Abstract
Uncertainty is an inherent feature of strategies to contain animal disease. In this paper, an interdisciplinary framework for representing strategies of containment, and analysing how uncertainties are embedded and propagated through them, is developed and illustrated. Analysis centres on persistent, periodic and emerging disease threats, with a particular focus on cryptosporidiosis, foot and mouth disease and avian influenza. Uncertainty is shown to be produced at strategic, tactical and operational levels of containment, and across the different arenas of disease prevention, anticipation and alleviation. The paper argues for more critically reflexive assessments of uncertainty in containment policy and practice. An interdisciplinary approach has an important contribution to make, but is absent from current real-world containment policy.
Keywords: animal disease, containment, uncertainty, policy, interdisciplinarity
1. Introduction
This paper examines uncertainties associated with strategies to contain animal disease. In general terms, uncertainty analysis is a way of assessing, to varying degrees of statistical and analytical precision, limits to reasoning and understanding [1,2]. Uncertainty is an inherent and inescapable attribute of decision-making processes that aim to prevent, anticipate and alleviate animal disease. Encompassing a range of procedures and priorities, governance arrangements for containment are both institutionally and scientifically complex. The extensive, open and highly unstructured character of disease threats means that interventions come with few guarantees.
A range of techniques, originating from within the sciences, are available to decision makers to explain the character and significance of uncertainty across different aspects of disease containment. These include, for instance, probabilistic and qualitative assessments of emerging threats, outbreak behaviour and the efficacy of mitigation measures. In principle, therefore, uncertainly analysis is a way of informing decision makers about the extent to which particular outcomes can be inferred from available knowledge, hedged by cautions against unrealistic aspirations for science within procedurally rational decision making.
Important though these techniques are, they cannot reveal how and why uncertainties come to be embedded in the policy and practice of containment, and indeed, what role institutional arrangements for animal disease governance may play in perpetrating them. An understanding of these issues requires a much broader treatment of the priorities and functions of containment systems and how scientific and other forms of knowledge are viewed, interpreted and deployed in relation to them. This paper provides a framework for such an approach. It examines how and why uncertainties emerge in the arenas of disease prevention, surveillance and control, and examines their strategic, tactical and operational expressions.
The origins of this paper are in interdisciplinary research. Its insights arise from an initial analysis of expert interviews, policy documentation and scientific evidence from a 3 year study of uncertainties in animal disease containment, undertaken by a research team of veterinary scientists, sociologists, biologists, geographers and political scientists. The general framework we develop emerged from a process of group discussion and learning between researchers working from different theoretical and empirical starting points: examining the procedures and assumptions that guide recognition of uncertainty in natural scientific terms and assessing the institutional context and circumstances in which knowledge is created and deployed for particular containment ends. The framework is not designed to encompass all aspects of uncertainty analysis in disease containment, but rather to function as a heuristic for thinking about uncertainty in an integrated and cross-disciplinary way.
The framework is illustrated primarily by reference to three animal diseases: cryptosporidiosis, foot and mouth disease (FMD) and avian influenza (AI). Each exemplifies different epidemiological characteristics in a UK context: cryptosporidiosis is endemic and zoonotic; FMD is exotic, notifiable and non-zoonotic; AI is notifiable, exotic, newly emerging and potentially zoonotic. Each differs markedly from the others in terms of pathogenicity, rates of evolution and transmission routes. The governance arrangements for each are distinct. However, in this paper, we aim to develop a framework designed to identify generic—cross disease—parameters for the analysis of uncertainty in containment practice.
The paper begins by presenting a general conceptualisation of strategies of containment and their associated uncertainties. An overview of the key theoretical terms related to uncertainty analysis is then provided, drawing on examples from each of the diseases. Using this framework a detailed analysis of some the uncertainties associated with strategies of containment is developed and illustrated in the context of three key arenas of practice: prevention, anticipation and alleviation. The paper concludes by highlighting practical learning responses from this analysis for policy development and the related role of interdisciplinary research.
2. Strategies for containing animal disease: general conceptualization
In this paper, containment is interpreted broadly. It is taken to encompass the whole cycle of disease containment, from issues of prevention and surveillance to those of recovery and control. Alongside issues of disease morbidity and mortality in non-human populations, containment is also understood to incorporate the wider zoonotic and non-zoonotic burdens of animal disease, including human livelihoods, health and well-being, and more generally, political and institutional capabilities and reputations. In particular, our conceptualization encompasses three key arenas of action:
— Prevention, or reducing the occurrence of animal disease. The focus here is on taking pre-emptive forms of action that reduce the chances of a disease outbreak, such as regulating zoosanitary practices on farms, investing in new technical infrastructures to limit disease transmission within livestock populations or changing livestock management practices.
— Anticipation, or acknowledging a potential animal disease threat and predicting and preparing for disease outbreaks. This arena of practice includes building capacities to identify failures of prevention through earliest possible disease surveillance. It also encompasses experimental modelling of disease scenarios and the design and testing of contingency planning arrangements.
— Alleviation, or the process of responding to disease occurrence. The focus here is on the procedures adopted to control and eradicate disease in real-world circumstances. This includes associated technical functions such as modelling and projecting outbreak behaviour and restricting the wider burdens and legacies of disease, including managing the long-term repercussions of outbreaks for affected individuals and communities.
Furthermore, our conceptualization is designed to recognize that each of these strategies has different forms of expression according to the level of policy practice. In particular, we distinguish between:
— The strategic level, structures and processes that directly or indirectly shape underpinning principles of containment. This can include policy activities and networks with formal responsibilities to produce these strategies, as well as the wider political, economic, regulatory arrangements prescribing the scope, ambition and remit of containment practice. The use of legislation to mandate stakeholders to act on disease risks, such as the continuous sampling of oocysts in the UK under the 1999 Cryptosporidium Regulations, or to extend state powers to act on disease, such as the preventive and control powers under the UK's Avian Influenza Order 2006, would be examples of high-level strategic processes.
— The tactical level, where strategic level goals are translated into practical rules, procedures and tools for decision making. Tactical level activities are essentially a context in which underpinning rationales for containment are given procedural expression. For instance, making decisions regarding how water should in practice be monitored, such as the design of sampling arrangements, or use of particular types of technical instrument, are examples of tactical processes. Another is the development of criteria for intervening in AI disease outbreaks, such as the creation of surveillance protection zones, or the design of preventive measures, such as the compulsory registration of poultry owners.
— The operational level, practical contexts of disease containment, in all their variety. Operational level activities are variegated systems of technological and human practice. In principle, they should be the outcomes/repercussions of strategic decisions for containment and the practical expression of tactics. Examples of operational practices include activities in diagnostic laboratories, the process of vaccinating birds or livestock, the implementation of biosecurity measures at livestock markets or the technical process of providing and handling water samples.
