Abstract
The terms ‘placebo’ and ‘placebo effects’ cause confusion among patients, practitioners and scientists. This confusion results in both the adoption of practices that have no evidence of specificity yet considerable risk (such as surgery for low back pain) or the elimination of clinical practices proven to facilitate healing because they are not ‘better than placebo’ (such as acupuncture for low back pain). In this article, I discuss these issues and introduce the concept of optimal healing environment as a framework for disentangling what is useful from placebo research for adopting into clinical practice in a manner that is ethical and evidence-based.
Keywords: placebo, meaning, context
1. Introduction
Placebo is a term widely used by medical and research professionals and the general public, often with confidence that they know what it means. And yet, no other medical term has been more misused and has caused more confusion for professionals and the public alike. In this paper, I describe the dilemma that occurs from the definitions and misuse of the term ‘placebo effect’, which I claim obscures scientific investigation and obstructs the clinical delivery of optimal healing. I will suggest that we deconstruct placebo into its different components and begin to replace ‘placebo’ with terms such as ‘the meaning and context response’ and ‘conditioning and learning’. In clinical practice, I suggest the concept of ‘better than placebo’ be replaced by addressing components of the placebo effect (the context, ritual and learning) to create an ‘optimal healing environment’ (OHE). I'll then explore what the implications of this reframing are for evidence-based practices and for applying a science that builds on the components of the placebo response.
2. The dilemma
Let me illustrate the dilemma that the word placebo creates with a clinical case: Mr Raymond (not the patient's real name) was a 57-year-old retired government worker with chronic low back pain. The pain had been getting progressively worse and was determined by physicians to be mechanical, musculoskeletal and non-surgical. Mr Raymond had tried a number of treatments, including analgesics, non-steroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, antidepressants and facet injections. He'd been told to engage in bed rest, physical activity, exercise, physical therapy and traction. He had also sought out chiropractic and osteopathic manipulation—all with only temporary relief. Mr Raymond came into my office seeking information about acupuncture, which given the history of treatments he'd had and the refractory nature of his condition led him to ask me point-blank, ‘Doc, is acupuncture worth my while or is it just all placebo?’ Since acupuncture was not covered by Mr Raymond's insurance—and he would therefore pay for it out of his own pocket—he wanted to know if it was worth the time and cost. Mr Raymond's wife had also highly recommended acupuncture; she had told him that her family had used it extensively in Korea with much success for conditions like his.
3. An evidence-based approach
As a practitioner who takes pride in practising evidence-based medicine, I sought out answers to four questions in order to give Mr Raymond good advice. They were the following: (i) Is acupuncture better than placebo? (ii) Is it better than no treatment? (iii) Is it better than or equal to a proven treatment? (iv) What are its adverse effects and costs? These questions are basic information that a clinician needs to know in order to make evidence-based decisions about any medical information for any condition. In order to compare the evidence of acupuncture with that of other possible treatments, I created an evidence table with the treatments Mr Raymond had already had along the left-hand side and the four questions I needed to answer along the top (table 1). An assessment of the current evidence for those treatments indicates that only one, NSAIDs, had proven to be better than placebo and better than many of the other treatments including analgesics. However, the NSAID had over a 10 per cent gastrointestinal side effect rate and it was contraindicated with other anticoagulants. Unfortunately, Mr Raymond was on Coumadin (an anticoagulant) for atrial fibrillation and NSAIDs were contraindicated. The evidence for acupuncture indicated that it was no better than placebo, but was probably better than no treatment and other proven treatments with very low side effects. Mr Raymond would have to pay for it out of his own pocketbook. One of the recent studies that led me to believe it could be useful for him was an article published by Haake et al. [1]. This study indicated that the success rate for traditional acupuncture was almost double that for standard guideline-based therapy for chronic pain problems such as Mr Raymond's. And yet, the sham acupuncture—needles placed in non-acupuncture sites—produced equally good effect and was no better than the ‘real’ acupuncture. Thus, I was faced with a paradox. Acupuncture is a treatment that is certainly better than no treatment, it is significantly better than the standard treatment that Mr Raymond had received, and yet it is no better than its own placebo, or sham. Should I recommend against it because it was not ‘better than placebo’, or recommend it because it produces such a large effect compared with conventional care. If acupuncture is largely owing to the ritual, expectation and context of delivery (its ‘environment’), should I not focus on determining which components produce this effect when treating Mr Raymond?
Table 1.
Balance sheet for the treatment of chronic low back pain.
| treatment | better than placebo? | better than no treatment? | equal or better than a proven treatment? | side effects and contraindications? |
|---|---|---|---|---|
| analgesicsa | unknown | probably | less effective than acupuncture and NSAIDs | up to 50% especially in the elderly |
| NSAIDsa | yes | yes | better than analgesics | up to 10% gastrointestinal side effects but contraindicated with Coumadin |
| muscle relaxantsa | unknown | unknown | probably not | up to 70% and may be addictive |
| antidepressantsa | doubtful | unknown | unknown | up to 80% but often mild |
| injections (facet joint and trigger point) | doubtful | unknown | unknown | tissue damage, infection and bleeding possible. |
| advice to stay activea | unknown | unknown | unknown | not addressed |
| bed resta | unknown | no | no, looks worse than other treatments | increases risk of chronic disability and other problems |
| biofeedback | doubtful | unknown | doubtful | very low but not addressed |
| exercisesa | unknown | possibly | probably better than physical therapies and back schools | possible increased ‘spine stress’ |
| physical therapiesa | unknown | unknown | unknown | unknown |
| traction | no | no | unknown | unknown |
| manipulationa | possibly | probably | probably as good as standard care | low but strokes post-manipulation have been reported |
| acupuncture | no | probably | probably as good as standard care and all other interventions | very low but infection and trauma reported |
aAlready used by the patient on the first visit.
