Abstract
This comprehensive review provides an overview about placebo and nocebo phenomena in antidepressant trials. Improvements in the placebo groups may partly be explained through methodological issues such as natural course of depression and regression to the mean, but also fundamentally reflect investigators' and participants' expectations. A meta-analysis by our group of 96 randomized placebo-controlled trials showed large placebo responses to antidepressant medication. Moderator analyses revealed substantially larger placebo responses in observer ratings compared with self-report. Effect sizes in observer ratings showed strong increase with publication year while this effect was not found for patients' self-ratings. This reflects the strong influence of investigators' expectations. The analysis of ‘nocebo effects’, e.g. adverse effects in placebo groups of antidepressant trials also confirms the impact of expectations: nocebo symptoms reflected the typical side-effect patterns expected in the drug group, with higher symptoms rates in the placebo groups of tricyclic antidepressant trials compared with placebo groups of trials testing selective serotonin reuptake inhibitors. While the placebo response seems to be similar for women and men, gender differences were found for nocebo rates. In the conclusion, we discuss potential implications for clinical trial designs and argue for interventions aimed at optimizing positive expectations of treatment benefit while minimizing the impact of adverse effects.
Keywords: placebo, nocebo, antidepressant, depression, meta-analysis, expectations
1. Introduction
Improvements that take place in the placebo group of clinical trials account for a substantial part of the effect of active drugs in many medical conditions [1]. In antidepressant drug trials, improvements in placebo groups seem to be notably powerful. As many as 30 per cent of the patients receiving placebo pills respond to treatment, compared with 50 per cent in the drug group [2]. Thus, the placebo response accounts for up to 75 per cent of the positive effects of antidepressant medication [3,4]. In other areas of research, the placebo response is estimated to comprise up to 50 per cent of the response to pain medication [5] or in generalized anxiety disorder [6]. However, not all changes occurring in the placebo group can be attributed to the ‘placebo effect’. In the same way that drug response and drug effect are distinguished, it is worth to distinguish between placebo response and placebo effect. The placebo response refers to changes that occur in the placebo group of clinical trials, while the placebo effect, more specifically, is defined as the difference between the placebo response and changes that occur without the administration of a placebo [4].
Kirsch et al. [7] noted that statistically significant differences between antidepressant medication and placebo might not necessarily translate into clinically significant benefit. In their recent meta-analysis, they found a clinical benefit for selective serotonin reuptake inhibitors (SSRIs) over placebo only for patients with high initial depression severity, owing to a decreased responsiveness to placebo in highly depressed patients rather than an increased responsiveness to medication. This conclusion has recently been questioned by Horder et al. [8], who criticized methodological aspects of the Kirsch study (e.g. the use of effect sizes; the computation of overall effect sizes for placebo groups and drug groups instead of computing effect sizes of drug–placebo differences within each trial). However, the approach of Kirsch et al. is considered appropriate in current methodological recommendations for meta-analyses. In fact, the alternatives suggested by Horder et al. only shed marginally better light on drug effects, without invalidating the major findings of the Kirsch et al. study.
In any case, high placebo responses and small differences between drug and placebo outcomes have even led to refusal of drug approval [9]. In recent years it has become increasingly difficult to prove drug efficacy against placebo, suggesting that the response to placebo might have increased [2,10].
Beside the positive effects of placebos, adverse effects that occur in placebo groups of clinical trials need to be considered. Placebos are known to induce side effects (the ‘nocebo’ phenomenon; [11]) that might lead to non-adherence and discontinuation of drug use [12]. Therefore, adverse effects in placebo groups have important implications for the risk–benefit assessment in clinical trials.
In this paper, we aim to give an overview about placebo and nocebo phenomena in antidepressant trials. We examine the placebo effect in trials testing antidepressant medication and review possible moderators. We argue that a substantial part of the placebo response is caused by expectations as a basic mechanism of the placebo effect. However, methodological issues play an important role and need to be taken into consideration. Furthermore, we examine side-effect patterns in the placebo groups of antidepressant trials. We argue that expectations about drug side effects substantially influence reported side effects in the placebo arms. We also consider gender differences in placebo and nocebo effects. It is necessary to point out that some of the mechanisms considered in this review have been derived from non-depression trials, and therefore need to be confirmed as valid for antidepressant trials in future.
2. The placebo response in antidepressant trials
A meta-analysis by our group estimated effect sizes of placebo groups in antidepressant trials and reviewed possible moderators [13]. The analysis included data from 96 randomized controlled trials, with 9566 patients in the placebo groups. Besides depression as primary outcome, effect sizes were calculated for anxiety, general psychopathology and quality of life. Medications in the active drug groups included SSRIs, tri- or tetracyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), herbal remedies, and others (for a more detailed description of the study protocol see [13]). Within-group effect sizes (Cohen's d) for the drug and placebo groups were computed. These effect sizes are defined as the mean difference from pre- to post-treatment divided by the pooled standard deviation and have been used in several studies to compare improvements in the drug group to those in the placebo group [4]. As a main result, a large placebo response of d = 1.69 (95% CI = 1.54–1.85) was documented, compared with d = 2.50 (95% CI = 2.30–2.69) in the drug groups. Thus, 67.6 per cent of the improvements in the drug groups can also be found in the placebo groups. In other words, only 32.4 per cent of the improvement can be considered as specific pharmacologic effects of antidepressant medication. The placebo response was highest for depression as a primary outcome. However, there were also substantial placebo responses for secondary outcomes such as anxiety, general psychopathology and quality of life [13]. The results are in line with the findings from other meta-analyses and confirm the strong placebo response to antidepressant medication [2,4,7,14–16].
Effect sizes in placebo groups were highly correlated with those in drug groups (r = 0.69, p < 0.001), as shown in previous studies [4]. A potential explanation could be that different types of antidepressants show different levels of effectiveness, and expectations about benefits might also influence effects in the placebo groups. However, mean placebo responses did not differ substantially (d = 1.65 in SSRI trials versus d = 1.73 in other antidepressant trials). The strong relation might be explained by non-specific variables (e.g. context effects). Studies set up certain psychosocial contexts or include unidentified parallel interventions (e.g. amount of time spent with the patient, provision of a supportive relationship, etc.) that differ across studies but have a similar impact both on treatment and placebo groups in the same trial [5,17]. These context effects might explain common variability within trials.
