Abstract
Current placebo research postulates that conditioning processes are one of the major mechanisms of the placebo response. Behaviourally conditioned changes in peripheral immune functions have been demonstrated in experimental animals, healthy subjects and patients. The physiological mechanisms responsible for this ‘learned immune response’ are not yet fully understood, but some relevant afferent and efferent pathways in the communication between the brain and the peripheral immune system have been identified. In addition, possible benefits and applicability in clinical settings have been demonstrated where behaviourally conditioned immunosuppression attenuated the exacerbation of autoimmune diseases, prolonged allograft survival and affected allergic responses. Here, we summarize data describing the mechanisms and the potential clinical benefit of behaviourally conditioned immune functions, with particular focus on learned placebo effects on allergic reactions.
Keywords: behavioural conditioning, immune system, placebo response, allergy
1. Introduction
A placebo is commonly defined as a sham pharmaceutical, routinely employed in clinical studies to verify the effectiveness of a drug or a treatment, and is lacking any defined active component. However, the results of clinical and experimental studies have clearly shown that placebos can indeed affect symptomatology, as demonstrated, for example in patients with depression [1,2], Parkinson's disease [3–6], pain [7,8] and asthma [9]. How is it that substances without any defined active component affect not only psychological but more importantly physiological functions?
Current scientific research focuses on two major neuropsychological mechanisms for the placebo response: the patient's or subject's expectation of the impact of the drug or treatment, and classical conditioning procedures [10–12]. The fact that learning processes are mediating the placebo response has been reported in early experiments in which a placebo medication was more potent after the experience of an effective drug therapy [13,14]. These observations were later confirmed by a number of experimental data demonstrating that associative learning processes are one important factor mediating the placebo response [9,15–17].
In this review, we will focus on classical conditioning as a major mechanism steering the placebo response, specifically on the classical conditioning of immune functions, and will summarize data demonstrating learned placebo effects on allergic responses.
The phenomenon of the conditioning of immune functions was first studied by Metalnikov & Chorine [18]. They injected guinea pigs with the plant extract Tapioka (the unconditioned stimulus, US), which increased peripheral leucocyte numbers. Together with the injection, the skin of the animals was either heated or slightly slit (the conditioned stimulus, CS). After several CS–US pairings, the stimulation of the skin alone increased the leucocyte numbers, indicating a conditioned immune response.
However, this line of research was not followed up until Ader & Cohen [19] demonstrated a behaviourally conditioned immunosuppression employing a conditioned taste aversion paradigm in rats. This early work led to a number of experimental approaches considering the conditioning of immune functions in experimental animals, yielding results showing behaviourally conditioned effects on humoral immune functions like antibody responses [20,21] and effects on cellular immune functions such as mitogen-induced lymphocyte proliferation [22], leucocyte number [23], the circulation of specific lymphocyte subpopulations [24], the activity of natural killer cells [25] and acute phase reactions [26,27]. By employing the immunosuppressive drug cyclosporin A as a US in a taste aversion paradigm in rats, a conditioned immunosuppression could be repeatedly demonstrated reflected by a reduction in spleen and thymus weight [28], a reduced proliferation rate of lymphocytes in the spleen [28–30] and decreased interleukin-2 (IL-2) and γ-interferon (γ-IFN) concentrations [29,31].
In parallel to the experimental evidence of a conditioned suppression of immune functions, other studies demonstrated a conditioned enhancement of immune responses. Solvason et al. [32] induced a conditioned increase in natural killer cell activity in mice by pairing the odour of camphor with an injection of poly I:C, a substance that stimulates natural killer cell activity via the release of γ-IFN. The activity of cytotoxic T-lymphocytes, which can be enhanced through immunization with allogeneic spleen cells, can also be conditioned by pairing the immunization procedure with the camphor odour [33].
In summary these studies demonstrate across different drugs employed as a US and different conditioning models as well as distinct cellular and humoral immune parameters used as read-out systems that immune functions can be modified by associative learning processes [34].
2. How conditioning of peripheral immune functions works: principles and mechanisms
Basically, a conditioning procedure requires the pairing of an initially neutral stimulus (the CS) with a US, for example a drug that elicits a physiological response within the organism. After this association phase, the presentation of the CS alone will induce a conditioned response that is comparable with the unconditioned response formerly elicited by the US. A prerequisite for the classical conditioning of immune functions is the functional interaction between the central nervous system (CNS) and the peripheral immune system [35–37]. Although the classical conditioning of immune functions is a fascinating example of the communication between the CNS and the immune system, experimental evidence of how these systems interact during the immune function conditioning process is rare. The basic model assumes that there are three important steps in the acquisition and evocation of a conditioned immune response. First, the US (e.g. an immunomodulatory drug) must be either directly sensed by the CNS or indirectly recognized via changes in the immune response. Second, the CNS must now integrate and associate signals caused by the US and the sensory information provided by the CS (generally a taste or odour). Third, in the evocation phase, the re-exposure to the CS must activate those brain areas which integrated the CS/US association, and subsequently modify the immune response via efferent pathways [34].
(a). Afferent pathways: how the central nervous system receives signals from the peripheral immune system
It is as yet not thoroughly understood how the CNS receives information about the immunological status change caused by the US. Two possible pathways have been proposed: a systemic or humoral pathway and a neural pathway. For the systemic or humoral pathway, it is assumed that the relevant messengers that are produced by immune cells in the periphery (e.g. cytokines or prostaglandins) reach the brain by crossing the blood–brain barrier directly [38] or by crossing the circumventricular organs [39]. The activation of the neural pathway requires that the information from a relevant messenger is turned into a neural signal. The vagus nerve may play a major role in this kind of CNS–immune system communication [40–42], a view which is supported by the fact that many effects in the CNS induced by the immune system can be depleted or even abolished by prior vagotomy [43,44].
(b). Brain structures and neurotransmitters involved in the integration of the conditioned stimulus and the unconditioned stimulus
Studies concerning taste aversion learning demonstrated that the insular cortex is particularly relevant for the acquisition and retention of the associative learning process [45,46]. Furthermore, it was found that the insular cortex is also needed for the evocation process, whereas the amygdala seemed to be necessary for the acquisition phase only [47,48].
Regarding the role of central neurotransmission on the conditioned effect, it was shown that central catecholamines were needed for the association process, whereas glutamate was also required for the evocation of a conditioned increase in natural killer cell activity [49,50]. However, the role of these neurotransmitters remains to be analysed across different conditioning models.
