Abstract
The hypothesis put forth is that expectations of treatment effects reduce negative emotions and thereby reduce symptoms, e.g. pain. Negative emotions increase pain, and it is hypothesized that placebos reduce pain by reducing negative emotions, i.e. feelings of nervousness, fear and anxiety. Placebo analgesia has been shown to be mediated via opioid activity, and relaxation increases opioid activity. The placebo acquires its relaxing effect due to verbal information that pain will be reduced, or due to associations between the placebo and the reduction in pain after effective treatment. Thus, the placebo signals that unpleasantness will be less after administration of the placebo. This involves negative reinforcement which is due to activation of a dopaminergic system that has been found to be activated during placebo analgesia and is involved in positive emotions. The nocebo effect of increased pain is, consistent with this model, because of increased fear and anxiety. The new aspect of the presented model is the hypothesis that expectations reduce negative emotions, and that negative reinforcement that involves the dopaminergic reinforcement system should be a contributor to placebo responses.
Keywords: placebo effect, placebo analgesia, pain, emotion, classical conditioning, operant conditioning
1. Introduction
An individual experiencing pain may self-administer acetaminophen or ibuprofen, or may receive a dose of morphine from health personnel. In both instances, effective treatment is administered, and at the same time an expectation is generated that the pain will be reduced [1]. Experimental studies have shown that administration of inactive treatment, e.g. sugar pills, inactive cream, inactive devices and inactive sham surgery, may reduce pain as long as the participant believes that the treatment is effective [2,3]. Thus, information that a painkiller has been administered induces an expectation that pain will be reduced, with a subsequent reduction in pain. This is termed placebo analgesia. Some of the psychophysiological mechanisms that mediate the effect of expectations on pain have been identified, and in the following a hypothesis is put forth that seeks to complement this knowledge.
It is argued that expectations reduce pain via a reduction in negative emotions. Pain is a sensation, but is often highly correlated with negative emotions like fear and anxiety. This is reflected in the neuroanatomy of pain where the pain pathways reach cerebral areas serving both sensory/cognitive and emotional/motivational functions. Thus, emotional activation is part of the response to pain. In the following, a hypothesis is presented that describes how expectations may change emotions during anticipation of pain. There are different placebo responses in different diseases, and even different placebo responses in the expectation of treatment for pain [3]. However, modulation of emotional processes may be a common factor in many placebo responses.
2. Theoretical background
A systematic description and review of the literature discussing the role of emotions in placebo responding have, to our knowledge, not been published. However, several authors have related reduced anxiety or stress to increased placebo responding [1,4–7]. The general idea is that placebos reduce anxiety or other negative emotions, and thereby reduce symptoms.
The argument put forth here is that the placebo reduces negative emotions which in turn decrease pain. The inert placebo modulates emotions via verbal information that pain-relieving treatment has been administered, or via associations with the beneficial effects of a drug or other treatment. The administration of the placebo has two effects: it reduces negative emotions (or induces a positive emotional state), and maintains self-administration via reinforcement of drug-taking behaviour. The model proposes that the placebo response involves modulation of emotional processes, and that this is a common component in many placebo responses. Emotional modulation is of a general nature and implies that the placebo response involves unspecific processes. Finally, the model is mechanistic in the sense that the placebo response is seen as an emotional reaction to signals that an unpleasant state is being remedied. The two new aspects of the model are that emotional processes and the associated neurobiological changes are seen as mediators of placebo analgesia. Furthermore, it is argued that operant reinforcement is involved in the reduction of negative emotions (or induction of positive emotions). To describe the model in more detail, pain will be used as an example, as the field of placebo analgesia has received a large amount of research and more is known about its psychology and neurobiology compared with placebo responding in other response systems.
3. Pain
The intensity of pain is often measured by a visual analogue scale consisting of a 10 cm line anchored with the words ‘no pain’ and ‘worst pain imaginable’. Alternatively, pain can be indicated by having the subject state vocally a number between 0 and 10, indicating the intensity of pain. The unpleasantness of pain is recorded in the same way.
The reliability in the reporting of pain has been brought into question by findings that the social context may modulate pain report (and reports of other subjective states). Aslaksen et al. [8] found that males reported less pain and stress to female experimenters compared with males who reported pain and stress to male experimenters. However, heart rate variability during the painful stimuli was the same in males tested by female and male experimenters, suggesting that the pain was the same in both groups, and that only the reporting was modulated by the social context. This response bias is a serious problem for the reliability of subjective report, and needs to be considered when recording pain. Especially in the field of placebo analgesia, where response bias has been put forth as an explanation for the reduced pain report after administration of a placebo [9,10], the use of objective methods is especially important, and such methods are being increasingly used [11–13].
Pain has several dimensions [14]. The sensory-discriminative dimension is served by lateral nuclei in the thalamus that project to the primary and secondary somatosensory cortices. This dimension is related to pain intensity. The affective-motivational dimension is served by medial nuclei in the thalamus, and projects to the insula and the anterior cingulate cortex. This dimension is related to pain unpleasantness. Pain intensity and unpleasantness are often highly correlated in experimental studies.
4. Emotions
Two of the basic dimensions of emotions are valence and arousal. Valence describes whether the emotion is good or bad, appetitive or aversive, and different emotions can be classified along this one axis. Emotional valence may be induced by videos or photos depicting situations with emotional content, and is often registered on a five- or seven-point scale, where lower scores indicate more negative emotions.
Another important dimension is the continuum from low to high arousal, which represents the intensity of an emotion. An emotion with negative valence, e.g. fear, may thus be described as nervousness, fear or panic depending on the level of arousal. The continua of valence and arousal are not independent, as there is a tendency for very negative or very positive emotions to be associated with high arousal [15].
Fear and anxiety are the emotions that have received most research in the field of pain. The anticipation of pain or pain itself can induce these emotions. In the present context, negative emotions will often mean these two emotions. The concept of stress is often used to describe a state of negative emotional activation that persists over time. Stress has been linked to a number of different health-related problems, including pain. Pain increases stress, but stress, i.e. negative emotional activation, may also increase pain. There is considerable overlap between the concepts of negative emotions and stress, and they are often used interchangeably, e.g. questionnaires asking for how nervous and tense a person is may describe this as stress [16]. Accordingly, no clear distinction is made between negative emotions and stress here.
5. The effect of emotions on pain
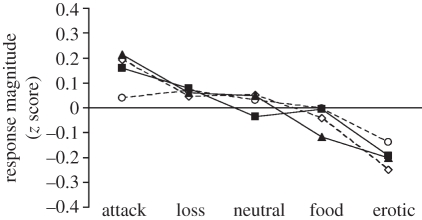
The literature on the effect of emotions on pain is not consistent, as it is necessary to control for other processes, e.g. attention to or away from the emotional stimulus, to assess how emotions may modulate pain. Most studies, but not all, have found that negative emotions increase pain, whereas positive emotions reduce pain [17]. Rhudy et al. [18] have shown in a series of experiments that negative emotional valence increases pain and positive emotions decrease pain (figure 1). Emotions have been induced by photos from the International Affective Picture System that have been presented for several seconds, and pain has been induced experimentally a few seconds after offset of the photos to control for the confounding effect of attention to the slides. Rhudy et al. [18] found that pain report, as well as the nociceptive flexion reflex and skin conductance responses, decreased as a function of increasing positive emotional valence. Thus, not only subjective report, but also physiological pain responses displayed the same trend towards reduced pain in the presence of positive emotions. The effect is not large, but is reliable.
