Abstract
Recent advances in stem cell technology have generated enthusiasm for their potential to study and treat a diverse range of human disease. Pluripotent human stem cells for therapeutic use may, in principle, be obtained from two sources: embryonic stem cells (hESCs), which are capable of extensive self-renewal and expansion and have the potential to differentiate into any somatic tissue, and induced pluripotent stem cells (iPSCs), which are derived from differentiated tissue such as adult skin fibroblasts and appear to have the same properties and potential, but their generation is not dependent upon a source of embryos. The likelihood that clinical transplantation of hESC- or iPSC-derived tissues from an unrelated (allogeneic) donor that express foreign human leucocyte antigens (HLA) may undergo immunological rejection requires the formulation of strategies to attenuate the host immune response to transplanted tissue. In clinical practice, individualized iPSC tissue derived from the intended recipient offers the possibility of personalized stem cell therapy in which graft rejection would not occur, but the logistics of achieving this on a large scale are problematic owing to relatively inefficient reprogramming techniques and high costs. The creation of stem cell banks comprising HLA-typed hESCs and iPSCs is a strategy that is proposed to overcome the immunological barrier by providing HLA-matched (histocompatible) tissue for the target population. Estimates have shown that a stem cell bank containing around 10 highly selected cell lines with conserved homozygous HLA haplotypes would provide matched tissue for the majority of the UK population. These simulations have practical, financial, political and ethical implications for the establishment and design of stem cell banks incorporating cell lines with HLA types that are compatible with different ethnic populations throughout the world.
Keywords: immunology, pluripotent stem cells, stem cell banking
1. Introduction
Technological advances in stem cell research have promoted confidence in the potential use of stem cells for treating and studying a range of human diseases. These include neurodegenerative diseases, such as Parkinson's and Alzheimer's disease, autoimmune diseases such as diabetes and multiple sclerosis, cardiovascular disease and some forms of haematopoietic malignancy. In the UK, there are around 250 000 patients with type 1 insulin-dependent diabetes and 120 000 with Parkinson's disease that could potentially benefit from regenerative stem cell therapy [1,2]. For patients with autoimmune disease, stem cell-derived tissue replacement may be used in conjunction with additional therapy to control disease recurrence and progression. Worldwide there are several million potential beneficiaries of stem cell therapy and the question arises concerning how best to realize the full potential and offer affordable and durable treatment on such a large scale.
Haematopoietic stem cell (HSC) transplantation for the treatment of certain haematological deficiencies and malignancies has been in use for many years, and more recently, mesenchymal stem cell therapy has been applied clinically for the control of graft-versus-host disease (GVHD) [3,4]. The attraction of pluripotent stem cell therapy is the potential for deriving any type of replacement tissue from a single donor source. This review focuses on immunological considerations of pluripotent stem cell research, therapy and banking.
Human pluripotent stem cells for therapeutic use may, in principle, be obtained from two sources. Embryonic stem cells (hESCs) are derived from the inner cell mass of the blastocyst arising from fertilized oocytes and are capable of extensive self-renewal and expansion; they have the potential to differentiate into any somatic tissue, such as insulin-producing pancreatic beta cells, cardiomyocytes that form heart muscle and neurons that form nerve and brain tissue. Induced pluripotent stem cells (iPSCs) appear to have the same properties and potential but are derived from differentiated tissue such as adult skin fibroblasts, and their generation is not dependent upon a source of embryos. There are, however, concerns that the most robust techniques for reprogramming iPSC precursors currently require viral vectors that may preclude clinical application, but future research will undoubtedly overcome this hurdle [5].
The likelihood that clinical transplantation of embryonic or iPSC-derived tissues from a genetically unrelated donor may generate an immune response remains unresolved. Hitherto, experimental studies using embryonic or adult stem cells and their derivatives have generally used immuno-incompetent recipients, or have transplanted to an immunologically privileged site such as the brain, or have failed to either report whether immunosuppression was used or to consider whether failure of engraftment may be attributed to immunological rejection. Experience of clinical HSC transplantation, however, clearly demonstrates that the contribution of an immune response to transplant and patient outcome cannot be ignored.
The human immune system has evolved in a hostile environment populated by pathogens that aim to invade and colonize our bodies. To prevent potentially pathogenic micro-organisms, including bacteria, viruses and protozoa, from causing disease and death, the human immune system has developed a sophisticated and highly effective series of defence mechanisms, both innate and adaptive, that recognize, isolate and eliminate invading pathogens. These same immune defence mechanisms also have the potential to recognize and destroy stem cell-derived tissue (hESCs and iPSCs) when transplanted into an unrelated recipient.
This paper reviews the likely role of the immune system in regenerative medicine and considers strategies for avoiding immunological rejection, as well as practical aspects of establishing a resource of hESCs and iPSCs that would be of most benefit to a majority of patients who might be treated by regenerative medicine.