(c). Efferent pathways: how the central nervous system affects the immune system
In the case of behaviourally conditioned immunosuppression employing cyclosporin A as a US, sympathetic nervous system activity could be identified as one of the major mechanisms mediating the conditioned immune response on the efferent pathway from the CNS to the peripheral immune system. A surgical denervation of the spleen completely inhibited the conditioned suppression of proliferation of lymphocytes in the spleen and IL-2 and γ-IFN production [29,51]. In parallel, noradrenaline seemed to be the predominant neurotransmitter mediating the learned immune response. Moreover, it was demonstrated that this effect seemed to be β-adrenoreceptor-dependent, as the application of β-adrenoreceptor antagonists blocked the learned immunosuppression [52,53]. However, the splenic nerve does not seem to be the only efferent pathway that is activated during evocation, as splenic denervation does not affect the conditioned suppression of the contact hypersensitivity reaction [30]. These results indicate that multiple efferent mechanisms are activated during the behaviourally conditioned effects on immunity.
The efferent and afferent communication pathways employed by the CNS and the peripheral immune system as well as the central processing of the learned immune response are currently not entirely understood. They are partially described for some selective paradigms of conditioned immune responses only, without knowing yet whether and to what extent these experimental data are reflecting mechanisms of behaviourally conditioned immune responses in general.
3. Clinical implications of the behaviourally conditioned immune response
The paradigm of the classical conditioning of immune functions is a fascinating example of the communication between the CNS and the peripheral immune system. A number of studies in experimental animals and humans have addressed the possibility that behaviourally conditioned changes in immune function are of clinical relevance and can be employed to affect the disease outcome.
In a rodent model of the autoimmune disease lupus erythematosus, behaviourally conditioned mice showed a significantly prolonged latency and survival time when compared with the control animals [54]. Similarly, in rats with experimentally induced rheumatoid arthritis, re-exposure to a saccharin-vanilla-flavoured solution that had previously been paired with the immunosuppressant cyclophosphamide resulted in a reduction of the inflammatory processes [55,56]. Conditioned responses also proved to be therapeutically effective in grafting experiments, expressed by a delayed tissue rejection after allogeneic skin transplantation [57]. Similarly, studies on heart allografts demonstrated that behavioural conditioning prolonged transplant survival [58,59].
A longer survival time in mice that were conditioned with poly I:C and camphor and then re-exposed to the CS after the transplantation of a myeloma was demonstrated [60,61]. Likewise, tumour growth could be delayed [62] and lifespan prolonged [63] through conditioned immune responses after the transplantation of T-cell lymphomas. These results in experimental animals document the potential clinical relevance of classical conditioning of immune functions and disease outcome in chronic inflammatory diseases, organ transplant survival or tumour development.
Beyond data from animal studies, there are reports that classical conditioning of immune functions is also possible in humans [11,34]. A prolonged elevated level of immune activation was demonstrated after repeated exposure to an oral stimulus (CS) that was initially paired with γ-IFN injections (US). The activation was assessed by measuring the serum concentration of quinolinic acid and neopterin and the expression of Fc receptors on peripheral blood mononuclear cells [64]. A transient conditioned decrease in white blood cell level was demonstrated in patients with multiple sclerosis after re-exposure to anise-flavoured syrup (CS) and a subtherapeutic dose (10 mg) of cyclophosphamide [65]. In another experimental approach in healthy humans, cyclosporin A (US) was repeatedly paired with a novel-tasting drink (CS) [15]. A conditioned inhibition of cytokine release and cytokine mRNA expression for IL-2 and γ-IFN and a decreased lymphocyte proliferation rate were observed when subjects were re-exposed to the CS. A more recent study established a protocol that may indicate a possible application for the conditioned immune response in clinical settings [66]. Patients suffering from psoriasis were treated with a corticosteroid ointment. However, the experimental group received the real drug only at 25–50% of the treatment settings, and was otherwise treated with a placebo cream. After eight weeks, those patients who received the drug at the reduced dosage (25–50%) and the rest of the time the placebo showed symptom scores comparable with the group that received the standard therapy (100%), while patients treated with a reduced dose throughout the study showed worsening of symptoms.
Collectively, these data are demonstrating a ‘proof of principle’ that behaviourally conditioned immune responses are possible in humans, with the next step being to document that these learning procedures are robust enough to be employed in clinical routine treatment [34].
4. Behaviourally conditioned responses in cases of allergy
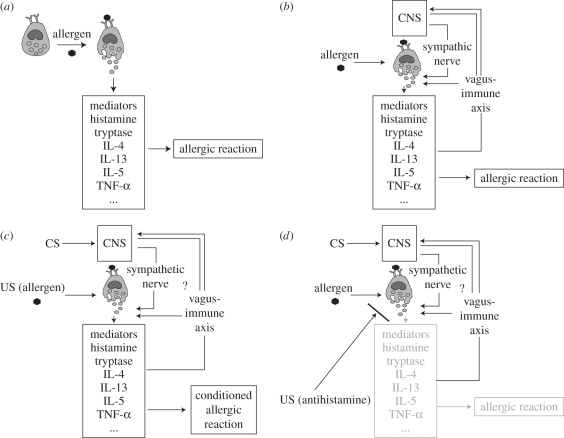
Allergic diseases are widespread and their incidence has increased over the past 50 years, for example by 12–16% for atopic dermatitis, by 15 per cent for allergic rhinoconjunctivitis and by 9–10% for bronchial asthma [67]. Allergic responses (figure 1a) can be enhanced experimentally by stress and anxiety [70] and be modulated by interventions other than conventional drug therapy, for example via hypnosis [71]. This indicates a responsiveness to psychological factors, and an interaction between the CNS and the immune cells that are relevant for the allergic reaction, e.g. mast cells (figure 1b). Thus, allergic responses, including asthmatic attacks, seem to be a promising model to study the mechanisms and clinical relevance of placebo responses in general and, more specifically, the effects of associative learning processes on immune function.
Figure 1.
(a,b) The principle of an allergic reaction: the IgE-primed mast cell releases mediators, such as histamine and several cytokines, when confronted with an allergen, leading to the allergic reaction (a). Mast cells have been shown to be in close interaction with the CNS. The vagus nerve may be a bidirectional pathway for CNS–mast cell communication [68]. In addition, contact formation of mast cells and sympathetic nerves were demonstrated in vitro [69] (b). (c,d) Model of allergic reactions affected by the classical conditioning paradigm: after one or several pairings of the CS with the US during the acquisition phase, re-exposure to the CS during the evocation phase induces an allergic reaction with increased release of mediators (c). It was also demonstrated that anti-allergic reactions can be affected by classical conditioning. The exact pathways and mediators for the conditioned response are not known (d).