Figure 1.
Subjective and physiological responses to painful stimulation as a function of emotional valence. Open circles, nociception flexion reflex; open diamonds, pain; filled triangles, skin conductance response; filled squares, heart rate. Adapted from Rhudy et al. [18] with kind permission from the publisher.
The relationship between pain and emotional valence suggests that arousal could play a role, as highly positive and negative emotions are associated with larger arousal. At the high end of arousal and valence, in the case of severe stress, pain is reduced, called stress-induced analgesia. Flor & Grusser [19] have shown that conditioned stress, induced by presenting a conditioned stimulus that has been paired with a difficult task and a distracter, can reduce pain via opioid mechanisms. Thus, the linear relationship of emotional valence and/or arousal to pain holds only for positive emotions and moderately intense negative emotions. When levels of stress are high, stress-induced analgesia reverses the incremental effect that negative emotions have on pain.
6. The neurobiology of emotional modulation of pain
Studies using neuroimaging techniques have suggested that the cerebral representation of pain involves two parallel subsystems; the lateral system processing sensory-discriminative information and the medial system that process the affective-cognitive component of pain [20]. The lateral system projects sensory information to the primary and secondary somatosensory cortices via the lateral nuclei of the thalamus. The medial system projects through the medial thalamic nuclei to the anterior cingulate cortex, insula and the prefrontal cortices [21,22]. Consequently, the medial pain system should be central in the emotional modulation of pain.
Even if cortical pain pathways are well explored, there are surprisingly few neuroimaging studies that directly address the influence of emotions on pain. Studies employing functional magnetic resonance imaging have revealed that the entorhinal cortex of the left hippocampal formation, the perigenual cingulate cortex and the mid-insula show increased activity when painful stimulation was administered after induction of negative emotions [23], and these regions amplify pain experience in chronic pain patients with depression [24,25]. Trait-like individual differences in pain-related fear might also have an impact on neural processing of pain. Ochsner et al. [26] found that subjects with high scores on the Fear of Pain Questionnaire displayed increased activity in the anterior and the posterior cingulate cortex and in the orbitofrontal cortex during painful stimulation compared with non-painful stimulation.
7. Similarities between the reduction of stress and negative emotions, and placebo analgesia
Stress and negative emotions are general processes and involve many neurobiological changes that have been reviewed elsewhere [27]. In the present context, however, the finding that relaxation training is mediated via opioid mechanisms is of special importance. McCubbin et al. [28] subjected one group of males with mildly elevated mean arterial blood pressure to relaxation training, whereas a control group did not receive training. The group that received relaxation training displayed decreased blood pressure reactivity to mental stress compared with the control group. Naltrexone, an opioid antagonist, antagonized the effect of relaxation training, showing that the effect of relaxation training was mediated by opioid mechanisms.
Increased opioid activity has been hypothesized to increase positive emotions and/or decrease negative emotions, as opioids have been implicated in sex [29], intake of food and addictive drugs [30], and pain relief [31], which all involve positive and negative emotions. In a study using positron emission tomography, Koepp et al. [32] induced positive emotions via a film clip, music and positive statements, and contrasted this with neutral emotions. They found reduced binding of a µ-receptor agonist during positive emotions, indicating that positive emotions were associated with increased opioid activity.
Several studies have shown that placebo analgesic responding is also mediated via opioid release. Levine & Gordon [31] showed that an injection of the opioid antagonist naloxone partly reversed the placebo analgesic effect, and other studies have shown similar results [33,34]. Brain-imaging studies have shown activity in the descending pain-inhibitory opioid system in the brain and in the spinal cord during placebo analgesia [35,36]. These findings indicate that placebo analgesia is partly due to reduced pain transmission at the level of the spinal cord owing to opioid release.
Taken together, these findings suggest that opioid mechanisms are involved in the modulation of emotions, as well as in placebo analgesia.
8. Reduction in pain and stress, and negative reinforcement
Behaviour that has pleasant consequences will occur more frequently in the future, termed positive reinforcement [37]. The pleasant stimulus produced by the behaviour is called a ‘reinforcer’, and is any stimulus that the organism will behave in a way to obtain. Typical primary reinforcers are food, sex and drugs of abuse. Similarly, negative reinforcement occurs when an overt response removes or reduces an unpleasant stimulus, e.g. pain. In both instances, behaviour is reinforced meaning that it will occur more frequently in the future.
Behaviour may also be reinforced by money, the attention of others, praise or a ‘thank you’. For these stimuli, their reinforcing effect is because of their association with a primary reinforcer, and they are termed ‘conditioned reinforcers’. Money becomes a conditioned reinforcer as money is instrumental in obtaining primary reinforcers. Praise is also a conditioned reinforcer as it will occasionally be accompanied by a primary reinforcer, or another conditioned reinforcer like money or attention.
The initially neutral conditioned reinforcer comes to induce positive emotions because of its association with a primary reinforcer. Thus, money not only signals that food may be obtained, but money may also induce positive emotions, as money is associated with food and other appetitive stimuli. This is an obvious fact as long as money is used as an example, but the same model may be used to understand placebo effects.
9. Placebo responding and negative reinforcement
Pain is unpleasant and humans and other animals seek to avoid pain. A reduction in pain can be considered a negative reinforcer, and any act that reduces pain can be reinforced. Thus, when pain occurs, any act that previously has been associated with a reduction in pain is likely to be emitted [37], e.g. self-administration of a painkiller.
The presence of pain, or presence of stimuli associated with pain, should increase the probability of occurrence of behaviour that has reduced pain in the past. Thus, self-administration of painkillers will occur in the presence of pain, or to cues that pain is about to occur. A person who, for example, often suffers headaches in the evenings may ingest a painkiller earlier in the evening to avoid the pain. A person who is about to perform movements or tasks that cause pain may ingest a painkiller prior to performing the task to avoid the pain. Stimuli that heighten the probability of self-administration may be considered conditioned reinforcers, a stimulus that informs the individual that a response may be performed which avoids the unpleasant unconditioned stimulus. This is different from a conditioned stimulus that elicits physiological and subjective conditioned responses (CRs) which are independent from any overt behaviour.
Negative reinforcement describes the occurrence of behaviour that in the past has reduced pain, but a purely behavioural analysis cannot explain placebo analgesia, i.e. the experience of reduced pain. To explain placebo analgesia, the neurobiology of reinforcement must be taken into account, as it involves positive emotions (pleasure) that have been shown to reduce pain.