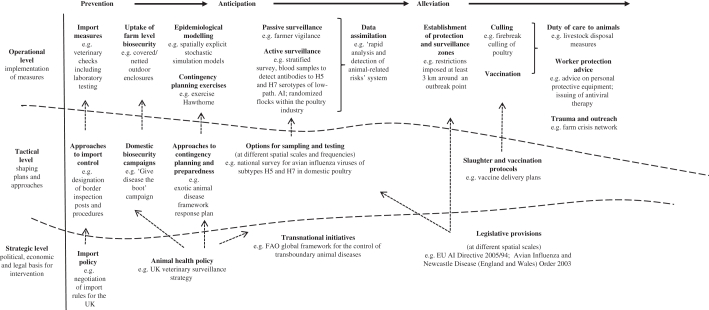
The generalized nature of this conceptualization should be emphasized. Making the analytical distinction between ‘arenas’ and ‘levels’, for instance, is likely to be readily identifiable to policy and decision makers, and indeed, is sufficiently generic to be relevant to both different categories of animal disease, for instance endemic and exotic, and different spatial and temporal scales of containment, such as a localized outbreak of cryptosporidiosis or a national outbreak of FMD. A visualization of these dimensions of containment, and how they interact, is provided in figure 1, taking the example of AI.
Figure 1.
Example application of conceptual framework: containment of avian influenza in the UK.
It is by following the interactions between these arenas and levels that many of the uncertainties associated with strategies of containment can be identified and accounted for. First, uncertainty may be situated within a particular level/arena. For example, at the operational level of anticipation, veterinary practitioners may fail to recognize clinical signs in animals affected by FMD. Second, uncertainties may emerge as we move between different levels of policy practice. For example tactics may be ignored, circumnavigated or misunderstood at the operational level, such as moving animals when restrictions are in place. Third, uncertainties may emerge as we move between different arenas of the containment cycle, such as uncertainties of alleviation being amplified because of delays in disease notification; that is, because of failures of anticipation.
In the following sections of the paper, we provide a non-exhaustive treatment of these dimensions of uncertainty. To begin approaching this task, we provide an overview of the different ways uncertainty can be interpreted, drawing on simple illustrations from each of the case study diseases.
3. Uncertainty: general theoretical propositions
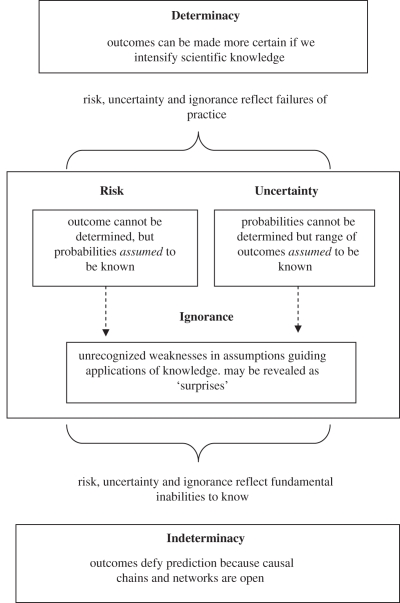
A range of taxonomies and accounts of uncertainty have emerged within the scientific and social scientific literature [1,3–5] (figure 2). A common theoretical proposition of this work is that uncertainties can be distinguished according to the degree that reasoning about a given problem or issue departs from a de facto scientific ideal of determinate (i.e. certain) knowledge. Thus, in the context of infectious disease, we know in the most general sense that agents including viruses, bacteria and parasites cause disease when they come into contact with a suitable host; but it is not certain that they will cause disease in every case. This may be due to a range of factors characteristic of both the host and the pathogen, such as natural or acquired immunity, genetic variability and so on.
Figure 2.
General theoretical conceptualization of uncertainty.
An important distinction within uncertainty analysis concerns whether an uncertainty can be expressed in probabilistic terms, that is, where frequency distributions can be inferred for a known set of outcomes. Probabilistic uncertainty is sometimes referred to as ‘statistical uncertainty’ or ‘weak uncertainty’, but most commonly, ‘risk’. There are numerous examples in disease of factors that lend themselves to some form of probabilistic treatment. So, for example, in the case of Cryptosporidium, it is possible to calculate a theoretical risk of exposure posed by drinking a glass of contaminated water, provided we know basic parameters such as how many oocysts per litre are present in the water supplied, their viability and the volume of water in the glass.
This type of uncertainty can be contrasted with situations in which a range of possible outcomes are known, but probabilities are not. Here, decision making proceeds on the basis of broader approximations and best guesses. This latter type of uncertainty is sometimes referred to as ‘strong uncertainty’, ‘scenario uncertainty’, but most commonly, simply ‘uncertainty’. For instance, during an outbreak of FMD, policy makers may reasonably ask: ‘how long will this disease outbreak last?’; or in the case of an outbreak of AI, ‘what is the risk of the emergence of zoonotic genotypes?’ Researchers may not be able to respond to these questions in probabilistic terms, but experience may grant them some understanding or ‘feel’ for the types of outcomes more or less likely to occur.
Importantly, both ‘strong’ and ‘weak’ uncertainty may be driven by assumptions that may be exposed as fallible by way of surprising and unanticipated results. In other words, there may be unrecognized shortcomings in the capacity of available knowledge to identify outcomes, or describe systems effectively, regardless of whether they can be expressed probabilistically. This form of unrecognized uncertainty is commonly termed ‘ignorance’. So for example when the first cases of bovine spongiform encephalopathy (BSE) in UK cattle arose, former unquestioned assumptions were broken by the emergence of a new paradigm by which an infectious disease could be spread in the food chain independently of viruses, bacteria or parasites. Only when the role of prion disease became better understood was it possible to re-engage probabilistic assessments in the building of animal and public health policies with respect to transmissible spongiform encephalopathies.
Risk, uncertainty and ignorance may elicit two types of reactions/responses. First, they may be thought to reflect practical failures in the way information is acquired (such as measurement uncertainty, owing to sampling errors, inaccuracy or imprecision). These are often collectively referred to as epistemic or reducible uncertainties; the assumption being that, by overcoming shortcomings in techniques and methods, risk will be better represented and controlled, uncertainty will narrow and ignorance diminish (i.e. systems will become more determinate). For example, an epistemic practice in disease containment would be to improve methods of surveillance, such as endeavouring to reduce human errors in oocyst identification in water treatment works as the basis for improving detection rates for Cryptosporidium. Another would be improving calibration methodologies within epidemiological modelling to validate further the trajectories of hypothetical FMD and AI outbreaks. Second, these risks, uncertainties and ignorances may be assumed to be the product of systems that exceed scientific capacities to rationalize them. These are often collectively referred to as ontological or irreducible uncertainties. The dynamics of weather patterns and their influence on airborne transmissions of FMD would be an example of indeterminacy. Indeterminacy emphasizes that causal chains and networks of complex social and technological systems, such as disease containment, are often open, emergent and highly context specific, and therefore persistently defy prediction and control (i.e. systems are indeterminate). In practical terms, it is an idea closely associated with the arguments for adaptive management; that is, approaches to disease control that are responsive to local contingencies and changing conditions.