2. Immune recognition
Innate and adaptive immune mechanisms have evolved that confer non-specific immediate protection against foreign organisms and allow time for a more powerful and specific immune response to develop that will neutralize pathogens. The immune system is strongly programmed to recognize tissue compatibility (termed MHC restriction [6]), which is a requirement for raising an effective immune response against pathogens and, eo ipso, is able to adapt and respond to the presence of incompatible allogeneic tissues. Thus, the immunological principles that challenge successful organ, tissue and bone marrow transplantation are likely to apply equally to transplantation of hESC- and iPSC-derived tissue. Tissue incompatibility between a transplant recipient and their donor arises from allelic disparities at genetic loci that encode the ABO blood group system, the major histocompatibility complex (MHC) antigens and minor histocompatibility (mHC) antigens.
Humans and higher primates express ABO blood group antigens that are abundantly displayed on the surface of red blood cells, epithelial cells and vascular endothelial cells [7,8]. Human ABO blood group antigens comprise a homologous family of oligosaccharide structures carried on glycoprotein and glycolipid components of the cell surface. Similar repetitive carbohydrate structures are also displayed by bacteria colonizing the gastrointestinal tract that stimulate the production of natural immunoglobulin (Ig) M and IgG antibodies to confer innate host immunity against a range of micro-organisms [9]. These natural antibodies, however, cross-react with human blood group A and B antigens: blood group A individuals have circulating anti-B antibodies, blood group B individuals have circulating anti-A antibodies and those who are blood group O have antibodies to both A and B antigens. Only blood group AB individuals do not have circulating anti-ABO antibodies. Because the antibodies are already present, they bind rapidly to ABO-incompatible transplanted tissues and cells, activate the complement cascade and the coagulation response, and cause widespread red cell lysis and extensive tissue damage through the process known as hyperacute rejection [10,11]. Recent research has found that not only hESCs, but also cell types differentiated in vitro from hESCs, including hepatocyte- and cardiomyocyte-like cells, express ABO antigens, indicating a requirement for ABO matching for regenerative medicine [12]. Additional blood group antigens, such as Kell, Duffy and Lewis, may also be relevant particularly for transplantation in ethnically diverse populations, although currently they have no known role in vascularized organ allografts and in HSC transplantation.
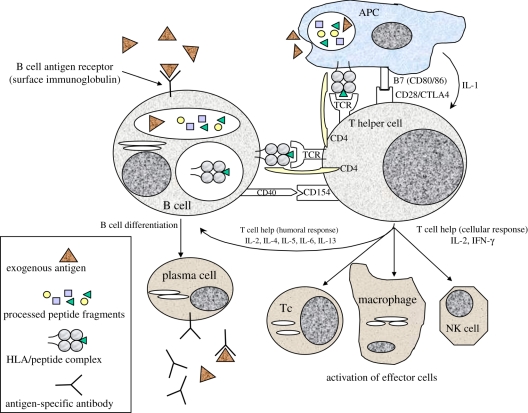
The MHC region contains around 200 genes that are central to immune recognition. In humans it is called the human leucocyte antigen (HLA) system and it encodes, among others, two major classes of highly polymorphic cell surface glycoproteins whose key role is to bind peptide fragments derived from self proteins and foreign pathogens for presentation on antigen-presenting cells (APCs) to T lymphocytes (figure 1). The HLA system comprises six principal loci encoding two classes of molecules; HLA-A, -B and -C are HLA class I molecules and their primary function is to present peptides derived predominantly from intracellular and viral proteins for recognition by CD8 cytotoxic T lymphocytes (CTLs). The HLA-DR, -DQ and -DP class II molecules predominantly bind peptides derived from the processing of extracellular proteins and pathogenic material, and are recognized by CD4 helper T cells. The two classes of molecules have widely different cellular distribution, which reflects their disparate functions. HLA class I molecules are ubiquitously expressed on nucleated cells of the body, whereas HLA class II molecules are constitutively expressed on bone marrow-derived APCs and thymic epithelial cells. During inflammatory conditions, the presence of the cytokine interferon-γ (IFN-γ) results in de novo expression of HLA class II on many types of endothelial and epithelial cells, as well as upregulation of both class I and class II expression on APCs. The effect of this is an increase in the ability of the immune system to respond to an antigenic stimulus through increased antigen-presenting capacity.
Figure 1.