10. The neurobiology of reinforcement
The mechanism that most consistently has been found to underlie reinforcement is the mesolimbic system, which originates in the ventral tegmental area in the midbrain, and projects to forebrain areas involving especially the nucleus accumbens, a part of the basal ganglia. This area is activated by reinforcing stimuli, and dopamine is secreted in response to reinforcers like food and sex, cocaine, amphetamine, ethanol, nicotine and heroine. A number of other neurotransmitters or neuromodulators are also released in response to these reinforcers, but the common element among reinforcers is dopamine activation [38]. Human brain-imaging studies have also shown nucleus accumbens activation to a reinforcer [39].
Many reinforcers also activate opioid mechanisms in the nucleus accumbens and the ventral tegmental area [30,38]. Activation of opioid receptors in the ventral tegmental area increases dopamine secretion in the nucleus accumbens. Thus, blockade of opioid activity in the ventral tegmental area may block reinforcement.
Importantly, brain-imaging studies have shown that the nucleus accumbens is activated also in response to stimuli that signal primary reinforcers [40], i.e. conditioned reinforcers. Thus, a stimulus with no inherent reinforcing properties may, via associations with a reinforcing stimulus, come to elicit dopaminergic activity and reinforce behaviour. Money is one example, as money has no inherent reinforcing properties, but still arouses the dopamine system.
11. Similarities between the neurobiology of reinforcement and the neurobiology of placebo effects
Dopaminergic activity in the nucleus accumbens has been observed under placebo analgesia [41,42]. Administration of treatment to a person in pain, with a resultant reduction in pain, can be considered negative reinforcement. The role of negative reinforcement is to increase the probability of taking medication in the future, and negative reinforcement is associated with dopamine release that increases positive emotions. Several authors have put forth the idea that placebo responding is because of a reduction in negative emotions [6,43], which implicates a role for negative reinforcement and the dopamine system in placebo responding. Negative reinforcement has been implicated as a mechanism for placebo analgesia [42], but has not been subjected to systematic treatment and review.
Termination of unpleasant stimuli also increases dopaminergic activity, and behaviour that reduces or terminates unpleasant stimuli, for example pain, is reinforced [44]. Thus, it is hypothesized that ingesting a painkiller activates the mesolimbic reinforcement system, with a consequent increase in positive emotions.
Stimuli associated with reinforcement may also activate the mesolimbic dopaminergic system, as noted, even in the absence of unconditioned stimuli that unconditionally activate the system. Thus, conditioned reinforcers may increase dopamine secretion in the nucleus accumbens. The colour and shape of a tablet may be considered a conditioned reinforcer, as the tablet is associated with a negative reinforcer, i.e. reduction in pain. Thus, it can be hypothesized that ingestion of the tablet should elicit dopaminergic activation which in turn should increase feelings of pleasure and decrease negative emotions.
The proposed model thus works as follows: When pain occurs, or when a situation where pain has been experienced in the past occurs, negative emotions are induced. (i) The patient worries about the pain, thinks about things she cannot do, and sadness, nervousness and anger are common reactions. (ii) The individual ingests a painkiller. The tablet or capsule reduces some of the negative emotions induced by pain or stimuli associated with pain. (iii) Pain is reduced via the reduction in negative emotions (or increase in positive emotions). Research that addresses steps (ii) and (iii) in the model are reviewed below.
12. Do emotions modulate the placebo response, or does the placebo response modulate emotions?
Aslaksen & Flaten [45] administered experimental heat pain at several points in time before and after administration of capsules containing corn starch with information that it was a powerful painkiller. A natural history control condition was also employed, where the same subjects received only the painful stimulation. Placebo analgesia was observed as significantly lower pain report when the subjects received information that they had been administered a painkiller. Interestingly, the reported stress was also reduced after placebo (figure 2), and reduced stress after placebo predicted the placebo analgesic response. Because of the long duration of the painful stimuli, heart rate variability could be computed. Heart rate variability indexes sympathetic and parasympathetic influences on the heart, and this measure mirrored the stress data and indicated lower sympathetic activation to painful stimulation after administration of a placebo. These data suggest that administration of a placebo reduces negative emotions, with a subsequent reduction in pain. However, measures of stress and heart rate variability were obtained during and not prior to painful stimulation, and it could not be ascertained whether reduced stress was a cause or a consequence of the placebo analgesic response of reduced pain. Reduced sympathetic response to painful stimulation after placebo administration was also seen by Pollo et al. [46].
Figure 2.
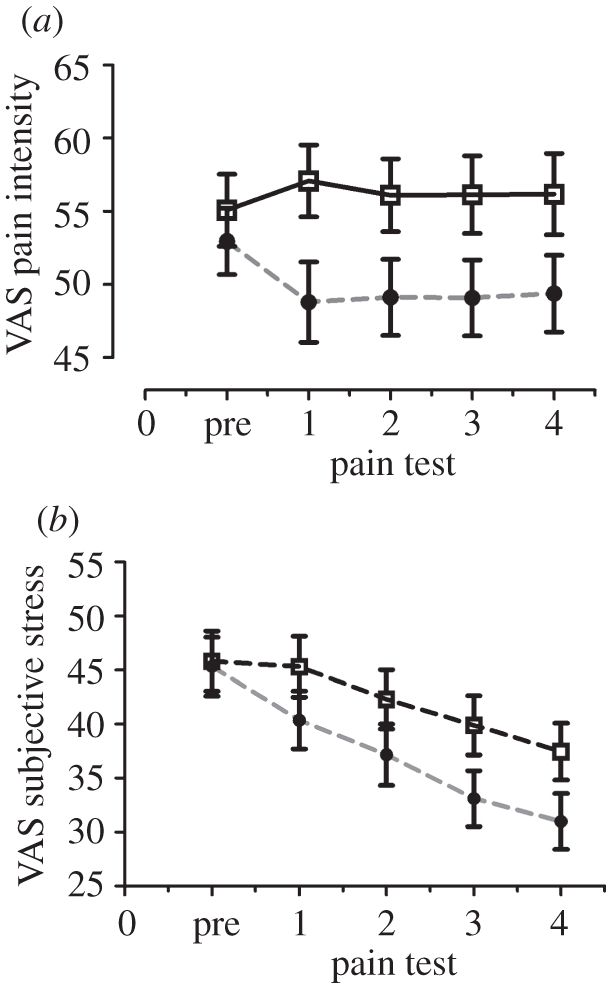
(a) Reported pain intensity before and after administration of a placebo with information that it was a painkiller in the placebo condition. In the control condition, pain was applied five times to control for the natural history of pain. The placebo analgesic response is the difference between the conditions in pain tests one to four. (b) Reported subjective stress levels in the placebo and natural history conditions. Open squares, control; filled circles, placebo. Adapted from Aslaksen & Flaten [45] with kind permission from the publisher.
On the other hand, placebo analgesic responding has been observed in the absence of reduced stress, as assessed by cortisol, in Johansen et al. [47]. In Flaten et al. [7], a placebo analgesic response was observed, but subjective stress and cortisol were not significantly reduced by the information that a powerful painkiller had been administered. Thus, placebo analgesia can be elicited without a concomitant decrease in stress. However, stress levels in Flaten et al. [7] were low prior to pain onset, which could explain that the information that a painkiller had been administered did not significantly reduce pain.
