Abstract
Pluripotency is a transient cellular state during early development which can be recreated in vitro by direct reprogramming. The molecular mechanisms driving entry into and exit from the pluripotent state are the subject of intense research interest. Here, we review the role of the homeodomain-containing transcription factor Nanog in mammalian embryology and induced pluripotency. Nanog was originally thought to be confined to the maintenance of pluripotency, but recent insights from genetic studies uncovered a new biological function. Embryonic stem cells deficient in Nanog alleles are more prone to differentiate but do not lose pluripotency per se. Instead, Nanog is transiently required for the specification of the naive pluripotent epiblast and development of primordial germ cells. Nanog is also essential to finalize somatic cell reprogramming during induction of pluripotency. We propose that this unique transcription factor acts as a molecular switch to turn on the naive pluripotent programme in mammalian cells. In this context, the capacity of Nanog to resist differentiation can be regarded as recapitulation of effects normally associated with the specification of pluripotency. Pertinent questions are how Nanog specifies naive pluripotency and whether this mechanism is evolutionarily conserved.
Keywords: Nanog, pluripotency, reprogramming, ES cells, iPS cells, molecular evolution
1. Nanog counteracts differentiation but is ultimately dispensable for maintenance of pluripotency in embryonic stem cells
Named after the land of the ever young, Nanog was discovered by two groups based on its ability to maintain mouse embryonic stem (ES) cell self-renewal independently of the cytokine leukaemia inhibitory factor (LIF) [1] and by comparing expressed sequence tag libraries from mouse ES cells with various somatic tissues [2]. Chambers and colleagues [1] screened an ES cell cDNA library for factors capable of maintaining the self-renewal of ES cells deficient in the LIF-receptor. Nanog transfectants continued to proliferate as undifferentiated ES cells in the absence of cytokines and resisted chemical induction of differentiation in monolayer cultures. This study also showed that cytokine-dependence and normal differentiation responsiveness were restored upon excision of the Nanog cDNA by Cre recombinase. Cre-reverted ES cells contributed to adult chimeras upon blastocyst injection, demonstrating formally that Nanog can maintain the pluripotency of ES cells without LIF [1].
ES cells deficient in Nanog, generated by Yamanaka and colleagues after targeted disruption of both alleles, expressed markers of differentiation but also maintained some expression of pluripotency genes including Oct4 and Rex1 [2]. No homozygous mutant pups were born from crossings between heterozygous mutant mice. At embryonic day (E) 5.5 Nanog−/− embryos consisted of disorganized extraembryonic tissues with no discernible epiblast. Analysis of mutant embryos suggested that Nanog deficiency causes embryonic lethality subsequent to the formation of the inner cell mass (ICM) at E3.5. ICMs deficient in Nanog did not persist as undifferentiated masses in vitro, indicating that Nanog is required for the successful derivation of ES cells [2].
Several gain- and loss-of-function studies in ES cells demonstrated that Nanog counteracts differentiation-inducing cues. In the absence of serum, ES cells normally require bone morphogenetic proteins (BMPs) in combination with LIF to maintain self-renewal. Forced expression of Nanog, however, could bypass the requirements for both BMP/serum and LIF [3]. This phenotype was directly attributable to constitutive expression of Nanog, as Cre-reverted Nanog transfectants underwent rapid neural differentiation in the absence of BMP and LIF. Thus, constitutive expression of Nanog confers the capacity for autonomous self-renewal to ES cells. Nanog+/− ES cells were reported to be caught in a labile undifferentiated state that could only be propagated in optimal culture conditions [4]. Upon withdrawal from feeders, Nanog+/− ES cells differentiated into endodermal, mesodermal and ectodermal derivatives even in the continued presence of LIF. This suggested that Nanog is a global regulator that represses differentiation into multiple lineages. In agreement with this hypothesis, Ivanova and colleagues found that short hairpin RNA (shRNA)-mediated knockdown of Nanog expression in ES cells caused derepression of markers for various lineages, including endoderm, trophectoderm and epiblast-derived lineages [5]. Conversely, forced expression of Nanog could compensate for the loss of several other pluripotency regulators in shRNA-transduced ES cells [5]. These studies consolidated an emerging view that Nanog, much like Oct4 [6] and Sox2 [7], is central to the maintenance of pluripotency.
The dogma that Nanog performs an essential role in the housekeeping machinery of pluripotency was challenged when Chambers and colleagues observed that Nanog protein is undetectable in a fraction of ES cells that express Oct4 [8]. Moreover, individually seeded Nanog-negative cells could give rise to Nanog-positive cells. This suggested that transient downregulation of Nanog may predispose ES cells to differentiation, but does not mark irreversible commitment. It also prompted re-examination of the requirement of Nanog in ES cell self-renewal using a conditional deletion approach [8]. In agreement with previous descriptions of homozygous and heterozygous mutant ES cells [2,4] and results from RNA interference studies [5,9], differentiated cells appeared upon removal of Nanog from ES cells. Crucially, however, undifferentiated Nanog−/− ES cells persisted during repeated passaging. By applying selection for drug resistance expressed from the endogenous Nanog locus, it was possible to propagate pure populations of Nanog−/− ES cells. These cells retained expression of other pluripotency markers, but expanded more slowly than wild-type cells and had a reduced capacity to form undifferentiated colonies. To determine the potential for multi-lineage differentiation, fluorescent Nanog−/− ES cells were aggregated with wild-type morulae. Extensive contribution of Nanog−/− cells to mid-gestation and adult chimeras demonstrated that Nanog is strictly dispensable for the maintenance of pluripotency in established ES cells. However, Nanog−/− ES cells were prone to differentiate, suggesting that fluctuating Nanog expression renders individual ES cells susceptible to lineage commitment [8].
