing behaviours (table 2).
Table 2.
Framework for situated risk communication populated with respect to precautions around tick bites and Lyme borreliosis.
|
when? the points in time at which behaviour may be influenced and specific actions taken |
||||||
|---|---|---|---|---|---|---|
| pre-visit | visit | post-visit | post-bite | post-infection | ||
| what? the possible behaviours that can minimise risk of acquiring the disease | obtain appropriate clothing and repellent | wear appropriate clothing and repellent; consider route and activity | check for ticks | prompt removal and subsequent monitoring of bite location | prompt help-seeking and appropriate treatment | |
| who? who might need to consider precautionary behaviours | potential visitor (public or employee); those encouraging or supervising visits or activities | visitor engaged in specific activities (on path/off path) (public or employee) | visitor engaged in specific activities (on path/off path) (public or employee) | visitor engaged in specific activities (on path/off path) (public or employee) | patient (public or employee) medical staff | |
| where? the extent to which the risk and risk communication is place specific | hot-spots (if they exist) and particular routes and vegetation types | on path/off path; different vegetation types | place of stay | place of stay | GP surgery | |
| how? the way in which the influencing actions are configured to encourage precautionary behaviours | Encourage (give the right signals) | information on web | notices in car park | de-briefing of conducted visits | information at health sites | ensure GP awareness |
| Exemplify (lead by example) | staff identifying risks in briefings | staff wearing appropriate clothing | ||||
| Engage (get people involved) | share best practice information with key stakeholders; involve publics in communication design and evaluation | share best practice information with key stakeholders | share best practice information with key stakeholders | share best practice information with key stakeholders | share best practice information with key stakeholders | |
| Enable (make it easier) | routing of path; location of picnic sites | provision of tick removal device | provision of tick removal device | provision of tick removal device | improve diagnostic tools and keys | |
To what extent do existing risk communication practices reflect the proposed framework of influencing behaviour?
Who? Many land managers do not consider Lyme borreliosis to be a major risk, compared with other hazards on their land. Despite this, the policies, procedures and practices for communicating risks to staff are formalized and adhered to closely, such that there is a strong safety culture within, for example, the Forestry Commission. By contrast, policies, procedures and practices with respect to the public vary across the country with much less consistency than the approach for staff. Despite evident concern for risk and safety, warning leaflets reveal a tendency to consider the public as lacking knowledge and being unresponsive to information, and are not tailored to those involved in particular activities. This may be a consequence of the local staff's perception of the incidence of Lyme borreliosis, but also reflect some reluctance for fear of the implications of accepting ownership of the public problem as discussed above. Although the environment affords some similar risks to the workforce and visitors, the duty of care that land-based organizations have towards each is different and is reflected in risk communication.
Where? At a small number of locations, signs are reportedly placed at key access points to habitats known to harbour ticks, and some leaflets refer to vegetation types that might have high tick abundance (although these did not accord precisely with those shown by our surveys to have high tick abundance). From our interviews with forestry staff, the presence of ticks and Lyme borreliosis was often seen to be ‘elsewhere’ (i.e. ‘not here’) and in particular places, such as the New Forest. As a consequence, these places were stigmatized with reputations as ‘hot spots’ for Lyme borreliosis. However, the notion of hot spots has to be challenged. If the density of infected ticks is no greater in a particular place, the risk per individual is no higher than average, even though total numbers of Lyme disease cases may be high owing to intense human recreational use. The notion of hot spots is problematic because not only does it over-represent the prevalence of infected ticks and the incidence of Lyme borreliosis in some places, but also under-represents it in others. Thus, when considered against the backdrop of expert biological assessments of risk, public concerns may be intensified in some instances and attenuated in others.
When? Much of the information currently provided does not distinguish particular timing of risk, nor is it made available selectively through the risk season. In contrast to the action groups that focus on tick-awareness week as a way of highlighting the spring increase in tick numbers, very few locations provide seasonal signage to alert the public to the seasonal onset of the risk of ticks.
What? There was little enthusiasm for wholesale change in behaviour from members of the general public. Even among those who had previously acquired the disease, more than 90 per cent disagreed with the notion of avoiding the countryside in the future. Some of the recommended mitigation behaviours may be at variance with the behaviours that people expect and want to engage in when in the countryside. Although patients and visitors with a range of links to the countryside indicated positive support for both those precautions to be taken during the visit (such as wearing long sleeves and tucking trousers in socks) and those taken afterwards (such as checking the skin for ticks), there was significantly greater preference for post-visit precautions.