In order to assess the causal relation between emotions and placebo effects, emotions must be recorded in the absence of pain or other symptoms. A placebo analgesic response will be correlated with reduced negative emotions, when emotions are recorded during the painful stimulation as pain levels are decreased. However, the reduced pain is probably the reason for the reduction in negative emotions, and a design where emotions and pain are measured at the same time does not allow conclusions about causality.
Three studies have, to our knowledge, tested for emotional reactions, in the absence of pain, after a placebo manipulation. Aslaksen et al. [11] recorded stress and arousal in the absence of pain to observe whether stress was reduced by information that a painkiller was administered. Experimental heat pain was induced before, and at 10–15 and at 25–30 min after administration of a painkiller with information that it was a potent painkiller. Administration of the placebo reduced stress in both post-placebo measurements, and the reduced stress explained 17 and 26 per cent of the placebo analgesic response in the two tests, respectively.
Scott et al. [41] observed that administration of a placebo reduced pain, and also reduced negative affect and fear. Importantly, the reduction in negative affect and fear occurred after placebo administration but prior to pain administration, which shows that the reduction in negative emotions was not confounded with the reduction in pain. Thus, the placebo reduced negative emotions prior to and independent of the subsequent reduction in pain, suggesting a causal link between the two. Another interesting finding in Scott et al. [41] was that positive emotions tended to increase after administration of the placebo. It is not known whether the increase in positive emotions was because of the reduction in negative emotions (or vice versa) or whether the changes in negative and positive emotions were independent of each other.
Vase et al. [6] also recorded emotions in the absence of pain. They first presented phasic painful stimuli for 20 min, and after this first phase of study, expectations of pain levels, anxiety and desire for pain relief were recorded in the absence of painful stimuli. Thereafter, a second phase of phasic painful stimulation was in effect for about 20 min. They showed that the expected pain levels, desire for pain relief and anxiety accounted for 58 per cent of the placebo effect in the second phase of the experiment. However, a change in the expected pain was the only unique predictor of placebo analgesic responding.
In sum, these findings suggest that administration of a placebo may decrease stress and negative emotions that mediate the decrease in pain, i.e. placebo analgesia. The decrease in stress and negative emotions occur in the absence of reduced pain and prior to the recording of the placebo analgesic response, suggesting a causal connection from the reduced stress to the reduced pain, i.e. the placebo analgesic response.
13. Individual differences in emotions: state and trait
The fact that emotions modulate pain may be linked to individual differences in placebo responding. The field of individual differences is problematic, especially for methodological reasons. Firstly, causal inference cannot be made on the observation of a correlation between placebo analgesic responding and a low or high score on a measure of emotion. Secondly, a placebo response must be observed in each individual in order for the response to be correlated to other variables. A within-subjects design where each participant takes part in the placebo and the natural history conditions must, therefore, be employed. This design may induce variability owing to the order of the conditions. The subjects are more nervous at the beginning of the first session, and pain may be higher in that session. This can interfere with the placebo response. Thus, the placebo response can be underestimated in subjects where the placebo session is run before the natural history session.
Only one study has looked at whether a trait measure of emotional responding can affect placebo analgesia. Lyby et al. [48] showed that increased fear of pain, a trait measure indexing how fearful an individual is for different forms of painful stimulation, abolished the placebo analgesic response. A placebo analgesic response was observed only in the participants with lower scores on the Fear of Pain Questionnaire. Moreover, individuals high in fear of pain also reacted with more anticipatory stress prior to the administration of painful stimulation, and reacted with increased pain. Taken together, these findings suggest that increased levels of stress or negative emotions reduce the placebo analgesic response.
14. Expectations of drug effects can modulate emotions
Several studies have shown that expectations about drug effects can modulate the arousal levels of the participants. Flaten [49] and Flaten et al. [50] told subjects that they received a drug that would increase or decrease alertness, and found that self-report and skin conductance responses behaved in accordance with the drug-related information. Subjects who were told they received a stimulant drug were found to have increased reported and autonomic arousal [50], and subjects who were told that they received a relaxant drug showed decreased levels of arousal when compared with a control group [49]. Several other studies have also made the observation that verbal information can increase or decrease arousal levels [51,52].
Petrovic et al. [53] administered a benzodiazepine and observed that the anxiolytic reduced the rated unpleasantness of pictures presented to the subjects. Thereafter, in a second session, the subjects were given placebo but told that they received the same drug as in the first session. They observed a placebo response of reduced anxiety and reduced ratings of the negative emotions displayed in the pictures. Interestingly, the placebo reduced the processing of negative emotions in the brain, which parallels the findings of reduced processing in pain-related areas in the brain during placebo analgesia [12].
Studies where no drug-related information has been provided and where stimuli that have been reliably paired with the stimulant caffeine have been presented have yielded the same results. Caffeine is a centrally acting stimulant that exerts its effect via antagonism of the inhibitory transmitter adenosine, thereby producing increased alertness and autonomic arousal. Flaten & Blumenthal [54] had caffeine-deprived subjects drink decaffeinated coffee that has no stimulating effect by itself. All the subjects were coffee drinkers, and the smell and taste of coffee had been paired hundreds and probably thousands of times with the stimulating effect of caffeine. Thus, the decaffeinated coffee was a conditioned stimulus, the presentation of which increased subjective and autonomic arousal.
Oken et al. [55] administered placebos to elderly subjects with information that they received a cognitive enhancer. This information increased performance on cognitive tests, and mostly so in subjects who had increased stress at the start of the session. Unfortunately, stress was not measured after administration of the capsules, and it is not known whether the placebo reduced stress, and whether stress mediated the effect of the placebo on cognitive performance.
These findings are evidence that expectations and/or conditioned responses to drug effects may modulate general bodily arousal, which may be a central mechanism in placebo analgesia.
15. The nocebo response: negative placebo effect or separate process?
Expectations that a procedure will hurt, or information that a stimulus will be even more painful, have been found to increase pain [56]. In this case, the expectation that pain is about to occur or that pain will increase induces negative emotions like nervousness and fear, which increases pain. This is a logical extension of the hypothesis that placebo effects are due to reduced negative emotions. The nocebo is not specific to the information provided, but is dependent on the anxiety-inducing content of the information, the same way that placebo effects are hypothesized to be dependent on the anxiety-reducing content of the information.
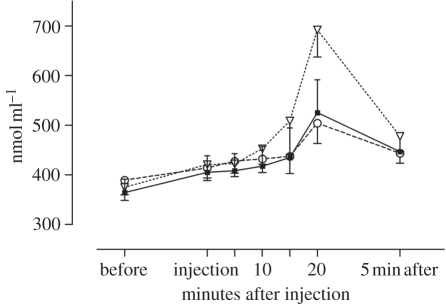
Information that a substance or a procedure increases pain has been found to increase stress as measured by cortisol secretion [47] (figure 3). In the study by Johansen et al. [47], the pain levels were already at seven when the stress-inducing information was provided, and a further increase in pain was not observed. However, other studies have shown that induction of fear or anxiety increases pain. Schweiger & Parducci [57] told healthy subjects that an electrical current was being passed through their heads, while no stimulation was administered, and found that most subjects responded with feelings of pain. A note of caution is in place here, however, as studies on the nocebo effect have not, to our knowledge, recorded the expectations of the subjects. The studies have assumed that information that a certain procedure will increase pain have generated the corresponding expectation.