A number of studies on experimental animals reported the effects of classical conditioning on allergic reactions such as allergic asthma, immediate hypersensitivity reactions or delayed-type hypersensitivity reactions (table 1). Early studies employed the paradigm of inducing asthmatic attacks in guinea pigs, resembling bronchial asthma in humans, by sensitizing the animals to substances such as bovine serum albumin (BSA) or egg albumin [72–74,89]. These early studies reported behaviourally conditioned effects on the strength of the asthmatic attack, documented by the increased use of accessory muscles, gasping, coughing and pronounced respiratory distress [73] or by increased pressure between inspiratory and expiratory peak, which is larger during an asthmatic attack [74].
Table 1.
Summary of studies concerning behavioural conditioning of allergic and anti-allergic responses.
| conditioned stimulus |
unconditioned stimulus | conditioned response/target parameter | animals/subjects | reference | |
|---|---|---|---|---|---|
| animal studies | |||||
| auditory cue | egg white | asthmatic attack | guinea pigs | [72] | |
| environment (chamber) | egg white | asthmatic attack | guinea pigs | [73] | |
| environment, features of the challenge | egg albumin | asthmatic attack | guinea pigs | [74] | |
| odour (sulphur smell) | BSA | histamine release | guinea pigs | [75] | |
| audiovisual cue | egg albumin | mast cell protease II | rats | [76] | |
| odour | ovalbumin | histamine release | guinea pigs | [77] | |
| odour (dimethylsulphide) | ovalbumin | histamine release | guinea pigs | [78,79] | |
| odour (dimethylsulphide) | ovalbumin | histamine release | guinea pigs | [80] | |
| odour | BSA | histamine release | guinea pigs | [81] | |
| odour | BSA | histamine release | guinea pigs | [82] | |
| saccharin solution | cyclosporin A | ear swelling | rats | [30] | |
| human studies | |||||
| inhalation situation; inhalation procedure | inhaled allergens (grass pollen, house dust mite) | vital capacity | asthmatic subjects | [83] | |
| environment, coloured vials, nurse… | tuberculin applied on arm | erythema size, induration size | tuberculin-positive subjects | [84] | |
| environment, coloured vials, nurse… | allergens (pollen, house dust mite, cat or dog hair applied on arm) | weal size | patients with a common allergy (pollen, house dust mite, cat or dog hair) | [85] | |
| novel flavoured drink | house dust mite allergen (nasal challenge) | mast cell tryptase | patients with house dust mite allergy | [86] | |
| olfactory cue | grass allergens | subjective symptoms, histamine release, nasal airflow | patients with seasonal allergic rhinitis | [87] | |
| coloured and flavoured drink | allergens (house dust mite) | erythema and weal size, subjective symptom score, basophil activation | patients with house dust mite allergy | [88] | |
The hypothesis that histamine release could not only be the consequence of external stimuli that directly affects immune functions but could also occur as a learned response was tested in guinea pigs [75]. The animals were sensitized and subsequently exposed to BSA which was presented together with a sulphur odour as a conditioned stimulus (CS+). Using a discriminative conditioning protocol, the animals were exposed to another odour stimulus (CS−) which had not previously been paired with albumin and thus should not elicit an allergic reaction when presented after the association phase. As hypothesized, the animals showed a more pronounced histamine release in the plasma when re-exposed to the CS+ than to the CS−. Furthermore, the conditioned histamine release was affected by stress exposure and could not be observed in anaesthesized animals when re-exposed to the CS, indicating that this ‘learned’ histamine release was mediated by the CNS [77–82]. Employing a similar paradigm, the cellular basis of this conditioned allergic response was investigated [76]. Protease II, which is specific to mucosal mast cell activity, was analysed after pairing egg albumin injections with an audiovisual cue. Rats that were exposed to the CS again showed a significantly greater release of protease compared with the non-conditioned control animals, indicating that the CNS effects on mast cell activity play a major role in the conditioned response in allergy.
In an experimental model of contact hypersensitivity, rats were conditioned by pairing injections of cyclosporin A (US) with a saccharin solution (CS) [30]. The animals of the experimental and control groups were sensitized to 2,4-dinitrochlorobenzene to elicit an allergic reaction, which was subsequently applied to the ear. The strength of the allergic response was measured by the degree of ear swelling and leucocyte infiltration. Conditioned rats showed a significant inhibition of ear swelling comparable with the effect of cyclosporin A treatment. These results indicate a potential clinical relevance of behaviourally conditioned immune responses in the inhibition of allergic reactions.
There are few human studies on the behavioural conditioning of allergic reactions. In 1886, McKenzie [90] published a case study in which he described a woman who showed asthmatic reactions when exposed to an artificial rose. This interesting but rather anecdotal finding was followed by experimental data more than 50 years later. Two asthmatic patients who were allergic to house dust extract and grass pollen were exposed to these allergens by inhalation [83]. After several conditioning trials in which the environment and the act of inhalation were supposed to serve as a CS, both subjects showed a conditioned response in the form of allergic attacks after inhaling a neutral solvent.
In an approach to behaviourally condition allergic reactions in tuberculin-positive subjects, the response to tuberculin was analysed by assessing the size of erythema and induration [84]. Subjects received a tuberculin skin test on one arm and a saline control on the other arm for six months. On the last trial, the contents of the two differently coloured vials that contained the substances were switched unknown to the subjects. As hypothesized, the reaction to what the subjects' thought was saline was weaker than their reaction to the drug previously. In this study, it is not clear whether this effect can be ascribed to conditioning processes—with the vials and other contextual stimuli serving as CS—or to the subjects' expectations of a certain response after the application of an assumed known substance. Another attempt employing a similar conditioning paradigm focusing on the immediate hypersensitivity reaction to common allergens failed to replicate the data obtained by Smith & McDaniel [85].