How? The focus of current practice is overwhelmingly on information provision (a form of ‘encourage’), typically in the form of leaflets at visitor centres, risk assessments and some signage; at least one land manager had taken steps to inform local doctors of the occurrence of Lyme borreliosis. Several potential methods (table 2) were rarely reported: including enabling measures (provision of tick removal devices to staff, routing of paths or activities away from habitats with high tick abundance), engaging (working with stakeholders) and exemplifying (actions by staff, e.g. in leading visits).
6. Discussion
(a). Integration of natural and social sciences
The recent upsurge of interdisciplinary research has led to new ways of framing problems and examining issues of public importance [51]. A number of frameworks have been developed to examine the interactions between ecological and social systems [52]. Many of these explore the negative impacts of human activities upon ecosystem condition and integrity [53,54], or the dependence of human well-being upon the goods and services of ecosystems [55]. Ecosystem services are considered to be ‘the benefits people obtain from ecosystems’ and health an important component of human well-being [55]. The Millennium Ecosystem Assessment considered that degradation of ecosystems could compromise regulating services, and that deterioration of disease regulation could lead to impacts upon human health. Responses were identified across a range of scales and institutions, with constraints of lack of knowledge and of failure to use adequately the information that does exist in support of management decisions; supporting frameworks included cost–benefit analysis, risk assessment and multi-criteria analysis. Our study also considers the interactions between humans and nature, but focuses more upon a negative aspect of the interaction, the risk of zoonotic disease, and places emphasis on social and behavioural factors in response options. It develops decision frameworks necessary to identify and progress responses to disease incidence, accepting that countryside users enter habitats containing complex enzootic cycles, and that increases in disease transmission may be due to changes of behaviour as well as any loss of regulating service. Our second framework emphasizes how responses may need to be found within society (i.e. via influencing behaviour), when a focus on ecosystem management is less appropriate. Arguably this emphasis on human behaviour to some extent reflects the focus on the managed landscapes of the UK rather than more pristine ecosystems, but also the focus on land management rather than those responsible for global or regional strategies.
The development of the two frameworks and their application to Lyme borreliosis has been possible by, and emphasizes the value of, integration of natural and social sciences. Zoonoses present particular problems to those seeking to reduce their impact, or potential impact, upon human health owing to their diverse character and the complex cycles within which they circulate in the environment. The elaboration of the P–S–R model emphasizes that the responses to disease threat are not exclusively those concerned with medical intervention or biological control of host, vector and reservoir (figure 1). The example of Lyme borreliosis illustrates that neither response may be adequate or feasible to protect human health. Influencing behaviour of those who might be at risk may well be the most feasible and likely response, yet it is far from straightforward.
Successful influencing will depend upon sound understanding of the biology of the disease but also, as our risk communication framework highlights, the social practices of the human population at risk and an understanding of how various groups react to a variety of risk communication actions. Despite widespread rejection of an information deficit model in academic studies, the view that information provision will bring about appropriate behaviour was common in our discussions with a range of responsible organizations. Provision of accurate and comprehensive biological information will not guarantee that precautionary behaviour is adopted. Nor is comprehensive information always necessary or timely. Greater attention is required around the timing, place, audience and content of communication—for example, a recommendation to apply repellent is unhelpful if only displayed at the car park and the visitor does not carry the product with them. Consideration of risk communication by point of intervention (table 2) suggests that as yet targeting by time, place or audience is relatively unsophisticated. Attempts to target risk communication to particular locations and seasons is infrequently observed, and, with the exception of a distinction between an employee and a non-employee (where the motivation may in part be legalistic), there is little sign of audience segmentation. Land managers and action groups hold divergent views on the extent of risk, yet information from the groups is provided to the public by the land-based organizations.