Figure 3.
Plasma cortisol before and after an injection of saline with information that it was a painkiller (placebo group, filled squares) or that it would increase pain (nocebo group, open inverted triangles). The natural history group (open circles) did not receive any information and no injection. Adapted from Johansen et al. [47] with kind permission of the publisher.
Benedetti et al. [58] showed that administration of proglumide, a cholecystokinin antagonist, cancelled the nocebo response of increased pain. Cholecystokinin is an anti-opioid peptide that induces subjective and physiological stress [59,60], and thereby increases pain. Interestingly, administration of proglumide also enhanced the placebo analgesic response. In sum, a reduction in stress enhanced placebo analgesia. An experimental situation where pain is induced is stressful to the participants, and by reducing some of the stress a beneficial effect on pain is obtained. It is not clear whether administration of proglumide increased positive feelings, via indirect effects on the endogenous opioid system, or whether proglumide decreased negative feelings, via its effect on cholecystokinin. However, a process of emotional modulation seems to underlie both the placebo and nocebo responses. This conclusion is supported by the finding that the cholecystokinin agonist pentagastrin has been found to counteract placebo analgesia [59]. Thus, activation of cholecystokinin receptors, implicated in the induction of negative emotions, completely disrupted placebo analgesia.
16. Why not a compensatory response?
Administration of a drug may induce a disturbance to homoeostatic systems in the body, and signals of impending drug administration may elicit conditioned compensatory responses that counteract the drug response [61]. In one of the few human studies in this field, Flaten et al. [62] administered the muscle relaxant carisoprodol once per week for three weeks to healthy volunteers. Carisoprodol reduces blink reflex magnitudes, and tolerance was observed after repeated administration of carisoprodol, i.e. the drug had less inhibitory effects on blink reflex amplitudes. Flaten et al. [62] showed that conditioned stimuli that signalled carisoprodol administration increased blink reflexes, i.e. signals of carisoprodol administration, elicited a conditioned compensatory response. Cue-elicited drug-compensatory responses are suggestive of a homoeostatic mechanism: the effect of the drug represents a perturbation of homoeostatic levels, and physiological reactions that compensate for and reduce the drug response are elicited by the drug or stimuli that signal the drug [63,64].
Compensatory conditioned responses represent the opposite of placebo responses, and the question of why compensatory drug-antagonistic responses are sometimes observed, and under other circumstances drug-agonistic responses are seen has received much attention [65,66]. One important difference is that placebo effects are observed when the placebo is administered to reduce a symptom, as is the case when pain is induced in healthy volunteers, and the placebo is administered with information that it reduces pain. Compensatory responses, on the other hand, are often observed when a drug has been administered but where a symptom has not been induced, as in Flaten et al. [61]. In the only brain-imaging study that has separated the effects of expectations from the effects of the drug, Gundersen et al. [67] administered a drink containing alcohol or placebo and crossed this with information that the drink contained alcohol or no alcohol. Information that the subjects received alcohol increased feelings of intoxication, replicating several previous studies [68]. Furthermore, alcohol decreased neural activation in the dorsal anterior cingulate cortex and in prefrontal areas, whereas expectation alone increased activity in the same areas. Thus, even when a placebo response was observed in the subjective response to alcohol, there was evidence of a compensatory response at the level of the central nervous system.
17. Evidence not supporting the hypothesis
There are findings indicating specificity in placebo responding that are not compatible with the hypothesis that placebo effects are due to general emotional changes. Benedetti et al. [69] and Montgomery & Kirsch [70] applied placebo cream to one extremity and not to others, and observed placebo analgesic responding only in the placebo-treated extremity. A placebo response involving only global changes in emotion cannot explain these results. However, at least one other study found that this procedure, in about one-third of the subjects, induced placebo responses in both extremities, and in one-third of the subjects induced placebo responses in only one extremity [13].
Meissner [71] induced placebo responses in gastric function, and found that this was not accompanied by other responses in autonomic function or in subjective feelings of arousal. Thus, there are findings of specificity in placebo responses that are not compatible with a general mechanism for placebo responding.
Furthermore, there is evidence of opioid-insensitive placebo analgesic responses. Vase et al. [6] observed large placebo analgesic responses in a sample of patients with irritable bowel disease that were unaffected by naloxone administration and thus not mediated by endogenous opioid release. Similar conclusions were reached by Amanzio & Benedetti [72] who used several different painkillers as unconditioned stimuli in conditioning procedures. When opioid painkillers were associated with the ingestion of a pill, the subsequent placebo response to that pill was completely blocked by naloxone, suggesting that the placebo response was completely mediated by endogenous opioid release. However, when non-opiate painkillers were used as unconditioned stimuli, the placebo analgesic response was either partly blocked or unaffected by the administration of naloxone. Thus, some of these placebo responses were not mediated by endogenous opioid activity. The same conclusion was reached by Kupers et al. [73] in a study of a single patient over a period of 50 days. The patient displayed placebo responses of over 90 per cent pain reduction, which were unaffected by naloxone. Thus, placebo analgesic responses are, under some conditions, not mediated by endogenous opioid release. The role of emotional modulation for these placebo responses, however, has not been assessed.
Placebo effects have been observed in the absence of reduced stress [7,47]. In both these studies, pain was induced by the submaximum tourniquet technique where the pain stimulus is highly stressful. Placebo effects on pain intensity were small in these studies, and most importantly, stress was slightly but not significantly reduced after information that a painkiller had been administered. As the drug-related information did not reduce stress, an effect on pain should not be expected.
18. Conclusions
Administration of a placebo has been shown to reduce negative emotions. The reduction in negative emotions correlates with a reduction in pain. This hypothesis is further supported by findings that reduction in stress and induction of positive emotions increase opioid activity, a process similar to that underlying placebo analgesia. Participants who are fearful of pain do not display placebo responses, further suggesting that negative emotions are counteracting the placebo response. Behaviour that reduces stress and negative emotions is reinforced, and reinforcement is dependent on a dopaminergic midbrain system that has been found to be activated during placebo responding, although evidence is still scarce on the role of dopamine in placebo responding. Finally, the nocebo effect, the opposite of the placebo effect, is due to an increase in fear and anxiety.
On the other hand, a placebo may not be effective in reducing negative emotions if stress levels are high, as in individuals with high levels of fear of pain. Furthermore, findings of specificity in the placebo response are incompatible with the hypothesis that emotions mediate placebo responses. Thus, changes in emotions after administration of a placebo cannot explain the entire placebo effect.
Further research could focus on the role of positive emotions in placebo responses. It is not clear whether placebos induce or increase positive emotions, or reduces negative emotions. Studies are needed where it is possible to disentangle the causal role of emotions on pain. Furthermore, the relatively few studies performed until now have involved healthy participants, and studies involving patients are needed. Finally, the hypothesis that reduction in pain and negative emotions activates the dopaminergic reinforcement system needs more research.