Adaptive immune response to foreign antigen. Exogenous particulate or soluble antigens (e.g. glycoproteins) are taken up by antigen-presenting cells (APCs) and processed into peptide fragments that are translocated to the HLA class II peptide-binding groove. Exogenous antigen may also be recognized and internalized by antigen-specific B cells through their surface immunoglobulin (Ig) receptor. CD4 positive (HLA class II restricted) T ‘helper’ cells bearing antigen-specific T cell receptors (TCR) recognize self HLA/foreign peptide complex on APCs (signal 1) and engage with costimulatory molecules (e.g. CD80/CD28, CD40/CD154) (signal 2), resulting in T cell activation. Activated T helper cells secrete cytokines including IL-2 and IFN-gamma to recruit and activate antigen-specific CD8 (HLA class I restricted) cytotoxic T lymphocytes (Tc), and non-antigen-specific effector cells (macrophages and natural killer (NK) cells). Alternatively, T helper cells may secrete cytokines, such as IL-4, IL-5 and IL-13, that stimulate differentiation of antigen-specific B cells into antibody-producing plasma cells.
Early in vitro studies of MHC restriction demonstrated the exquisite specificity of antigen-reactive T cells: for example, CTLs were able to recognize and kill virally infected target cells that shared MHC class I alleles with the CTL responders but would not respond to the same viral peptide presented by allogeneic MHC molecules that were distinct from those expressed by the CTLs [6]. However, in the case of clinical transplantation, responding T cells can readily recognize and reject tissues expressing allogeneic HLA. The expression of allogeneic HLA molecules by tissues differentiated from hESCs and iPSCs would render them equally susceptible to recognition by the host immune system.
mHC antigens exist as allelic variants in different individuals but they are not expressed as cell surface molecules. Peptides derived from these molecules may be presented by APCs following transplantation but because they do not form a readily accessible target they do not elicit a powerful rejection response against mHC-mismatched vascularized allografts. In an otherwise un-primed individual, any response to mHC antigens is readily controlled by standard immunosuppressive therapy. However, in HLA-identical sibling bone marrow transplantation, combinations of multiple mHC antigens are a significant cause of GVHD, which occurs in around 40 per cent of patients. Well-known examples of mHC antigens that contribute to clinical HSC transplant rejection and GVHD include peptides derived from HA-1, and the male H-Y gene product, recognized by female immune cells as foreign [13,14]. Mitochondrial gene products are another example of mHC antigens that may be relevant to regenerative medicine when the ESC line is derived from an embryo created by nuclear transfer, depending on the origin of the host oocyte.
3. Hla structure
Peptide presentation by HLA class I and II molecules is achieved through common elements of their molecular structure. They are both highly polymorphic heterodimeric molecules tethered in the cell membrane, with membrane-proximal conserved regions that form docking sites for the CD4 and CD8 chains adjacent to the T cell receptor on lymphocytes, together with membrane-distal regions that have highly variable areas enabling the binding of an extensive array of different peptides. HLA class I molecules consist of the heavy (α) chain of approximately 44 kDa comprising an intracellular tail, transmembrane region and three extracellular Ig-like domains, complexed with the non-polymorphic, 12 kDa β2-microglobulin chain that is not inserted in the cell membrane. The membrane-proximal α3 domain of the heavy chain bears the CD8 docking region while the α1 and α2 distal domains each consist of a β-pleated sheet surmounted by an α-helix, together forming the peptide binding cleft [15]. HLA class II molecules are composed of an α and a β chain of 32 and 28 kDa, each having an intracellular tail, a transmembrane region and two extracellular Ig-like domains. The distal α1 and β1 domains, as for class I, form the peptide-binding cleft and the β2 domain contains the CD4 docking region. HLA gene polymorphism encodes amino acid variability principally in the β-pleated sheet and α-helices, permitting binding of a vast assortment of peptides for presentation.
4. Initiating allograft rejection
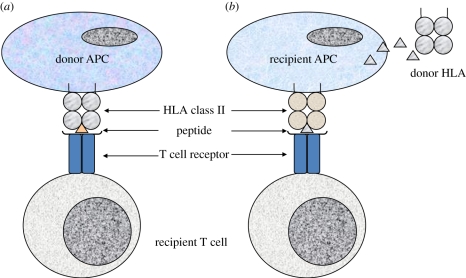
T lymphocytes emerging from the thymus populate the secondary lymphoid tissue where they remain latent on encounter with self peptide/HLA complexes on APCs, but become activated if they encounter foreign peptide bound to self HLA (figure 1) [16]. Following transplantation, host T lymphocytes residing in lymph nodes in the vicinity of the transplant may be exposed to APCs (dendritic cells) that migrate from the donor tissue or organ within the first few hours and days following transplantation. T cells that normally recognize, and are unresponsive to, self HLA/endogenous peptide complexes on self APCs are activated, paradoxically, by foreign HLA/endogenous peptide complexes on donor APCs on the basis of molecular mimicry, or recognition of altered self, such that the complex overall is sufficiently similar to a self complex for the T cell to recognize it but sufficiently different for the T cell to respond to it, by becoming activated, as though it were a self HLA/foreign peptide complex. This type of T cell activation is unique to transplantation and is known as the direct recognition pathway (figure 2). It is a very potent pathway for initiating a rapid graft rejection response since it has been estimated that around 1–5% of an individual's T cells are able to respond to foreign HLA in this way [17,18].