There is scope for considering a wider range of techniques in risk communication; again our framework (table 2) emphasizes that it is not simply by providing information, but also by enabling, exemplifying and engaging. Applying the specific knowledge of a disease within the framework will identify which of the suite of actions is possible. In the case of Lyme borreliosis, post-visit precautions were preferred by visitors and are effective if carried out thoroughly and promptly. However, other zoonoses may demand a different balance of action—if transfer of the zoonoses happens more rapidly, if it is incurable and if the transmission occurs via contact with water or soil. For example, Weil's disease (leptospirosis) is a bacterial infection contracted through skin abrasions or ingestion, typically following exposure to water contaminated by urine from infected animals such as rats [56]. Desirable responses to the threat of Weil's disease might include encouraging the covering of cuts and wounds prior to a visit, enabling the washing of equipment at visit sites, engaging terrier clubs to control rodents in the vicinity, and exemplifying appropriate behaviours by actions of the guides of conducted visits.
(b). Responses and responsibility
Our P–S–R framework (figure 1) does not seek to allocate responsibility for responding, though it is possible to identify organizations likely to be involved in the initiation or execution of a response (table 1). In managing a disease of domestic livestock, the responsibilities are relatively well understood (e.g. Animal Health Strategy), even if the costs of action are not as uniformly accepted. Plant health is also relatively clear. The recent observation that the management of wildlife diseases is not joined up [57] has been echoed in the complexity of responsibility for the management of wild animals [58]. In the same way, no one actor appears to have an overview or overall responsibility for zoonotic disease management.
Our analysis identified lack of clarity over who is responsible for management of ticks and Lyme borreliosis and who should be involved in influencing appropriate precautionary behaviours. There was little connection between public health bodies, which might be considered to be responsible for and benefit from preventing disease, and land-based organizations, which have more direct contact with the population of countryside users at risk. The latter organizations may provide information, but this may be partial or incorrect. The motivation for provision of information was diverse, with suggestions that some was linked to ‘duty of care’ legislation, whereas others (but not all) felt a more general responsibility to provide information. In our interviews with the public and in focus group discussions, there was no clear or consistent view of responsibility for communicating the risk of Lyme borreliosis. Sources of advice that were considered were not necessarily those of the public bodies; information from family and friends and via the internet (where searches do not necessarily return official sites) was mentioned frequently. Some information may have been recycled, with the best of intentions from a diverse range of sources in a way that misses opportunities for targeting particular behaviours and may also propagate myths and mis-information.
The P–S–R framework (figure 1) identified here may have merit in structuring thoughts and fostering dialogue with a range of stakeholders. Although there appears to be a potential role for government to stimulate such responses, and in particular to consider the wider range of actors potentially involved in implementing the full range of responses, this is at odds with the prevailing political climate. Instead, the framework could be used by fora (e.g. loose affiliations of several organizations from governmental and charitable sectors around a common interest), such as the Visitor Safety in the Countryside Group, the Outdoor Health Forum, Countryside Recreation Network to structure deliberation and identify responses in the absence of central direction. The framework could be used to identify explicitly which actors have a role in managing a disease, thereby forming a collaborative group or confirming the need for central support. A further use of the framework could be to structure thinking for an individual landowner who assesses the local disease incidence (or threat) to be unacceptable, and wishes to target action to best effect.
It would be instructive to explore its use for a number of other diseases where the particular circumstances (biological, situational) may place emphasis on different elements of the framework. Preliminary discussions with our Practitioner Panel indicate that the framework has merit and could be adapted to other threats, thereby identifying different outcomes depending upon the organizational linkages, political profile and the gravity of the situation.
(c). Participants in risk communication
Our risk communication framework emphasizes the need to customize information and other communication to the circumstances and the audience, and engage stakeholders in dialogue. Arguably, habituation and desensitization can arise from being given undifferentiated information that does not recognize variability in exposure to risk that relates place, time and people and their practices. Gathering evidence for making meaningful differentiations is not, of course, straightforward. The biological basis may require detailed and labour-intensive site-specific studies, as has been achieved in this project (figure 2), but the sociological basis may demand wide-ranging considerations of audiences comprising visitors and potential visitors. For example, if we consider this in relation to the ‘who’ dimension, while market segmentation has proved useful in some domains, it tends to be associated with either socio-demographic profile or purchasing power. This may have some applicability; for example, vaccination is available for some diseases such as tick-borne encephalitis in mainland Europe, but uptake is variable and, in poorer regions, related to economic status and education [59]. However, these may not be the most appropriate dimensions on which to segment audiences for influencing visitor behaviour. Visitors to forests and other places where ticks are present may be looking for particular kinds of experiences so that matching risk communication to different experience-seeking profiles may be more efficacious. Work to segment the audience for climate change risk information could be useful [60] as these capture aspects of values and attitudes, enabling a more nuanced guide to communication. Thus, there is considerable scope for improving the response focused on influencing behaviour. This could also include refining the place and timing of risk communication and taking the attitudes, preferences and practices of the intended audiences into account in encouraging proportionate precautionary action. For example, there may be scope for the use of social groups (e.g. the Mountaineering Council of Scotland) to communicate such precautions and greater involvement of scientists and public health professionals in engaging in dialogue with them.