4. Words as treatment
Further investigation into the reasons for this revealed that acupuncture did have some specific effects, but they interacted significantly with a person's expectation. For example, a study published by Pariente et al. [2] showed that sticking needles in acupuncture points without any expectation induced a stimulus response over the sensory cortical areas. And yet, if the same needles were stuck in the same point with the expectation that they were supposed to be therapeutic, they would stimulate not only in the sensory cortex, but also areas in the limbic system associated with expectation. More recent studies such as that by Richter et al. [3] indicated that the very words used when communicating about a treatment or pain can activate different parts of the brain. Negative affective words that had equivalent emotional valance to pain words produced different activation in the pain area if those words had pain content. Thus, not only did the effect of words and expectations have impact, but the specific content of those words seemed to produce different neurological effects on pain. Remarkably, the differences in the neuroprocessing of pain-related words compared with non-pain-related words are specific to the pain relevance of the words and cannot simply be explained by their emotional valance or arousal effect. Thus, it appeared that whatever recommendations I made to Mr Raymond, I would need to be very careful as to the content of the words as well as the emotional tone I used to communicate the evidence to him.
The evidence created an even further dilemma as illustrated in figure 1. It appeared that I had in acupuncture a treatment such as that depicted under the graph in treatment A—a treatment in which the meaning and context (MAC) effects (in white in the graph) were very large and yet the specific effects (in grey) were small. Analgesics and NSAIDs—that is, writing him a prescription—have smaller MAC effects and yet the specific effects of these treatments are larger. Thus, the science directs me to give him treatment B, but Mr Raymond's maximum benefit for pain directs me to give him treatment A. I was stuck in a dilemma between person-centred care and evidence-based medicine.
Figure 1.
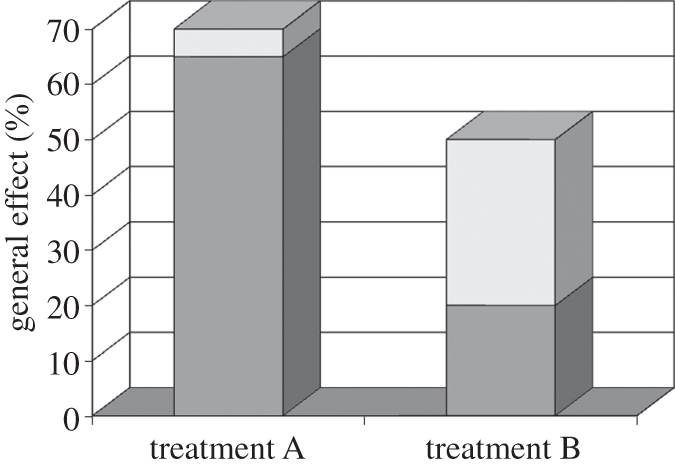
Hypothetical example showing differential importance of MAC effects. The histograms show two treatments for the same condition with different proportion of context and specific effects. Treatment A has a large proportion of effect provided by means of MAC effects. Conversely, treatment B has a small contribution by MAC and a larger part by specific effects. White bars, specific; grey bars, MAC.
5. The content and variation of placebo
Further investigations indicated that what was determined to be evidence-based medicine had a lot to do with the context and environment where a study is done, in addition to the words that are being used. Dan Moerman [4] reviewed 117 placebo-controlled trials of ulcers across multiple countries and showed that the response rate for ulcer healing in the placebo groups varied from 0 per cent to 100 per cent. There was wide variation from country to country, such as the response rate in Germany being high and the response rate in The Netherlands and Denmark being low [4]. Thus, what was proven in The Netherlands to have specific effects could never be proven in Germany. The evidence varied by country, context, delivery and the interpretation of that delivery by the patient and his or her culture [4].
No wonder that reports of the magnitude of placebo effect are literally all over the map—anywhere from 0 to 100% and not the traditional one-third of effects as originally reported by Beecher [5] or the two-thirds as reported by Roberts et al. [6]. In other words, I could not make generalizations about the magnitude or duration of placebo effects, but I had found evidence that it markedly influenced what I determined to be scientifically valid information. The way I delivered this information to Mr Raymond and the way he interpreted it could make the large difference as to whether he got better or not. Was this why the multiple treatments that Mr Raymond had undergone for his back pain had only been temporarily effective? Or, was there another reason? When Mr Raymond first came to me, I was prepared to use the evidence to indicate that acupuncture should not be used (to tell him in was mostly placebo), but now it was unclear what advice I should give him.
The more we examine placebo, the more it appears that the term ‘placebo’ is often used for political rather than scientific reasons and the underlying scientific contributions to the literature about the placebo effect are often covered up using this term. In the case of acupuncture, I could easily justify and find good evidence to discount it as quack medicine or to recommend it as scientifically proven. The confusion over the term placebo not only makes it difficult to make a decision about how to treat Mr Raymond, but it confuses the public in general. For example, a recent article that systematically reviewed the adverse effects in placebo groups in anti-migraine clinical trials [7] was interpreted in a full page article in Time Magazine with the title ‘The Flip Side of Placebo: The Nocebo Effect’ [8]. The author of the original article said that was a misrepresentation of the findings. The sugar pill itself did not ‘heal or harm’, and this illustrated the public dilemma of how the word ‘placebo’ is widely used in a confusing manner [8].
6. One word: opposite uses
The confusion that arises both in the public and among professionals occurs because the term placebo is used for at least two completely different reasons. One description of placebo is that it is a response to the context and meaning of a treatment, in which case we wish to use it to enhance these effects for any therapy. The term is also used to indicate the response from an inert agent such as a pill, needle or knife used in the treatment, in which case we want to avoid it because it is inert. By ‘inert’ here I mean that the needle, pill or knife is a delivery device and not in itself meant to be the specific treatment. My search for evidence-based information on acupuncture led me to a dilemma in which there was lack of an adequate definition to answer Mr Raymond's question when he asked, ‘Doc, is it just all placebo?’ Had he asked me if it was just all magic or a miracle, I could have indicated that those words are not scientifically based. Instead of magic, we seem to use the term placebo as if in some way it defines science, when it does not. Yet, to ground talk about placebo effects in science, we must disentangle the term into its specific components. We must take a positivist and reductionist approach to identifying the components underlying the term to better understand their specific contribution to healing and recovery. This requires reframing the term placebo, and I suggest that this reframing needs to occur both in research and practice. For research purposes, I and others have proposed that rather than use the term placebo effect, we should talk about the ‘meaning, context and learning response’ or what I call MAC effects, for short. We could then define placebo effects as the physiological, psychological and clinical effects of MAC [9]. Sometimes inert substances, which we would then term placebos, are used to help understand the therapeutic aspects of the meaning, context and learning response. In clinical practice, I suggest that we reframe placebo delivery as seeking to create an OHE [10].