Figure 2.
Direct and indirect pathways of alloantigen recognition. (a) Direct recognition pathway, donor APCs (e.g. dendritic cells) that are present in the transplanted tissue migrate from the graft to nearby recipient lymphoid tissue where they encounter host lymphocytes. Mature APCs express high levels of HLA class II molecules and other accessory molecules that are required to stimulate T cell activation. Recipient CD4 (HLA class II restricted) T cells directly recognize foreign (donor) HLA as ‘altered self’, which can occur with or without peptide recognition. Such HLA alloantigen-specific T cells exist at high precursor frequency and initiate a rapid and vigorous immune response. Activated alloantigen-specific T cells recognizing donor HLA migrate from recipient lymphoid tissue to the allograft where they orchestrate graft rejection.(b) Indirect pathway of alloantigen recognition, soluble and particulate foreign protein antigens (e.g. donor HLA) released from transplanted allogeneic tissue are taken up by recipient APCs and processed into peptide fragments that are presented by recipient HLA to recipient T cells. Self HLA-restricted T cells recognize the allo-peptide as foreign and initiate an immune response to the allograft.
The alternative pathway of T cell activation to an unrelated transplant, known as indirect recognition, is analogous to the way in which the immune system responds to any potentially dangerous exogenous protein or pathogen (figure 2). The foreign material, which may be soluble donor HLA molecules or membrane fragments released from the transplant both in the first few hours and in subsequent weeks and months, is picked up and processed by professional recipient APCs including dendritic cells and B lymphocytes. The resulting peptides are presented principally by class II HLA molecules on APCs within draining lymph nodes to recipient CD4 T lymphocytes which become activated. Because there is no place for molecular mimicry in this situation, only peptide-specific T cells are able to respond and it is estimated that because of their extensive repertoire, the frequency of naive T cells in a non-primed individual able to respond to any given peptide is 100–1000-fold lower than that of direct pathway T cells (figure 2).
5. Immunogenicity of embryonic stem cells and their differentiated progeny
The relative contribution of the direct and indirect allorecognition pathways to allograft rejection is an important and topical subject of research and has significant implications for regenerative medicine, relating to the distribution of APCs that are present in the blood circulation and secondary lymphoid tissues, but also in parenchymal tissues throughout the body [19]. It is now widely supposed that direct recognition plays a major role in acute organ allograft rejection, which occurs most commonly in the first few weeks following transplantation as donor APCs migrate from the graft and activate the recipient adaptive immune system. All transplant patients receive effective maintenance immunosuppression and consequently few grafts are now lost through acute rejection since it responds well to a temporary boost of immunosuppressive drug therapy.
Experimental models of transplantation in which donor dendritic cells have been depleted from the tissue prior to transplantation show prolonged allograft survival [20,21]. Stem cell-derived tissues, however, are unlikely to contain APCs unless they are derived from mesodermally differentiated stem cells and include haematopoietic tissue. They would therefore be unable, after transplantation, to initiate allograft rejection via direct recognition and so may be considered to be relatively non-immunogenic [16]. This remains an important consideration for tissue engineering research. As stem cell-derived tissues develop and differentiate in vitro, they will reach a critical size after which the deeper layers of cells die through poor diffusion of nutrients and removal of waste products. This problem may be overcome by encouraging angiogenesis—the formation of a network of vascular tissue, but while they may not contain APCs, the resulting endothelial cells have the potential to express HLA class II under inflammatory conditions. After transplantation, shed necrotic endothelial cells may activate graft rejection through indirect recognition and, moreover, the intact endothelium expressing both HLA class I and class II molecules would act as a target for a rejection response.
Clearly, any transplanted stem cell-derived tissue that is not genetically identical to the recipient has the potential to induce allograft rejection via indirect recognition and the controversy surrounding the immunogenicity of stem cell-derived tissues relates largely to the stringency of early studies that have attempted to document HLA (or MHC) expression by ESCs. This is an important matter because the experience of clinical and experimental organ and tissue transplantation has shown that indirect recognition has the potential to continue to contribute to drug-resistant chronic graft rejection indefinitely, and ultimately results in transplant failure.
6. HLA expression by embryonic stem cells and their differentiated progeny
Optimism that ESC-derived cells are, somehow, immunologically privileged originates from studies showing low levels of HLA class I expression and a complete absence of class II expression on undifferentiated hESCs, even in the presence of IFN-γ, and further studies showing that human T lymphocytes failed to proliferate when co-cultured with either differentiated or undifferentiated hESCs in vitro, and in vivo using a humanized mouse as recipient [22–24]. Countering this optimism, other studies have provided evidence that both the innate and adaptive immune responses may be strongly activated by the transfer of allogeneic mouse ESCs, and enhanced by upregulation of MHC expression on the ESCs, while even mHC antigen disparity in stem cells is able to provoke a rejection response [25–27].