Tryptase release, a specific marker for mast cell activation in nasal fluid, can be analysed as an indicator of the intensity of an allergic reaction [91]. Subjects underwent a conditioning protocol which comprised a single learning trial in which a novel-tasting drink (CS) was paired with an intranasal challenge of house dust mite allergens. When re-exposed to the gustatory stimulus, subjects showed a conditioned allergic reaction, documented by an increase in tryptase levels in their nasal fluid compared with the control groups [86]. In parallel to the conditioned increase in tryptase release, a behaviourally conditioned increase in histamine release and a decrease in nasal airflow were reported in patients suffering from seasonal allergic rhinitis [87]. In addition, in this study, a ‘training’ effect was demonstrated reflected by a more strongly conditioned effect if subjects underwent three CS/US pairings (olfactory stimulus + allergen) instead of a single CS/US pairing [92]. Moreover, the conditioned effect was smaller after a second re-exposure to the CS, indicating an extinction of this ‘learned’ immune response.
While the reports mentioned above document that an allergic reaction can be induced by learning paradigms, a more recent study demonstrated a behaviourally conditioned anti-allergic effect in patients with allergic rhinitis [88]. Patients were exposed to a novel-tasting drink (CS) that they received together with the H1-receptor antagonist desloratadine on five consecutive days. After a single evocation trial, subjects showed a conditioned reduction of subjective symptoms, a reduced response to the skin prick test and a reduced percentage of activated basophile granulocytes.
In summary, there is substantial evidence that both allergic and anti-allergic responses can be influenced through classical conditioning processes as a ‘learned’ placebo response. The exact biochemical mechanisms involved are as yet unknown (figure 1c,d).
5. Perspectives
The experimental data from animals and humans summarized above clearly documents that conditioning protocols can not only affect peripheral immune functions but can also affect disease symptoms and disease progress, such as chronic inflammatory autoimmune diseases, or the prevention of recurrent symptoms in patients with psoriasis, as described above. Thus the question arises whether and to what extent behavioural conditioning protocols may be used in a clinical setting as a supplement to an immunomodulatory drug regimen, with the aim to reduce the dose of medication required, thereby possibly limiting adverse drug effects and saving costs.
However, before seriously considering conditioning protocols as a treatment option, several open questions must be elucidated. It is unknown to date whether all immune-related diseases and symptoms are subject to conditioned placebo responses and, in particular, which substances have immunopharmacological properties that may be subject to conditioning and to what extent. In order to answer this question, a better understanding of the reciprocal CNS–immune system communication on a physiological and molecular level is essential.
Furthermore, it is not known how long the conditioned effects on the immune response last and whether these effects are reproducible. In addition, the optimal amount of learning and re-exposure trials to maximize the conditioned effect are not yet understood, as this may vary depending on the drug and/or disease. To establish conditioning protocols for clinical settings, it will be crucial to assess the intervals of appropriate reinforcement and the extinction patterns of the learned immune response.
With a more thorough knowledge about the behavioural, neurological and immunological mechanisms that underlie these processes, it is conceivable that classical conditioning, as part of a systematically applied placebo response, could serve as a supportive therapy in many immune-related diseases [11,34,93].
Acknowledgements
This work was supported by the Volkswagen Foundation (I/83806).
Footnotes
One contribution of 17 to a Theme Issue ‘Placebo effects in medicine: mechanisms and clinical implications’.
References
- 1.Dworkin R. H., Katz J., Gitlin M. J. 2005. Placebo response in clinical trials of depression and its implications for research on chronic neuropathic pain. Neurology 65(Suppl. 4), 7–19 [DOI] [PubMed] [Google Scholar]
- 2.Kirsch I. 2009. Antidepressants and the placebo response. Epidemiol. Psychiatr. Soc. 18, 318–322 [DOI] [PubMed] [Google Scholar]
- 3.De la Fuente-Fernandez R., Ruth T. J., Sossi V., Schulzer M., Calne D. B., Stoessl A. J. 2001. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science 293, 1164–1166 10.1126/science.1060937 (doi:10.1126/science.1060937) [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F., Colloca L., Torre E., Lanotte M., Melcarne A., Pesare M., Bergamasco B., Lopiano L. 2004. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat. Neurosci. 7, 587–588 10.1038/nn1250 (doi:10.1038/nn1250) [DOI] [PubMed] [Google Scholar]
- 5.De la Fuente-Fernandez R., Lidstone S., Stoessi A. J. 2006. Placebo effect and dopamine release. J. Neural Transm. Suppl. 70, 415–418 10.1007/978-3-211-45295-0-62 (doi:10.1007/978-3-211-45295-0-62) [DOI] [PubMed] [Google Scholar]
- 6.Lidstone S. C., Schulzer M., Dinelle K., Mak E., Sossi V., Ruth T. J., de la Fuente-Fernandez R., Phillips A. G., Stoessl A. J. 2010. Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch. Gen. Psychiatry 67, 857–865 10.1001/archgenpsychiatry.2010.88 (doi:10.1001/archgenpsychiatry.2010.88) [DOI] [PubMed] [Google Scholar]