Both ontological uncertainty and epistemic uncertainty have an ethical dimension as well [6], opening up science and policy to deeper philosophical uncertainties of principle and conduct. Ontological uncertainty raises the questions: what do we seek to achieve and why? For instance, what priorities should dictate the policy significance of disease, and accompanying commitments of resource to containment systems? On what basis do we assign relative significance to AI, FMD and cryptosporidiosis, and more broadly, to biological risks over other potential sources of harm: radiological, chemical and so forth? The answers to these questions are less than clear cut. Epistemic uncertainty, in turn, raises further questions: in particular, how do we arbitrate on the fairness of a potential intervention when faced with contingent knowledge, scientific or otherwise, and a range of known and unknown outcomes, and where it is inevitable that there will be both winners and losers?
Often these questions are interpreted through technocratic processes, such as policy appraisal and regulatory impact assessment in government, where the costs and benefits of action are assessed. A useful example of this type of approach to reasoning would be the use of numerical scoring and weighting procedures to rank diseases against different criteria of significance [7] and thereby establish priorities for resource allocation. In the UK, for instance, this approach is used by the responsible government department as part of its disease prioritization. It has assessed diseases on the basis of 39 different criteria, each assigned varying importance within the overall scoring scheme, and spanning such epidemiological, economic and institutional questions as public health and animal welfare, consequences for industry and economy, the scale of government effort involved, as well as legal obligations and ramifications [8].
At the practical level of assessment, these methodologies are uncertain because they typically produce judgements of overall disease importance by blending together available scientific evidence with surrogate—expert-informed—datasets. The latter are employed in (the many) situations where scientific understanding is weak, or indeed, entirely absent. As Krause [9] shows in an overview of approaches taken in different national settings, elicitation involves methodologies for collecting ‘opinion’—usually by way of survey and group techniques. However, the general point is that the composite and numerical nature of the scoring process creates an illusion of confidence about priorities where irreducible uncertainties and contingencies may actually be in play. This applies even where judgements appear to be based on competent scientific knowledge, since any given criterion in the prioritization process is itself open to different types of interpretation. Take for instance, the criterion of ‘severity’ as a marker of significance, and consider this in relation to the diarrhoeal disease of cryptosporidiosis. As one interviewee in our research suggested, official medical literature persistently characterizes this as a ‘mild self-limiting illness’, but: ‘if you spoke to someone who had had clinical cryptosporidiosis …[ ]… and said “you have got a mild illness”, they would slap you because people can get very poorly’. In other words, these approaches are based on a pragmatic calculus that often belies the deeper ethical complexity of policy choices.
4. Uncertainties of prevention
In this section, we consider how uncertainties are embedded in the strategic, tactical and operational dimensions of prevention. By definition, preventive measures extend patterns of innovation and action in disease containment beyond that of preparedness and control. At an operational level, these measures may be applied at a variety of spatial scales, such as promoting zoosanitary practices on farms to mitigate the emergence of AI or FMD or instituting barrier controls, such as import control measures, at the national level. Part of the strategic reasoning behind the use of preventive measures is that they reduce resource burdens felt elsewhere in the containment cycle. In many, but by no means all cases, costs associated with alleviation and recovery will be orders of magnitude higher than investments in preventive measures. Relatedly, because prevention extends patterns of knowledge acquisition, innovation and action beyond the issue of post-outbreak measures alone, new communities of interest in animal disease containment may be revealed, and overall costs and responsibilities of containment therefore further diffused.
While commitments to prevention are a logical aspiration for policy and decision makers, it does not follow that prevention ensures that containment systems are more resilient to disease outbreaks. The reverse may actually be the case if the preventive tactics employed are too simplistic, such as those that apply measures at a single operational level (for instance, import controls only) or those undertaken to the neglect of developing other facets of the containment system (such as the preparation of contingency plans).
The recent emergence of biosecurity as a strategic and organizing agenda to prevent the introduction and spread of disease agents is a good example of an unfolding interest in animal disease prevention, one that has found currency at international [10] and national levels [11]. This has resulted in a range of tactical measures—mandatory and voluntary—to cultivate preventive attitudes, behaviours and responsibilities at the operational level. In the UK, for instance, biosecurity has been promoted as matter of routine good practice on farms by government through a recent ‘Give disease the boot’ campaign and accompanying advice networks [12]. It has also been instituted into Farm Health Planning in the UK, a set of tactical initiatives designed to promote and foster good practice in managing livestock health and welfare risks.
Preventive measures create new possibilities for scientific innovation and research within containment, but science often struggles to determine measurable operational ‘outcomes’ as increasingly demanded by audit cultures in policy. Prevention can therefore remain elusive to objective standard-setting, but these uncertainties are embedded into containment practice because often they arise out of political, rather than scientific, forms of calculation. As Donaldson [13] explains, in the case of the UK, biosecurity emerged as a public policy term at the height of the 2001 FMD outbreak and as a way of explaining practical measures that could be deployed to alleviate disease spread at the farm level. It was invented to govern and describe appropriate operational conduct, and failings therein, during crisis. Part of his argument is that biosecurity has had to be placed on a scientific footing post hoc.
A useful example of the type of problems scientists face in measuring and thereby rendering accountable the efficacy of preventive processes is provided in Cryptosporidium management. Here, a range of upstream land, manure and livestock management options, implying varying degrees of capital investment, income foregone, and of practical competence on the part of land managers, are emerging as a means of minimizing potential outbreaks of cryptosporidiosis among water consumers [14]. This may include employing biosecurity practices on farms (such as cleaning and disinfection) as well as undertaking more fundamental changes to the farm system, such as investing in new technical infrastructures to limit disease transmission within livestock populations, and changing patterns of livestock management.
There is a good understanding of the biological variables controlling these systems, but not how they interact especially with human social variables to allow reliable assessment, with probabilistic confidence, of the relationship between measures and risks in any given practical context [15]. Source-tracking technologies, for instance, are rarely employed in real-world settings as an active preventive practice, and are operationally difficult even in experimental terms [16]. Moreover, this science is being applied further away from measurable human health outcomes. While measures to prevent cryptosporidiosis in livestock may well reduce incidences of cryptosporidiosis in humans, intervening variables and steps in the containment process (for instance, raw water treatment) make these relationships impossible to align, and account for, precisely.