(d). Concluding remarks
In conclusion, we return to the two challenges that were presented at the outset: The first challenge is how to encourage precaution without alarm. The second challenge is how to encourage participation in the countryside rather than avoidance of the countryside. Our two frameworks should assist the development of appropriate responses to the risk of zoonotic diseases by giving greater clarity over organizational roles. A more sophisticated appreciation of mechanisms for influencing behaviour should make it possible to foster appropriate precaution and encourage countryside use by many who would benefit. Framework analysis of specific situations, disease or place-based, could provide an object for dialogue by responsible or concerned parties and, over time, the basis for agreement over consistent and collective actions. This would engender confidence that the countryside, like the built environment, contains hazards with unpleasant consequences but for which the likelihood can be reduced to acceptable levels by precautionary actions rather than avoidance.
Acknowledgements
The research was conducted as part of the UK Research Councils' Rural Economy and Land Use (Relu) Programme (project RES-229-25-0007). Relu is funded jointly by the Economic and Social Research Council, the Biotechnology and Biological Sciences Research Council and the Natural Environment Research Council, with additional funding from the Department of Environment, Food and Rural Affairs and the Scottish Government. We are grateful for the inputs of members of the Project Advisory Board and Practitioner Panel, to all those who answered questionnaires and participated in focus groups, and to Lexi Thompson and Norman Dandy who made early contributions to the project. The paper benefitted from the comments of a number of referees. This paper is catalogued by the project steering committee as RELU_ADR_001.
Footnotes
One contribution of 11 to a Theme Issue ‘Interdisciplinary perspectives on the management of infectious animal and plant diseases'.
References
- 1.Wallwork J., Dixon J. A. 2004. Foxes, green fields and Britishness: on the rhetorical construction of place and national identity. Br. J. Soc. Psychol. 43, 21–39 10.1348/014466604322915962 (doi:10.1348/014466604322915962) [DOI] [PubMed] [Google Scholar]
- 2.Cowie H. A., Soutar C. A., Graveling R. A., Cattermole T. J., Cherrie J. W., Graham M. K., Mulholland R. M. Baseline incidence of ill health in agriculture in Great Britain. Sudbury, UK: HSE Books; 2005. [Google Scholar]
- 3.Forestry Commission 2009. Forestry facts and figures 2009: a summary of statistics about woodlands and forests. Edinburgh, UK: Forestry Commission [Google Scholar]
- 4.TNS Travel and Tourism. Leisure day visits. Report of the 2002–3 Great Britain Day Visits Survey. Edinburgh, UK: TNS Travel and Tourism; 2004. [Google Scholar]
- 5.Forestry Commission 2009. Public opinion of forestry 2009: UK. Edinburgh, UK: Forestry Commission [Google Scholar]
- 6.Defra 2008. Outdoors for all? An action plan to increase the number of people from under-represented groups who access the natural environment. London, UK: Defra [Google Scholar]
- 7.Rehman H., Gervais C. 2005. ‘What about us?’ Diversity Review evidence: challenging perceptions: under-represented groups' visitor needs. Cheltenham, UK: Countryside Agency [Google Scholar]
- 8.Department of Health 2004. At least five a week—evidence on the impact of physical activity and its relationship to health. London, UK: DoH [Google Scholar]
- 9.World Health Organization 2010. Depression. Geneva, Switzerland: WHO [Google Scholar]
- 10.Pretty J., Peacock J., Sellens M., Griffin M. 2005. The mental and physical health outcomes of green exercise. Int. J. Environ. Health Res. 15, 319–337 10.1080/09603120500155963 (doi:10.1080/09603120500155963) [DOI] [PubMed] [Google Scholar]