- 7.Colloca L., Benedetti F. 2005. Placebos and painkillers: is mind as real as matter? Nat. Rev. Neurosci. 6, 545–552 10.1038/nrn1705 (doi:10.1038/nrn1705) [DOI] [PubMed] [Google Scholar]
- 8.Colloca L., Petrovic P., Wager T. D., Ingvar M., Benedetti F. 2010. How the number of learning trials affects placebo and nocebo responses. Pain 151, 430–439 10.1016/j.pain.2010.08.007 (doi:10.1016/j.pain.2010.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemeny M. E., Rosenwasser L. J., Panettieri R. A., Rose R. M., Berg-Smith S. M., Kline J. 2007. Placebo response in asthma: a robust and objective phenomenon. J. Allergy Clin. Immunol. 119, 1375–1381 10.1016/j.jaci.2007.03.016 (doi:10.1016/j.jaci.2007.03.016) [DOI] [PubMed] [Google Scholar]
- 10.Price D. D., Finniss D. G., Benedetti F. 2008. A comprehensive review of the placebo effect: recent advances and current thought. Annu. Rev. Psychol. 59, 565–590 10.1146/annurev.psych.59.113006.095941 (doi:10.1146/annurev.psych.59.113006.095941) [DOI] [PubMed] [Google Scholar]
- 11.Enck P., Benedetti F., Schedlowski M. 2008. New insights into the placebo and nocebo responses. Neuron 59, 195–206 10.1016/j.neuron.2008.06.030 (doi:10.1016/j.neuron.2008.06.030) [DOI] [PubMed] [Google Scholar]
- 12.Finniss D. G., Kaptchuk T. J., Miller F., Benedetti F. 2010. Biological, clinical, and ethical advances of placebo effects. Lancet 375, 686–695 10.1016/S0140-6736(09)61706-2 (doi:10.1016/S0140-6736(09)61706-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batterman R. C. 1965. Methodology of analgesic evaluation: experience with orphenadrine citrate compound. Curr. Ther. Res. 7, 639–647 [PubMed] [Google Scholar]
- 14.Ader R. 2000. The role of conditioning in pharmacotherapy. In The placebo effect. An interdisciplinary exploration (ed. Harrington A.), pp. 138–165 Cambridge, UK: Harvard University Press [Google Scholar]
- 15.Goebel M. U., et al. 2002. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 16, 1869–1873 10.1096/fj.02-0389com (doi:10.1096/fj.02-0389com) [DOI] [PubMed] [Google Scholar]
- 16.Benedetti F., Pollo A., Lopiano L., Lanotte M., Vighett S., Rainero I. 2003. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J. Neurosci. 23, 4315–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedetti F., Mayberg H. S., Wager T. D., Stohler C. S., Zubieta J. K. 2005. Neurobiological mechanisms of the placebo effect. J. Neurosci. 25, 390–402 10.1523/JNEUROSCI.3458-05.2005 (doi:10.1523/JNEUROSCI.3458-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metalnikov S., Chorine V. 1926. The role of conditioned reflexes in immunity. In Foundations of psychoneuroimmunology (eds Locke S., Ader R., Besedovsky H., Hall N., Solemon G., Strom T.), pp. 263–267 New York, NY: Aldine [Google Scholar]
- 19.Ader R., Cohen N. 1975. Behaviorally conditioned immunosuppression. Psychosom. Med. 37, 333–340 [DOI] [PubMed] [Google Scholar]
- 20.Cohen N., Ader R., Green N., Bovbjerg D. 1979. Conditioned suppression of thymus independent antibody response. Psychosom. Med. 41, 487–491 [DOI] [PubMed] [Google Scholar]
- 21.Gorczynski R. M., Macrea S., Kennedy M. 1984. Factors involved in the classical conditioning of antibody responses in mice. In Breakdown in human adaptation to ‘stress’: towards a multidisciplinary approach (eds Ballieux R. E., Fielding J. F., L'Abbate A.), pp. 704–712 Hingham, MA: Martinus Nijhof [Google Scholar]
- 22.Lysle D. T., Cunnik J. E., Fowler H., Rabin B. S. 1988. Pavlovian conditioning of shock-induced suppression of lymphocyte reactivity: acquisition, extinction, and preexposure effect. Life Sci. 42, 2185–2194 10.1016/0024-3205(88)90369-4 (doi:10.1016/0024-3205(88)90369-4) [DOI] [PubMed] [Google Scholar]
- 23.Klosterhalfen S., Klosterhalfen W. 1987. Classically conditioned effects of cyclophosphamide on white blood cell counts in rats. Ann. NY Acad. Sci. 496, 569–577 10.1111/j.1749-6632.1987.tb35815.x (doi:10.1111/j.1749-6632.1987.tb35815.x) [DOI] [PubMed] [Google Scholar]
- 24.Husband A. J., King M. G., Brown R. 1987. Behaviorally conditioned modification of T cell subset ratios in rats. Immunol. Lett. 14, 91–94 10.1016/0165-2478(87)90085-X (doi:10.1016/0165-2478(87)90085-X) [DOI] [PubMed] [Google Scholar]
- 25.Ghanta V. K., Hiramoto N. S., Solvason H. B., Spector N. H. 1985. Neural and environmental influences on neoplasia and conditioning of NK activity. J. Immunol. 135, 848–852 [PubMed] [Google Scholar]
- 26.Exton M. S., Bull D. F., King M. G., Husband A. J. 1995. Modification of body temperature and sleep states using behavioral conditioning. Physiol. Behav. 57, 723–729 10.1016/0031-9384(94)00314-9 (doi:10.1016/0031-9384(94)00314-9) [DOI] [PubMed] [Google Scholar]
- 27.Janz L. J., Green-Johnson J., Murray L., Vrind C. Y., Nance D. M., Greenberg A. H., Dyck D. 1996. Pavlovian conditioning of LPS-induced responses: effects on corticosterone, splenic NE, and IL-2 production. Physiol. Behav. 59, 1103–1109 10.1016/0031-9384(95)02171-X (doi:10.1016/0031-9384(95)02171-X) [DOI] [PubMed] [Google Scholar]
- 28.Exton M. S., von Hörsten S., Vöge J., Westermann J., Schult M., Nagel E., Schedlowski M. 1998. Conditioned taste aversion produced by cyclosporine A: concomitant reduction in lymphoid organ weight and splenocyte proliferation. Physiol. Behav. 63, 241–247 10.1016/S0031-9384(97)00432-0 (doi:10.1016/S0031-9384(97)00432-0) [DOI] [PubMed] [Google Scholar]