In any case, scientific uncertainty in measuring the efficacy of these practical initiatives is itself embedded in a less-than-perfect world of operational practice. For example, the practical viability of any given measure depends on individuals possessing and deploying skills in ways concurrent with an effective scientific measure, but this cannot be assured. Informal and self-organizing networks of knowledge exchange, for instance among farmers [17], is one way of ameliorating these problems operationally. Indeed, the practical cost of systematically enforcing measures, or simply providing advice, involves the law of diminishing returns: required efforts may simply outweigh the level of perceived risk.
5. Uncertainties of anticipation
Anticipatory containment is about recognizing and planning for potential disease outbreaks. Its broad purpose is to cultivate systems that can respond to disease threats in a timely fashion.
A significant aspect of anticipation is the development of disease-monitoring systems that embed localized surveillance into national, and ultimately international, assessments of disease emergence. However, comparative national standards for surveillance are highly varied, from real-time disease control and prevention to cumbersome manual procedures with a high capacity for human error [18]. Even within well-resourced systems, information of use to surveillance may not be integrated effectively at the tactical and operational levels. For example, a recent account of the UK veterinary surveillance strategy by Lowe [19] has pointed to national level surveillance of animal disease risks as disinclined to incorporate data from ‘on-the-ground’ clinical observations, such as from veterinarians and industry stakeholders, relying instead on laboratory testing and reporting arrangements.
Furthermore, because surveillance data often pass through a range of monitoring and reporting stages, patterns of disease emergence may be underestimated. Take, for example, reporting procedures surrounding cryptosporidiosis. In the UK, reporting of this disease depends on information transmission through a complex architecture of self-reporting, stool sampling, laboratory testing and notification. Infections may remain undiagnosed because individuals choose not to report symptoms. In turn, a general practitioner in the UK may not choose to take faecal samples, so nothing would be reported to laboratory surveillance. Not all hospital laboratories will test for Cryptosporidium, and where they do, there is no guarantee that all samples will be tested. Moreover, methods of detection typically involve the staining and microscopic examination of faecal specimens, but it has been suggested that this fails to detect about half of all Cryptosporidium infections [20].
In this particular case, general technological innovations in containment practice could change case ascertainments significantly. For example, molecular techniques in laboratory testing would increase reported rates of infection, while technological innovations in water monitoring, such as the use of real-time polymerase chain reaction amplification of DNA, could improve rates of detection, thereby enhancing overall system preparedness. Yet, not only would such innovations imply commitments of resource, they depend on readiness for uptake. For Cryptosporidium, though, it is questionable whether water industries would readily innovate in systems that are producing low detection rates in treated water.
An important further point of note about anticipation is that surveillance systems inevitably reflect a restricted body of knowledge on disease behaviour and therefore may be fundamentally ignorant of emergent risks. This may be unimportant where the risks are relatively low. For instance, in 2008, a previously unrecognized Cryptosporidium rabbit genotype was linked to an outbreak of cryptosporidiosis in Northamptonshire, UK, and this has been incorporated into existing containment practices as a potential low-level, background threat [21]. In other instances, the consequences of ignorance can be more paradigmatic. The recent emergence of influenza A (H1N1) virus—‘swine flu’—is a good example, as was the emergence of the neurodegenerative disease BSE in cattle in the 1980s and the variant Creutzfeldt–Jakob disease in humans. In these circumstances of revealed ignorance and surprise, institutional ability to recognize and adapt becomes paramount as the new disease scenario unfolds. For instance, the emergence of H1N1 brings the potential for recombination with highly pathogenic AI (H5N1) and the development of new, and more virulent, strains with a wider host range. New—often hybrid—platforms for anticipatory research therefore begin to emerge as ex ante priorities are reassessed.
While strategic level prioritization regularly seeks to update priorities (i.e. tactics) on the basis of research and intelligence precisely to avoid this ignorance of system change within anticipatory arenas, the point still holds that emergent conditions for disease can pass entirely unrecognized until they have gone past tipping points. This is as true for ostensibly ‘known’ diseases as it is for system ‘surprises’. Take, for instance, changing attitudes to FMD in the UK. It is now widely recognized that the intensification and concentration of market systems for livestock in the years preceding the 2001 outbreak had not only exposed the UK system to greater unnoticed risk of disease emergence, but by orders of magnitude higher than for previous crises. However, these systemic and gradual developments took place during an extended ‘disease-free’ period in the UK. Not only did the visibility of the disease within political, public and expert discourses tacitly wane [22], but institutions and society ‘forgot’ the necessary skills and capacities needed to identify and cope with a future outbreak [23].
In principle, randomized surveillance would be one means by which system ignorance may be engaged with proactively, and at the same time, allow the prevalence of known disease threats to be rechecked. However, what constitutes effective randomized surveillance in biological terms is itself unclear, and may in any case be implausible on the wider grounds of proportionality, not least the costs entailed.
To some extent, uncertainties in disease identification may be anticipated through systems of quality audit and control. In these cases, anticipation is more effective because the system is already predicated on uncertainty. Technological systems for surveillance and the sampling methodologies that accompany them may, for example, observe risks at one step removed from disease occurrence, such as through the use of ‘indicator organisms’ to demonstrate potential pathogenic presences in waterborne disease. For example, faecal indicator organisms (FIOs) such as generic Escherichia coli are used in water quality assessment as an ‘indicator’ of the ingress of sewage/wastewater into drinking water. But FIOs are not themselves a significant health hazard; rather they suggest the potential presence of pathogenic micro-organisms (whether bacterial, protozoan or viral). Debates consequently prevail on the validity of some FIOs as surrogates for bacterial, protozoan and viral pathogens [24], but many regulatory bodies across the world currently use these indicators to monitor microbial water quality.
Even if we were to accept that what is being monitored may lead to disease, acting on this information to protect human health may be impeded by the context in which information is interpreted. Consider again Cryptosporidium in the UK. Until recently, it was the privatized water industry working in the context of water quality standards (i.e. arenas of prevention and anticipation), rather than public health outcomes (i.e. arenas of alleviation and recovery) that defined priorities for a significant area of the containment system. Yet, although water quality standards may be used as analogues for public health outcomes, they are not substitutes. For example, before 2008, water industry regulations stipulated that, on average, no more than one oocyst per 10 l of water sampled was allowable in treated water. However, under this system, oocysts could be detected above the threshold but not necessarily result in a disease outbreak; just as detection below this threshold could lead to disease despite being permissible. Partly for this reason, these Cryptosporidium monitoring requirements were revoked, requiring the water industry to create ‘water safety plans’ in which comprehensive risk assessments are undertaken, thereby aligning priorities more directly to health outcomes.