(a). Mechanisms underlying the placebo effect in depression
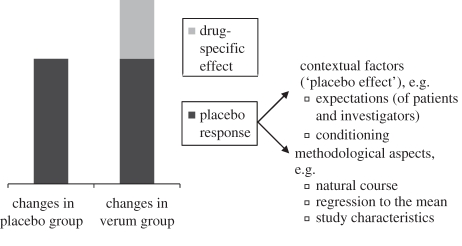
In order to understand mechanisms underlying the placebo effect in depression, it is helpful to look at two basic mechanisms of the placebo phenomenon: conditioning and expectation (figure 1). The effect of classical conditioning depends on prior experiences of combining drug features (e.g. a pill) with specific effects (e.g. antidepressant effect). In antidepressant trials, conditioning might facilitate the placebo response, but it is unlikely that all participants have been exposed to conditioning processes. The second and more important mechanism involves expectancy. Patients involved in a clinical study develop expectations about the benefits (and the risks) of the studied drug. Expectations are formed through several pathways. They can be elicited by verbal cues given by the study investigator, such as the information that a pill is a powerful pain killer or in contrast an inert substance [18]. Expectations are also formed through general knowledge about the effectiveness of a certain drug. Though placebo effects might be larger when conditioning processes and expectations interact [18], they can also be triggered by expectations alone [19]. Not only patients' expectancies but also those of study investigators constitute an essential element of the psychosocial context in which treatment takes place [20]. For instance, if a clinician promotes confidence in a certain drug, it might affect the patient's response to the medication. A single-blind randomized controlled trial in 262 patients with irritable bowel syndrome showed that placebo treatment is more effective when combined with a supportive patient–clinician relationship [21]. So generally, conditioning and expectations have been identified as important mechanisms of the placebo effect.
Figure 1.
Aspects of the placebo response in clinical trials.
3. Moderators of the placebo response
The data suggest that there is a large variation in the size of the placebo response. Reviews have pointed out several factors that influence the placebo phenomenon. These factors include the assessment method, the year of study publication, the type of placebo applied and the severity of depression.
(a). Assessment methods: observer rating versus self-report
Outcome in antidepressant trials can be assessed in different ways. The majority of trials in depression use observer ratings such as the Hamilton Depression Scale (HAMD). Some studies also apply additional self-rating scales such as the Beck Depression Inventory (BDI). Little is known whether changes in placebo groups also occur in self-ratings. When comparing placebo responses in observer ratings with those in self-report measures, considerably higher effect sizes in the observer ratings were found (d = 1.85; 95% CI = 1.69–2.01; 93 studies) compared with the self-report (d = 0.67; 95% CI = 0.49–0.85; 28 studies). The same was found in the drug group (observer ratings d = 2.89 versus self-report d = 1.12; [13]). Observer ratings and self-report were moderately correlated in those studies that reported both (Pearson's correlation r = 0.57; p < 0.01; 24 studies). These findings suggest that investigators' expectations are reflected in large placebo responses in observer ratings. However, it also raises questions about the validity of different assessment methods. Scientists doubt whether depressed patients are able to detect small mood changes, and therefore argue in favour of observer ratings. On the other hand, clinicians might tend to overestimate mood changes, which make additional assessment methods necessary [22].
(b). The publication year effect
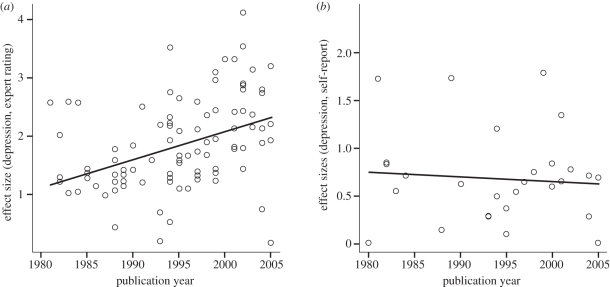
The placebo response in antidepressant trials has increased over the last decades. This so-called ‘publication year effect’ was first reported in the meta-analysis by Walsh et al. [2]. In our meta-analysis, a correlation of r = 0.41 (p < 0.001) between placebo effect size in the observer ratings and publication year was found, similar to r = 0.45 (p < 0.001) reported by Walsh. Interestingly, when changes were assessed via self-ratings, effect sizes in the placebo group were not related to publication year (r = −0.08; ns; figure 2). In the drug group, the same tendency was found, though the effect was less clear owing to more variance and failed to reach significance.
Figure 2.
Correlations of effect sizes with publication year: (a) observer ratings and (b) self-report. Adapted with permission from [13].
Expectations about the effectiveness of a drug depend—among other factors such as directly induced expectations—on previous experiences with the substance [1]. Since the beginning of the use of antidepressant drugs, millions of depressed patients have been treated. Thus, the belief in the effectiveness of antidepressant medication has probably increased over the last decades. Indeed, positive expectations and confidence in the drug have increased for study investigators, but not for study participants [13]. However, alternative explanations involve changes in the study populations, such as sample homogeneity. Since effect sizes are defined as the mean difference divided by the pooled standard deviation, more homogeneous samples lead to larger effects. The correlation of the standard deviation of baseline depression scores and publication year was significant and of moderate size (r = 0.30; p < 0.01). In other words, the tendency of including homogeneous patient groups has increased over time. However, the variation of standard deviations was less than 25 per cent of the values from 1980. Therefore, it can only partly account for the publication year effect. No changes in inclusion cut-off scores for depression or mean baseline depression severity over time could be found. As a conclusion, investigators' expectations about positive effects of antidepressant medication serve as a mechanism in explaining part of the growing placebo response in antidepressant trials. It seems unlikely that drug and placebo effects increased more than 100 per cent over the past two decades. Ultimately, this might lead to an overestimation of positive effects in placebo and drug groups and calls for careful selection of outcome variables and blinded assessment.
(c). Placebo condition: active placebos
Placebo pills are by definition inert substances that contain no active medication. However, this causes differences between placebo and drug groups not only in terms of efficacy, but also in terms of side effects. A few studies have applied so-called ‘active placebos’ that mimic side effects of the active medication. Moncrieff et al. [23] analysed eight studies that compared tricyclic antidepressants with active placebo conditions, usually atropine. The difference in effectiveness between TCAs and active placebos was small and non-significant (Hedge's g = 0.17; 95% CI = 0–0.34). An explanation for the decreased drug–placebo difference in this design is that side effects lead to unblinding both in patients and in raters, thereby strengthening the belief that the patient is in fact receiving the real drug. This belief seems to trigger positive expectations and enhances the placebo effect. In this case, the proportion of placebo effect in the drug group is larger than in the placebo group. Furthermore, the occurrence of side effects could also unblind raters and lead to biased observer ratings. Clinical trial data indicate that the ability of patients and investigators to correctly assume group assignment despite blinding exceeds chance levels and influences drug efficacy and safety [24,25]. Furthermore, side effects have been shown to highly correlate with patient and investigator outcome depression ratings [26].
4. Considering methodological issues
Expectations play a crucial role for the large placebo response in antidepressant drug trials. However, despite context factors like expectation and conditioning, other factors contributing to changes in placebo groups need to be considered. Those factors include the naturalistic course of the disease, regression-to-the-mean phenomena and selective study publication.