- 11.Milligan C., Bingley A. 2007. Restorative places or scary places? The impact of woodland on the mental well-being of young adults. Health Place 13, 799–811 10.1016/j.healthplace.2007.01.005 (doi:10.1016/j.healthplace.2007.01.005) [DOI] [PubMed] [Google Scholar]
- 12.Land Use Consultants in association with the Centre for Research into Environment and Health 2007. Delivering healthier communities in London. London, UK: HUDU [Google Scholar]
- 13.Wilson G. A., Memon P. A. 2005. Indigenous forest management in 21st century New Zealand: towards a postproductivist indigenous forest-farmland interface. Environ. Plan. A 37, 1439–1517 10.1068/a37144 (doi:10.1068/a37144) [DOI] [Google Scholar]
- 14.Breakwell G. M. 2007. The psychology of risk. Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Barnett J., Breakwell G. M. 2001. Risk perception and experience: hazard personality profiles and individual differences. Risk Anal. 21, 171–177 10.1111/0272-4332.211099 (doi:10.1111/0272-4332.211099) [DOI] [PubMed] [Google Scholar]
- 16.Timotijevic L., Barnett J. 2006. Managing the possible health risks of mobile telecommunications: public understandings of precautionary action and advice. Health Risk Soc. 8, 143–164 10.1080/13698570600677324 (doi:10.1080/13698570600677324) [DOI] [Google Scholar]
- 17.Jones K. E., Patel N. G., Levy M. A., Storeygard A., Balk D., Gittleman J. L., Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993 10.1038/nature06536 (doi:10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daszak P., Cunningham A. A., Hyatt A. D. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287, 443–449 10.1126/science.287.5452.443 (doi:10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 19.Kasperson R. E., Renn O., Slovic P., Brown H. S. 1988. The social amplification of risk: a conceptual framework. Risk Anal. 8, 177–178 10.1111/j.1539-6924.1988.tb01168.x (doi:10.1111/j.1539-6924.1988.tb01168.x) [DOI] [Google Scholar]
- 20.Pidgeon N., Henwood K. 2010. The social amplification of risk framework (SARF): theory, critiques, and policy implications. In Risk communication and public health (eds Bennett P., Calman K., Curtis S., Fischbacher-Smith D.), 2nd edn Oxford, UK: Oxford University Press [Google Scholar]
- 21.Bennett K., Phillipson J., Lowe P., Ward N., Cattermole A., Donaldson A., Elliot C., Midgley J., Thompson N. 2001. The impact of the foot and mouth crisis on rural firms: a survey of microbusinesses in the North East of England. Newcastle Upon Tyne, UK: Newcastle University Centre for Rural Economy [Google Scholar]
- 22.Defra 2001. Report of the Rural Task Force: tackling the impact of foot and mouth on the rural economy. London, UK: Defra [Google Scholar]
- 23.Linard C., Lamarque P., Heyman P., Ducoffre G., Luyasu V., Tersago K., Vanwambeke S. O., Lambin E. F. 2007. Determinants of the geographic distribution of Puumala virus and Lyme borreliosis infections in Belgium. Int. J. Health Geogr. 6, 15–29 10.1186/1476-072X-6-15 (doi:10.1186/1476-072X-6-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randolph S. E. 2008. Tick-borne encephalitis incidence in Central and Eastern Europe: consequences of political transition. Microbes Infect. 10, 209–216 10.1016/j.micinf.2007.12.005 (doi:10.1016/j.micinf.2007.12.005) [DOI] [PubMed] [Google Scholar]
- 25.Randolph S. E., et al. 2008. Variable spikes in tick-borne encephalitis incidence in 2006 independent of variable tick abundance but related to weather. Parasites Vectors 1, 44. 10.1186/1756-3305-1-44 (doi:10.1186/1756-3305-1-44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Organization for Economic Cooperation and Development 1993. OECD core set of indicators for environmental performance reviews. OECD Environment Directorate Report. Paris, France: Organization for Economic Cooperation and Development [Google Scholar]
- 27.Ledbury M., Miller N., Lee A., Fairman T., Clifton C.Understanding policy options. 2006. Home Office Online Report 06/06. London, UK: Home Office.