Anticipation and preparedness also encompass basic and applied scientific research into disease behaviour under future outbreak scenarios. In this vein, there is an emerging tradition of tactical research simulating animal diseases using modelling techniques, such as examining the propagation of H5N1 within the British poultry industry [25–27] and FMD in the livestock sector [28,29]. These approaches provide contexts in which policy options for disease control and risk assessment can be built into system preparedness, though from experience they are often poor decision-making tools for disease alleviation (see below). Perhaps not surprisingly, the specification and parametrization of these models, and their accompanying evidence base, have often been identified as highly uncertain [30,31]. Parameter estimation, an essential part of model development, is frequently based on data considered to be comparable to the system under study, while clearly being different. For example, because of the paucity of data specific for H5N1 transmission in the UK, models have relied upon extrapolation from other infectious agents, including those as different as bacteria [25]. Further, these models attempt to provide detailed representations of the potential transmission contacts between explicitly located populations of poultry (e.g. farms). However, such models are, by definition, incomplete and highly simplified system representations, and the requisite parameter values and data are uncertain.
An important related element of model building is therefore model evaluation, and in particular, the use of sensitivity analysis (where parameter values are varied across what is considered to be a plausible range). This can be used to explore ‘parameter space’ i.e. plausible values given current knowledge. However, what is less commonly acknowledged is the impact of model specification itself, including the interpretation of model sensitivity analysis. Hence, if a model is insensitive to variation in a particular parameter, it may be assumed that more detailed knowledge of this parameter is not needed; but it is not usually acknowledged that this inference relates only to the model in question, rather than some fundamental ‘reality’. Thus, there are important domains of usually unstated scientific ignorance that underlie policy and may compromise its robustness. An important point here is that decisions regarding model specification themselves inevitably reflect a restricted body of knowledge and represent judgements on what aspects should be included or excluded.
Relatedly, the research underpinning this paper is revealing the dilemmas faced by scientists when putting models into the policy domain of animal disease. As one puts it, there is a prevailing perception among policy makers that models are, ‘some kind of forecast …[like]…weather forecasts…[and]… econom[ic] forecasts…[ ]…and somehow that they're including everything’. And yet, as this respondent puts it, for a scientist working in these applied worlds of animal disease policy:
‘it's very dangerous to say you don't believe this model before you start. It's quite a hard trick to pull off to convince the policymaker that the model has value and should be believed and they should base their policy on it, and at the same time explain that actually the model, it's not true, is wrong.’
Modellers working on animal disease were, it was suggested, ‘engineers’ of the model—and thereby attentive to model faults—but simultaneously ‘salespeople’ wanting their work to assert influence on the consumer (i.e. policy users). It is hardly a novel observation within academic discourse to suggest that the value of model building lies as much in its capacity to assist ‘learning about’ uncertainty as it does to serve the creation of ‘predictive truth machines’ [32]. However, it was argued that the placement of highly experimental epidemiological modelling research within policy development often presumes the latter. As it was put by one respondent in our study ‘as far as [policy customers] are concerned you know, these models are reality in a computer [of] which you turn the handle and it tells you what's going to happen’.
6. Uncertainties of alleviation
A key uncertainty governing alleviation processes surrounds issues of purpose: what is it we seek to achieve and why? This is not only a matter for policy commitment, but also affects related knowledge development and selection. The answer to these questions is often ambiguous. In a purely epidemiological sense, aspirations for containment may be expressed around commitments to reduce or eradicate disease. Yet, this concern invariably accompanies a range of other needs: for minimal duration; to restrict burdens on industries and communities; to maintain trust in institutions; to minimize over-reaction; to be cost efficient; to share responsibility; to be humane; and so forth. In other words, disease containment is not reducible to a single notion of purpose or effective outcome, nor by implication to a single criterion against which uncertainties may be judged.
At the highest levels of strategic political discourse, this tension is in play. For instance the UK government plan for managing exotic animal disease [33] describes, with great procedural exactness, the strategic, tactical and operational roles of organizations and individuals during outbreak scenarios. But it contains inherent conflicts within it regarding what the containment process is trying to achieve. It suggests in one respect that, ‘[t]he Government's first objective in tackling outbreaks …[ ]… is to restore the UK's disease free status as quickly as possible’. Yet, it also states that it intends to ‘[c]ause the least possible disruption to the food, farming and tourism industries, to visitors to the countryside, and to rural communities in the wider economy’. These wider aims are, of course, in direct conflict to achieving the first objective, but this conflict is neither resolved, nor even substantially addressed, in the document. The implication is that these issues need to be—indeed, can only be—resolved in the specific context of a disease outbreak.
The dimensions of this issue are different in the context of cryptosporidiosis; in this case, alleviation of significant outbreak incidents is centred on the use of ‘boil-water’ notices. Here, the priorities are to an extent clearer—the delivery of public health outcomes is overriding and paramount. However, sustained boil-water notices are costly for industry and commerce and potentially damaging to consumer confidence in water supplies. A further complication is that the public health outcomes of alleviation are by no means clear. Not only does this containment strategy generate anxiety among publics, but it also has a further unintended health outcome, in that it increases the number of reported scalding incidents [34]. Thus, there are potentially competing public health priorities.
Alongside these uncertainties of purpose, it is the tactical and operational dimensions of science that are also significant to the propagation of uncertainty within alleviation practice. In the context of AI and FMD, the use of modelling is again particularly important, with the predictive weakness of models tending to be exposed during crises. The use of ‘real-time’ modelling to inform and guide FMD disease control in the 2001 UK disease outbreak was a watershed in this respect [35]. Using either deterministic or stochastic techniques, the models sought to build computer micro-simulations of the disease that could explain how it might transmit and progress through farms in space and time [28,36]. It is in the relationship between the tactical and operational level of these modelling practices that uncertainties are exposed. For instance, in the course of our research, it has been argued by some scientists involved in the 2001 crisis that basic information on the transmission characteristics of the disease was limited. In this instance, expert opinion was used to inform the initial parametrization of models:
[the]… data or knowledge out there was qualitative rather than quantitative and so what we tended to find was that a relatively small group of scientists worldwide had been working on FMD. They were the experts called upon historically to advise and control [of] the epidemic and they sort of had a kind of gut feeling of this; how it behaved and a lot of it wasn't really quantified in any, sort of, rigorous way.