(a). Naturalistic course of depression
Usually patients are included in clinical trials during peak intensity periods of their illness. Thus, many patients spontaneously improve without any treatment. In depression, 45 to 71 per cent of the patients diagnosed with major depression at baseline show remission 12 months later [27,28]. In order to rule out naturalistic course effects, a control group that receives no treatment would be needed. However, these groups are rarely included in depression trials owing to ethical concerns. In a critical analysis of placebo, Hróbjartsson & Gøtzsche [29] compared placebo with no treatment in different medical conditions and found substantial small placebo effects. For eight studies reporting continuous outcomes in depression, they reported a standardized mean difference of d = 0.25 (95% CI = −0.05–0.55; [30]). Kirsch & Sapirstein [4] estimated from their analysis of no-treatment groups and waiting-list-control groups in psychotherapy trials that natural history accounts for approximately 24 per cent of the medication effect, leaving another 51 per cent to the placebo effect. The placebo effect needs to be distinguished from spontaneous remission, life changes and simple passage of time.
(b). The placebo response: a regression to the mean phenomenon?
Another important factor is represented by the regression to the mean, a statistical phenomenon which assumes that repeated measures with extreme values at the first assessment tend to approximate the centre of the distribution at the second assessment. As mentioned above, patients seek treatment when their depression levels are likely to be near the peak. Owing to fluctuation, levels tend to be lower at a second assessment point [17]. Walsh et al. [2] reported a larger placebo response in trials with higher minimum entry scores (r = 0.27; 95% CI = 0.02–0.49), suggesting that higher entry scores increase the impact of regression to the mean which might partly explain the placebo response in antidepressant trials. By contrast, Kirsch et al. [7] showed that placebo response does not change with depression severity.
(c). Selective publication of antidepressant trials
The selective publication of significant results comprises a crucial problem for all clinical trials and secondary analyses. In the meta-analysis, publication bias was controlled by computing fail-safe-n rates. It was shown that publication bias was unlikely to account for substantial improvements in the placebo group [13]. However, in a recent meta-analysis of antidepressant drug trials submitted to the US Food and Drug Administration (FDA), Turner et al. [31] compared effect sizes in published versus unpublished studies. They showed that published studies reported larger mean drug–placebo differences (Hedge's g = 0.41 versus g = 0.17). A possible interpretation could be that the placebo effect might actually be even larger than assumed.
5. Nocebo effects in antidepressant trials
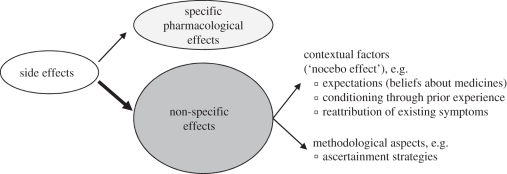
In routine clinical care, clinicians' decisions to prescribe antidepressant medication depend as much on minimizing adverse effects as on maximizing drug efficacy. Fifty to sixty per cent of patients taking antidepressant medications report side effects [32]. Side effects of antidepressant drugs have strong consequences on prescription behaviour, adherence and discontinuation of drug use. While SSRIs and TCAs are similarly effective [33], SSRIs are prescribed more often because of their favourable side-effect profile [34]. Rates of antidepressant treatment discontinuation can reach up to 60 per cent, and adverse effects are the most common reason for non-adherence and drug discontinuation [35]. Fifteen to twenty-seven per cent of patients discontinue medication use owing to perceived side effects [36]. Experience of at least one bothersome side effect makes patients three times more likely to discontinue drug use [37]. Adverse effects do not only occur in the medication group, about a quarter of the patients in the placebo group report side effects [11]. In analogy to placebo, side effects can be divided into specific pharmacokinetic drug effects and non-specific effects (i.e. clinical nocebo effects; figure 3). Nocebo effects are symptoms that cannot be explained on the basis of known pharmacology of the drug. In clinical studies, only a small proportion of reported side effects are actually pharmacologically related to the drug, while the majority of reported symptoms are non-specific side effects [11]. They influence adherence and discontinuation processes in a similar way to the ‘real’ drug side effects [12].
Figure 3.
Factors influencing medication side effects.
(a). Mechanisms underlying the nocebo effect
The crucial question is how patients develop side effects when they do not receive an active drug. Again, the two mechanisms underlying the nocebo phenomenon involve conditioning and expectations. First, conditioning processes might apply through prior experiences and learning processes [11,38]. Patients might manifest side effects not because of specific pharmacology of the medication, but because they have experienced side effects during former antidepressant treatment. Second, patients who expect to experience distressing side effects are more likely to develop them [39]. The mention of a certain side effect in the informed consent bears a higher risk of actually reporting it [40,41]. Link et al. [42] reported that participants who believed they received a herbal drug experienced more side effects compared with those who believed they received a placebo, although all participants received a placebo. Neuroimaging studies also confirm the importance of expectations for nocebo effects. Keltner et al. [43] found that the level of expected pain intensity changed the perceived pain intensity and also resulted in activation of different brain regions independent from the applied pain. In many cases, perceived side effects do not arise from newly experienced symptoms, but patients might attribute already existing symptoms to a newly prescribed medication. Thus, symptoms of the underlying disease for which the patient receives treatment, for instance, tiredness in depression, might be misattributed as side effects of the medication [11]. Moreover, unspecific somatic symptoms are extremely common in the general population [44,45], making it likely to misclassify already existing symptoms as drug-elicited side effects.
(b). Adverse effects in placebo groups of antidepressant trials
When taking antidepressant medication, patients might expect to experience a certain side-effect pattern depending on the type of the drug. While SSRIs are known to have more activating side effects (such as extrapyramidal symptoms), TCAs are supposed to have stronger sedating and cholinergic side effects such as dry mouth and dizziness [32]. Negative expectations about potential side effects are frequently elicited through informed consent procedures, medication package inserts or information through fellow patients and the media, and might lead to increased side effects reporting [18,46,47]. Little is known, however, how adverse effects in placebo groups are related to those in the drug group. There is recent evidence that side-effect patterns of the drug group strongly influence those in the placebo group in anti-migraine trials [48]. A systematic review by our group investigated adverse effect patterns in antidepressant trials [49]. Randomized controlled trials that tested SSRIs (122 trials) and TCAs (21 trials) in the treatment of depressive disorders and anxiety disorders were included (for further details see [49]). The data revealed that one quarter of the patients discontinued treatment, with similar discontinuation rates in drug and placebo group (24.8% versus 24.7%). Of those patients discontinuing explicitly because of perceived adverse effects more than 40 per cent were in the placebo group.
(c). The role of ascertainment strategies
In many clinical trials, the evaluation of adverse effects is often done with less valid and reliable instruments than the evaluation of drug benefits. The better the assessment quality, the more likely effects are detected [12]. In our review, systematic assessment yielded higher rates of reported symptoms for 14 of 17 symptoms than unstructured assessment methods, with a mean odds ratio of 2.6 (mean 95% CI = 2.1–3.3; [49]). In other words, the report of adverse effects is much more likely when assessed with systematic strategies.
(d). How ‘unspecific’ are unspecific side effects?