7. Meaning, context and learning
Once we begin to disentangle placebo into its specific parts, we see that placebo variations—or the MAC effects—have many sources, components and mechanisms. (See the recent review by Finniss et al. [11] summarizing these factors and mechanisms.) It has been shown, for example, that the colour [12] and number of pills [13] make a difference in outcomes, as also do the compliance with the treatment, the label on the treatment [14], the form of the treatment (i.e. pill, needle, heat, injection or laser), the location of the treatment (i.e. home, hospital or OHE) [15], the order and administration of treatments, the tone of treatment delivery [16], the authority of the physician or practitioner and the type of information provided with the delivery, including the social and cultural factors that shape and interpret that delivery [17,18]. All of these factors have been shown to affect outcome [19]. In addition, the actual effectiveness of a drug (or other drugs) makes a difference in the magnitude of the MAC effects [9,19]. When these factors are combined in a number of therapeutic settings they maximize the meaning, context and healing effects. For the clinician, it comes down to this question: How can we put all the meaning, context and learning influences together in an evidence-based and ethical way when we treat patients? To clarify this further, I would like to illustrate how one major area in current healthcare delivery may be maximizing MAC effects. This is the area of surgery for pain. This area is especially relevant for the clinical case I have presented, as surgery and surgery-like interventions are often used for the treatment of chronic back pain such as that experienced by Mr Raymond.
9. Surgery as placebo
Surgery is one of the greatest advances of modern medicine in the last 100 years. Modern surgery has been made possible primarily because of two non-surgical technological advances. First, sterile techniques have prevented and reduced infection rates that used to kill large numbers of post-operative patients. And secondly, the development of chemicals that can anaesthetize and produce analgesia has allowed surgery to be conducted on a wider range of conditions than ever before in the history of mankind. Surgery creates a combination of many of the placebo factors listed above, and so should be an excellent approach to induce meaning and context responses. Indeed, literature indicates that in most cases, surgical procedures for chronic pain may produce their effects largely because of MAC influences. Let me illustrate this with several examples.
Bilateral internal mammary artery ligation (BIMAL) was an effective and widely used treatment for coronary artery disease in the 1950s and 1960s. In this procedure, the internal mammary artery was exposed using a surgical procedure, ligated (tied off), and then the patient was sewn up. The rationale for this procedure was that the ligation of the mammary artery resulted in retrograde blood flow into the coronary vessels, increasing perfusion into the heart. Observational studies indicated that this procedure increased function and decreased chest pain from coronary arterial disease, or angina, by 70–80%. However, in laboratory experiments with dogs, ligation of the mammary artery did not result in retrograde flow. And so, two placebo-controlled trials in humans were conducted [20,21]. In these cases, patients were randomized to either obtain the full procedure, in which the mammary artery was ligated, or to simply have the artery exposed during surgery without ligation followed by closure of the chest wall. In both studies, relief from angina was significant in 70–80% of the patients whether they had had the sham or real procedure. Soon after these studies were performed, clinicians claimed that BIMAL was all placebo effect and the procedure was dropped from practice. It was around this time that the heart–lung bypass machine was developed, allowing the heart to be stopped for coronary artery bypass surgery—the procedure of choice today for many with coronary artery disease. However, coronary artery bypass surgery has never been tested in a placebo-controlled trial and ironically its effectiveness in treating angina is approximately the same as was BIMAL: 70–80%. Bypass and similar approaches are in widespread use for angina despite the lack of placebo-controlled studies to determine the extent of MAC effects from the procedure.
More recent examples include percutaneous laser myocardial revascularization. In this procedure, a laser catheter is inserted into the femoral artery and passed up against the wall of the myocardium. Holes are then punched into the myocardium with the laser in order to increase myocardial profusion. The effect is used primarily on individuals with advanced cardiac failure (class III or IV) and has been shown to be markedly effective in improving cardiac function. In a prophetic article titled ‘Surgery as Placebo’ in 1994, Alan G. Johnson stated that ‘electrical machines have great appeal to patients and I think doctors, and recently anything with the word laser attached to it has caught the imagination’ [22]. This appears to be the case with myocardial laser revascularization. Subsequently, a placebo-controlled study of 298 patients with class III or IV heart failure underwent high-dose laser treatment, low-dose laser treatment or placebo [23]. In the placebo intervention, the catheter was inserted but the laser was never turned on. All groups had significant improvement such that nearly 60 per cent of patients improved by an entire functional class. The benefit lasted over six months and was equal in all groups, including the placebo group.
Another illustration of long-term effects from a surgical intervention that are owing to placebo is in arthroscopic surgery for arthritis. In a study by Moseley et al. on laparoscopic surgery for osteoarthritis, published in The New England Journal of Medicine in 2002 [24], the placebo ‘debridement’ of osteoarthritis with arthroscopic surgery showed just as good pain relief as actual debridement for the same procedure. Two control conditions were created. One in which only laparoscopic lavage was used and another in which a skin incision was made without insertion of the laparoscope at all. Pain relief lasted over 2 years and was equal in all groups. A more recent example is vertebroplasty for osteoporotic spinal fractures. Two recent studies also published in The New England Journal of Medicine indicated that placebo vertebroplasty produced similar improvement for up to 24 weeks on all outcomes compared with a sham procedure in which a patient simply had topical anaesthesia applied without the vertebroplasty [25,26].