2. Nanog is required for the formation of naive pluripotency and germ cell development
Even though Nanog is dispensable for the maintenance of pluripotency in ES cells, disruption of Nanog resulted in peri-implantation lethality and loss of the post-implantation epiblast [2]. It was assumed that this reflected the failure of Nanog in maintaining the pluripotent compartment in the embryo. However, could it be that the pluripotent compartment had not been specified in Nanog−/− embryos? At E3.5, transcripts for Nanog and Gata6, a specification factor for the primitive endoderm, are distributed in a ‘salt-and-pepper-like’ manner throughout the ICM [10]. Gene-expression profiling of single ICM cells confirms the presence of two emerging populations of cells at E3.5 with one population of cells showing increased expression of Nanog and the other showing increased expression of Gata4 and Gata6 [11]. By E4.5, Nanog protein expression becomes spatially confined to a subset of ICM cells and is mutually exclusive with the Gata factors, which localize to the hypoblast/primitive endoderm lining the ICM towards the blastocoel [12]. In female embryos, Nanog expression at E4.5 correlates precisely with the subset of cells that show reactivation of the silenced X chromosome [12]. This can be visualized by immunostaining for Eed, a component of the PRC2 polycomb group complex which coats the inactive X chromosome at this stage [13]. The presence of two active X chromosomes in the pluripotent founder tissue is a precondition for random X chromosome inactivation in the embryo proper, and is a distinctive feature of female ES cells [14,15]. Experiments in ES cells suggested that Nanog may have a direct role in X chromosome reactivation through repression of the non-coding RNA Xist [16,17]. In agreement with this hypothesis, the inactive X chromosome persists in Oct4-positive/Gata4-negative E4.5 ICM cells in Nanog−/− embryos [12]. E4.5 Nanog−/− ICMs also showed apoptosis and failed to form a hypoblast, but a few cells retained a capacity to specify into trophoblast when cultured as outgrowths. Nanog−/− embryos failed to respond to selective inhibitors of mitogen-activated protein (MAP) kinase and GSK3 (2i) with LIF, a culture medium that induces the expansion of the naive pluripotent epiblast and that is optimal for the propagation of Nanog−/− ES cells [12,18]. These findings demonstrate that, in the absence of Nanog, pluripotency is not specified and as a result the ICM undergoes apoptosis. A small proportion of cells escapes this fate to become trophoblast, the only other available option.
A surprising aspect of the E4.5 ICM status in Nanog−/− blastocysts was the additional failure to form a hypoblast [12]. This left open the possibility that Nanog may have a separate role in the specification of the hypoblast, which gives rise to the extraembryonic endoderm layer of the visceral and parietal yolk sacs. However, this phenotype could also be explained by a requirement for paracrine signals from the epiblast during hypoblast development. Messerschmidt & Kemler [19] distinguished between these possibilities by injecting ES cells into β-galactosidase-expressing, Nanog-deficient E3.5 host blastocysts. X-gal staining and sectioning of chimeric embryos at E7.5 revealed that epiblast-derived tissues were exclusively X-gal-negative and thus ES cell-derived, confirming the requirement for Nanog in the specification of the epiblast lineage [12]. However, hypoblast-derived tissues in the chimeric embryos were X-gal-positive, indicating derivation from the Nanog−/− host blastocyst [19]. This revealed that Nanog−/− cells are in fact competent to form the hypoblast in the presence of a wild-type epiblast. Hence, Nanog is required for hypoblast formation through a non-cell autonomous mechanism, most likely paracrine support from the naive epiblast. The nature of this paracrine signal remains elusive, but recent evidence points in the direction of the fibroblast growth factor (FGF)–MAP kinase pathway. Treatment of embryos with a selective MAP kinase inhibitor induces the whole ICM to become naive pluripotent epiblast [18]. In contrast, treatment with recombinant Fgf4 results in the expansion of the hypoblast at the expense of the epiblast [20]. Auto-inductive Fgf4/MAP kinase signalling also poises ES cells for lineage commitment in vitro [21,22]. We surmise that the absence of an epiblast-derived signal, possibly Fgf4, is the reason why Nanog−/− ICM outgrowths fail to form extra-embryonic endoderm [12].
The emerging picture is that Nanog should be regarded as a bonafide specification factor for the naive pluripotent epiblast, orchestrating the transition of ICM cells to pluripotency between E3.5 and E4.5. This explains why Nanog−/− embryos cannot give rise to ES cells [2] even though Nanog can be deleted in established ES cells without compromising their pluripotency [8]. However, Nanog is also expressed in primordial germ cells (PGCs) migrating to the genital ridge from E7.75 to E11.5 [23]. Expression of Nanog is initiated after PGCs are specified and persists into the period of widespread epigenetic erasure between E11.5 and E12.5 [24]. Examination of chimeric embryos made from aggregation between Nanog−/− ES cells and wild-type morulae showed that Nanog−/− PGCs are not present beyond E11.5 [8]. This phenotype was directly attributable to the lack of Nanog expression as repair of one of the mutant alleles in Nanog−/− ES cells restored contribution to the germ lineage at E12.5. What is the fate of presumptive PGCs that lack Nanog? Yamaguchi and co-workers [25] addressed this question by conditional shRNA-mediated knockdown of Nanog mRNA in PGCs. Knockdown of Nanog in migrating PGCs resulted in the appearance of apoptotic cell death as early as E10.5. When E10.5 PGCs were cultured in vitro, apoptosis was observed within 24 h after Nanog depletion. Single-cell expression analysis in Nanog-depleted PGCs at E10.5 indicated significant up- or downregulation of several genes, including the repression of Id1 and the PRC2 subunit Suz12. However, major transcriptional regulators of germ cell fate were similarly expressed between Nanog knockdown and control PGCs [25]. These data suggest that lack of Nanog expression causes the loss of PGCs by E12.5 as a result of progressive apoptotic cell death, rather than trans-differentiation to a somatic cell fate.