- 29.Exton M. S., et al. 1998. Behaviorally conditioned immunosuppression using cyclosporine A: central nervous system reduces IL-2 production via splenic innervations. J. Neuroimmunol. 88, 182–191 10.1016/S0165-5728(98)00122-2 (doi:10.1016/S0165-5728(98)00122-2) [DOI] [PubMed] [Google Scholar]
- 30.Exton M. S., Elfers A., Jeong W. Y., Bull D. F., Westermann J., Schedlowski M. 2000. Conditioned suppression of contact sensitivity is independent of sympathetic splenic innervations. Am. J. Physiol. 279, 1310–1315 [DOI] [PubMed] [Google Scholar]
- 31.Von Hörsten S., et al. 1998. Behaviorally conditioned effects of Cyclosporine A on the immune system of rats: specific alterations of blood leukocyte numbers and decrease of granulocyte function. J. Neuroimmunol. 85, 193–201 10.1016/S0165-5728(98)00011-3 (doi:10.1016/S0165-5728(98)00011-3) [DOI] [PubMed] [Google Scholar]
- 32.Solvason H. B., Ghanta V. K., Hiramoto R. N. 1988. Conditioned augmentation of natural killer cell activity. Independence from nociceptive effects and dependence of interferon-beta. J. Immunol. 140, 661–665 [PubMed] [Google Scholar]
- 33.Hiramoto R. N., Hsueh C. M., Rogers C. F., Demissie S., Hiramoto N. S., Soong S. J., Ghanta V. K. 1993. Conditioning of the allogeneic cytotoxic lymphocyte response. Pharmacol. Biochem. 44, 275–280 10.1016/0091-3057(93)90462-3 (doi:10.1016/0091-3057(93)90462-3) [DOI] [PubMed] [Google Scholar]
- 34.Schedlowski M., Pacheco-Lopez G. 2010. The learned immune response: Pavlov and beyond. Brain Behav. Immunol. 24, 176–185 10.1016/j.bbi.2009.08.007 (doi:10.1016/j.bbi.2009.08.007) [DOI] [PubMed] [Google Scholar]
- 35.Dantzer R., O'Connor J. C., Freund G. G., Johnson R. W., Kelley K. W. 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 10.1038/nrn2297 (doi:10.1038/nrn2297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glaser R., Kiecolt-Glaser J. K. 2005. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 5, 243–251 10.1038/nri1571 (doi:10.1038/nri1571) [DOI] [PubMed] [Google Scholar]
- 37.Tracey K. J. 2009. Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428 10.1038/nri2566 (doi:10.1038/nri2566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banks W. A. 2005. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr. Pharm. Des. 11, 973–984 10.2174/1381612053381684 (doi:10.2174/1381612053381684) [DOI] [PubMed] [Google Scholar]
- 39.Goehler L. E., Erisir A., Gaykema R. P. 2006. Neural-immune interface in the rat area postrema. Neuroscience 140, 1415–1434 10.1016/j.neuroscience.2006.03.048 (doi:10.1016/j.neuroscience.2006.03.048) [DOI] [PubMed] [Google Scholar]
- 40.Goehler L. E., Gaykema R. P., Opitz N., Reddaway R., Badr N., Lyte M. 2005. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav. Immunol. 19, 334–344 10.1016/j.bbi.2004.09.002 (doi:10.1016/j.bbi.2004.09.002) [DOI] [PubMed] [Google Scholar]
- 41.Tracey K. J. 2002. The inflammatory reflex. Nature 420, 853–859 10.1038/nature01321 (doi:10.1038/nature01321) [DOI] [PubMed] [Google Scholar]
- 42.Rosas-Ballina M., Tracey K. J. 2009. The neurology of the immune system: neural reflexes regulate immunity. Neuron 64, 28–32 10.1016/j.neuron.2009.09.039 (doi:10.1016/j.neuron.2009.09.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konsman J. P., Luheshi G. N., Bluthe R. M., Dantzer R. 2000. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur. J. Neurosci. 12, 4434–4446 10.1046/j.0953-816X.2000.01319.x (doi:10.1046/j.0953-816X.2000.01319.x) [DOI] [PubMed] [Google Scholar]
- 44.Luheshi G. N., Bluthe R. M., Rushforth D., Mulcahy N., Konsman J. P., Goldbach M., Dantzer R. 2000. Vagotomy attenuates the behavioural but not the pyrogenic effects of interleukin-1 in rats. Auton. Neurosci. 85, 127–132 10.1016/S1566-0702(00)00231-9 (doi:10.1016/S1566-0702(00)00231-9) [DOI] [PubMed] [Google Scholar]
- 45.Bermúdez-Rattoni F., McGaugh J. L. 1991. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res. 549, 165–170 10.1016/0006-8993(91)90616-4 (doi:10.1016/0006-8993(91)90616-4) [DOI] [PubMed] [Google Scholar]
- 46.Cubero I., Thiele T. E., Bernstein I. L. 1999. Insular cortex lesions and taste aversion learning: effects of conditioning method and timing of lesion. Brain Res. 839, 323–330 10.1016/S0006-8993(99)01745-X (doi:10.1016/S0006-8993(99)01745-X) [DOI] [PubMed] [Google Scholar]
- 47.Ramírez-Amaya V., Alvarez-Borda B., Bermúdez-Rattoni F. 1998. Differential effects of NMDA-induced lesions into the insular cortex and amygdala on the acquisition and evocation of conditioned immunosuppression. Brain Behav. Immunol. 12, 149–160 10.1006/brbi.1998.0518 (doi:10.1006/brbi.1998.0518) [DOI] [PubMed] [Google Scholar]
- 48.Pacheco-López G., Niemi M. B., Kou W., Härting M., Fandrey J., Schedlowski M. 2005. Neural substrates for behaviorally conditioned immunosuppression in the rat. J. Neurosci. 25, 2330–2337 10.1523/JNEUROSCI.4230-04.2005 (doi:10.1523/JNEUROSCI.4230-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsueh C., Kuo J., Chen S., Huang H., Cheng F., Chung L., Lin R. 1999. Involvement of catecholamines in recall of the conditioned NK cell response. J. Neuroimmunol. 94, 172–181 10.1016/S0165-5728(98)00250-1 (doi:10.1016/S0165-5728(98)00250-1) [DOI] [PubMed] [Google Scholar]
- 50.Kuo J., Chen S., Huang H., Yang C., Tsai P., Hsueh C. 2001. The involvement of glutamate in recall of the conditioned NK cell response. J. Neuroimmunol. 118, 245–255 10.1016/S0165-5728(01)00340-X (doi:10.1016/S0165-5728(01)00340-X) [DOI] [PubMed] [Google Scholar]