- 28.Dolan P., Hallsworth M., Halpern D., King D., Vlaev I. 2010. MINDSPACE: influencing behaviour through public policy. London, UK: Institute for Government [Google Scholar]
- 29.Griffin R. J., Neuwirth K., Dunwoody S., Giese J. 2004. Information sufficiency and risk communication. Media Psychol. 6, 23–61 10.1207/s1532785xmep0601_2 (doi:10.1207/s1532785xmep0601_2) [DOI] [Google Scholar]
- 30.Weinstein N. D. 1989. Optimistic biases about personal risks. Science 246, 1232–1233 10.1126/science.2686031 (doi:10.1126/science.2686031) [DOI] [PubMed] [Google Scholar]
- 31.Darnton A. 2008. Reference report: an overview of behaviour change models and their uses. London, UK: Government Social Research [Google Scholar]
- 32.Thompson S. C., Schlehofer M. M., Bovin M. J. 2006. The measurement of threat orientations. Am. J. Health Behav. 30, 147–157 [DOI] [PubMed] [Google Scholar]
- 33.Defra 2008. A framework for pro-environmental procedures. London, UK: Defra [Google Scholar]
- 34.Thaler R. H., Sunstein C. R. 2008. Nudge: improving decisions about health, wealth, and happiness. New Haven, CT: Yale University Press [Google Scholar]
- 35.Breakwell G. M., Barnett J. 2003. The significance of uncertainty and conflict: developing a social psychological theory of risk communications. New Rev. Soc. Psychol. 2, 107–114 [Google Scholar]
- 36.Slovic P. 1987. Perception of risk. Science 236, 280–285 10.1126/science.3563507 (doi:10.1126/science.3563507) [DOI] [PubMed] [Google Scholar]
- 37.Slovic P. 1993. Perceived risk, trust and democracy. Risk Anal. 13, 675–682 10.1111/j.1539-6924.1993.tb01329.x (doi:10.1111/j.1539-6924.1993.tb01329.x) [DOI] [Google Scholar]
- 38.Steere A. C., Malawista S. E., Snydman D. R., Shope R. E., Andiman W. A., Ross M. R., Steele F. M. 1977. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 20, 7–17 10.1002/art.1780200102 (doi:10.1002/art.1780200102) [DOI] [PubMed] [Google Scholar]
- 39.Piesman J., Gern L. 2004. Lyme borreliosis in Europe and North America. Parasitology 129, S191–S220 10.1017/S0031182003004694 (doi:10.1017/S0031182003004694) [DOI] [PubMed] [Google Scholar]
- 40.O'Connell S. 2005. Lyme borreliosis. Medicine 33, 106–109 10.1383/medc.33.5.106.64958 (doi:10.1383/medc.33.5.106.64958) [DOI] [Google Scholar]
- 41.Humair P.-F., Gern L. 1998. Relationship between Borrelia burgdorferi sensu lato species, red squirrels (Sciurus vulgaris) and Ixodes ricinus in enzootic areas in Switzerland. Acta Trop. 69, 213–227 10.1016/S0001-706X(97)00126-5 (doi:10.1016/S0001-706X(97)00126-5) [DOI] [PubMed] [Google Scholar]
- 42.Scharlemann J. P. W., Johnson P. J., Smith A. A., Macdonald D. W., Randolph S. E. 2008. Trends in ixodid tick abundance and distribution in Great Britain. Med. Vet. Entomol. 22, 238–247 10.1111/j.1365-2915.2008.00734.x (doi:10.1111/j.1365-2915.2008.00734.x) [DOI] [PubMed] [Google Scholar]
- 43.O'Brien E. A., Morris J. 2009. Active England: the woodland project. Edinburgh, UK: Forestry Commission [Google Scholar]
- 44.Harrison A., Newey S., Gilbert L., Haydon D. T., Thirgood S. 2010. Culling wildlife hosts to control disease: mountain hares, red grouse and louping ill virus. J. Appl. Ecol. 47, 926–930 10.1111/j.1365-2664.2010.01834.x (doi:10.1111/j.1365-2664.2010.01834.x) [DOI] [Google Scholar]
- 45.Laurenson M. K., Hudson P. J., McGuire K., Thirgood S. J., Reid H. W. 1997. Efficacy of acaricidal tags and pour-on as prophylaxis against ticks and louping-ill in red grouse. Med. Vet. Entomol. 11, 389–393 10.1111/j.1365-2915.1997.tb00427.x (doi:10.1111/j.1365-2915.1997.tb00427.x) [DOI] [PubMed] [Google Scholar]