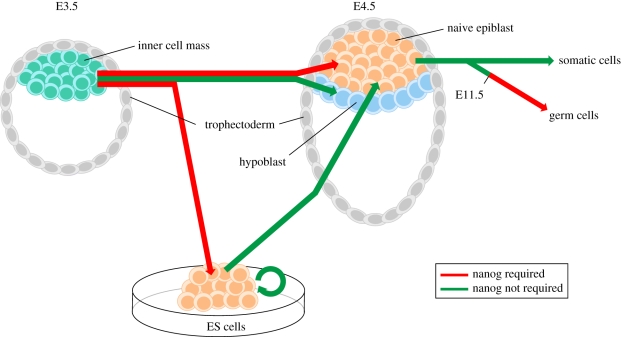
In summary, loss-of-function studies indicate two independent requirements for Nanog during embryonic development (figure 1). First, Nanog is required for specification of the naive pluripotent epiblast, the founder tissue of the embryo proper. Rapid downregulation of Nanog at the time of implantation marks the end of a brief expression window that coincides with the establishment of a pluripotent ground state. By in situ hybridization, Nanog mRNA can also be detected in the post-implantation proximal epiblast between E6.0 and E7.5 [26]. However, extensive contribution of Nanog−/− ES cells to adult chimeras questions the functional relevance of Nanog expression at this stage of development [8]. The second requirement of Nanog is in the development of germ cells beyond E11.5. It is noteworthy that pluripotent cells and germ cells, the two embryonic cell types functionally dependent on Nanog, are both characterized by unique epigenetic features. Between E11.5 and E12.5, PGCs undergo extensive epigenetic reprogramming, which includes reactivation of the inactive X chromosome (in female cells) [27] and genome-wide DNA demethylation [28]. As discussed here, the presence of two active X chromosomes is also a distinctive feature in the female naive pluripotent epiblast and derivative ES cells [12]. Thus, we conclude that Nanog is required during early embryonic development for the specification of two cell states with unique epigenomes.
Figure 1.
Requirement of Nanog in the specification of naive pluripotency and germ cell development. The inner cell mass cells can develop either into the naive epiblast in the pre-implantation embryo or generate embryonic stem (ES) cells in vitro. The former then gives rise to either somatic cells or germ cells, while the latter can be re-introduced back into embryonic development. Inner cell mass cells of embryonic day (E) 3.5 blastocysts, deficient in Nanog, fail to generate a naive pluripotent epiblast [12,19] and are not capable of giving rise to ES cells [2,12]. Deletion of Nanog in established ES cells however does not impair ES cell self-renewal, and upon re-introduction of these in development they are competent to efficiently contribute to all somatic lineages [8]. However, Nanog-deficient ES cells fail to contribute to the germ lineage beyond E11.5 [8,25].
3. Nanog finalizes molecular reprogramming
Takahashi & Yamanaka [29] reported in 2006 that somatic cells can be induced to acquire characteristics of ES cells by the ectopic expression of four transcription factors. Nanog was not among the minimal set of genes required for direct reprogramming. This came as a surprise as overexpression of Nanog enhances reprogramming of somatic cells after cell fusion with ES cells [30]. However, it subsequently became clear that selection for endogenous Nanog expression could distinguish bonafide induced pluripotent stem (iPS) cells [31,32]. Nanog was also included among the minimal set of factors involved in reprogramming of human somatic cells [33]. Indeed, in the absence of Nanog, reprogramming of mouse somatic cells does not occur, as was demonstrated by Silva and colleagues [12]. Infection of Nanog−/− neural stem cells with retroviral transgenes encoding Oct4, Klf4 and c-Myc resulted in the generation of a proliferative cell state referred to as pre-iPS [12,22]. This is marked by the loss of somatic markers but also failure to activate the full repertoire of pluripotency-associated genes. Additionally, Nanog−/− pre-iPS cells cannot transit to pluripotency in the presence of 2i/LIF medium and in fact die. This culture medium was demonstrated to shield ES cells from differentiation-inducing cues while maintaining robust self-renewal capacity [34]. Furthermore, this medium promotes the induction of pluripotency [22]. Only Nanog−/− cells transfected with a constitutive Nanog transgene generated iPS cells in 2i/LIF. After excision of the Nanog transgene, these iPS cells demonstrated a capacity to colonize the embryo and to contribute to the adult animal [12]. This shows that Nanog is not necessary in iPS cells once pluripotency is established, similar to the findings from conditional deletion of Nanog in ES cells [8].