- 51.Exton M. S., Schult M., Donath S., Strubel T., Bode U., del Rey A., Westermann J., Schedlowski M. 1999. Conditioned immunosuppression makes subtherapeutic cyclosporin effective via splenic innervation. Am. J. Physiol. 276, 1710–1717 [DOI] [PubMed] [Google Scholar]
- 52.Exton M. S., Gierse C., Meier B., Mosen M., Xie Y., Frede S., Goebel M. U., Limmroth V., Schedlowski M. 2002. Behavioral conditioned immunosuppression in the rat is regulated via noradrenaline and beta-adrenoceptors. J. Neuroimmunol. 131, 21–30 10.1016/S0165-5728(02)00249-7 (doi:10.1016/S0165-5728(02)00249-7). [DOI] [PubMed] [Google Scholar]
- 53.Xie Y., Frede S., Harnish M. J., Exton M. S., Schedlowski M. 2002. Beta-adrenoreceptor-induced inhibition of rat splenocyte proliferation: cytokine gene transcription as the target of action. Immunobiology 206, 345–353 10.1078/0171-2985-00185 (doi:10.1078/0171-2985-00185) [DOI] [PubMed] [Google Scholar]
- 54.Ader R., Cohen N. 1982. Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus. Science 215, 1534–1536 10.1126/science.7063864 (doi:10.1126/science.7063864) [DOI] [PubMed] [Google Scholar]
- 55.Klosterhalfen W., Klosterhalfen S. 1983. Pavlovian conditioning of immunosuppression modifies adjuvant arthritis in rats. Behav. Neurosci. 97, 663–666 [DOI] [PubMed] [Google Scholar]
- 56.Klosterhalfen W., Klosterhalfen S. 1985. Conditioned immunopharmacologic effects and adjuvant arthritis: further results. In Neuroimmodulation: Proc. of the First International Workshop on Neuroimmunomodulation. Papers from the workshop held by the International Group on Neuroimmunomodulation (IWGN), National Library of Medicine (of the National Institutes of Health), Bethesda, MD, USA, November 27–December 3, 1984 (eds Spector N. H., Bulloch K., Fox B. H., et al.), pp. 183–187 London, UK: Gordon and Breach [Google Scholar]
- 57.Gorczynski R. M., Kennedy M., Ciampi A. 1990. Conditioned enhancement of skin allografts in mice. Brain Behav. Immunol. 4, 85–92 10.1016/0889-1591(90)90011-E (doi:10.1016/0889-1591(90)90011-E) [DOI] [PubMed] [Google Scholar]
- 58.Grochowicz P. M., Schedlowski M., Husband A. J., King M. G., Hibberd A. D., Bowen K. M. 1991. Behavioral conditioning prolongs heart allograft survival in rats. Brain Behav. Immunol. 5, 349–356 10.1016/0889-1591(91)90030-E (doi:10.1016/0889-1591(91)90030-E) [DOI] [PubMed] [Google Scholar]
- 59.Exton M. S., Schult M., Donath S., Strubel T., Nagel E., Westermann J., Schedlowski M. 1998. Behavioral conditioning prolongs heart allograft survival in rats. Transpl. Proc. 30, 2033. 10.1016/S0041-1345(98)00522-3 (doi:10.1016/S0041-1345(98)00522-3) [DOI] [PubMed] [Google Scholar]
- 60.Ghanta V. K., Hiramoto R. N., Solvason B., Spector N. H. 1987. Influence of conditioned natural immunity on tumor growth. Ann. NY Acad. Sci. 496, 637–646 10.1111/j.1749-6632.1987.tb35824.x (doi:10.1111/j.1749-6632.1987.tb35824.x) [DOI] [PubMed] [Google Scholar]
- 61.Ghanta V. K., Miura T., Hiramoto N. S., Hiramoto R. N. 1988. Augmentation of natural immunity and regulation of tumor growth by conditioning. Ann. NY Acad. Sci. 521, 29–42 10.1111/j.1749-6632.1988.tb35263.x (doi:10.1111/j.1749-6632.1988.tb35263.x) [DOI] [PubMed] [Google Scholar]
- 62.Ghanta V. K., Hiramoto N. S., Solvason H., Soong S. J., Hiramoto R. N. 1990. Conditioning: a new approach to immunotherapy. Cancer Res. 50, 4295–4299 [PubMed] [Google Scholar]
- 63.Ghanta V. K., Hiramoto N. S., Soong S. J., Miller D. M., Hiramoto R. N. 1993. A multiple modality approach combining the effect of conditioning with adoptive chemoimmunotherapy. Int. J. Neurosci. 71, 251–265 [DOI] [PubMed] [Google Scholar]
- 64.Longo D. L., Duffey P. L., Kopp W. C., Heyes M. P., Alvord W. G., Sharfman W. H., Schmidt P. J., Rubinow D. R., Rosenstein D. L. 1999. Conditioned immune response to interferon-gamma in humans. Clin. Immunol. 90, 173–181 10.1006/clim.1998.4637 (doi:10.1006/clim.1998.4637) [DOI] [PubMed] [Google Scholar]
- 65.Giang D. W., Goodman A. D., Schiffer R. B., Mattson D. H., Petrie M., Cohen N., Ader R. 1996. Conditioning of cyclophosphamide-induced leukopenia in humans. J. Neuropsychiatry 8, 194–201 [DOI] [PubMed] [Google Scholar]
- 66.Ader R., Mercurio M. G., Walton J., James D., Davis M., Ojha V., Kimball A. B., Fiorentino D. 2010. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom. Med. 72, 192–197 10.1097/PSY.0b013e3181cbd38b (doi:10.1097/PSY.0b013e3181cbd38b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pamupura A. N. 2005. Prevalence of atopic diseases and the use of topical corticosteroids. Is there any connection? Med. Hypotheses 64, 575–578 10.1016/j.mehy.2003.12.059 (doi:10.1016/j.mehy.2003.12.059) [DOI] [PubMed] [Google Scholar]
- 68.Stead R. H., Colley E. C., Wang B., Partosoedarso E., Lin J., Stanisz A., Hillsley K. 2006. Vagal influences over mast cells. Auton. Neurosci. 125, 53–61 10.1016/j.autneu.2006.01.002 (doi:10.1016/j.autneu.2006.01.002) [DOI] [PubMed] [Google Scholar]
- 69.Blennerhassett M. G., Tomioka M., Bienenstock J. 1991. Formation of contacts between mast cells and sympathetic neurons in vitro. Cell Tissue Res. 265, 121–128 10.1007/BF00318146 (doi:10.1007/BF00318146) [DOI] [PubMed] [Google Scholar]
- 70.Kiecolt-Glaser J. K., Heffner K. L., Glaser R., Malarkey W. B., Porter K., Atkinson C., Laskowski B., Lemeshow S., Marshall G. D. 2009. How stress and anxiety can alter immediate and late phase skin test responses in allergic rhinitis. Psychoneuroendocrinology 34, 670–680 10.1016/j.psyneuen.2008.11.010 (doi:10.1016/j.psyneuen.2008.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Langewitz W., Izakovic J., Wyler J., Schindler C., Kiss A., Bircher A. J. 2005. Effect of self-hypnosis on hay fever symptoms—a randomised controlled intervention study. Psychother. Psychosom. 74, 165–172 10.1159/000084001 (doi:10.1159/000084001) [DOI] [PubMed] [Google Scholar]