The wider literature on this crisis has noted that at the operational level, detailed and accurate data on the spatial distribution of farms and livestock were not available to modellers. Moreover, all of the models avoided the use of important, if indeterminate, environmental variables relating to transmission by air, such as weather and topography. Models were highly insensitive to the great variability in the susceptibility of farms to infection, not least in the context of the infectiousness of different livestock. Little credence was given to the behaviour of farmers in adopting biosecurity measures. Together with the imposition of the 3 km/24–48 h culling policy, these operational deficiencies in modelling—much more than the perceived problem of being unable to quantify qualitatively known processes, indeed almost the opposite of this, a masking of ignorance by excessive quantification—led to a process described as ‘post-code slaughter’ or ‘carnage by computer’ [37]. As a result, the need has been emphasized to build governance structures that can enhance the efficacy and empirical realism of these scientific modelling practices for future outbreaks, and for more locally adapted determinations of slaughter tactics otherwise defined nationally and in the abstract.
Sociological evidence from the 2001 FMD crisis has provided analytical and qualitative insights into repercussions of the operational dimensions of the outbreak [38,39], and in particular how strategic approaches to alleviation, wedded to epidemiological models, ‘lacked common sense and alienated and marginalized local knowledge’ [40]. Local knowledge in this sense means bodies of expertise tied to the experience of disease in particular places and locales. It encompasses professional specialists occupying roles in the public, private and third sectors (such as veterinarians, mental health workers, teachers) but also non-professionalized (lay) forms of expertise (such as the practical ‘know-how’ of farmers and land managers). The proposition is that harnessing local understandings of the operational practice of animal disease alleviation may expose higher level weakness in containment practices, such as those embedded in necessarily more synthetic scientific models often reflect. Such knowledge is inevitably bounded by the particular circumstances of its production. It does not travel with great efficiency and often arrives in messy and unstructured forms. Yet, it is precisely because local knowledge is so ‘situated’ that it is authoritative at the point of outbreak. Strategic responsiveness to salient local knowledge is therefore important, though the emergent nature of this knowledge may mean it arrives too late to ameliorate weaknesses in tactics and strategy. There may be also fundamental mismatches between local and global understandings of an appropriate intervention, as Woolhouse [41] notes with regard to optimal culling rates in the 2001 FMD outbreak. A more practically effective overall containment system has to find ways of combining these different kinds and sources of knowledge and authority about animal disease. The testing of contingency arrangements in anticipatory arenas is one context for this. In the UK, for instance, simulated live exercises that rehearse the strategic, tactical and operational dimensions of alleviating outbreaks of exotic disease across distributed set of stakeholders are now being periodically conducted [42]. However, a more general precondition for this constructive reconciliation of divergent expertise is prior recognition of these multivalent conditions of uncertainty in prevailing policy-authoritative scientific knowledge.
7. Conclusions
History offers plenty of high-profile lessons where, during moments of crisis, the procedural rationality of decision making has been exposed as inadequate, and sometimes chronically unable to manage and mitigate disease occurrence in socially acceptable ways. The language of public ‘fear’, ‘dread’ and ‘panic’, which increasingly accompanies emergency situations in a range of fields, including animal diseases, signals a deeper sense of anxiety surrounding political and institutional capacities to cope, and thus by implication also, surrounding scientific advisory capacity to provide sound, practically attuned knowledge. Even where public anxieties are unfounded, there is a sense during outbreak situations that the governance of disease containment stands perpetually on the brink of running, quite literally, ‘out of control’—and not only biologically.
As Pretty [43] asks in the context of animal disease, ‘within scientific disciplines, uncertainty is an accepted norm …[·]… Yet how does this dynamic translate as the evidence rock is pushed towards the Sisyphean policy summit, or across to the public and media? Do policy makers and the public want evidence couched with uncertainties and probabilities? Or do they want simple answers?’ This paper has sought to develop a framework for thinking constructively and critically about the way these uncertainties can be handled for animal disease containment.
One plausible response to uncertainty is to redouble commitments to resolving inadequacies in relevant knowledge. This may mean enhancing precautionary measures within containment: for instance, investing in technical monitoring instruments of new filtration plants for public water supplies to anticipate better the occurrence of zoonotic waterborne pathogens such as Cryptosporidium, or to pre-empt ignorance and lack of downstream control by imposing more restrictive measures on animal movements and traceability to reduce the probability of the spread of FMD. Alternatively, it may mean experimenting in potentially paradigm-shifting innovations, such as through the introduction of novel technologies like faster and more precise diagnostics, new vaccines or improved epidemiological modelling.
Important though these efforts are, a key facet must also be ‘learning to live with’ systems that remain open (in relation to inadequate knowledge of them, or intrinsically) and contingent in character, that is, systems with a great capacity for unanticipated consequences and surprises. In the development of animal disease containment strategies, there is a need to recognize the essential creativity of complex hybrid behavioural systems in evading prediction and control. Two implications of this are: a need for appropriately distributed, as distinct from concentrated knowledge, agency and responsibility; and a readiness to acknowledge the essential contingency of any expert knowledge, so that it can be open to supplementary knowledge from other salient quarters. Neither of these is easily accommodated in typical institutional settings of science and policy, in any domain [44].
Acknowledging that there are by-definition incalculable ignorances and indeterminacies that affect applications of scientific knowledge in disease containment, and building this into policy process design, does not mean abandoning policy to ignorance. It does, however, mean opening all such bodies of expert knowledge to question as to often-hidden and taken-for-granted framing premises, which other bodies of knowledge, including non-scientific specialist (such as livestock experts—known as farmers), or social scientific research knowledge of relevant practices, may then be able to improve, or even correct.
Enabling policy actors to be alert to, and reflexive about, these inevitable shortcomings demands cross-disciplinary work at the interfaces of different disciplinary and policy discourse practice, and across different arenas of containment. The framework developed here is designed to enable this process: the basis for more formalized and robust analysis of where uncertainty may exist and hence assist in highlighting areas where greater, cross-disciplinary effort might actually lead to a better containment policy. To what extent current framings of natural and social scientific knowledge in disease containment policy can accommodate this is debatable. The use of different types of natural and social science within strategies of containment is highly asymmetric, with disease modelling and economics arguably providing dominant policy framings of each, respectively. Moreover, the relationship between natural and social science is also asymmetric, such that the former do not simply inform policy, but far more significantly, in effect end up by default defining the policy issues. For example, scientific models, taken to be representations of only natural systems, reflect tacit commitments to what factors are taken as beyond policy influence, and what are feasible (or desirable) points of policy intervention [45]. The associated tendency for policy to elaborate technical instruments instead of considering appropriate institutional changes that could help cultivate a policy culture more open to contingencies and lack-of-(predictive)-control has been discussed for risk management contexts, by Wynne [1]. In the UK, dimensions of how government may enable this culture through broader and deeper platforms of social research are now emerging [46].