Together, these experiments indicate that Nanog is dispensable initially after the introduction of reprogramming transgenes to generate the pre-iPS cell state, but becomes required later to generate bonafide iPS cells (figure 2). Nanog seems to act at a stage of reprogramming when other key factors, specifically Oct4, Sox2 and Klf4, are already present. This is similar to the situation in the E3.5 ICM, where Oct4 and Sox2 are ubiquitously expressed before the appearance of the naive epiblast [12]. In migrating PGCs, expression of Nanog is also initiated in the presence of Oct4 and Sox2 [24]. Oct4 and Sox2 may have a direct role in sustaining Nanog expression in these different contexts as the Nanog proximal promoter contains an evolutionarily conserved Oct–Sox motif, which is bound by the Oct4/Sox2 binary complex in ES cells [35,36]. This may explain why induction of pluripotency can be achieved in the absence of exogenous Nanog as long as endogenous Nanog alleles are functionally intact [31,32]. Another potential analogy to the sequence of events in vivo is that female pre-iPS cells retain an inactive X chromosome [22,37]. It will be of interest to determine whether appearance of Nanog protein during in vitro reprogramming precedes X chromosome reactivation in individual cells, as is observed in ICM cells between E3.5 and E4.5 [12].
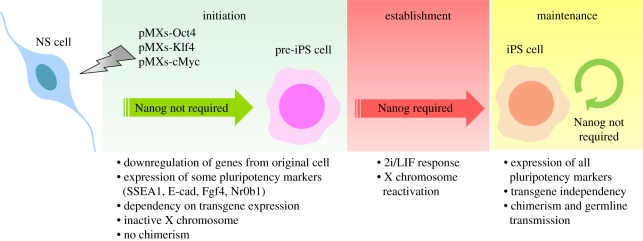
Figure 2.
Requirement of Nanog in induced pluripotency. The process of induction of pluripotency, for which Nanog is crucial, has three phases. The initiation phase comprises the transduction of somatic cells, in this particular example neural stem (NS) cells, with retroviruses (pMXs) containing the reprogramming transgenes Oct4, Klf4 and cMyc. This results in the appearance of a proliferative cell type (pre-iPS), where downregulation of genes from the original cell and expression of some markers of pluripotency occurs. This phase does not require the expression of Nanog [12]. Pre-iPS cells are dependent on the continuous expression of transgenes, are not pluripotent and their exposure to 2i/LIF culture conditions leads to the generation of induced pluripotent stem (iPS) cells [22]. This event marks the establishment phase and Nanog is required for its completion [12]. Properties of iPS cells include the ability to enter normal embryo development and to contribute to the adult animal (chimerism). In the maintenance phase, the last in the process of induction of pluripotency, Nanog is no longer required and can be deleted without compromising self-renewal or the ability of Nanog null iPS cells to contribute to the adult animal [12].
The evidence from genetics suggests that transcriptional activation of endogenous Nanog may be a rate-limiting step during the final stages of somatic cell reprogramming. Indeed, constitutive expression of Nanog was shown to accelerate reprogramming in a study using inducible lentiviral factors [38]. One hypothesis is that Nanog may be the watershed separating pre-iPS cells from bonafide iPS cells. In support of this, it was observed that endogenous Nanog mediates reprogramming downstream of kinase inhibition, and that constitutive expression of Nanog is sufficient to unblock the path to pluripotency in cooperation with LIF/STAT3 signalling [39].
But through what molecular mechanisms does Nanog establish pluripotency? Chromatin immunoprecipitation analysis in partially reprogrammed cells by Sridharan and colleagues has yielded an important clue [37]. This study revealed that cooperative binding by the reprogramming factors was particularly impaired at promoter targets that are also bound by Nanog in ES cells. This suggests that Nanog may be required as a cofactor to coordinate binding of the reprogramming factors to their cognate ES cell targets. In fact, it was shown that Nanog forms multiple protein–protein interactions with other pluripotency regulators in ES cells [40]. The reprogramming factors, Oct4, Sox2 and Klf4, have all been linked to the physical network surrounding Nanog through affinity purification of biotinylated protein complexes [41]. Promoters bound by multiple pluripotency factors tend to be expressed in ES cells and then switched off upon differentiation [42]. Thus, activation of such loci during reprogramming may be contingent with the presence of Nanog. However, microarray analysis after Nanog knockdown indicates that Nanog also represses many of its transcriptional targets in mouse and human ES cells [43,44]. Moreover, Nanog has been directly or indirectly linked with various co-repressor complexes in protein interaction studies [40,45]. Consequently, Nanog may also be required during the final stages of reprogramming to close down paths to alternative cell programmes.
4. Is the function of nanog in specification of naive pluripotency evolutionarily conserved?
Our understanding of the molecular mechanisms controlling pluripotency is largely the fruition of work in mouse ES cells, specifically from the permissive 129 strain. With the advent of 2i/LIF medium, ground state ES cells have recently been captured from non-permissive mouse strains [46] and rats [47,48]. Human ES cells, first described by Thomson in 1998 [49], differ from these rodent ES cells in important biological and molecular respects. These include differences in culture requirements, X chromosome status (in female cells) [50] and target promoter occupancy by the core pluripotency regulators [43]. It was thought for many years that these differences reflect variation between species. In 2007, however, two groups reported that self-renewing stem cell lines derived from the post-implantation epiblast of mouse embryos have properties similar to human ES cells [51,52]. This suggested that differences between mouse and human ES cells may be developmental, rather than species-specific. Mouse epiblast stem cells can be reprogrammed to ground state pluripotency by expression of defined factors and manipulation of the culture environment [53–55]. Could a similar strategy be applied to convert human ES cells into a pluripotent ground state that includes the unique features of X chromosome reactivation and LIF-dependence? Hanna and colleagues reported recently that ectopic induction of three factors in combination with 2i/LIF medium may be sufficient to achieve this [56]. This has prompted the intriguing question of whether ground state pluripotency may be a generic feature in mammals or even throughout evolution.