Footnotes
One contribution of 17 to a Theme Issue ‘Placebo effects in medicine: mechanisms and clinical implications’.
References
- 1.Price D. D., Milling L. S., Kirsch I., Duff A., Montgomery G. H., Nicholls S. S. 1999. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain 83, 147–156 10.1016/S0304-3959(99)00081-0 (doi:10.1016/S0304-3959(99)00081-0) [DOI] [PubMed] [Google Scholar]
- 2.Hoffman G., Harrington A., Fields H. 2005. Pain and the placebo—what we have learned. Perspect. Biol. Med. 48, 248–265 10.1353/pbm.2005.0054 (doi:10.1353/pbm.2005.0054) [DOI] [PubMed] [Google Scholar]
- 3.Benedetti F. 2009. Placebo effects. Understanding the mechanisms in health and disease. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Morton D. L., Brown C. A., Watson A., El-Deredy W., Jones A. K. P. 2010. Cognitive changes as a result of a single exposure to placebo. Neuropsychologia 48, 1958–1964 10.1016/j.neuropsychologia.2010.03.016 (doi:10.1016/j.neuropsychologia.2010.03.016) [DOI] [PubMed] [Google Scholar]
- 5.Vase L., Robinson M. E., Verne G. N., Price D. D. 2003. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients – an empirical investigation. Pain 105, 17–25 10.1016/s0304-3959(03)00073-3 (doi:10.1016/s0304-3959(03)00073-3) [DOI] [PubMed] [Google Scholar]
- 6.Vase L., Robinson M. E., Verne G. N., Price D. D. 2005. Increased placebo analgesia over time in irritable bowel syndrome (IBS) patients is associated with desire and expectation but not endogenous opioid mechanisms. Pain 115, 338–347 10.1016/j.pain.2005.03.014 (doi:10.1016/j.pain.2005.03.014) [DOI] [PubMed] [Google Scholar]
- 7.Flaten M. A., Aslaksen P. M., Finset A., Simonsen T., Johansen O. 2006. Cognitive and emotional factors in placebo analgesia. J. Psychosom. Res. 61, 81–89 10.1016/j.jpsychores.2005.12.004 (doi:10.1016/j.jpsychores.2005.12.004) [DOI] [PubMed] [Google Scholar]
- 8.Aslaksen P. M., Myrbakk I. N., Hoifodt R. S., Flaten M. A. 2007. The effect of experimenter gender on autonomic and subjective responses to pain stimuli. Pain 129, 260–268 10.1016/j.pain.2006.10.011 (doi:10.1016/j.pain.2006.10.011) [DOI] [PubMed] [Google Scholar]
- 9.Allan L. G., Siegel S. 2002. A signal detection theory analysis of the placebo effect. Eval. Health Prof. 25, 410–420 10.1177/0163278702238054 (doi:10.1177/0163278702238054) [DOI] [PubMed] [Google Scholar]
- 10.Clark W. C. 1969. Sensory-decision theory analysis of the placebo effect on the criterion for pain and thermal sensitivity (d′). J. Abnorm. Psychol. 74, 363–371 10.1037/h0027509 (doi:10.1037/h0027509) [DOI] [PubMed] [Google Scholar]
- 11.Aslaksen P. M., Bystad M., Vambheim S. M., Flaten M. A. 2011. Gender differences in placebo analgesia—event related potentials and emotional modulation. Psychosom. Med. 73, 193–199 10.1097/psy.0b013e3182080d73 (doi:10.1097/psy.0b013e3182080d73) [DOI] [PubMed] [Google Scholar]
- 12.Wager T. D., Rilling J. K., Smith E. E., Sokolik A., Casey K. L., Davidson R. J., Kosslyn S. M., Rose R. M., Cohen J. D. 2004. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science 303, 1162–1167 10.1126/science.1093065 (doi:10.1126/science.1093065) [DOI] [PubMed] [Google Scholar]
- 13.Watson A., El-Deredy W., Bentley D. E., Vogt B. A., Jones A. K. P. 2006. Categories of placebo response in the absence of site-specific expectation of analgesia. Pain 126, 115–122 10.1016/j.pain.2006.06.021 (doi:10.1016/j.pain.2006.06.021) [DOI] [PubMed] [Google Scholar]
- 14.Treede R.-D., Kenshalo D. R., Gracely R. H., Jones A. K. P. 1999. The cortical representation of pain. Pain 79, 105–111 10.1016/S0304-3959(98)00184-5 (doi:10.1016/S0304-3959(98)00184-5) [DOI] [PubMed] [Google Scholar]
- 15.Lang P., Greenwald M., Bradley M., Hamm A. 1993. Looking at pictures: affective, facial, visceral and behavioral reactions. Psychophysiology 30, 261–273 10.1111/j.1469-8986.1993.tb03352.x (doi:10.1111/j.1469-8986.1993.tb03352.x) [DOI] [PubMed] [Google Scholar]
- 16.O'Neill A. C., Parrott S. T. 1992. Stress and arousal in sedative and stimulant cigarette smokers. Psychopharmacology 107, 442–446 10.1007/BF02245173 (doi:10.1007/BF02245173) [DOI] [PubMed] [Google Scholar]
- 17.Keefe F. J., Lumley M., Anderson T., Lynch T., Studts J. L., Carson K. L. 2001. Pain and emotion: new research directions. J. Clin. Psychol. 57, 587–607 10.1002/jclp.1030 (doi:10.1002/jclp.1030) [DOI] [PubMed] [Google Scholar]
- 18.Rhudy J. L., Williams A. E., McCabe K. M., Russell J. L., Maynard L. J. 2008. Emotional control of nociceptive reactions (ECON): do affective valence and arousal play a role? Pain 136, 250–261 10.1016/j.pain.2007.06.031 (doi:10.1016/j.pain.2007.06.031) [DOI] [PubMed] [Google Scholar]
- 19.Flor H., Grusser S. M. 1999. Conditioned stress-induced analgesia in humans. Eur. J. Pain 3, 317–324 10.1016/S1090-3801(99)90013-7 (doi:10.1016/S1090-3801(99)90013-7) [DOI] [PubMed] [Google Scholar]
- 20.Apkarian A. V., Bushnell M. C., Treede R. D., Zubieta J. K. 2005. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 9, 463–484 10.1016/j.ejpain.2004.11.001 (doi:10.1016/j.ejpain.2004.11.001) [DOI] [PubMed] [Google Scholar]
- 21.Rainville P. 2002. Brain mechanisms of pain affect and pain modulation. Curr. Opin. Neurobiol. 12, 195–204 10.1016/S0959-4388(02)00313-6 (doi:10.1016/S0959-4388(02)00313-6) [DOI] [PubMed] [Google Scholar]
- 22.Vogt B. A. 2005. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 6, 533–544 10.1038/nrn1704 (doi:10.1038/nrn1704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ploghaus A., Narain C., Beckmann C. F., Clare S., Bantick S., Wise R., Matthews P. M., Rawlins J. N. P., Tracey I. 2001. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J. Neurosci. 21, 9896–9903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gundel H., Valet M., Sorg C., Huber D., Zimmer C., Sprenger T., Tolle T. R. 2008. Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain 137, 413–421 10.1016/j.pain.2007.10.003 (doi:10.1016/j.pain.2007.10.003) [DOI] [PubMed] [Google Scholar]