Thus, it appears that surgery is one of the most powerful placebos we have, combining many of the components of MAC effects. Despite this, clinicians who would otherwise have abandoned treatments because these studies show they are not evidence-based are now making changes in their interpretation. For example, in an editorial in The New England Journal of Medicine about the vertebroplasty studies, an interventional radiologist came to the following conclusion:
‘Given the increased use, limited benefit, and potential risk how often should vertebroplasty be performed? When faced with several choices for which the evidence is less clear than clear, patients and doctors must thoroughly review the options together. Informed choice helps to educate patients about the treatment options and allows them to recognize that a decision can be based on their values and preferences’.
[27, p. 620]
It appears that this clinician defers to patient-centred care by advocating that patient preferences and choices be the guiding principle, rather than evidence.
10. Making clinical decisions
What do we do with all this information for helping Mr Raymond make his decision about acupuncture? If I take the strict evidence-based medicine approach, I would tell him, ‘No, acupuncture appears to be a placebo’. If I take a basic science approach, however, I would tell him, ‘Yes, not only does acupuncture work, but we know the neurophysiological mechanisms whereby it works’. If I take a regulatory approach, I would say, ‘No, it does not meet the requirement for specific effects and so should not be paid for’. If I put on my skeptic hat, I say, ‘No’; but with my believer hat I say, ‘Yes’, because I interpret the meaning of the evidence differently. As a physician, I would probably say, ‘Yes’, but with conditions for its use depending on the cultural context, on the words and way I communicate the likely benefit to him, on my own beliefs about acupuncture, on Mr Raymond's expectations and beliefs, on his interactions with his family and their cultures and on whether the way I deliver the treatment allows for physiological or psychological learning with reference to his back pain.
11. Maximizing healing
What are those factors that I need to take into consideration in delivering this recommendation to Mr Raymond? How could we enhance his healing response based on the placebo literature in a scientifically based and ethically feasible way? [19] There is a long list of factors that need to be considered, many of which I was not taught in medical school. These include the following:
— Use more frequent dosing [28].
— Apply therapies in a therapeutic setting [15].
— Attend to the administration [15].
— Deliver the therapies in a warm and caring way [16].
— Deliver the therapies with confidence and in a credible way [29].
— Align all beliefs congruently to mine and my patient's cultures [4,30,34,35].
— Deliver a benign, but conditioned stimulus along with the therapy such as with a needle, touch, aroma or pill [32,36–42].
— Use the newest and most prominent treatment available [43,44].
— Use a well-known name brand identified with success by the culture or that has been frequently advertised [14].
— Cut, stick the skin or poke into an orifice when that is believed to be important [43].
— Use a light laser or electronic device to deliver and track the treatment [44].
— Incorporate reassurance, relaxation, suggestion and anxiety reduction methods into the treatment. [47–49].
— Provide suggestions for the specific physiological processes desired [51].
— Create a ritual using an agent such as a knife, pin, pill, light or twist [52].
— Attend to the price of the treatment making sure it is neither too high, nor too low [53].
12. A framework for translating placebo research into practice
This list demonstrates that MAC effects present a complex array of what appears to be a chaotic conglomeration of unconnected evidence. However, there may be a way to deliver these factors in an organized way and to create optimal clinical strategies for creating healing in behaviour and environment. By the word ‘environment’ here we mean key context and delivery aspects including the ‘inner’, ‘interpersonal’, and ‘outer’ and ‘behavioural’ components that contribute to healing. An OHE is described and defined as those components that enhance healing responses in clinical care. This description is very close to the reframing of placebo as meaning and context effects in the earlier part of this chapter [10,55,56]. Definitions and standards of that environment have also been described [57,58].
The goal of an OHE is to create healing, independent of or in parallel with a treatment. Healing is the process of recovery, repair, restoration and the experience of well-being in mind, body and spirit. It may or may not result in a disease cure, and it is most applicable in chronic illnesses and prevention when a cure is not possible. Thus, the effects of an OHE have both specific components (derived from placebo research on MAC) for healing and a non-specific relationship to the biological mechanisms of many diseases. An OHE is defined as a system and a place comprising people, behaviours, treatments, and their psychological and physical parameters. These are the meaning, context and learning aspects of therapy. The purpose of an OHE is to provide conditions that stimulate and support the inherent healing capacities of the participants. Thus, the OHE is a framework for the clinical application of the MAC (placebo) effects.
The components of an OHE go from the inner (psychological) aspects of the environment through the interpersonal (relationship) components to the behavioural components to the external (physical) components. We have reorganized the placebo or MAC effects into this framework to allow the practitioner to systematically examine those specific components that facilitate healing from a meaning and context perspective. The components of an OHE relevant to enhancing placebo effects are described in more detail below.
(a). The inner environment
First is the inner environment. This focuses on the expectation and belief aspects of placebo literature. Developing healing attention means the habitual practice of attention to the present in its full complexity—sometimes called mindfulness. Second, healing intention—the conscious determination to improve the health of another person. Third, expectation—the belief and anticipation of improvement and hope that a desired goal can be achieved. Let me illustrate how one can attend specifically to the alteration of healing expectations and intention as a therapeutic modality. Generally, a person who walks into a hospital setting immediately has a role defined for them—the sick role—in which they are disempowered. The belief, hope and meaning of their illness have immediate effects on their expectancy of healing, and belief congruency of those that they deal with (especially physicians) can impact those expectations. Often they seek the reason for the illness and the suffering of their person. By simply changing perception in a chronic illness, healing and pain can improve. An example of this is given an article published by Steen & Haugli [59]. Steen took a population of 60 patients with chronic refractory pain—these were patients with high drug use and frequent visits—and put them into a 12 week programme designed to change perceptions about themselves, but not to specifically treat the pain. In fact, they completely reversed the normal medical dialogue to avoid the idea that they were treating pain. They used techniques such as: (i) The body as a talking subject rather than focusing on the pain; (ii) attending to the wholeness of the participants' situation rather than the pain and refusing to discuss the pain as either physical or psychological; (iii) using everyday language rather than medicalizing their care in a dependent way; (iv) respected listening, and trusting the group participants to help educate each other; and (v) challenged the participants to evoke internal control rather than to use the healthcare personnel or system as the focus of control. This process, as you can imagine, had to go against the medical system's usual approach of eliciting and reinforcing (by detailed diagnostic and therapeutic focus) the pain story with patients. A year later, the participants had an increased awareness of self, more constructive ways of handling life situations and significantly less pain. Thus, attending to the inner aspects of the healing environment can produce significantly beneficial effects without attempting to medically treat the condition.