It therefore seems likely that ESC-derived tissues will be susceptible to immunological rejection, most probably triggered by indirect recognition, and may become more immunogenic following transplantation as they mature in vivo and express additional molecules. The contribution of the innate immune response that may accompany the inflammatory environment associated with the introduction of therapeutic cells cannot be ignored. The innate immune response is triggered by events such as ischaemic injury and reperfusion injury resulting in production of harmful free radicals and reactive oxygen species (ROS) expressing damage-associated molecular patterns, or by local infection by micro-organisms expressing pathogen-associated molecular patterns [28]. These patterns are recognized by Toll-like receptors on antigen-non-specific cells including macrophages, neutrophils, natural killer (NK) cells and dendritic cells, which respond by producing chemokines and inflammatory cytokines, resulting in recruitment of immune cells of the adaptive immune response.
It might be surmised that avoidance of immunological rejection could be achieved through the absence of expression of HLA molecules. However, that strategy has already been exploited by pathogenic viruses and oncogenes, which are able to inhibit peptide-loading mechanisms resulting in reduced HLA class I expression and evasion of attack by host CTLs. The immune system has evolved to respond to absence of HLA class I expression by activation of NK cells that are triggered both by failure of their killer immunoglobulin-like receptors (KIR) to engage with HLA molecules on the target cells, as well as by NK activatory receptors that engage with a range of cell surface molecules, resulting in lysis of the potentially infected or malignant target cell [29,30].
7. Mechanisms of immunological allograft rejection
Unless tissue derived from stem cells is HLA identical with the intended recipient, graft rejection is likely, for the reasons outlined above, to be a major barrier to transplantation and regenerative medicine. T cells recognizing allogeneic material become activated, undergo rapid clonal proliferation and initiate a number of responses that together culminate in destruction of the transplanted tissue. The CD4 T cell is central to the adaptive immune response: engagement of the T cell receptor and co-stimulatory molecules on T cells with the HLA/peptide complex and co-stimulatory counterparts on APCs initiates signalling pathways that result in gene transcription and production of a range of cytokines by activated T cells. Cytokines provide help for differentiation of effector CD8 CTLs and B lymphocytes, and recruit antigen-non-specific inflammatory cells and together, these cell types mediate graft damage by direct cytotoxic lysis of target cells, by production of antibodies and by a ‘delayed-type hypersensitivity’ (DTH) response mediated by inflammatory macrophages, neutrophils and NK cells (figure 1). At the same time, cytokines, particularly IFN-γ, induce local upregulation of HLA expression that further enhances the rejection response by increasing both antigen-presenting capacity and the density of target molecules.
CD8 T cells require help from CD4 T cells, in the form of the T helper 1 (Th1) cytokine interleukin-2 (IL-2), in order to mature into functional CTLs that recognize target cells expressing specific HLA class I/peptide complexes. Target cell killing is rapid and precise: CTLs make contact with their target cell via an immunological synapse, or supramolecular adhesion complex (SMAC) formed in both cells by rearrangement of the cytoskeleton to produce a ring of adhesion molecules encircling a pore [31]. Lytic granules pass from the CTL through the SMAC and lyse the cell membranes, releasing perforins and granzymes into the target cell where they trigger apoptosis. The CTL then disengages and moves on to kill another target cell.
B lymphocytes are effective APCs and also mature to become antibody-secreting plasma cells. B cells have surface Ig molecules that serve as antigen receptors via which exogenous antigenic proteins are sampled, processed and presented as peptides in the context of the B cell HLA class II molecules to responding CD4 T cells (figure 1). CD4 T cells are in turn activated and produce Th2 cytokines, including IL-2, IL-4 and IL-5, which induce B-cell clonal proliferation and maturation. The resulting plasma cells produce monoclonal antibodies targeting the original antigenic protein that is then destroyed by opsonization and by complement-mediated cell lysis.
The DTH response contributes to allograft rejection through non-specific mechanisms that include the release of chemokines and pro-inflammatory cytokines, such as IL-6 and TNF-α, as well as endothelial cell activation, increased vascular permeability and enhanced leucocyte migration.
The relative contribution of any of these effector mechanisms depends on variables such as the type of transplant, the sensitisation status of the recipient and the degree of HLA disparity between donor and recipient. The success of transplantation, in turn, depends on minimizing the contribution of these variables through several strategies, including improved HLA typing and donor and recipient HLA matching, improvements in treatment of the donor tissue and operative procedures to reduce the contribution of the innate immune response, and improvements in immunosuppressive treatment. The effector pathways described above, as well as strategies for minimizing the risk of rejection, are as relevant to stem cell transplantation as to organ and tissue transplantation, although there might be a different emphasis for different tissues [14]. There may be additional, as yet unidentified, pathways that would contribute particularly to rejection of pluripotent cell-derived tissues, such as epigenetic mechanisms that may regulate MHC and antigen presentation molecules in hESCs and iPSCs [32].