- 72.Noelpp B., Noelpp-Eschenhagen I. 1951. Experimental bronchial asthma in guinea pigs. II. The role of conditioned reflexes in the pathogenesis of bronchial asthma. Int. Arch. Allergy Appl. Immunol. 2, 321–329 [PubMed] [Google Scholar]
- 73.Ottenberg P., Stein M., Lewis J., Hamilton C. 1958. Learned asthma in the guinea pig. Psychosom. Med. 20, 395–400 [DOI] [PubMed] [Google Scholar]
- 74.Justesen D. R., Braun E. W., Garrison R. G., Pendleton R. B. 1970. Pharmacological differentiation of allergic and classically conditioned asthma in the guinea pig. Science 170, 864–866 10.1126/science.170.3960.864 (doi:10.1126/science.170.3960.864) [DOI] [PubMed] [Google Scholar]
- 75.Russell M., Dark K. A., Cummins R. W., Ellman G., Callaway E., Peeke H. V. 1984. Learned histamine release. Science 225, 733–734 10.1126/science.6205449 (doi:10.1126/science.6205449) [DOI] [PubMed] [Google Scholar]
- 76.MacQueen G., Marshall J., Perdue M., Siegel S., Bienenstock J. 1989. Pavlovian conditioning of rat mucosal mast cells to secrete rat mast cell protease II. Science 243, 83–85 10.1126/science.2911721 (doi:10.1126/science.2911721) [DOI] [PubMed] [Google Scholar]
- 77.Irie M., Maeda M., Nagata S. 2001. Can conditioned histamine release occur under urethane anesthesia in guinea pigs? Physiol. Behav. 72, 567–573 10.1016/S0031-9384(00)00438-8 (doi:10.1016/S0031-9384(00)00438-8) [DOI] [PubMed] [Google Scholar]
- 78.Irie M., Nagata S., Endo Y. 2002. Fasting stress exacerbates classical conditioned histamine release in guinea pigs. Life Sci. 72, 689–698 10.1016/S0024-3205(02)02219-1 (doi:10.1016/S0024-3205(02)02219-1) [DOI] [PubMed] [Google Scholar]
- 79.Irie M., Nagata S., Endo Y. 2002. Effect of isolation on classical conditioned histamine release in guinea pigs. Neurosci. Res. 44, 31–35 10.1016/S0168-0102(02)00081-0 (doi:10.1016/S0168-0102(02)00081-0) [DOI] [PubMed] [Google Scholar]
- 80.Irie M., Nagata S., Endo Y. 2004. Diazepam attenuates conditioned histamine release in guinea pigs. Int. J. Psychophysiol. 51, 231–238 10.1016/S0167-8760(03)00220-4 (doi:10.1016/S0167-8760(03)00220-4) [DOI] [PubMed] [Google Scholar]
- 81.Peeke H. V. S., Dark K., Ellman G., McCurry C., Salfi M. 1987. Prior stress and behaviorally conditioned histamine release. Physiol. Behav. 39, 89–93 10.1016/0031-9384(87)90349-0 (doi:10.1016/0031-9384(87)90349-0) [DOI] [PubMed] [Google Scholar]
- 82.Dark K., Peeke H. V., Ellman G., Salfi M. 1987. Behaviorally conditioned histamine release. Prior stress and conditionability and extinction of the response. Ann. NY Acad. Sci. 496, 578–582 10.1111/j.1749-6632.1987.tb35816.x (doi:10.1111/j.1749-6632.1987.tb35816.x) [DOI] [PubMed] [Google Scholar]
- 83.Dekker E., Pelser H. E., Groen J. 1957. Conditioning as a cause of asthmatic attacks: a laboratory study. J. Psychosom. Res. 2, 97–108 10.1016/0022-3999(57)90015-6 (doi:10.1016/0022-3999(57)90015-6) [DOI] [PubMed] [Google Scholar]
- 84.Smith G. R., McDaniel S. M. 1983. Psychologically mediated effect on the delayed hypersensitivity reaction to tuberculin in humans. Psychosom. Med. 45, 65–70 [DOI] [PubMed] [Google Scholar]
- 85.Booth R. J., Petrie K. J., Brook R. J. 1995. Conditioning allergic skin responses in humans: a controlled trial. Psychosom. Med. 57, 492–495 [DOI] [PubMed] [Google Scholar]
- 86.Gauci M., Husband A. J., Saxarra H., King M. G. 1994. Pavlovian conditioning of nasal tryptase release in human subjects with allergic rhinitis. Physiol. Behav. 55, 823–825 10.1016/0031-9384(94)90066-3 (doi:10.1016/0031-9384(94)90066-3) [DOI] [PubMed] [Google Scholar]
- 87.Barrett J. E., King M. G., Pang G. 2000. Conditioning rhinitis in allergic humans. Ann. NY Acad. Sci. 917, 853–859 10.1111/j.1749-6632.2000.tb05451.x (doi:10.1111/j.1749-6632.2000.tb05451.x) [DOI] [PubMed] [Google Scholar]
- 88.Goebel M. U., Meykadeh N., Kou W., Schedlowski M., Hengge U. R. 2008. Behavioral conditioning of antihistamine effects in patients with allergic rhinitis. Psychother. Psychosom. 77, 227–234 10.1159/000126074 (doi:10.1159/000126074) [DOI] [PubMed] [Google Scholar]
- 89.Noelpp B., Noelpp-Eschenhagen I. 1951. Role of conditioned reflex in bronchial asthma; experimental investigation on the pathogenesis of bronchial asthma. Helv. Med. Acta 18, 142–158 [PubMed] [Google Scholar]
- 90.McKenzie J. 1886. The production of the so-called rose effect by means of an artificial rose, with remarks and historical notes. Am. J. Med. Sci. 91, 45–57 [Google Scholar]
- 91.Castells M., Schwartz L. B. 1988. Tryptase levels in nasal-lavage fluid as an indicator of the immediate allergic response. J. Allergy Clin. Immunol. 82, 348–355 10.1016/0091-6749(88)90005-X (doi:10.1016/0091-6749(88)90005-X) [DOI] [PubMed] [Google Scholar]
- 92.Niemi M. B., Härting M., Kou W., Del Rey A., Besedovsky H. O., Schedlowski M., Pacheco-López G. 2007. Taste-immunosuppression engram: reinforcement extinction. J. Neuroimmunol. 188, 74–79 10.1016/j.jneuroim.2007.05.016 (doi:10.1016/j.jneuroim.2007.05.016) [DOI] [PubMed] [Google Scholar]
- 93.Pacheco-López G., Engler H., Niemi M. B., Schedlowski M. 2006. Expectations and associations that heal: immunomodulatory placebo effects and its neurobiology. Brain Behav. Immunol. 20, 430–446 10.1016/j.bbi.2006.05.003 (doi:10.1016/j.bbi.2006.05.003) [DOI] [PubMed] [Google Scholar]