(b). The interpersonal environment
The interpersonal environment means cultivating healing relationships. This consists of at least two domains—the social and clinical. The primary domain is social support and service that comes from the household, family, friends, support groups, school, work and community. This is where the patient spends most of his or her time, and it has the greatest effect. The second is the so-called therapeutic alliance, which is the embodied social and psychological interaction between the healer and the patient in the clinical encounter. Extensive literature illustrates social support as an important component of healing, but healing relationships can also enhance therapeutic benefit in clinical conditions. For example, a study by Smyth et al. [60] showed the results of two randomized control trials—one with asthma patients and one with rheumatoid arthritis patients—that dealt primarily with altering the social component of the clinical encounter. The intervention was storytelling. In one group, the patient described a significantly difficult conflict or secret about past traumas, injury, rape or abuse to the clinician in a single session. In another group, they simply talked about superficial things like the weather or what they had to eat the day before. At the four-month follow-up, asthma patients who had been randomized to the trauma stories showed significant improvements in forced expiratory volume compared with the control group. Those with rheumatoid arthritis showed significant improvements in pain compared with the controls [60]. Thus, enhancing the therapeutic alliance through storytelling can alter the meaning and context of a treatment and produce significant and objective clinical improvements.
Such effects need not be time consuming or elaborate, but simply require the clinician attend to how they manage the patient's expectancy. A classic study by K. B. Thomas was published in 1987 [16]. This large randomized controlled trial showed that a positive consultation by a physician produced 20–25% greater improvements in functional conditions compared with a negative consultation. The amount of time to conduct a positive or negative consultation was equal in both situations. The therapeutic relationship and bond is enhanced by the nature of communication. Extensive research has been done since 1987 on the therapeutic relationship; and I refer the reader to a summary of that literature published in the Journal on Alternative and Complementary Medicine [61], and a second one on relationship-centred care published in the Journal of General Internal Medicine [62].
(c). The external and behavioural environment
A third OHE component involves the physical and behavioural context. The physical context involves light, air, nature, colour, art, sound and music and their impact on function, flow, privacy, community and the neurophysiology of the patient. Light, for example, can make a significant difference in patient outcomes. Two studies demonstrated that patients who were placed in sunny rooms or close to windows had significantly reduced lengths of stay [63,64]. The average stay in a psychiatric unit was reduced from 19.5 to 16.9 days. Post-operative care was reduced by 1 day for patients assigned to rooms with windows versus no windows. Thus, the physical context can affect the rate of recovery in both chronic psychiatric and acute post-surgical conditions, perhaps by physiological psychological mechanisms [63].
The external context also involves behavioural rituals through which meaning, expectancy, conditioning and social learning are transmitted. We have described examples of these in previous sections of this paper, but one recent example in which the treatment ritual was studied explicitly involved a study by Kaptchuk and colleagues in the treatment of pain in patients with irritable bowel disorder [53]. In this study, placebo acupuncture (off point needling) was accompanied by a ritual that delivered only a few of the components listed from placebo studies or a ritual with many of the components. Those patients who received the placebo treatment with more of the ritual components had nearly twice the amount of pain relief as those receiving the minimal ritual. This study nicely illustrates how the components of MAC (placebo) effects can be reconstructed from the current evidence to enhance therapeutic benefit. This is the goal of studying these processes as MAC effects rather than as placebo effects and building them into an OHE for clinical delivery.
16. Applying placebo: using the context, learning and environment in healing
By reframing placebo research as studying the context, learning and environmental aspects of healing, we can use the evidence from placebo research in clinical application in a way that is ethically acceptable and effective. Rather than eliminating therapies because they are ‘no better than placebo’, we can now construct specific processes to deliver therapies in ways to maximize their benefit. This, of course, requires that we get to know the patient, their context, and provide the learning opportunities and arrange the environment to maximize those effects. If a patient has been conditioned with a number of cues—say the smell from food, smiles from family members, the touch of hands, prayers they are saying—these cues become tools for changing expectancy, experience and outcome. Over time, these social cues both mould expectations and can be used to create an OHE. If a medical practitioner comes into a patient's room and triggers those cues—with a touch, a smile, an odour or a prayer—the expectancy and conditioning from these acts can then facilitate recovery and/or well-being. The intelligent application of these components of a healing meaning and context may sometimes be the most important aspects of the clinician's role. By reframing the concept of ‘placebo effects’ into these components, we can deliver better healing that is evidence-based, ethical and effective.
13. Conclusions
In this article we have described how the continued use of the term ‘placebo effects’ to simplify a complex set of therapeutic processes in healthcare is confusing and misleading. We illustrated how this confusion occurs with a clinical case, how it distorts the concept of evidence-based medicine and results in the misuse of major systems of treatment such as acupuncture and surgery. We recommend that the term placebo effect be replaced with concepts such as the ‘meaning and context’ response components in research and the creation of OHEs in clinical care. By disentangling the components of what is called placebo into its constituents, we can re-construct a more rational approach to research and the clinical use of healing.
Footnotes
One contribution of 17 to a Theme Issue ‘Placebo effects in medicine: mechanisms and clinical implications’.