The aim of our review was to determine whether adverse effects in placebo groups reflect those in the antidepressant drug groups. Symptom report in the placebo group was highly related to symptom report in the drug group for seven of the eight most reported symptoms. For most symptoms (diarrhoea, dizziness, drowsiness, dry mouth, insomnia and headache) high correlations above r > 0.60 (all p < 0.001) were found. Higher side-effect rates were found in TCA placebo groups compared with SSRI placebo groups for 10 of 18 symptoms. Side-effect patterns in the placebo groups reflected those in the drug group. Symptoms typically associated with tricyclic antidepressants were more common in the TCA placebo groups. These symptoms included dry mouth (19.2% versus 6.4%), fatigue (17.3% versus 5.5%), drowsiness (16.8% versus 6.8%), constipation (10.7% versus 4.2%), vision/accommodation problems (6.9% versus 1.2%) and dizziness (13.8% versus 7.7%). These differences could not be attributed to differences in study characteristics between SSRI and TCA trials. These results lead to the conclusion that ‘unspecific’ adverse effects are highly drug specific and reflect expected side-effect patterns of the antidepressant drug to which placebo is compared.
6. Gender differences in placebo and nocebo effects
Studies suggest that women and men respond differently to antidepressant treatment. While men seem to present more favourable outcome on TCAs, women seem to respond better to SSRIs and MAOIs [50]. These differences are relevant for the prescription behaviour and success of antidepressant treatment. However, these differences do not seem to be reflected in the placebo groups. Several studies did not find any gender differences in the response to placebo [50–52].
Few studies have addressed gender differences in the nocebo effect. Women experience adverse drug reactions more often than do men [53], and evidence suggests that women are more prone to experience nocebo effects [54]. In a study evaluating the SSRI fluoxetine, women reported slightly more nocebo effects than men (38.5% versus 30.1%, p < 0.01) [52]. Experimental studies suggest that different mechanisms might apply. Employing a motion sickness paradigm, Klosterhalfen et al. [55] reported that women responded predominantly to conditioning, while men showed greater nocebo responses when verbal suggestions were used. Gender differences in nocebo responses might have an impact on study trial design and prescription behaviour. In a recent review by our group, gender differences in antidepressant trials were examined [56]. Since most of the studies did not analyse gender differences, trials with at least two-thirds of male subjects were compared with those that included predominantly women (at least 66.6%). Results differed drastically for trials of depression and trials of anxiety disorders. In depression, against expectation, trials that included predominantly male subjects reported more nocebo effects for nine of 11 symptoms (e.g. nausea, sweating and dizziness). In anxiety, however, the effect was reversed. Trials that included more women reported higher nocebo effects for eight of 11 symptoms (table 1). Though owing to methodology only proportions could be compared, the data suggest that different mechanisms might apply for men and women. Possibly, expectation processes have a higher impact in depression while subjects with anxiety disorders show stronger response to conditioning processes. Thus, male subjects with depression could be more prone to report adverse effects than female subjects with depression, while relations are vice versa in anxiety. Experimental research is needed in order to identify underlying mechanisms. However, the meta-analytical approach does not allow separation of the role of gender from other potentially influencing factors such as demographic variables or differences in study characteristics. Moreover, there are other influencing factors such as neuroticism [57], somatization [58] or beliefs about medicines [39] that predict the report of unspecific side effects and that might interact with gender and clinical diagnosis.
Table 1.
Gender comparison of nocebo rates in pharmacological trials of depression and anxiety disorders. n, total sample size in the placebo group; ktotal, total number of placebo groups reporting the symptom; 95% CI, confidence interval of odds ratio; modified from Nestoriuc & Rief [56].
| symptom | diagnosis: depression |
diagnosis: anxiety disorder |
||||
|---|---|---|---|---|---|---|
| n (ktotal) | odds ratioa | 95% CI | n (ktotal) | odds ratioa | 95% CI | |
| loss of appetite | 505 (9) | 13.2 | (6.6; 47.9) | 264 (5) | 0.3 | (0.02; 1.3) |
| sexual problems | 803 (5) | 4.0 | (1.6; 9.8) | 451 (8) | 0.2 | (0.09; 0.6) |
| sweating | 549 (5) | 12.5 | (6.0; 26.1) | 309 (5) | 0.2 | (0.06; 0.5) |
| dry mouth | 310 (12) | 0.6 | (0.1; 2.6) | 527 (13) | 0.7 | (0.42; 1.2) |
| nausea | 1291 (18) | 2.3 | (1.18; 4.7) | 791 (14) | 0.5 | (0.3; 0.8) |
| dizziness | 773 (11) | 10.5 | (6.2; 18.0) | 269 (7) | 0.3 | (0.2; 0.6) |
| drowsiness | 1062 (10) | 6.7 | (4.1; 11.1) | 551 (8) | 0.5 | (0.3; 0.9) |
| insomnia | 1009 (13) | 1.9 | (0.6; 5.6) | 701 (11) | 0.4 | (0.2; 0.5) |
| nervousness | 197 (6) | 3.4 | (1.1; 10.1) | 299 (7) | 0.3 | (0.2; 0.7) |
| diarrhoea | 944 (7) | 4.1 | (2.6; 6.5) | 406 (6) | 0.8 | (0.5; 1.5) |
| headache | 830 (12) | 3.1 | (1.3; 7.6) | 586 (11) | 0.6 | (0.4; 0.9) |
aNocebo rates from studies with high rates of female patients (greater than 66.6%) in relation to studies with high rates of male patients (greater than 66.6%); odds ratios higher than one denote higher nocebo rates in men, odds ratios smaller than one denote higher nocebo rates in women; significant effects are denoted in bold.
7. Implications for clinical research and practice
In this comprehensive review, we investigated the placebo and nocebo effects in antidepressant trials. A meta-analysis by our group confirmed the large placebo response in antidepressant trials [13]. Moderator analyses have revealed patients' and investigators' expectations as basic mechanisms of the placebo effect. Investigators' expectations are reflected in larger placebo responses in observer ratings than in self report, as well as in increasing placebo responses over time (‘publication year effect’). The importance of patients' expectations can be demonstrated in larger placebo responses when ‘active placebos’ that mimic side-effect patterns of the active drug are used as comparators [23]. The ‘unblinding’ properties of side effects might result in larger placebo effects in the drug group than in the placebo group. Though neurophysiological correlates of change are similar in drug and placebo, data suggest that placebo response in depression goes along with specific changes in brain function [59,60]. In analgesia, Colloca & Benedetti [5] propose two different neurophysiological pathways: a drug-induced pain pathway and a placebo-induced expectation pathway. Since both pathways are activated when a drug is given, the pharmacological effects of a certain drug need to be disentangled. Thus, different mechanisms might apply in medication and placebo groups that are not detected with standard clinical trial designs.
Beneath the fact that side effects might serve as a cue and thereby enhance response to treatment, adverse events can be very bothersome and lead to discontinuation of drug use [49]. In antidepressant trials, placebos induce side effects that strongly resemble the pattern of the active medication to which they are compared [49]. Different side-effect patterns were found for SSRIs and TCAs. Nocebo rates were higher in trials including predominantly men in depression, while anxiety trials reported higher nocebo rates in trials with more female subjects. Adverse effects influenced discontinuation, thus suggesting that nocebo effects play an important role for non-adherence to antidepressant medication in clinical practice.