8. Avoiding rejection
Allograft rejection may be avoided entirely by transplantation of autologous tissue, which is commonly practised in bone marrow transplantation for treatment of selected haematological malignancies, or of tissue from an identical twin donor. Most transplant recipients are managed, instead, with a combination of the best possible donor HLA match at transplantation and lifetime treatment with immunosuppressive drugs.
While the first organ transplant patients were effectively managed by treatment with azathioprine, to inhibit lymphocyte proliferation, and steroids as anti-inflammatory agents, improved understanding of the mechanisms of graft rejection together with drug development has resulted in a much greater choice of immunosuppressive agents that can, to some extent, be tailored to the patient's needs. The calcineurin inhibitors, cyclosporine and tacrolimus, are the mainstay of most immunosuppressive regimens, but patients are now treated with a variety of drugs ranging from newer anti-proliferative agents such as mycophenolate mofetil to monoclonal antibodies such as basiliximab that targets the CD25 molecule (the α-chain of the high affinity IL-2 receptor) expressed by activated lymphocytes. Other agents such as sirolimus target intracellular signalling pathways to inhibit gene transcription and protein synthesis, while a newer antibody, rituximab, developed to target the CD20 molecule on developing and mature B lymphocytes, is an attempt to minimize alloantibody production.
In clinical practice, stem cell-derived tissue created from the intended recipient offers the possibility of personalized stem cell therapy in which graft rejection would not occur. A potential source of such individualized stem cells might be hESCs obtained using somatic cell nuclear transfer, in which the nucleus extracted from an adult somatic cell of the intended recipient is injected into the enucleated human oocyte from an altruistic egg donor [33]. With the exception of maternal mitochondrial DNA present in the oocyte cytoplasm, the somatic cell derivatives are genetically identical to the intended recipient and immunological rejection is unlikely to occur. In practice, however, this requires an abundant source of human eggs obtained following super-ovulation and the merits of such an approach are questionable on both ethical and practical grounds.
In the case, however, of hESC-derived tissues transplanted into an unrelated recipient, there is potential for allograft rejection by the new host. To minimize the risk of immunological rejection, an alternative solution is to use individualized iPSCs derived from the patient as a source of replacement tissue, but the logistics of achieving this on a large scale are problematic owing to the relatively low success rate and, consequently, the high cost of inducing pluripotency. Modern robotic technology is likely to make large-scale production of individualized iPSCs possible in the future but this approach is expensive and may only benefit wealthy individuals who are able to pay for their treatment, or patients who are resident in wealthy countries with well-resourced healthcare systems, but it is unlikely to help a significant proportion of potential beneficiaries worldwide. Existing personalized stem cell gene therapies may, however, continue to offer the best approach for treatment for inherited disorders such as certain haemoglobinopathies and metabolic disorders, where the pathogenic gene would be corrected in personalized iPSCs that are subsequently differentiated into therapeutic tissues for transfer back to the patient [34].
An alternative approach is to establish stem cell banks that contain a range of stem cell lines derived from both iPSCs and hESCs, selected to treat a potentially unlimited number of patients on the basis that any given patient may expect to receive stem cell-derived tissue that is closely matched or compatible (as distinct from identical) for HLA. A resulting low-grade rejection response can be managed with lifelong immunosuppressive therapy or, ideally, through induction of antigen-specific immunological tolerance [16,34,35].
9. Inducing tolerance
On the basis that a stem cell bank will provide a resource of tissues ideally suited to research into strategies for inducing specific tolerance, a range of different approaches may be definitively explored. One approach, which has already proved successful in large animal models, would be to induce mixed haematopoietic chimerism by myeloablation of the patient and reconstitution with HSCs derived from hESCs or iPSCs, such that the thymus becomes re-populated with bi-directionally tolerant thymocytes. The patient would subsequently receive therapeutic tissue (e.g. renal, cardiac, hepatic, etc.) derived from the same hESCs or iPSCs that were used to generate the re-populating HSCs, obviating a requirement for immunosuppression [35]. The toxicity associated with myeloablative therapy, however, may limit the clinical application of such an approach to patients with life-threatening malignancy.
A second approach is to generate therapeutic tissue for transplantation and at the same time and using the same stem cell source, generate ‘tolerogenic’ immature dendritic cells that express low levels of HLA molecules but are able to present immunogenic peptides derived from the therapeutic tissue in the absence of co-stimulatory molecules, thus driving a tolerogenic rather than an immunogenic response in the patient's immune system. Although the dendritic cells themselves are ‘foreign’ to the recipient, there is a precedent for this approach in successful experiments in mice where donor-derived immature dendritic cells were used to generate regulatory T cell-dependent tolerance to a mHC-mismatched skin graft from the dendritic cell donor [34].