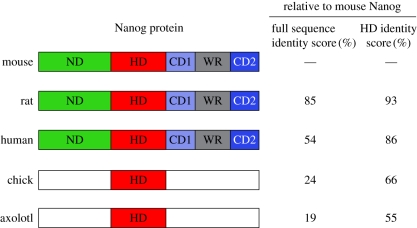
One way to address this question is to ask whether the molecular building blocks of ground state pluripotency, specifically its core transcriptional circuitry, are evolutionarily conserved. This can be done by (i) identifying putative orthologues of pluripotency regulators through sequence alignment and genomic synteny, and (ii) functionally testing whether a given orthologue can replace the mouse gene in a defined pluripotency assay. Nanog is an exceptional candidate for such an approach as it exclusively marks the pluripotent compartment of the ICM at E4.5. In addition, Nanog is required for the specification of pluripotency both during mouse embryogenesis and in vitro reprogramming [12]. Nanog is related to the NK homeobox genes first described by Kim & Nirenberg in Drosophila [57] and shows close sequence alignment with the transcription factors Msx1, Nkx2.5 and Barx1 (50% amino acid identity in the homeodomain). C-terminal to the homeodomain, Nanog contains a tryptophan repeat (WR) domain in which every fifth residue is a tryptophan. Two laboratories reported that the WR domain mediates Nanog dimerization and is required to confer LIF-independent self-renewal in ES cells [58,59]. A Nanog mutant bearing an alteration of 20 tryptophans to alanines within the WR domain lost the capacity to interact with several other pluripotency network proteins, including Sall4, Zfp281, Zfp198 and Dax1 [59]. The WR domain is conserved in the placental mammalian orthologues of Nanog (figure 3). Orthologues of Nanog have also been isolated in non-mammalian vertebrates, including chick [60] and axolotl [61]. The latter was shown to bind to chromatin in the vicinity of the Oct4 and Nanog genes in mouse ES cells and could delay differentiation in embryoid bodies. Unlike reports for human and chick Nanog [1,60], however, axolotl Nanog was not able to support LIF-independent self-renewal.
Figure 3.
Molecular conservation of Nanog in tetrapods. Coloured boxes represent the regions of Nanog orthologue proteins showing conservation relative to mouse Nanog. Identity scores between full-length proteins or homeodomain (HD) only are indicated on the right. The identity scores were calculated using ClustalW2 software. ND, N-terminal domain; CD1, C-terminal domain 1; WR, tryptophan repeat; CD2, C-terminal domain 2.
An important caveat in these studies is that all complementation experiments with Nanog orthologs or deletion mutants were performed in mouse ES cells where endogenous mouse Nanog was present. This raises the question of whether observed phenotypes can be unambiguously assigned to an ectopically introduced Nanog variant. More fundamentally, as we have argued in this review, the evidence from genetics indicates that the primary role of Nanog is to specify the pluripotent ground state. Therefore, only an assay that interrogates establishment of pluripotency in the absence of endogenous Nanog can reveal the full extent of Nanog conservation and required structural elements. A pertinent question is whether orthologues of Nanog retain an autonomous capacity to generate ground state iPS cells during direct reprogramming. Given the lack of structural conservation, it is questionable whether non-mammalian orthologues of Nanog can mediate full reprogramming.
5. Conclusions
Since the discovery of Nanog in 2003, its biological function has been redefined. Nanog was originally accorded a role in the housekeeping machinery that supports ES cell self-renewal, but conditional deletion showed that Nanog is dispensable for the maintenance of pluripotency [8]. Instead, Nanog is transiently required during mouse embryogenesis for the specification of the naive pluripotent epiblast and development of the germ cell lineage [8,12,25]. Nanog deletion also results in a defect in primitive endoderm development, but this phenotype can be explained by a non-cell autonomous dependence on pluripotent epiblast [19]. Despite the capacity to enhance fusion-induced reprogramming [30], Nanog is not included in the quartet of exogenous factors required to induce direct reprogramming [29]. Crucially, the original iPS cell experiments were performed in somatic cells with functional Nanog alleles. With the use of Nanog−/− somatic cells, it was shown that Nanog is dispensable for the initiation of dedifferentiation and generation of a pre-iPS cell state, but becomes required at the final stages of reprogramming to drive transition to full pluripotency [12]. Thus, loss-of-function studies indicate that Nanog is required for the formation of pluripotency, both embryonic and induced, and for PGC development during the period of global epigenetic remodelling.
There are striking parallels between the biological requirements of Nanog during embryogenesis and iPS cell generation. First, expression of Nanog is primed by a pre-existing transcriptional network, including Oct4 and Sox2. This is the case in the ICM at E3.5, migrating PGCs at E7.75 and pre-iPS cells in vitro. Second, Nanog action results in the formation of cell states with unique epigenetic features. This is best exemplified by the phenomenon of X chromosome reactivation in female epiblast/ES/iPS cells and PGCs, which may be directly dependent on Nanog binding to regulatory sites within the Xist gene [16,17]. We conclude that Nanog has evolved specifically to switch on an uncommitted ground state in mammalian cells, but acts only when other key factors are already present.
How can this perspective on the developmental function of Nanog be reconciled with the potent effects of Nanog in resisting differentiation? [1,3]. It is important to separate gain-of-function phenotypes in ES cell culture from the biological role of Nanog in embryonic development. In fact, Nanog expression seems to be tightly restricted during the lifespan of naive pluripotent epiblast cells precisely to avoid interference with differentiation after implantation. Therefore, the phenotype resulting from forced expression of Nanog in ES cells likely recapitulates effects normally associated with the specification of pluripotency. It is conceivable that repression of alternative cell states is part of the mechanism through which Nanog specifies cell fate to ground state pluripotency during embryogenesis and reprogramming. A second, non-mutually exclusive possibility is that Nanog coordinates targeting of the pluripotency machinery, and that constitutive expression of Nanog in ES cells makes the pluripotency network impervious to perturbation by external cues. The challenge now is to elucidate how Nanog specifies pluripotency and determine whether its capacity to switch on pluripotency predates the origin of mammals.