Recognizing and working effectively with these different qualities of calculable and incalculable uncertainties in animal disease containment thus depend on rethinking some central assumptions about the role of natural and social sciences in real-world policy design. This is not only a question of cross-disciplinary relations, but crucially, of the readiness of policy cultures to develop and enact new interdisciplinary understandings of the roles of knowledge, uncertainty and inherent limits of intellectual control, in realistic and credible policy practice.
Acknowledgements
The insights informing this paper arise from funding under the UK research councils' Rural Economy and Land Use (RELU) programme (Project Code: RES-229-25-0015). RELU is funded jointly by the Economic and Social Research Council, the Biotechnology and Biological Sciences Research Council and the Natural Environment Research Council, with additional funding from the Department for Environment, Food and Rural Affairs and the Scottish Government. This project received additional support from Defra. The programme's support is gratefully acknowledged.
Footnotes
One contribution of 11 to a Theme Issue ‘Interdisciplinary perspectives on the management of infectious animal and plant diseases'.
References
- 1.Wynne B. 1992. Uncertainty and environmental learning: reconceiving science and policy in the preventive paradigm. Global Environ. Change 2, 111–127 10.1016/0959-3780(92)90017-2 (doi:10.1016/0959-3780(92)90017-2) [DOI] [Google Scholar]
- 2.Harris G. 2008. Seeking sustainability in an age of complexity. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Zadeh L. 2005. Towards a generalised theory of uncertainty—an outline. Inform. Sci. 172, 1–40 10.1016/j.ins.2005.01.017 (doi:10.1016/j.ins.2005.01.017) [DOI] [Google Scholar]
- 4.Walker W. E., Harremoes P., Rotmans J., van der Sluijs J. P., van Asselt M. B. A., Janssen P., Krayer von Krauss M. P. 2003. Defining uncertainty: a conceptual basis for uncertainty management in model-based decision support. Integr. Assess. 4, 5–17 10.1076/iaij.4.1.5.16466 (doi:10.1076/iaij.4.1.5.16466) [DOI] [Google Scholar]
- 5.Ascough J. C., II, Maier H. R., Ravalico J. K., Strudley M. W. 2008. Future research challenges for incorporation of uncertainty in environmental and ecological decision-making. Ecol. Model. 219, 383–399 10.1016/j.ecolmodel.2008.07.015 (doi:10.1016/j.ecolmodel.2008.07.015) [DOI] [Google Scholar]
- 6.Tannert C., Elvers H. D., Jandrig B. 2007. The ethics of uncertainty. EMBO Rep. 8, 892–896 10.1038/sj.embor.7401072 (doi:10.1038/sj.embor.7401072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause G. 2008. How can infectious diseases be prioritized in public health? A standardized prioritization scheme for discussion. EMBO Rep. 9, S22–S27 10.1038/embor.2008.76 (doi:10.1038/embor.2008.76) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defra 2010. Veterinary surveillance: prioritisation project. London, UK: Defra; See http://www.defra.gov.uk/foodfarm/farmanimal/diseases/vetsurveillance/strategy/programme/prioritisation.htm (last accessed 17 November 2010) [Google Scholar]
- 9.Krause G. 2008. Prioritisation of infectious diseases in public health. Eurosurveillance 13, 1–6 [DOI] [PubMed] [Google Scholar]
- 10.FAO 2008. Biosecurity for highly pathogenic avian influenza: issues and options. Rome, Italy: FAO [Google Scholar]
- 11.Defra 2008. Biosecurity guidance to prevent the spread of animal diseases. London, UK: Defra [Google Scholar]
- 12.Defra 2008. Give disease the boot. London, UK: Defra; See http://www.webarchive.nationalarchives.gov.uk/20080609145742/http://defra.gov.uk/animalh/diseases/default.htm (last accessed 17 November 2010) [Google Scholar]
- 13.Donaldson A. 2008. Biosecurity after event: risk politics and animal disease. Environ. Plann. A 40, 1552–1567 10.1068/a4056 (doi:10.1068/a4056) [DOI] [Google Scholar]
- 14.Chalmers R. M., Giles M. 2010. Zoonotic cryptosporidiosis in the UK—challenges for control. J. Appl. Microbiol. 109, 1487–1497 10.1111/j.1365-2672.2010.04764.x (doi:10.1111/j.1365-2672.2010.04764.x) [DOI] [PubMed] [Google Scholar]
- 15.Fish R. D., Winter M., Oliver D. M., Chadwick D. R., Selfa T. L., Heathwaite A. L., Hodgson C. J. 2009. Unruly pathogens: eliciting values for environmental risk in the context of heterogeneous expert knowledge. Environ. Sci. Pol. 12, 281–296 10.1016/j.envsci.2009.02.002 (doi:10.1016/j.envsci.2009.02.002) [DOI] [Google Scholar]
- 16.Stapleton C. M., Kay D., Wyer M. D., Davies C., Watkins J., Kay C., McDonald A. T., Porter J., Gawler A. 2009. Evaluating the operational utility of Bacteroidales quantitative PCR-based MST approach in determining the source of faecal indicator organisms at a UK bathing water. Wat. Res. 43, 4888–4899 10.1016/j.watres.2009.09.015 (doi:10.1016/j.watres.2009.09.015) [DOI] [PubMed] [Google Scholar]
- 17.Waterton C., Norton L., Morris J. 2006. Understanding Loweswater: interdisciplinary research in practice. J. Agric. Econ. 57, 277–293 10.1111/j.1477-9552.2006.00052.x (doi:10.1111/j.1477-9552.2006.00052.x) [DOI] [Google Scholar]
- 18.Butler D. 2006. Disease surveillance needs a revolution. Nature 440, 6–7 10.1038/440006a (doi:10.1038/440006a) [DOI] [PubMed] [Google Scholar]
- 19.Lowe P. 2009. Unlocking potential: a report on veterinary expertise in food animal production. London, UK: Defra [Google Scholar]
- 20.Nichols G., Chalmers R., Lake I., Sopwith W., Regan M., Hunter P., Grenfell P., Harrison F., Lane C. 2006. Cryptosporidiosis: a report on the surveillance and epidemiology of Cryptosporidium infection in England and Wales. London, UK: Drinking Water Directorate [Google Scholar]
- 21.Chalmers R., Robinson G., Elwin K., Hadfield S., Xiao L., Ryan U., Modha D., Mallaghan C. 2009. Cryptosporidium rabbit genotype, a newly identified human pathogen. Emerg. Infect. Dis. 15, 829–830 10.3201/eid1505.081419 (doi:10.3201/eid1505.081419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods A. 2004. A manufactured plague: the history of foot-and-mouth disease in Britain. London, UK: Earthscan [Google Scholar]