References
- 1.Haake M., Muller H., Schade-Brittinger C., Basler H. D., Schafer H., Maier C., Endres H. G., Trampisch H. J. 2007. German acupuncture trials (GERAC) for chronic low back pain. Arch. Intern. Med. 167, 1892–1898 10.1001/archinte.167.17.1892 (doi:10.1001/archinte.167.17.1892) [DOI] [PubMed] [Google Scholar]
- 2.Pariente J., White P., Frackowiak R. S., Lewith G. 2005. Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture. Neuroimage 25, 1161–1167 10.1016/j.neuroimage.2005.01.016 (doi:10.1016/j.neuroimage.2005.01.016) [DOI] [PubMed] [Google Scholar]
- 3.Richter M., Eck J., Straube T., Miltner W. H., Weiss T. 2009. Do words hurt? Brain activation during the processing of pain-related words. Pain 148, 198–205 10.1016/j.pain.2009.08.009 (doi:10.1016/j.pain.2009.08.009) [DOI] [PubMed] [Google Scholar]
- 4.Moerman D. E. 2000. Cultural variations in the placebo effect: ulcers, anxiety, and blood pressure. Med. Anthropol. Q. 14, 51–72 10.1525/maq.2000.14.1.51 (doi:10.1525/maq.2000.14.1.51) [DOI] [PubMed] [Google Scholar]
- 5.Beecher H. K. 1955. The powerful placebo. JAMA 159, 1602–1606 [DOI] [PubMed] [Google Scholar]
- 6.Roberts A. H., Kewman D. G., Mercier L., Hovell M. 1993. The power of nonspecific effects in healing: implications for psychological and biological treatments. Clin. Psychol. Rev. 13, 375–391 10.1016/0272-7358(93)90010-J (doi:10.1016/0272-7358(93)90010-J) [DOI] [Google Scholar]
- 7.Amanzio M., Corazzini L. L., Vase L., Benedetti F. 2009. A systematic review of adverse events in placebo groups of anti-migraine clinical trials. Pain 146, 261–269 10.1016/j.pain.2009.07.010 (doi:10.1016/j.pain.2009.07.010) [DOI] [PubMed] [Google Scholar]
- 8.Cloud J. 2009. The flip side of placebos: the nocebo effect. Time Mag. NY: See http://www.time.com/time/health/article/0,8599,1929869,00.html [Google Scholar]
- 9.Moerman D. E., Jonas W. B. 2002. Deconstructing the placebo effect and finding the meaning response. Ann. Intern. Med. 136, 471–476 [DOI] [PubMed] [Google Scholar]
- 10.Chez R., Pelletier K., Jonas W. 2004. Toward optimal healing environments in health care. A Supplement to Second American Samueli Symposium. J. Altern. Complement Ther. 10 [DOI] [PubMed] [Google Scholar]
- 11.Finniss D. G., Kaptchuk T. J., Miller F., Benedetti F. 2010. Biological, clinical, and ethical advances of placebo effects. Lancet 375, 686–695 10.1016/S0140-6736(09)61706-2 (doi:10.1016/S0140-6736(09)61706-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Craen A. J., Roos P. J., de Vries A., Kleijnen J. 1996. Effect of colour of drugs: systematic review of perceived effect of drugs and of their effectiveness. Br. Med. J. 313, 1624–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Craen A., Moerman D., Heisterkamp S., Tytgat G., Tijssen J., Kleijnen J. 1999. Placebo effect in the treatment of duodenal ulcer. Br. J. Clin. Pharmacol. 48, 853–860 10.1046/j.1365-2125.1999.00094.x (doi:10.1046/j.1365-2125.1999.00094.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margo C. E. 1999. The placebo effect. Surv. Ophthalmol. 44, 31–44 10.1016/S0039-6257(99)00060-0 (doi:10.1016/S0039-6257(99)00060-0) [DOI] [PubMed] [Google Scholar]
- 15.de Craen A., Tijssen J., De Gans J., Kleijnen J. 2000. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J. Neurol. 247, 183–188 10.1007/s004150050560 (doi:10.1007/s004150050560) [DOI] [PubMed] [Google Scholar]
- 16.Thomas K. 1987. General practice consultations: is there any point in being positive? Br. Med. J. (Clin. Res. Ed.) 294, 1200–1202 10.1136/bmj.294.6581.1200 (doi:10.1136/bmj.294.6581.1200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gracely R., Dubner R., Deeter W. R., Wolskee P. J. 1985. Clinicians' expectations influence placebo analgesia. Lancet 1, 43. 10.1016/S0140-6736(85)90984-5 (doi:10.1016/S0140-6736(85)90984-5) [DOI] [PubMed] [Google Scholar]
- 18.Bergmann J., Chassany O., Gandiol J., Deblois P., Kanis J. A., Segrestaa J. M., Caulin C., Dahan R. 1994. A randomised clinical trial of the effect of informed consent on the analgesic activity of placebo and naproxen in cancer pain. Clin. Trials Meta-Anal. 29, 41–47 [PubMed] [Google Scholar]
- 19.Walach H., Jonas W. B. 2004. Placebo research: the evidence base for harnessing self-healing capacities. J. Altern. Complement Med. 10(Suppl. 1), S103–S112 10.1089/1075553042245773 (doi:10.1089/1075553042245773) [DOI] [PubMed] [Google Scholar]
- 20.Cobb L. A., Thomas G. I., Dillard D. H., Merendino K. A., Bruce R. A. 1959. An evaluation of internal-mammary-artery ligation by a double-blind technic. N. Engl. J. Med. 260, 1115–1118 10.1056/NEJM195905282602204 (doi:10.1056/NEJM195905282602204) [DOI] [PubMed] [Google Scholar]
- 21.Dimond E. G., Kittle C. F., Crockett J. E. 1960. Comparison of internal mammary artery ligation and sham operation for angina pectoris. Am. J. Cardiol. 5, 483–486 10.1016/0002-9149(60)90105-3 (doi:10.1016/0002-9149(60)90105-3) [DOI] [PubMed] [Google Scholar]