Finally, it may be possible to use a transgenic approach to induce tolerance or to minimize immunogenicity [16]. A number of genes are known to have a ‘protective’ function: cells and tissues that have been genetically modified to over-express such genes as A20, Bcl-xL, HO-1, FasL or IDO are less susceptible to rejection. Other target genes whose expression might be modified are those, such as the RFX complex of genes, which control aspects of MHC molecule expression, although complete deletion of MHC expression would probably render cells susceptible to NK-cell mediated killing, as discussed earlier. The inclusion, within the transferred gene construct, of elements enabling conditional gene expression may provide a better alternative to invariable gene expression or deletion as a strategy for avoiding rejection. Pluripotent stem cells are easily genetically modified in vitro, and it is unlikely that such modification would impair their ability to subsequently differentiate into therapeutic tissues for transplantation.
10. Tissue histocompatibility and HLA matching
The allocation of deceased donor kidneys to patients with end-stage renal failure is, in most transplant centres, undertaken on the basis of ABO compatibility and the best possible HLA match between donor and recipient. An understanding of matching criteria is likely to be of use in the design of stem cell banks and provides a rational basis for international variability in the range of stem cells stocked for treatment of ethnically diverse populations.
The primary consideration is to ensure ABO compatibility and there is an argument for stocking a UK stem cell bank only with blood group O stem cells, to entirely remove the concern of hyperacute rejection from blood group incompatibility [16]. Next, kidney transplant patients are matched as closely as possible at three of the HLA loci: HLA-A, HLA-B and HLA-DR, which appear to exert the strongest influence on graft outcome. This is a pragmatic approach to achieving the best match, which is driven by the practicalities of a small pool of recipients for each deceased donor and time constraints to ensure that the function of the organ is not compromised by delaying the transplant operation. With stem cell banking, it may be appropriate to attempt to match at additional HLA loci.
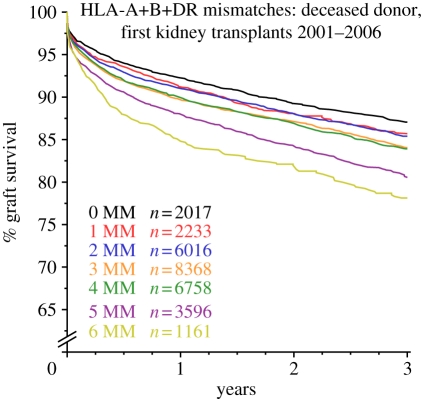
Each individual inherits, in Mendelian fashion, one set of HLA genes from each parent, known as a haplotype, so that their cells express two alleles at each of the HLA-A, -B and -DR loci. The theoretical number of possible combinations of alleles would make HLA matching untenable, but because of linkage disequilibrium and the rarity of chromosomal crossover within HLA haplotypes, certain patterns of HLA-A, -B and -DR alleles are represented at higher than random frequency. Thus, a white European UK national who has inherited a haplotype where the HLA-A allele is A1 has an increased chance of expressing HLA-B8 and -DR3 at two of the other loci of the haplotype, and similarly, if the HLA-A allele on the other chromosome is A2, then the two other alleles are more likely to be HLA-B12 and -DR4. This means that kidney allocation on the basis of best HLA match is a practical solution that translates to fewer rejection episodes and improved transplant survival (figure 3; [36]).
Figure 3.
Influence of HLA mismatches on the outcome of deceased donor kidney transplants. Kaplan–Meier plot of deceased donor kidney graft survival stratified by the number of HLA mismatches (MM) between donor and recipient. Recipients that receive a kidney transplant with zero HLA-A, -B and -DR mismatched donor antigens (0 MM, i.e. HLA matched) benefit from improved 3 year graft survival compared with transplants undertaken with one or more mismatched donor antigens (1 MM to 6 MM). Data from the Collaborative Transplant Study (www.ctstransplant.org), reproduced with kind permission from Prof. Gerhard Opelz, University of Heidelberg.
11. Stem cell banking
The development of national and international pluripotent stem cell tissue banks comprising tissues expressing a range of HLA types that are representative of different geographical populations and ethnic groups worldwide should be designed to provide a worthwhile match for a reasonable percentage of the target population. National and international umbilical cord blood stem cell banks are already well established. They contain units of cord blood, obtained at the time of neonate delivery, that are HLA-typed and cryopreserved for future use in unrelated HSC transplantation. Umbilical cord blood contains a high proportion of CD34-positive multi-potent HSCs and may be used for the treatment of acute leukaemia: providing an adequate number of cells are used, cord blood stem cell transplantation requires less stringent donor–recipient HLA matching compared with HSC transplantation using peripheral blood and bone marrow-derived HSCs [37].