However, placebo and nocebo effects need to be understood in the light of methodological issues. First, studies on the natural course of depression show high remission rates [27]. Data that compare placebos to natural course groups confirm the large impact of spontaneous fluctuation over time [29]. Regression to the mean might artificially inflate placebo and drug responses. Selective publication of antidepressant trials might limit the interpretation of the findings [31]. Concerning the nocebo effect, structured assessment leads to considerably higher report of symptoms than unstructured methods [49].
(a). Do we need to reconsider clinical trial designs?
Since the beginning of placebo use, scientists have expressed ethical concerns, stating that their use contradicts the Declaration of Helsinki [61]. It is claimed that, with effective antidepressants on the market, placebo control groups might not be necessary anymore and new drugs should be compared with interventions with established efficacy [62]. On the other hand, it has been argued in favour of placebo that the comparison of a new medication with placebo reduces the risk to public health of approving ineffective medications. Evidence does not support an increased suicide risk in placebo groups of antidepressant trials [63]. Our data suggest that the placebo effect in antidepressant trials is a highly variable phenomenon that depends as much on patients' and investigators' expectations as on study methodology. If a new medication is approved on the basis of equal efficacy to an approved one, it is unclear how much of the improvement is caused by the substantial placebo effect and other ‘unspecific’ factors. Therefore, neither the use of historical placebo benchmarks nor the mere comparison with an approved treatment can be recommended. The claim by Horder et al. [8] for improving antidepressant research is highly supported. Moreover, effect sizes of antidepressants tend to be larger in active comparator trials compared with placebo-controlled trials [64,65]. When a patient expects to receive a real treatment, he improves better than under the 50 per cent chance of receiving an inert substance. This fact provides further evidence of the importance of patients' expectations, but it does not provide further evidence of the real impact of antidepressant medication.
With the standard randomized controlled trial design, it is difficult to estimate the impact of the placebo effect. There is a need for more creative designs capable of disentangling the placebo effect. A crucial question is how pharmacological effects of a drug can be separated from expectation pathways. One solution results from the open–hidden paradigm where medication is given covertly [66]. Hidden administration of medication has been proved to be less effective than open administration when treatment is combined with a ‘healing’ procedure. Colloca et al. suggest that the efficacy of a treatment can be estimated through the difference between open and hidden application, thus estimating the placebo effect without the use of an actual placebo group. This is difficult, however, to apply in antidepressant treatment. A more practical possibility is the ‘balanced placebo design’ [4]. Subjects receive either drug or placebo, and half of each group is receiving false information about treatment. Thus, the group that is receiving the real medication but believes to receive placebo serves to estimate the ‘true’ drug effect. However, the balanced placebo design applies deception and therefore raises major ethical concerns in clinical trials involving patients. To overcome these ethical concerns, Miller et al. [67] have proposed to apply ‘authorized deception’ that has proved to be equally effective in inducing placebo responses in experimental research [68]. Further studies are needed to examine the effect of authorized deception in antidepressant trials.
Another point involves the importance of perceived assignment. The perceived group assignment might be more important than the real assignment to intervention or placebo group [69]. A double-blind sham-surgery controlled trial of human embryonic dopamine neurons in patients with Parkinson's disease revealed that the perceived treatment had more impact on motor function improvement and the quality of life than did the actual treatment (real or sham surgery; [70]). In a clinical trial, assessing expectations about perceived group assignment could be useful to understand treatment responses. Moreover, ‘active placebos’ might control for the unblinding properties of side effects and should be applied in clinical trials. Natural course groups in antidepressant trials could help to distinguish the placebo effect from passage of time. The ethical problem of not treating patients in natural course groups, however, remains unresolved. In order to understand the mechanisms of non-specific treatment effects, it is necessary to assess those effects with structured assessment methods. Since clinicians tend to overestimate changes in patients, clinician bias has to be managed through the addition of self-report measures, the administration of both clinician- and patient-rated measures, and systematic rater training [22].
Concerning the nocebo effect, our data strongly indicate the importance of systematic side-effect assessment. We have recently proposed a new instrument, the Generic Assessment of Side Effects scale (GASE [71]). When adverse effects are assessed with valid structured assessment methods, a better risk–benefit calculation is possible. Furthermore, base rates of somatic complaints should be assessed in order to control for attribution processes [45]. The fact that gender influences the experience of nocebo effects argues for balancing and controlling gender in clinical trial designs. Further research needs to shed light on the interaction of gender and diagnosis in the nocebo effect.
(b). Conclusions and future directions
The findings on placebo and nocebo effects in antidepressant trials have important implications for clinical practice. The high response rate of depressive symptoms to placebo reflects the therapeutic responsiveness of depression in general, thus highlighting the importance of multi-dimensional treatment approaches. In sum, pharmacological effects of antidepressant medication account for a minor part of the observed effects in antidepressant trials. Instead of minimizing non-specific therapeutic effects, placebo effects should be enhanced in order to optimize patients' response to both placebo and medication. This could be achieved by providing an optimal healing context; in other words, providing a treatment context in which social, psychological, and medical treatment components are designed to enhance the healing process [72]. Patients' expectations predict the success of antidepressant medication [73]. Optimizing patients' expectations might be a promising way of enhancing the positive effects of antidepressant treatment. Trials are needed that systematically vary both patients' and researchers' expectations.
With respect to the nocebo phenomenon, researchers should closely consider the way in which negative suggestions are delivered (e.g. through informed consent procedures). Though detailed information about adverse effects is necessary, negative suggestions inducing anxiety probably enhance the nocebo effect, and providing exhaustive lists of unproven side effects in drug leaflets is not warranted. It might be helpful to identify patients at high risk of developing nocebo effects [11,39]. Discussing the nocebo phenomenon explicitly with the patient might help to become more aware of self-fulfilling prophecies induced by misattribution. Tailored prevention programmes are needed that aim at helping patients tolerates adverse effects and reduces perceived disability.
Footnotes
One contribution of 17 to a Theme Issue ‘Placebo effects in medicine: mechanisms and clinical implications’.