- 25.Schweinhardt P., Kalk N., Wartolowska K., Chessell L., Wordsworth P., Tracey I. 2008. Investigation into the neural correlates of emotional augmentation of clinical pain. Neuroimage 40, 759–766 10.1016/j.neuroimage.2007.12.016 (doi:10.1016/j.neuroimage.2007.12.016) [DOI] [PubMed] [Google Scholar]
- 26.Ochsner K. N., Ludlow D. H., Knierim K., Hanelin J., Ramachandran T., Glover G. C., Mackey S. C. 2006. Neural correlates of individual differences in pain-related fear and anxiety. Pain 120, 69–77 10.1016/j.pain.2005.10.014 (doi:10.1016/j.pain.2005.10.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis J., Haviland-Jones J. M. 2000. Handbook of emotions, 2nd edn. London, UK: Guilford [Google Scholar]
- 28.McCubbin J. A., Wilson J. F., Bruehl S., Ibarra P., Carlson C. R., Norton J. A., Colclough G. W. 1996. Relaxation training and opioid inhibition of blood pressure response to stress. J. Consult. Clin. Psychol. 64, 593–601 10.1037/0022-006X.64.3.593 (doi:10.1037/0022-006X.64.3.593) [DOI] [PubMed] [Google Scholar]
- 29.Agmo A., Berenfeld R. 1990. Reinforcing properties of ejaculation in the male-rat: role of opioids and dopamine. Behav. Neurosci. 104, 177–182 10.1037/0735-7044.104.1.177 (doi:10.1037/0735-7044.104.1.177) [DOI] [PubMed] [Google Scholar]
- 30.Koob G. F., Volkow N. D. 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 10.1038/npp.2009.110 (doi:10.1038/npp.2009.110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine J. D., Gordon N. C. 1984. Influence of the method of drug administration on analgesic response. Nature 312, 755–756 10.1038/312755a0 (doi:10.1038/312755a0) [DOI] [PubMed] [Google Scholar]
- 32.Koepp M. J., Hammers A., Lawrence A. D., Asselin M. C., Grasby P. M., Bench C. J. 2009. Evidence for endogenous opioid release in the amygdala during positive emotion. Neuroimage 44, 252–256 10.1016/j.neuroimage.2008.08.032 (doi:10.1016/j.neuroimage.2008.08.032) [DOI] [PubMed] [Google Scholar]
- 33.Benedetti F. B. 1996. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain 64, 535–543 10.1016/0304-3959(95)00179-4 (doi:10.1016/0304-3959(95)00179-4) [DOI] [PubMed] [Google Scholar]
- 34.Grevert P., Albert L. H., Goldstein A. 1983. Partial antagonism of placebo analgesia by naloxone. Pain 16, 129–143 10.1016/0304-3959(83)90203-8 (doi:10.1016/0304-3959(83)90203-8) [DOI] [PubMed] [Google Scholar]
- 35.Eippert F., Bingel U., Schoell E. D., Yacubian J., Klinger R., Lorenz J., Buchel C. 2009. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543 10.1016/j.neuron.2009.07.014 (doi:10.1016/j.neuron.2009.07.014) [DOI] [PubMed] [Google Scholar]
- 36.Eippert F., Finsterbusch J., Bingel U., Buchel C. 2009. Direct evidence for spinal cord involvement in placebo analgesia. Science 326, 404. 10.1126/science.1180142 (doi:10.1126/science.1180142) [DOI] [PubMed] [Google Scholar]
- 37.Skinner B. F. 1953. Science and human behavior. New York, NY: Macmillan [Google Scholar]
- 38.Koob G. F., Nestler E. J. 1997. The neurobiology of drug addiction. J. Neuropsychiatry Clin. Neurosci. 9, 482–497 [DOI] [PubMed] [Google Scholar]
- 39.Cooper J. C., Knutson B. 2008. Valence and salience contribute to nucleus accumbens activation. Neuroimage 39, 538–547 10.1016/j.neuroimage.2007.08.009 (doi:10.1016/j.neuroimage.2007.08.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knutson B., Adams C. M., Fong G. W., Hommer D. 2001. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 21, RC159.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott D. J., Stohler C. S., Egnatuk C. M., Wang H., Koeppe R. A., Zubieta J. K. 2007. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 55, 325–336 10.1016/j.neuron.2007.06.028 (doi:10.1016/j.neuron.2007.06.028) [DOI] [PubMed] [Google Scholar]
- 42.Scott D. J., Stohler C. S., Egnatuk C. M., Wang H., Koeppe R. A., Zubieta J. K. 2008. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry 65, 220–231 10.1001/archgenpsychiatry.2007.34 (doi:10.1001/archgenpsychiatry.2007.34) [DOI] [PubMed] [Google Scholar]
- 43.Flaten M. A. 2009. Drug effects: agonistic and antagonistic processes. Scand. J. Psychol. 50, 652–659 10.1111/j.1467-9450.2009.00776.x (doi:10.1111/j.1467-9450.2009.00776.x) [DOI] [PubMed] [Google Scholar]
- 44.McCullough L. D., Sokolowski J. D., Salamone J. D. 1993. A neurochemical and behavioral investigation of the involvement of nucleus-accumbens dopamine in instrumental avoidance. Neuroscience 52, 919–925 10.1016/0306-4522(93)90538-Q (doi:10.1016/0306-4522(93)90538-Q) [DOI] [PubMed] [Google Scholar]
- 45.Aslaksen P. M., Flaten M. A. 2008. The roles of physiological and subjective stress in the effectiveness of a placebo on experimentally induced pain. Psychosom. Med. 70, 811–818 10.1097/PSY.001361818105ed (doi:10.1097/PSY.001361818105ed) [DOI] [PubMed] [Google Scholar]
- 46.Pollo A., Vighetti S., Rainero I., Benedetti F. 2003. Placebo analgesia and the heart. Pain 102, 125–133 10.1016/s0304-3959(02)00345-7 (doi:10.1016/s0304-3959(02)00345-7) [DOI] [PubMed] [Google Scholar]
- 47.Johansen O., Brox J., Flaten M. A. 2003. Placebo and nocebo responses, cortisol, and circulating beta-endorphin. Psychosom. Med. 65, 786–790 10.1097/01.psy.0000082626.56217.cf (doi:10.1097/01.psy.0000082626.56217.cf) [DOI] [PubMed] [Google Scholar]
- 48.Lyby P. S., Aslaksen P. M., Flaten M. A. 2010. Is fear of pain related to placebo analgesia? J. Psychosom. Res. 68, 369–377 10.1016/j.jpsychores.2009.10.009 (doi:10.1016/j.jpsychores.2009.10.009) [DOI] [PubMed] [Google Scholar]