- 46.Gould L. H., Nelson R. S., Griffith K. S., Hayes E. B., Piesman J., Mead P. S., Cartter M. L. 2008. Knowledge, attitudes, and behaviors regarding Lyme disease prevention among Connecticut residents, 1999–2004. Vector Borne Zoonotic Dis. 8, 769–776 10.1089/vbz.2007.0221 (doi:10.1089/vbz.2007.0221) [DOI] [PubMed] [Google Scholar]
- 47.Miller N. J., Thomas W. A., Mather T. N. 2009. Evaluating a deer-targeted acaricide applicator for area-wide suppression of blacklegged ticks, Ixodes scapularis (Acari: Ixodidae), in Rhode Island. Vector Borne Zoonotic Dis. 9, 401–406 10.1089/vbz.2008.0164 (doi:10.1089/vbz.2008.0164) [DOI] [PubMed] [Google Scholar]
- 48.Nigrovic L. E., Thompson K. M. 2007. The Lyme vaccine: a cautionary tale. Epidemiol. Infect. 135, 1–8 10.1017/S0950268806007096 (doi:10.1017/S0950268806007096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feder H. M., Johnson B. J. B., O'Connell S., Shapiro E. D., Steere A. C., Wormser G. P. & the Ad Hoc International Lyme Disease Group 2007. A critical appraisal of ‘chronic Lyme disease’. New Engl. J. Med. 357, 1422–1430 10.1056/NEJMra072023 (doi:10.1056/NEJMra072023) [DOI] [PubMed] [Google Scholar]
- 50.Ronn S. J. D. 2009. In the Lymelight: law and clinical practice guidelines. Southern Med. J. 102, 626–630 [DOI] [PubMed] [Google Scholar]
- 51.Lowe P., Whitman G., Phillipson J. 2009. Ecology and the social sciences. J. Appl. Ecol. 46, 297–305 10.1111/j.1365-2664.2009.01621.x (doi:10.1111/j.1365-2664.2009.01621.x) [DOI] [Google Scholar]
- 52.Rounsevell M., Dawson T., Harrison P. 2010. A conceptual framework to assess the effects of environmental change on ecosystem services. Biodivers. Conserv. 19, 2823–2842 10.1007/s10531-010-9838-5 (doi:10.1007/s10531-010-9838-5) [DOI] [Google Scholar]
- 53.Borja A., Galparsoro I., Solaun O., Muxika I., Tello E. M., Uriarte A., Valencia V. 2006. The European Water Framework Directive and the DPSIR, a methodological approach to assess the risk of failing to achieve good ecological status. Estuar. Coast. Shelf Sci. 66, 84–96 10.1016/j.ecss.2005.07.021 (doi:10.1016/j.ecss.2005.07.021) [DOI] [Google Scholar]
- 54.Organization for Economic Cooperation and Development 2001. Key environmental indicators. Paris, France: Organization for Economic Cooperation and Development [Google Scholar]
- 55.Millennium Ecosystem Assessment 2005. Ecosystems and human well-being: synthesis. Washington, DC: Island Press [Google Scholar]
- 56.World Health Organization 2003. Human leptospirosis: guidance for diagnosis, surveillance and control. Geneva, Switzerland: WHO [Google Scholar]
- 57.Parliamentary Office of Science and Technology 2008. Wildlife diseases. London, UK: POST [Google Scholar]
- 58.Phillip S., Dandy N., Gill R., MacMillan D. C. 2009. Is legislation a barrier to the sustainable management of game species? A case study of wild deer in Britain. J. Environ. Plan. Manag. 52, 993–1012 10.1080/09640560903327351 (doi:10.1080/09640560903327351) [DOI] [Google Scholar]
- 59.Sumilo D., Asokliene L., Avsic-Zupanc T., Bormane A., Vasilenko V., Lucenko I., Golovljova I., Randolph S. E. 2008. Behavioural responses to perceived risk of tick-borne encephalitis: vaccination and avoidance in the Baltics and Slovenia. Vaccine 26, 2580–2588 10.1016/j.vaccine.2008.03.029 (doi:10.1016/j.vaccine.2008.03.029) [DOI] [PubMed] [Google Scholar]
- 60.Leiserowitz A., Maibach E., Roser-Renouf C. 2008. Global warming's ‘Six Americas’: an audience segmentation. Fairfax, VA: George Mason University, Center for Climate Change Communication [Google Scholar]