References
- 1.Rief W., Hofmann S. G., Nestoriuc Y. 2008. The power of expectation—understanding the placebo and nocebo phenomenon. Soc. Pers. Psychol. Compass 2, 1624–1637 10.1111/j.1751-9004.2008.00121.x (doi:10.1111/j.1751-9004.2008.00121.x) [DOI] [Google Scholar]
- 2.Walsh B. T., Seidman S. N., Sysko R., Gould M. 2002. Placebo response in studies of major depression: variable, substantial, and growing. JAMA 287, 1840–1847 10.1001/jama.287.14.1840 (doi:10.1001/jama.287.14.1840) [DOI] [PubMed] [Google Scholar]
- 3.Kirsch I., Moore T. J., Scoboria A., Nicholls S. S. 2002. The emperor's new drugs: an analysis of antidepressant medication data submitted to the US Food and Drug Administration. Prevent. Treat. 5, 23 [Google Scholar]
- 4.Kirsch I., Sapirstein G. 1998. Listening to Prozac but hearing placebo: a meta-analysis of antidepressant medication. Prevent. Treat. 1, 0002a [Google Scholar]
- 5.Colloca L., Benedetti F. 2005. Placebos and painkillers: is mind as real as matter? Nat. Rev. Neurosci. 6, 545–552 10.1038/nrn1705 (doi:10.1038/nrn1705) [DOI] [PubMed] [Google Scholar]
- 6.Andrews G. 2001. Placebo response in depression: bane of research, boon to therapy. Br. J. Psychiatry 178, 192–194 10.1192/bjp.178.3.192 (doi:10.1192/bjp.178.3.192) [DOI] [PubMed] [Google Scholar]
- 7.Kirsch I., Deacon B. J., Huedo-Medina T. B., Scoboria A., Moore T. J., Johnson B. T. 2008. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 5, 260–268 10.1371/journal.pmed.0050045 (doi:10.1371/journal.pmed.0050045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horder J., Matthews P., Waldmann R. In press Placebo, Prozac and PLoS: significant lessons for psychopharmacology. J. Psychopharmacol. (doi:10.1177/0269881110372544) [DOI] [PubMed] [Google Scholar]
- 9.Enserink M. 1999. Psychopharmacology: can the placebo be the cure? Science 284, 238–240 10.1126/science.284.5412.238 (doi:10.1126/science.284.5412.238) [DOI] [PubMed] [Google Scholar]
- 10.Dworkin R. H., Turk D. C., Peirce-Sandner S., McDermott M. P., Farrar J. T., Hertz S., Katz N. P., Raja S. N., Rappaport B. A. 2010. Placebo and treatment group responses in postherpetic neuralgia vs. painful diabetic peripheral neuropathy clinical trials in the REPORT database. Pain 150, 12–16 10.1016/j.pain.2010.02.002 (doi:10.1016/j.pain.2010.02.002) [DOI] [PubMed] [Google Scholar]
- 11.Barsky A. J., Saintfort R., Rogers M. P., Borus J. F. 2002. Nonspecific medication side effects and the nocebo phenomenon. JAMA 287, 622–627 10.1001/jama.287.5.622 (doi:10.1001/jama.287.5.622) [DOI] [PubMed] [Google Scholar]
- 12.Rief W., Avorn J., Barsky A. J. 2006. Medication-attributed adverse effects in placebo groups: implications for assessment of adverse effects. Arch. Intern. Med. 166, 155–160 10.1001/archinte.166.2.155 (doi:10.1001/archinte.166.2.155) [DOI] [PubMed] [Google Scholar]
- 13.Rief W., Nestoriuc Y., Weiss S., Welzel E., Barsky A. J., Hofmann S. G. 2009. Meta-analysis of the placebo response in antidepressant trials. J. Affect. Disord. 118, 1–8 10.1016/j.jad.2009.01.029 (doi:10.1016/j.jad.2009.01.029) [DOI] [PubMed] [Google Scholar]
- 14.Joffe R., Sokolov S., Streiner D. 1996. Antidepressant treatment of depression: a metaanalysis. Can. J. Psychiatry 41, 613–616 [DOI] [PubMed] [Google Scholar]
- 15.Khan A., Warner H. A., Brown W. A. 2000. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: an analysis of the Food and Drug Administration database. Arch. Gen. Psychiatry 57, 311. 10.1001/archpsyc.57.4.311 (doi:10.1001/archpsyc.57.4.311) [DOI] [PubMed] [Google Scholar]
- 16.Fournier J. C., DeRubeis R. J., Hollon S. D., Dimidjian S., Amsterdam J. D., Shelton R. C., Fawcett J. 2010. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303, 47–53 10.1001/jama.2009.1943 (doi:10.1001/jama.2009.1943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst E., Resch K. L. 1995. Concept of true and perceived placebo effects. Br. Med. J. 311, 551–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finniss D. G., Kaptchuk T. J., Miller F., Benedetti F. 2010. Biological, clinical, and ethical advances of placebo effects. Lancet 375, 686–695 10.1016/S0140-6736(09)61706-2 (doi:10.1016/S0140-6736(09)61706-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedetti F., Pollo A., Lopiano L., Lanotte M., Vighetti S., Rainero I. 2003. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J. Neurosci. 23, 4315–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Blasi Z., Harkness E., Ernst E., Georgiou A., Kleijnen J. 2001. Influence of context effects on health outcomes: a systematic review. Lancet 357, 757–762 10.1016/S0140-6736(00)04169-6 (doi:10.1016/S0140-6736(00)04169-6) [DOI] [PubMed] [Google Scholar]
- 21.Kaptchuk T. J., et al. 2008. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 336, 999–1003 10.1136/bmj.39524.439618.25 (doi:10.1136/bmj.39524.439618.25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fava M., Evins A. E., Dorer D. J., Schoenfeld D. A. 2003. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother. Psychosom. 72, 115–127 10.1159/000069738 (doi:10.1159/000069738) [DOI] [PubMed] [Google Scholar]
- 23.Moncrieff J., Wessely S., Hardy R. 2004. Active placebos versus antidepressants for depression. Cochrane Database Syst. Rev. 1, CD003012. 10.1002/14651858.CD003012.pub2 (doi:10.1002/14651858.CD003012.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabkin J. G., Markowitz J. S., Stewart J., McGrath P., Harrison W., Quitkin F. M., Klein D. F. 1986. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 19, 75–86 10.1016/0165-1781(86)90094-6 (doi:10.1016/0165-1781(86)90094-6) [DOI] [PubMed] [Google Scholar]
- 25.Holroyd K. A., Tkachuk G., O'Donnell F., Cordingley G. E. 2006. Blindness and bias in a trial of antidepressant medication for chronic tension-type headache. Cephalalgia 26, 973–982 10.1111/j.1468-2982.2006.01139.x (doi:10.1111/j.1468-2982.2006.01139.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberg R. P., Bornstein R. F., Fisher S., Zborowski M. J., Greenberg M. D. 1994. A meta-analysis of fluoxetine outcome in the treatment of depression. J. Nerv. Ment. Dis. 182, 547–555 10.1097/00005053-199410000-00003 (doi:10.1097/00005053-199410000-00003) [DOI] [PubMed] [Google Scholar]