Acknowledgements
We would like to thank Yael Costa and Jennifer Nichols for discussions and critical reading of the manuscript. T.W.T. is a Wellcome Trust PhD Fellow and J.C.R.S is a Wellcome Trust Career Development Fellow.
References
- 1.Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. 2003. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643–655 10.1016/S0092-8674(03)00392-1 (doi:10.1016/S0092-8674(03)00392-1) [DOI] [PubMed] [Google Scholar]
- 2.Mitsui K., Tokuzawa Y., Itoh H., Segawa K., Murakami M., Takahashi K., Maruyama M. i, Maeda M., Yamanaka S. 2003. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631–642 10.1016/S0092-8674(03)00393-3 (doi:10.1016/S0092-8674(03)00393-3) [DOI] [PubMed] [Google Scholar]
- 3.Ying Q. L., Nichols J., Chambers I., Smith A. 2003. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115, 281–292 10.1016/S0092-8674(03)00847-X (doi:10.1016/S0092-8674(03)00847-X) [DOI] [PubMed] [Google Scholar]
- 4.Hatano S. Y., Tada M., Kimura H., Yamaguchi S., Kono T., Nakano T., Suemori H., Nakatsuji N., Tada T. 2005. Pluripotential competence of cells associated with Nanog activity. Mech. Dev. 122, 67–79 10.1016/j.mod.2004.08.008 (doi:10.1016/j.mod.2004.08.008) [DOI] [PubMed] [Google Scholar]
- 5.Ivanova N., Dobrin R., Lu R., Kotenko I., Levorse J., DeCoste C., Schafer X., Lun Y., Lemischka I. R. 2006. Dissecting self-renewal in stem cells with RNA interference. Nature 442, 533–538 10.1038/nature04915 (doi:10.1038/nature04915) [DOI] [PubMed] [Google Scholar]
- 6.Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Schöler H., Smith A. 1998. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95, 379–391 10.1016/S0092-8674(00)81769-9 (doi:10.1016/S0092-8674(00)81769-9) [DOI] [PubMed] [Google Scholar]
- 7.Avilion A. A., Nicolis S. K., Pevny L. H., Perez L., Vivian N., Lovell-Badge R. 2003. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17, 126–140 10.1101/gad.224503 (doi:10.1101/gad.224503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers I., et al. 2007. Nanog safeguards pluripotency and mediates germline development. Nature 450, 1230–1234 10.1038/nature06403 (doi:10.1038/nature06403) [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Theunissen T. W., Orkin S. H. 2007. Site-directed, virus-free, and inducible RNAi in embryonic stem cells. Proc. Natl Acad. Sci. USA 104, 20 850–20 855 10.1073/pnas.0710565105 (doi:10.1073/pnas.0710565105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chazaud C., Yamanaka Y., Pawson T., Rossant J. 2006. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell 10, 615–624 10.1016/j.devcel.2006.02.020 (doi:10.1016/j.devcel.2006.02.020) [DOI] [PubMed] [Google Scholar]
- 11.Kurimoto K., Yabuta Y., Ohinata Y., Ono Y., Uno K. D., Yamada R. G., Ueda H. R., Saitou M. 2006. An improved single-cell cDNA amplification method for efficient high-density oligonucleotide microarray analysis. Nucleic Acids Res. 34, e42. 10.1093/nar/gkl050 (doi:10.1093/nar/gkl050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva J., et al. 2009. Nanog is the gateway to the pluripotent ground state. Cell 138, 722–737 10.1016/j.cell.2009.07.039 (doi:10.1016/j.cell.2009.07.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva J., et al. 2003. Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of Eed-Enx1 polycomb group complexes. Dev. Cell 4, 481–495 10.1016/S1534-5807(03)00068-6 (doi:10.1016/S1534-5807(03)00068-6) [DOI] [PubMed] [Google Scholar]
- 14.Martin G. R. 1981. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA 78, 7634–7638 10.1073/pnas.78.12.7634 (doi:10.1073/pnas.78.12.7634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rastan S., Robertson E. J. 1985. X-chromosome deletions in embryo-derived (EK) cell lines associated with lack of X-chromosome inactivation. J. Embryol. Exp. Morphol. 90, 379–388 [PubMed] [Google Scholar]
- 16.Navarro P., Chambers I., Karwacki-Neisius V., Chureau C., Morey C., Rougeulle C., Avner P. 2008. Molecular coupling of Xist regulation and pluripotency. Science 321, 1693–1695 10.1126/science.1160952 (doi:10.1126/science.1160952) [DOI] [PubMed] [Google Scholar]
- 17.Navarro P., Avner P. 2009. When X-inactivation meets pluripotency: an intimate rendezvous. FEBS Lett. 583, 1721–1727 10.1016/j.febslet.2009.03.043 (doi:10.1016/j.febslet.2009.03.043) [DOI] [PubMed] [Google Scholar]
- 18.Nichols J., Silva J., Roode M., Smith A. 2009. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development 136, 3215–3222 10.1242/dev.038893 (doi:10.1242/dev.038893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messerschmidt D. M., Kemler R. 2010. Nanog is required for primitive endoderm formation through a non-cell autonomous mechanism. Dev. Biol. 344, 129–137 10.1016/j.ydbio.2010.04.020 (doi:10.1016/j.ydbio.2010.04.020) [DOI] [PubMed] [Google Scholar]