- 22.Johnson A. 1994. Surgery as placebo. Lancet 344, 1140–1142 10.1016/S0140-6736(94)90637-8 (doi:10.1016/S0140-6736(94)90637-8) [DOI] [PubMed] [Google Scholar]
- 23.Leon M. B., et al. 2005. A blinded, randomized, placebo-controlled trial of percutaneous laser myocardial revascularization to improve angina symptoms in patients with severe coronary disease. J. Am. Coll. Cardiol. 46, 1812–1819 10.1016/j.jacc.2005.06.079 (doi:10.1016/j.jacc.2005.06.079) [DOI] [PubMed] [Google Scholar]
- 24.Moseley J., O'Malley K., Petersen N., Menke T., Brody B., Kuykendall D., Hollingsworth J., Ashton C., Wray N. 2002. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N. Engl. J. Med. 347, 81–88 10.1056/NEJMoa013259 (doi:10.1056/NEJMoa013259) [DOI] [PubMed] [Google Scholar]
- 25.Buchbinder R., Osborne R. H., Ebeling P. R., Wark J. D., Mitchell P., Wriedt C., Graves S., Staples M. P., Murphy B. 2009. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N. Engl. J. Med. 361, 557–568 10.1056/NEJMoa0900429 (doi:10.1056/NEJMoa0900429) [DOI] [PubMed] [Google Scholar]
- 26.Kallmes D. F., et al. 2009. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N. Engl. J. Med. 361, 569–579 10.1056/NEJMoa0900563 (doi:10.1056/NEJMoa0900563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein J. N. 2009. Balancing science and informed choice in decisions about vertebroplasty. N. Engl. J. Med. 361, 619–621 10.1056/NEJMe0905889 (doi:10.1056/NEJMe0905889) [DOI] [PubMed] [Google Scholar]
- 28.de Craen A. J., Moerman D. E., Heisterkamp S. H., Tytgat G. N., Tijssen J. G., Kleijnen J. 1999. Placebo effect in the treatment of duodenal ulcer. Br. J. Clin. Pharmacol. 48, 853–860 10.1046/j.1365-2125.1999.00094.x (doi:10.1046/j.1365-2125.1999.00094.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uhlenhuth E. H., Rickels K., Fisher S., Park L. C., Lipman R. S., Mock J. 1966. Drug, doctor's verbal attitude and clinic setting in the symptomatic response to pharmacotherapy. Psychopharmacologia 9, 392–418 10.1007/BF00406450 (doi:10.1007/BF00406450) [DOI] [PubMed] [Google Scholar]
- 30.Cassidy C. M. 1998. Chinese medicine users in the United States. Preferred aspects of care. J. Altern. Complement Med. 4, 189–202 10.1089/acm.1998.4.189 (doi:10.1089/acm.1998.4.189) [DOI] [PubMed] [Google Scholar]
- 31.Frank J. 1961. Persuasion and healing: a comparative study of psychotherapy. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 32.Kirsch I. 1985. Response expectancy as a determinant of experience and behavior. Am. Psychol. 40, 1189–1202 10.1037/0003-066X.40.11.1189 (doi:10.1037/0003-066X.40.11.1189) [DOI] [Google Scholar]
- 33.Gracely R. H., Dubner R., Deeter W. R., Wolskee P. J. 1985. Clinicians' expectations influence placebo analgesia. Lancet 1, 43. 10.1016/S0140-6736(85)90984-5 (doi:10.1016/S0140-6736(85)90984-5) [DOI] [PubMed] [Google Scholar]
- 34.Phillips D. P., Ruth T. E., Wagner L. M. 1993. Psychology and survival. Lancet 342, 1142–1145 10.1016/0140-6736(93)92124-C (doi:10.1016/0140-6736(93)92124-C) [DOI] [PubMed] [Google Scholar]
- 35.Uexkull T. 1995. Biosemiotic research and not further molecular analysis is necessary to describe pathways between cells, personalities, and social systems. Adv. J. Mind-Body Health 11, 24–27 [Google Scholar]
- 36.Ader R. 1983. Behavioural conditioning and immunity. In Immunoregulation (eds Fabris N., Garaci E., Hadden J.), pp. 283–313 New York, NY: Plenum Press [Google Scholar]
- 37.Ader R., Cohen N. 1975. Behaviorally conditioned immunosuppression. Psychosom. Med. 37, 333–340 [DOI] [PubMed] [Google Scholar]
- 38.Montgomery G. H., Kirsch I. 1997. Classical conditioning and the placebo effect. Pain 72, 107–113 10.1016/S0304-3959(97)00016-X (doi:10.1016/S0304-3959(97)00016-X) [DOI] [PubMed] [Google Scholar]
- 39.Voudouris N. J., Peck C. L., Coleman G. 1985. Conditioned placebo responses. J. Pers. Soc. Psychol. 48, 47–53 10.1037/0022-3514.48.1.47 (doi:10.1037/0022-3514.48.1.47) [DOI] [PubMed] [Google Scholar]
- 40.Voudouris N. J., Peck C. L., Coleman G. 1989. Conditioned response models of placebo phenomena: further support. Pain 38, 109–116 10.1016/0304-3959(89)90080-8 (doi:10.1016/0304-3959(89)90080-8) [DOI] [PubMed] [Google Scholar]
- 41.Voudouris N. J., Peck C. L., Coleman G. 1990. The role of conditioning and verbal expectancy in the placebo response. Pain 43, 121–128 10.1016/0304-3959(90)90057-K (doi:10.1016/0304-3959(90)90057-K) [DOI] [PubMed] [Google Scholar]
- 42.Wickramasekera I. 1985. A conditioned response model of the placebo effect: predictions from the model. In Placebo–theory, research, mechanisms (eds White L., Tursky B., Schwartz G. E.), pp. 255–287 New York, NY: Guilford Press [Google Scholar]
- 43.Johnson A. G. 1994. Surgery as a placebo. Lancet 344, 1140–1142 10.1016/S0140-6736(94)90637-8 (doi:10.1016/S0140-6736(94)90637-8) [DOI] [PubMed] [Google Scholar]