- 49.Flaten M. A. 1998. Information about drug effects modify arousal–an investigation of the placebo response. Nord. J. Psychiatrry 52, 147–151 10.1080/08039489850139012 (doi:10.1080/08039489850139012) [DOI] [Google Scholar]
- 50.Flaten M. A., Simonsen T., Olsen H. 1999. Drug-related information generates placebo and nocebo responses that modify the drug response. Psychosom. Med. 61, 250–255 [DOI] [PubMed] [Google Scholar]
- 51.Brodeur D. W. 1965. Effects of stimulant and tranquilizer placebos on healthy subjects in a real-life situation. Psychopharmacologia 7, 444–452 10.1007/BF00402366 (doi:10.1007/BF00402366) [DOI] [PubMed] [Google Scholar]
- 52.Jensen M. P., Karoly P. 1991. Motivation and expectancy factors in symptom perception—a laboratory study of the placebo-effect. Psychosom. Med. 53, 144–152 [DOI] [PubMed] [Google Scholar]
- 53.Petrovic P., Dietrich T., Fransson P., Andersson J., Carlsson K., Ingvar M. 2005. Placebo in emotional processing—induced expectations of anxiety relief activate a generalized modulatory network. Neuron 46, 957–969 10.1016/j.neuron.2005.05.023 (doi:10.1016/j.neuron.2005.05.023) [DOI] [PubMed] [Google Scholar]
- 54.Flaten M. A., Blumenthal T. D. 1999. Caffeine associated stimuli elicit conditioned responses: an experimental model of the placebo effect. Psychopharmacology 145, 105–112 10.1007/s002130051038 (doi:10.1007/s002130051038) [DOI] [PubMed] [Google Scholar]
- 55.Oken B. S., Flegal K., Zajdel D., Kishiyama S., Haas M., Peters D. 2008. Expectancy effect: impact of pill administration on cognitive performance in healthy seniors. J. Clin. Exp. Neuropsychol. 30, 7–17 10.1080/13803390701775428 (doi:10.1080/13803390701775428) [DOI] [PubMed] [Google Scholar]
- 56.Enck P., Benedetti F., Schedlowski M. 2008. New insights into the placebo and nocebo responses. Neuron 59, 195–206 10.1016/j.neuron.2008.06.030 (doi:10.1016/j.neuron.2008.06.030) [DOI] [PubMed] [Google Scholar]
- 57.Schweiger A., Parducci A. 1981. Nocebo: the psychologic induction of pain. Pavlovian J. Biol. Sci. 16, 140–143 [DOI] [PubMed] [Google Scholar]
- 58.Benedetti F., Amanzio M., Vighetti S., Asteggiano G. 2006. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J. Neurosci. 26, 12 014–12 022 10.1523/jneurosci.2947-06.2006 (doi:10.1523/jneurosci.2947-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benedetti F., Amanzio M., Thoen W. 2011. Disruption of opioid-induced placebo responses by activation of cholecystokinin type-2 receptors. Psychopharmacology 213, 791–797 See http://dx.doi.org/10.1007/s00213-010-2037-y [DOI] [PubMed] [Google Scholar]
- 60.Shlik J., Zhou Y. P., Koszycki D., Vaccarion F. J., Bradwejn J. 1999. Effects of CCK-4 infusion on the acoustic eye-blink startle and psychophysiological measures in healthy volunteers. J. Psychopharmacol. 13, 385–390 10.1177/026988119901300409 (doi:10.1177/026988119901300409) [DOI] [PubMed] [Google Scholar]
- 61.Siegel S. 2008. Learning and the wisdom of the body. Learn. Behav. 36, 242–252 10.3758/lb.36.3.242 (doi:10.3758/lb.36.3.242) [DOI] [PubMed] [Google Scholar]
- 62.Flaten M. A., Simonsen T., Waterloo K., Olsen H. 1997. Pharmacological classical conditioning in humans. Hum. Psychopharmacol. Clin. Exp. 12, 369–377 (doi:10.1002/(SICI)1099-1077(199707/08)12:4<369::AID-HUP881>3.0.CO;2-D) [DOI] [Google Scholar]
- 63.Siegel S. 1975. Evidence from rats that morphine-tolerance is a learned response. J. Comp. Physiol. Psychol. 89, 498–506 10.1037/h0077058 (doi:10.1037/h0077058) [DOI] [PubMed] [Google Scholar]
- 64.Siegel S. 1976. Morphine analgesic tolerance—situation specificity supports a pavlovian conditioning model. Science 193, 323–325 10.1126/science.935870 (doi:10.1126/science.935870) [DOI] [PubMed] [Google Scholar]
- 65.Eikelboom R., Stewart J. 1982. Conditioning of drug-induced physiological-responses. Psychol. Rev. 89, 507–528 10.1037/0033-295X.89.5.507 (doi:10.1037/0033-295X.89.5.507) [DOI] [PubMed] [Google Scholar]
- 66.Ramsay D. S., Woods S. C. 1997. Biological consequences of drug administration: implications for acute and chronic tolerance. Psychol. Rev. 104, 170–193 10.1037//0033-295X.104.1.170 (doi:10.1037//0033-295X.104.1.170) [DOI] [PubMed] [Google Scholar]
- 67.Gundersen H., Specht K., Gruner R., Ersland L., Hugdahl K. 2008. Separating the effects of alcohol and expectancy on brain activation: an fMRI working memory study. Neuroimage 42, 1587–1596 10.1016/j.neuroimage.2008.05.037 (doi:10.1016/j.neuroimage.2008.05.037) [DOI] [PubMed] [Google Scholar]
- 68.Laberg J. C. 1986. Alcohol and expectancy: subjective, psychophysiological and behavioral-respones to alcohol stimuli in severely, moderately and non-dependent drinkers. Br. J. Addict. 81, 797–808 10.1111/j.1360-0443.1986.tb00407.x (doi:10.1111/j.1360-0443.1986.tb00407.x) [DOI] [PubMed] [Google Scholar]
- 69.Benedetti F., Arduino C., Amanzio M. 1999. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J. Neurosci. 19, 3639–3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montgomery G., Kirsch I. 1996. Mechanisms of placebo pain reduction: an empirical investigation. Psychol. Sci. 7, 174–176 10.1111/j.1467-9280.1996.tb00352.x (doi:10.1111/j.1467-9280.1996.tb00352.x) [DOI] [Google Scholar]
- 71.Meissner K. 2009. Effects of placebo interventions on gastric motility and general autonomic activity. J. Psychosom. Res. 66, 391–398 10.1016/j.jpsychores.2008.09.004 (doi:10.1016/j.jpsychores.2008.09.004) [DOI] [PubMed] [Google Scholar]
- 72.Amanzio M., Benedetti F. 1999. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J. Neurosci. 19, 484–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kupers R., Maeyaert J., Boly M., Faymonville M.-E., Laureys S. 2007. Naloxone-insensitive epidural placebo analgesia in a chronic pain patient. Anesthesiology 106, 1239–1242 10.1097/01.anes.0000265418.68005.8a (doi:10.1097/01.anes.0000265418.68005.8a) [DOI] [PubMed] [Google Scholar]