- 20.Yamanaka Y., Lanner F., Rossant J. 2010. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 137, 715–724 10.1242/dev.043471 (doi:10.1242/dev.043471) [DOI] [PubMed] [Google Scholar]
- 21.Kunath T., Saba-El-Leil M. K., Almousailleakh M., Wray J., Meloche S., Smith A. 2007. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 134, 2895–2902 10.1242/dev.02880 (doi:10.1242/dev.02880) [DOI] [PubMed] [Google Scholar]
- 22.Silva J., Barrandon O., Nichols J., Kawaguchi J., Theunissen T. W., Smith A. 2008. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 6, e253. 10.1371/journal.pbio.0060253 (doi:10.1371/journal.pbio.0060253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi S., Kimura H., Tada M., Nakatsuji N., Tada T. 2005. Nanog expression in mouse germ cell development. Gene Exp. Patterns 5, 639–646 10.1016/j.modgep.2005.03.001 (doi:10.1016/j.modgep.2005.03.001) [DOI] [PubMed] [Google Scholar]
- 24.Surani M. A., Hayashi K., Hajkova P. 2007. Genetic and epigenetic regulators of pluripotency. Cell 128, 747–762 10.1016/j.cell.2007.02.010 (doi:10.1016/j.cell.2007.02.010) [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi S., Kurimoto K., Yabuta Y., Sasaki H., Nakatsuji N., Saitou M., Tada T. 2009. Conditional knockdown of Nanog induces apoptotic cell death in mouse migrating primordial germ cells. Development 136, 4011–4020 10.1242/dev.041160 (doi:10.1242/dev.041160) [DOI] [PubMed] [Google Scholar]
- 26.Hart A. H., Hartley L., Ibrahim M., Robb L. 2004. Identification, cloning and expression analysis of the pluripotency promoting Nanog genes in mouse and human. Dev. Dyn. 230, 187–198 10.1002/dvdy.20034 (doi:10.1002/dvdy.20034) [DOI] [PubMed] [Google Scholar]
- 27.Monk M., McLaren A. 1981. X-chromosome activity in foetal germ cells of the mouse. J. Embryol. Exp. Morphol. 63, 75–84 [PubMed] [Google Scholar]
- 28.Reik W., Dean W., Walter J. 2001. Epigenetic reprogramming in mammalian development. Science 293, 1089–1093 10.1126/science.1063443 (doi:10.1126/science.1063443) [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K., Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 10.1016/j.cell.2006.07.024 (doi:10.1016/j.cell.2006.07.024) [DOI] [PubMed] [Google Scholar]
- 30.Silva J., Chambers I., Pollard S., Smith A. 2006. Nanog promotes transfer of pluripotency after cell fusion. Nature 441, 997–1001 10.1038/nature04914 (doi:10.1038/nature04914) [DOI] [PubMed] [Google Scholar]
- 31.Okita K., Ichisaka T., Yamanaka S. 2007. Generation of germline-competent induced pluripotent stem cells. Nature 448, 313–317 10.1038/nature05934 (doi:10.1038/nature05934) [DOI] [PubMed] [Google Scholar]
- 32.Wernig M., Meissner A., Foreman R., Brambrink T., Ku M., Hochedlinger K., Bernstein B. E., Jaenisch R. 2007. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448, 318–324 10.1038/nature05944 (doi:10.1038/nature05944) [DOI] [PubMed] [Google Scholar]
- 33.Yu J., et al. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 10.1126/science.1151526 (doi:10.1126/science.1151526) [DOI] [PubMed] [Google Scholar]
- 34.Ying Q. L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. 2008. The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 10.1038/nature06968 (doi:10.1038/nature06968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda T., Tada M., Kubota H., Kimura H., Hatano S. Y., Suemori H., Nakatsuji N., Tada T. 2005. Octamer and Sox elements are required for transcriptional cis-regulation of Nanog gene expression. Mol. Cell. Biol. 25, 2475–2485 10.1128/MCB.25.6.2475-2485.2005 (doi:10.1128/MCB.25.6.2475-2485.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodda D. J., Chew J. L., Lim L. H., Loh Y. H., Wang B., Ng H. H., Robson P. 2005. Transcriptional regulation of nanog by OCT4 and SOX2. J. Biol. Chem. 280, 24 731–24 737 10.1074/jbc.M502573200 (doi:10.1074/jbc.M502573200) [DOI] [PubMed] [Google Scholar]
- 37.Sridharan R., Tchieu J., Mason M. J., Yachechko R., Kuoy E., Horvath S., Zhou Q., Plath K. 2009. Role of the murine reprogramming factors in the induction of pluripotency. Cell 136, 364–377 10.1016/j.cell.2009.01.001 (doi:10.1016/j.cell.2009.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanna J., Saha K., Pando B., van Zon J., Lengner C. J., Creyghton M. P., van Oudenaarden A., Jaenisch R. 2009. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462, 595–601 10.1038/nature08592 (doi:10.1038/nature08592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theunissen T. W., van Oosten A. L., Castelo-Branco G., Hall J., Smith A., Silva J. C. 2011. Nanog overcomes reprogramming barriers and induces pluripotency in minimal conditions. Curr. Biol. 21, 65–71 10.1016/j.cub.2010.11.074 (doi:10.1016/j.cub.2010.11.074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J., Rao S., Chu J., Shen X., Levasseur D. N., Theunissen T. W., Orkin S. H. 2006. A protein interaction network for pluripotency of embryonic stem cells. Nature 444, 364–368 10.1038/nature05284 (doi:10.1038/nature05284) [DOI] [PubMed] [Google Scholar]