- 44.Lange R. A., Hillis L. D. 1999. Transmyocardial laser revascularization. N. Engl. J. Med. 341, 1075–1076 10.1056/NEJM199909303411410 (doi:10.1056/NEJM199909303411410) [DOI] [PubMed] [Google Scholar]
- 45.Bergmann J. F., Chassany O., Gandiol J., Deblois P., Kanis J. A., Segrestaa J. M., Caulin C., Dahan R. 1994. A randomised clinical trial of the effect of informed consent on the analgesic activity of placebo and naproxen in cancer pain. Clin. Trials Metaanal. 29, 41–47 [PubMed] [Google Scholar]
- 46.Skovlund E. 1991. Should we tell trial patients that they might receive placebo? Lancet 337, 1041. 10.1016/0140-6736(91)92701-3 (doi:10.1016/0140-6736(91)92701-3) [DOI] [PubMed] [Google Scholar]
- 47.Gheorghiu V., Netter P., Eysenck H., Rosenthal R. (eds) 1989. Suggestion and suggestibility: theory and research. Berlin, Germany: Springer [Google Scholar]
- 48.Stefano G. B., Fricchione G. L., Slingsby B. T., Benson H. 2001. The placebo effect and relaxation response: neural processes and their coupling to constitutive nitric oxide. Brain Res. Brain Res. Rev. 35, 1–19 10.1016/S0165-0173(00)00047-3 (doi:10.1016/S0165-0173(00)00047-3) [DOI] [PubMed] [Google Scholar]
- 49.Vase L., Robinson M. E., Verne G. N., Price D. D. 2003. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain 105, 17–25 10.1016/S0304-3959(03)00073-3 (doi:10.1016/S0304-3959(03)00073-3) [DOI] [PubMed] [Google Scholar]
- 50.Brody H. 2000. The placebo response. Recent research and implications for family medicine. J. Fam. Pract. 49, 649–654 [PubMed] [Google Scholar]
- 51.Meissner K. 2009. Effects of placebo interventions on gastric motility and general autonomic activity. J. Psychosom. Res. 66, 391–398 10.1016/j.jpsychores.2008.09.004 (doi:10.1016/j.jpsychores.2008.09.004) [DOI] [PubMed] [Google Scholar]
- 52.Kaptchuk T. J. 2002. The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance? Ann. Intern. Med. 136, 817–825 [DOI] [PubMed] [Google Scholar]
- 53.Waber R. L., Shiv B., Carmon Z., Ariely D. 2008. Commercial features of placebo and therapeutic efficacy. JAMA 299, 1016–1017 10.1001/jama.299.9.1016 (doi:10.1001/jama.299.9.1016) [DOI] [PubMed] [Google Scholar]
- 54.Cherkin D., Eisenberg D., Sherman K., Barlow W., Kaptchuk T. J., Street J., Deyo R. A. 2001. Randomized trial comparing traditional Chinese medical acupuncture, therapeutic massage, and self-care education for chronic low back pain. Arch. Intern. Med. 161, 1081–1088 10.1001/archinte.161.8.1081 (doi:10.1001/archinte.161.8.1081) [DOI] [PubMed] [Google Scholar]
- 55.Jonas W., Chez R. 2004. Toward optimal healing environments in health care. J. Altern. Complement Ther. Med. 10, S1–S6 10.1089/1075553042245818 (doi:10.1089/1075553042245818) [DOI] [PubMed] [Google Scholar]
- 56.Jonas W., Chez R., Duffy B., Strand D. 2003. Investigating the impact of optimal healing environments. Altern. Ther. Health Med. 9, 36–40 [PubMed] [Google Scholar]
- 57.Jonas W., Chez R. 2003. The role and importance of definitions and standards in healing research. Altern. Ther. Health Med. 9, A5–A7 [PubMed] [Google Scholar]
- 58.Jonas W., Chez R. 2003. Definitions and standards in healing research: a supplement to First American Samueli symposium. Altern. Ther. Health Med. 9 [PubMed] [Google Scholar]
- 59.Steen E., Haugli L. 2001. From pain to self-awareness–a qualitative analysis of the significance of group participation for persons with chronic musculoskeletal pain. Patient Educ. Couns. 42, 35–46 [DOI] [PubMed] [Google Scholar]
- 60.Smyth J., Stone A., Hurewitz A., Kaell A. 1999. Effects of writing about stressful experiences on symptom reduction in patients with asthma or rheumatoid arthritis: a randomized trial. JAMA 281, 1304–1309 10.1001/jama.281.14.1304 (doi:10.1001/jama.281.14.1304) [DOI] [PubMed] [Google Scholar]
- 61.Chez R. A., Jonas W. 2005. Developing healing relationships: a supplement to Third American Samueli Symposium. J. Altern. Complement Med. 11, S1–S2 10.1089/acm.2005.11.s-1 (doi:10.1089/acm.2005.11.s-1) [DOI] [PubMed] [Google Scholar]
- 62.Safran D., Miller W., Beckman H. 2006. Organizational dimensions of relationship-centered care: theory, evidence and practice. J. Gen. Intern. Med. 21, S9–S15 10.1111/j.1525-1497.2006.00303.x (doi:10.1111/j.1525-1497.2006.00303.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beauchemin K. M., Hays P. 1996. Sunny hospital rooms expedite recovery from severe and refractory depressions. J. Affect. Disord. 40, 49–51 10.1016/0165-0327(96)00040-7 (doi:10.1016/0165-0327(96)00040-7) [DOI] [PubMed] [Google Scholar]
- 64.Brandon D. H., Holditch-Davis D., Belyea M. 2002. Preterm infants born at less than 31 weeks' gestation have improved growth in cycled light compared with continuous near darkness. J. Pediatr. 140, 192–199 10.1067/mpd.2002.121932 (doi:10.1067/mpd.2002.121932) [DOI] [PubMed] [Google Scholar]