- 27.Rhebergen D., Beekman A. T. F., Graaf R. d., Nolen W. A., Spijker J., Hoogendijk W. J., Penninx B. W. J. H. 2009. The three-year naturalistic course of major depressive disorder, dysthymic disorder and double depression. J. Affect. Disord. 115, 450–459 10.1016/j.jad.2008.10.018 (doi:10.1016/j.jad.2008.10.018) [DOI] [PubMed] [Google Scholar]
- 28.Wells K. B., Burnam M. A., Rogers W., Hays R., Camp P. 1992. The course of depression in adult outpatients. Arch. Gen. Psychiatry 49, 788–794 [DOI] [PubMed] [Google Scholar]
- 29.Hróbjartsson A., Gøtzsche P. C. 2001. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N. Engl J. Med. 344, 1594–1602 10.1056/NEJM200105243442106 (doi:10.1056/NEJM200105243442106) [DOI] [PubMed] [Google Scholar]
- 30.Hróbjartsson A., Gøtzsche P. C. 2004. Placebo interventions for all clinical conditions. Cochrane Database Syst. Rev. 2, CD003974. 10.1002/14651858.CD003974.pub2 (doi:10.1002/14651858.CD003974.pub2) [DOI] [PubMed] [Google Scholar]
- 31.Turner E. H., Matthews A. M., Linardatos E., Tell R. A., Rosenthal R. 2008. Selective publication of antidepressant trials and its influence on apparent efficacy. N. Engl J. Med. 358, 252–260 10.1056/NEJMsa065779 (doi:10.1056/NEJMsa065779) [DOI] [PubMed] [Google Scholar]
- 32.Brambilla P., Cipriani A., Hotopf M., Barbui C. 2005. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry 38, 69–77 10.1055/s-2005-837806 (doi:10.1055/s-2005-837806) [DOI] [PubMed] [Google Scholar]
- 33.Cipriani A., Brambilla P., Furukawa T., Geddes J., Gregis M., Hotopf M., Malvini L., Barbui C., Cipriani A. M. 2005. Fluoxetine versus other types of pharmacotherapy for depression. Cochrane Database Syst. Rev. 4, CD004185. (doi:10.1002/14651858.CD004185.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrenson R. A., Tyrer F., Newson R. B., Farmer R. D. T. 2000. The treatment of depression in UK general practice: selective serotonin reuptake inhibitors and tricyclic antidepressants compared. J. Affect. Disord. 59, 149–157 10.1016/S0165-0327(99)00147-0 (doi:10.1016/S0165-0327(99)00147-0) [DOI] [PubMed] [Google Scholar]
- 35.Ashton A. K., Jamerson B. D. 2005. Antidepressant-related adverse effects impacting treatment compliance: results of a patient survey. Curr. Ther. Res. Clin. Exp. 66, 96–106 10.1016/j.curtheres.2005.04.006 (doi:10.1016/j.curtheres.2005.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montgomery S. A., Henry J., McDonald G., Dinan T., Lader M., Hindmarch I., Clare A., Nutt D. 1994. Selective serotonin reuptake inhibitors: meta-analysis of discontinuation rates. Int. Clin. Psychopharmacol. 9, 47–54 10.1097/00004850-199400910-00008 (doi:10.1097/00004850-199400910-00008) [DOI] [PubMed] [Google Scholar]
- 37.Bull S. A., Hu X. H., Hunkeler E. M., Lee J. Y., Ming E. E., Markson L. E., Fireman B. 2002. Discontinuation of use and switching of antidepressants: influence of patient–physician communication. JAMA 288, 1403–1409 10.1001/jama.288.11.1403 (doi:10.1001/jama.288.11.1403) [DOI] [PubMed] [Google Scholar]
- 38.Colloca L., Sigaudo M., Benedetti F. 2008. The role of learning in nocebo and placebo effects. Pain 136, 211–218 10.1016/j.pain.2008.02.006 (doi:10.1016/j.pain.2008.02.006) [DOI] [PubMed] [Google Scholar]
- 39.Nestoriuc Y., Orav E. J., Liang M. H., Horne R., Barsky A. J. 2010. Prediction of nonspecific side effects in rheumatoid arthritis patients by beliefs about medicines. Arthritis Care Res. 62, 791–799 10.1002/acr.20160 (doi:10.1002/acr.20160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mondaini N., Gontero P., Giubilei G., Lombardi G., Cai T., Gavazzi A., Bartoletti R. 2007. Finasteride 5mg and sexual side effects: how many of these are related to a nocebo phenomenon? J. Sex Med. 4, 1708–1712 10.1111/j.1743-6109.2007.00563.x (doi:10.1111/j.1743-6109.2007.00563.x) [DOI] [PubMed] [Google Scholar]
- 41.Myers M. G., Cairns J. A., Singer J. 1987. The consent form as a possible cause of side effects. Clin. Pharmacol. Ther. 42, 250–253 10.1038/clpt.1987.142 (doi:10.1038/clpt.1987.142) [DOI] [PubMed] [Google Scholar]
- 42.Link J., Haggard R., Kelly K., Forrer D. 2006. Placebo/nocebo symptom reporting in a sham herbal supplement trial. Eval. Health Prof. 29, 394–406 10.1177/0163278706293403 (doi:10.1177/0163278706293403) [DOI] [PubMed] [Google Scholar]
- 43.Keltner J. R., Furst A., Fan C., Redfern R., Inglis B., Fields H. L. 2006. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J. Neurosci. 26, 4437–4443 10.1523/jneurosci.4463-05.2006 (doi:10.1523/jneurosci.4463-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiller W., Rief W., Brähler E. 2006. Somatization in the population: from mild bodily misperceptions to disabling symptoms. Soc. Psychiatry Psychiatr. Epidemiol. 41, 704–712 10.1007/s00127-006-0082-y (doi:10.1007/s00127-006-0082-y) [DOI] [PubMed] [Google Scholar]
- 45.Rief W., Hessel A., Braehler E. 2001. Somatization symptoms and hypochondriacal features in the general population. Psychosom. Med. 63, 595–602 [DOI] [PubMed] [Google Scholar]
- 46.Enck P., Benedetti F., Schedlowski M. 2008. New insights into the placebo and nocebo responses. Neuron 59, 195–206 10.1016/j.neuron.2008.06.030 (doi:10.1016/j.neuron.2008.06.030) [DOI] [PubMed] [Google Scholar]
- 47.Sohl S. J., Schnur J. B., Montgomery G. H. 2009. A meta-analysis of the relationship between response expectancies and cancer treatment-related side effects. J. Pain Symptom Manage. 38, 775–784 10.1016/j.jpainsymman.2009.01.008 (doi:10.1016/j.jpainsymman.2009.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amanzio M., Corazzini L. L., Vase L., Benedetti F. 2009. A systematic review of adverse events in placebo groups of anti-migraine clinical trials. Pain 146, 261–269 10.1016/j.pain.2009.07.010 (doi:10.1016/j.pain.2009.07.010) [DOI] [PubMed] [Google Scholar]
- 49.Rief W., Nestoriuc Y., Anna von L.-T., Dogan I., Schreiber F., Hofmann S. G., Barsky A. J., Avorn J. 2009. Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: a systematic review and meta-analysis. Drug Saf. 32, 1041–1056 10.2165/11316580-000000000-00000 (doi:10.2165/11316580-000000000-00000) [DOI] [PubMed] [Google Scholar]