- 41.Orkin S. H., Wang J., Kim J., Chu J., Rao S., Theunissen T. W., Shen X., Levasseur D. N. 2008. The transcriptional network controlling pluripotency in ES cells. Cold Spring Harb. Symp. Quant. Biol. 73, 195–202 10.1101/sqb.2008.72.001 (doi:10.1101/sqb.2008.72.001) [DOI] [PubMed] [Google Scholar]
- 42.Kim J., Chu J., Shen X., Wang J., Orkin S. H. 2008. An extended transcriptional network for pluripotency of embryonic stem cells. Cell 132, 1049–1061 10.1016/j.cell.2008.02.039 (doi:10.1016/j.cell.2008.02.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyer L. A., et al. 2005. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 10.1016/j.cell.2005.08.020 (doi:10.1016/j.cell.2005.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loh Y. H., et al. 2006. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 38, 431–440 10.1038/ng1760 (doi:10.1038/ng1760) [DOI] [PubMed] [Google Scholar]
- 45.Liang J., et al. 2008. Nanog and Oct4 associate with unique transcriptional repression complexes in embryonic stem cells. Nat. Cell Biol. 10, 731–739 10.1038/ncb1736 (doi:10.1038/ncb1736) [DOI] [PubMed] [Google Scholar]
- 46.Nichols J., Jones K., Phillips J. M., Newland S. A., Roode M., Mansfield W., Smith A., Cooke A. 2009. Validated germline-competent embryonic stem cell lines from nonobese diabetic mice. Nat. Med. 15, 814–818 10.1038/nm.1996 (doi:10.1038/nm.1996) [DOI] [PubMed] [Google Scholar]
- 47.Buehr M., et al. 2008. Capture of authentic embryonic stem cells from rat blastocysts. Cell 135, 1287–1298 10.1016/j.cell.2008.12.007 (doi:10.1016/j.cell.2008.12.007) [DOI] [PubMed] [Google Scholar]
- 48.Li P., et al. 2008. Germline competent embryonic stem cells derived from rat blastocysts. Cell 135, 1299–1310 10.1016/j.cell.2008.12.006 (doi:10.1016/j.cell.2008.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S., Jones J. M. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 10.1126/science.282.5391.1145 (doi:10.1126/science.282.5391.1145) [DOI] [PubMed] [Google Scholar]
- 50.Silva S. S., Rowntree R. K., Mekhoubad S., Lee J. T. 2008. X-chromosome inactivation and epigenetic fluidity in human embryonic stem cells. Proc. Natl Acad. Sci. USA 105, 4820–4825 10.1073/pnas.0712136105 (doi:10.1073/pnas.0712136105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesar P. J., Chenoweth J. G., Brook F. A., Davies T. J., Evans E. P., Mack D. L., Gardner R. L., McKay R. D. G. 2007. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 10.1038/nature05972 (doi:10.1038/nature05972) [DOI] [PubMed] [Google Scholar]
- 52.Brons I. G., et al. 2007. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 10.1038/nature05950 (doi:10.1038/nature05950) [DOI] [PubMed] [Google Scholar]
- 53.Guo G., Yang J., Nichols J., Hall J. S., Eyres I., Mansfield W., Smith A. 2009. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063–1069 10.1242/dev.030957 (doi:10.1242/dev.030957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanna J., et al. 2009. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell 4, 513–524 10.1016/j.stem.2009.04.015 (doi:10.1016/j.stem.2009.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greber B., et al. 2010. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell 6, 215–226 10.1016/j.stem.2010.01.003 (doi:10.1016/j.stem.2010.01.003) [DOI] [PubMed] [Google Scholar]
- 56.Hanna J., Cheng A. W., Saha K., Kim J., Lengner C. J., Soldner F., Cassady J. P., Muffat J., Carey B. W., Jaenisch R. 2010. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl Acad. Sci. USA. 10.1073/pnas.1004584107 (doi:10.1073/pnas.1004584107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim Y., Nirenberg M. 1989. Drosophila NK-homeobox genes. Proc. Natl Acad. Sci. USA 86, 7716–7720 10.1073/pnas.86.20.7716 (doi:10.1073/pnas.86.20.7716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mullin N. P., Yates A., Rowe A. J., Nijmeijer B., Colby D., Barlow P. N., Walkinshaw M. D., Chambers I. 2008. The pluripotency rheostat Nanog functions as a dimer. Biochem. J. 411, 227–231 10.1042/BJ20080134 (doi:10.1042/BJ20080134) [DOI] [PubMed] [Google Scholar]
- 59.Wang J., Levasseur D. N., Orkin S. H. 2008. Requirement of Nanog dimerization for stem cell self-renewal and pluripotency. Proc. Natl Acad. Sci. USA 105, 6326–6331 10.1073/pnas.0802288105 (doi:10.1073/pnas.0802288105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lavial F., et al. 2007. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development 134, 3549–3563 10.1242/dev.006569 (doi:10.1242/dev.006569) [DOI] [PubMed] [Google Scholar]
- 61.Dixon J. E., et al. 2010. Axolotl Nanog activity in mouse embryonic stem cells demonstrates that ground state pluripotency is conserved from urodele amphibians to mammals. Development 137, 2973–2980 10.1242/dev.049262 (doi:10.1242/dev.049262) [DOI] [PMC free article] [PubMed] [Google Scholar]