- 23.Doering M., Nerlich B. 2009. From mayhem to meaning: an introduction to the cultural meaning of the 2001 outbreak of foot and mouth disease in the UK. In The social and cultural impact of foot and mouth disease in the UK in 2001 (eds Doering M., Nerlich B.), pp. 3–18 Manchester, UK: Manchester University Press [Google Scholar]
- 24.Wilkes G., et al. 2009. Seasonal relationships among indicator bacteria, pathogenic bacteria, Cryptosporidium oocysts, Giardia cysts and hydrological indices for surface waters within an agricultural landscape. Wat. Res. 43, 2209–2223 10.1016/j.watres.2009.01.033 (doi:10.1016/j.watres.2009.01.033) [DOI] [PubMed] [Google Scholar]
- 25.Dent J. E., Kao R. R., Kiss I. Z., Hyder K., Arnold M. 2008. Contact structures in the poultry industry in Great Britain: exploring transmission routes for a potential avian influenza virus epidemic. Vet. Res. 4, 27. 10.1186/1746-6148-4-27 (doi:10.1186/1746-6148-4-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharkey K. J., Bowers R. G., Morgan K. L., Robinson S. E., Christley R. M. 2007. Epidemiological consequences of an incursion of highly pathogenic H5N1 avian influenza into the British poultry flock. Proc. R. Soc. B 275, 19–28 10.1098/rspb.2007.1100 (doi:10.1098/rspb.2007.1100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Truscott J., Garske T., Chris-Ster I., Guitian J., Pfeiffer D., Snow L., Wilesmith J., Ferguson N. M., Ghani A. C. 2007. Control of highly pathogenic H5N1 avian influenza outbreak in the GB poultry flock. Proc. R. Soc. B 274, 2287–2295 10.1098/rspb.2007.0542 (doi:10.1098/rspb.2007.0542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kao R. 2002. The role of mathematical modelling in the control of the 2001 FMD epidemic in the UK. Trends Microbiol. 10, 279–286 10.1016/S0966-842X(02)02371-5 (doi:10.1016/S0966-842X(02)02371-5) [DOI] [PubMed] [Google Scholar]
- 29.Keeling M. J. 2005. Models of foot-and-mouth disease. Proc. R. Soc. B 272, 1195–1202 10.1098/rspb.2004.3046 (doi:10.1098/rspb.2004.3046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitching R. P., Thrusfield M. V., Taylor N. M. 2006. Use and abuse of mathematical models: an illustration from the 2001 foot and mouth disease epidemic in the United Kingdom. Rev. Sci. Tech. 25, 294–311 [DOI] [PubMed] [Google Scholar]
- 31.Guitain J., Pfeiffer D. 2006. Should we use models to inform policy development? Vet. J. 172, 393–395 10.1016/j.tvjl.2006.03.001 (doi:10.1016/j.tvjl.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 32.Wynne B., Shackley S. 1994. Environmental models: truth machines or social heuristics? The Globe 21, 6–8 [Google Scholar]
- 33.Defra 2009. Contingency plan for exotic diseases of animals: framework response plan. London, UK: Defra [Google Scholar]
- 34.O'Donnell M., Platt C., Aston R. 2000. Effect of a boil water notice on behaviour in the management of a water contamination incident. Commun. Dis. Public Health 3, 56–59 [PubMed] [Google Scholar]
- 35.Bickerstaff K., Simmons P. 2004. The right tool for the job? Modelling, spatial relationships, and styles of scientific practice in the UK foot and mouth crisis. Environ. Plann. D Soc. Space 22, 393–412 10.1068/d344t (doi:10.1068/d344t) [DOI] [Google Scholar]
- 36.Green L. E., Medley G. F. 2002. Mathematical modelling of the foot and mouth disease epidemic of 2001: strengths and weaknesses. Res. Vet. Sci. 73, 201–205 [DOI] [PubMed] [Google Scholar]
- 37.Campbell D., Lee R. 2003. Carnage by computer: the blackboard economics of the 2001 foot and mouth epidemic. Soc. Legal Stud. 12, 425–459 10.1177/0964663903012004002 (doi:10.1177/0964663903012004002) [DOI] [Google Scholar]
- 38.Convery I., Bailey C., Mort M., Baxter J. 2005. Death in the wrong place? Emotional geographies of the UK 2001 foot and mouth disease epidemic. J. Rural Stud. 21, 99–109 10.1016/j.jrurstud.2004.10.003 (doi:10.1016/j.jrurstud.2004.10.003) [DOI] [Google Scholar]
- 39.Bailey C., Convery I., Mort M., Baxter J. 2006. Different public health geographies of the 2001 foot and mouth disease epidemic: ‘citizen’ versus ‘professional’ epidemiology. Health Place 12, 157–166 10.1016/j.healthplace.2004.11.004 (doi:10.1016/j.healthplace.2004.11.004) [DOI] [PubMed] [Google Scholar]
- 40.Convery I., Mort M., Bailey C., Baxter J. 2008. Animal disease and human trauma. Basingstoke, UK: Palgrave Macmillan [Google Scholar]
- 41.Woolhouse M. 2011. How to make predictions about future infectious disease risks. Phil. Trans. R. Soc. B 366, 2045–2054 10.1098/rstb2010.0387 (doi:10.1098/rstb2010.0387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Defra 2010. Disease control: contingency planning exercises. See http://www.defra.gov.uk/foodfarm/farmanimal/diseases/control/exercises.htm; http://www.defra.gov.uk/foodfarm/farmanimal/diseases/control/exercises.htm (last accessed 17 November 2010)
- 43.Pretty J. 2009. Speaking truth to power: FMD and the future of agriculture and its communities. In The social and cultural impact of foot and mouth disease in the UK in 2001 (eds Doering M., Nerlich B.), pp. 245–258 Manchester, UK: Manchester University Press [Google Scholar]
- 44.Felt U., et al. 2007. Science and governance: taking European knowledge society seriously. Luxembourg: Office for Official Publications of the European Communities [Google Scholar]
- 45.Taylor P. 1995. Re/constructing socioecologies: system dynamics modeling of nomadic pastoralists in sub-Saharan Africa. In The right tools for the job: at work in the 20th century life-sciences (eds Clarke A., Fujimura J.), pp. 3–44 Princeton, NJ: Princeton University Press [Google Scholar]
- 46.Defra 2007. Social research in Defra. London, UK: Defra [Google Scholar]