- 50.Kornstein S. G., McEnany G. 2000. Enhancing pharmacologic effects in the treatment of depression in women. J. Clin. Psychiatry 61, 18–27 [PubMed] [Google Scholar]
- 51.Quitkin F. M., Stewart J. W., McGrath P. J., Taylor B. P., Tisminetzky M. S., Petkova E., Chen Y., Ma G., Klein D. F. 2002. Are there differences between women's and men's antidepressant responses? Am. J. Psychiatry 159, 1848–1854 10.1176/appi.ajp.159.11.1848 (doi:10.1176/appi.ajp.159.11.1848) [DOI] [PubMed] [Google Scholar]
- 52.Casper R. C., Tollefson G. D., Nilsson M. E. 2001. No gender differences in placebo responses of patients with major depressive disorder. Biol. Psychiatry 49, 158–160 10.1016/S0006-3223(00)00966-5 (doi:10.1016/S0006-3223(00)00966-5) [DOI] [PubMed] [Google Scholar]
- 53.Zopf Y., Rabe C., Neubert A., Gassmann K. G., Rascher W., Hahn E. G., Brune K., Dormann H. 2008. Women encounter ADRs more often than do men. Eur. J. Clin. Pharmacol. 64, 999–1004 10.1007/s00228-008-0494-6 (doi:10.1007/s00228-008-0494-6) [DOI] [PubMed] [Google Scholar]
- 54.Liccardi G., et al. 2004. Evaluation of the nocebo effect during oral challenge in patients with adverse drug reactions. J. Investig. Allergol. Clin. Immunol. 14, 104–107 [PubMed] [Google Scholar]
- 55.Klosterhalfen S., Kellermann S., Braun S., Kowalski A., Schrauth M., Zipfel S., Enck P. 2009. Gender and the nocebo response following conditioning and expectancy. J. Psychosom. Res. 66, 323–328 10.1016/j.jpsychores.2008.09.019 (doi:10.1016/j.jpsychores.2008.09.019) [DOI] [PubMed] [Google Scholar]
- 56.Nestoriuc Y., Rief W. 2010. Gender differences in the nocebo response in antidepressant trials. Z Med. Psychol. 19, 175–179 [Google Scholar]
- 57.Davis C., Ralevski E., Kennedy S. H., Neitzert C. 1995. The role of personality factors in the reporting of side effect complaints to moclobemide and placebo: a study of healthy male and female volunteers. J. Clin. Psychopharmacol. 15, 347–352 10.1097/00004714-199510000-00007 (doi:10.1097/00004714-199510000-00007) [DOI] [PubMed] [Google Scholar]
- 58.Agosti V., Quitkin F. M., Stewart J. W., McGrath P. J. 2002. Somatization as a predictor of medication discontinuation due to adverse events. Int. Clin. Psychopharmacol. 17, 311–314 10.1097/00004850-200211000-00007 (doi:10.1097/00004850-200211000-00007) [DOI] [PubMed] [Google Scholar]
- 59.Leuchter A. F., Cook I. A., Witte E. A., Morgan M., Abrams M. 2002. Changes in brain function of depressed subjects during treatment with placebo. Am. J. Psychiatry 159, 122–129 10.1176/appi.ajp.159.1.122 (doi:10.1176/appi.ajp.159.1.122) [DOI] [PubMed] [Google Scholar]
- 60.Mayberg H. S., Silva J. A., Brannan S. K., Tekell J. L., Mahurin R. K., McGinnis S., Jerabek P. A. 2002. The functional neuroanatomy of the placebo effect. Am. J. Psychiatry 159, 728–737 10.1176/appi.ajp.159.5.728 (doi:10.1176/appi.ajp.159.5.728) [DOI] [PubMed] [Google Scholar]
- 61.Enserink M. 2000. Are placebo-controlled drug trials ethical? Science 288, 416. 10.1126/science.284.5412.238 (doi:10.1126/science.284.5412.238) [DOI] [PubMed] [Google Scholar]
- 62.Michels K. B. 2000. The placebo problem remains. Arch. Gen. Psychiatry 57, 321. 10.1001/archpsyc.57.4.321 (doi:10.1001/archpsyc.57.4.321) [DOI] [PubMed] [Google Scholar]
- 63.Hammad T. A., Laughren T. P., Racoosin J. A. 2006. Suicide rates in short-term randomized controlled trials of newer antidepressants. J. Clin. Psychopharmacol. 26, 203–207 10.1097/01.jcp.0000203198.11453.95 (doi:10.1097/01.jcp.0000203198.11453.95) [DOI] [PubMed] [Google Scholar]
- 64.Sneed J. R., Rutherford B. R., Rindskopf D., Roose S. P. 2008. Design makes a difference: antidepressant response rates in placebo-controlled versus comparator trials in late life depression. Am. J. Geriatr. Psychiatry 16, 65–73 10.1097/JGP.0b013e3181256b1d (doi:10.1097/JGP.0b013e3181256b1d) [DOI] [PubMed] [Google Scholar]
- 65.Rutherford B. R., Sneed J. R., Roose S. P. 2009. Does study design influence outcome? Psychother. Psychosom. 78, 172–181 10.1159/000209348 (doi:10.1159/000209348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colloca L., Lopiano L., Lanotte M., Benedetti F. 2004. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet Neurol. 3, 679–684 10.1016/S1474-4422(04)00908-1 (doi:10.1016/S1474-4422(04)00908-1) [DOI] [PubMed] [Google Scholar]
- 67.Miller F. G., Wendler D., Swartzman L. C. 2005. Deception in research on the placebo effect. PLoS Med. 2, e262. 10.1371/journal.pmed.0020262 (doi:10.1371/journal.pmed.0020262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin A. L., Katz J. 2010. Inclusion of authorized deception in the informed consent process does not affect the magnitude of the placebo effect for experimentally induced pain. Pain 149, 208–215 10.1016/j.pain.2009.12.004 (doi:10.1016/j.pain.2009.12.004) [DOI] [PubMed] [Google Scholar]
- 69.Bausell R. B., Lao L., Bergman S., Lee W. L., Berman B. M. 2005. Is acupuncture analgesia an expectancy effect?: preliminary evidence based on participants' perceived assignments in two placebo-controlled trials. Eval. Health Prof. 28, 9–26 10.1177/0163278704273081 (doi:10.1177/0163278704273081) [DOI] [PubMed] [Google Scholar]
- 70.McRae C., et al. 2004. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch. Gen. Psychiatry 61, 412–420 10.1001/archpsyc.61.4.412 (doi:10.1001/archpsyc.61.4.412) [DOI] [PubMed] [Google Scholar]
- 71.Rief W., Glombiewski J. A., Barsky A. J. 2009. GASE: generic assessment of side effects in clinical trials. See www.gase-scale.com
- 72.Jonas W. B., Chez R. A. 2004. Toward optimal healing environments in health care. J. Altern. Complement. Med. 10, 1–6 [DOI] [PubMed] [Google Scholar]
- 73.Krell H. V., Leuchter A. F., Morgan M., Cook I. A., Abrams M. 2004. Subject expectations of treatment effectiveness and outcome of treatment with an experimental antidepressant. J. Clin. Psychiatry 65, 1174–1179 10.4088/JCP.v65n0904 (doi:10.4088/JCP.v65n0904) [DOI] [PubMed] [Google Scholar]