A study to estimate the number of hESC lines that would be needed to provide tissue representing a worthwhile HLA match for a reasonable percentage of the UK population found that approximately 150 random donors, or 100 selected blood group O donors would provide an ABO compatible and full HLA-A, -B and -DR match for only a minority (less than 20%) of potential recipients and a beneficial match (defined as a single HLA-A or -B mismatch or better) for around 38 per cent, but good HLA matching was observed in a higher proportion of white Europeans than in those of Asian and black ethnicity [38]. Extending the number of donors beyond 150 conferred only marginal additional benefit with respect to HLA matching. Of note was the observation that the chance occurrence of a common HLA haplotype that is homozygous at HLA-A, -B and -DR (expressing only one HLA specificity at each locus) strongly increased the degree of HLA matching, and it was found that a panel of only 10 highly selected donors homozygous for conserved HLA haplotypes would be expected to provide a complete HLA-A, -B and -DR match for around 38 per cent of recipients and a beneficial match for 67 per cent [38]. Of 10000 consecutive deceased organ donor ABO blood groups and HLA types studied (considered as representative of the HLA types of potential ESC donors in the UK population), 40 (0.4%) were identified as blood group O and bearing the conserved homozygous HLA-A1, -B8 and -DR3 haplotype. When comparing this homozygous HLA type with the ABO blood groups and HLA types of all 6577 patients registered on the UK kidney transplant waiting list, 13 per cent of the patients were found to be ABO blood group and HLA compatible. These data suggest that stem cell banks comprising cell lines obtained from blood group O donors with conserved homozygous HLA haplotypes would provide maximum utility for selecting ABO-compatible and HLA-matched tissue for treating the majority of individuals in a given population [39].
Similar simulation studies of the potential for HLA matching performed in Japanese and Chinese populations reported broadly similar results, although a higher level of HLA matching was observed in Japanese recipients, reflecting the relatively low ethnic diversity of the Japanese population [40,41].
The above simulations have practical, financial, political and ethical implications for the establishment and design of stem cell banks incorporating cell lines with ABO blood groups and HLA types that are compatible with different ethnic populations throughout the world. A problem with this approach is the requirement to screen many thousands of potential stem cell donors in order to identify the small number of individuals that fulfil these criteria [42]. The limited availability of hESCs derived from surplus embryos donated following IVF treatment is unlikely to provide sufficient numbers to populate a highly selected cell bank. Nakatsuji et al. [41] calculated that to find at least one HLA homozygous donor for each of 50 different HLA haplotypes in the Japanese population would require a database of 24 000 individuals.
One theoretical solution that avoids the ethically contentious use of spare embryos, but still depends on egg donation, would be to populate a bank with selected HLA homozygous hESC lines generated by parthenogenesis. MHC homozygous ESC lines have been derived in mice and non-human primates from parthenogenetic embryos, where an unfertilized oocyte is activated by electrical or chemical stimulation to undergo a first meiotic division, generating two identical daughter chromatids from the single parental chromosome of the oocyte [43–47]. If successful in humans, the diploid blastocyst resulting from parthenogenesis could yield hESCs that are homozygous at the HLA gene region (and throughout the genome) and are able, in principle, to contribute to all cells of the human body, as has been shown for parthenogenetic mouse ESCs [48]. The inclusion in future stem cell banks of parthenogenetic homozygous hESC lines expressing a single-conserved HLA haplotype would greatly increase the utility of providing HLA compatible tissue for a large number of people. The clinical utility, however, of parthenogenetic hESCs may be limited owing to potential epigenetic instability arising from the exclusively maternal origin of the genome [49].
12. Identifying and banking homozygous HLA induced pluripotent stem cells lines
More recently, the prospect of generating iPSCs from adult donors offers new opportunities to establish stem cell banks populated with highly selected HLA-typed lines that would provide HLA matched tissue for the target population. The precedent for this approach is already well established in clinical HSC transplantation, where HLA matched unrelated donors are identified from national and international panel registries of volunteer bone marrow donors. When a suitable HLA matched donor for a given patient is identified on a registry, the volunteer donor is contacted and, subject to satisfactory confirmatory tests and medical clearance, is invited to donate either peripheral blood (containing HSCs) or bone marrow. Adopting a targeted approach of identifying volunteer stem cell donors with selected HLA types known to provide maximum utility for stem cell banking circumvents the problem of HLA typing existing collections of hESC lines that are likely to yield very few useful cell lines for banking.
The international registry ‘Bone Marrow Donors Worldwide’ (BMDW) contains HLA-typing information for nearly 15 million volunteer stem cell donors and cord blood units, registered from 44 countries, representing White, Black (Afro-Caribbean), Asian and Oriental ethnic groups worldwide (http://www.bmdw.org). The BMDW registry contains over 20 000 volunteers with the conserved homozygous HLA haplotype HLA-A1, -B8, -C7, -DR17(3), -DQ2 and over 2500 with the conserved homozygous HLA haplotype HLA-A2, -B44(12), -Cw5, -DR4 and -DQ8(3). Table 1 shows that, assuming one iPSC line is capable of unlimited expansion in vitro, two such homozygous donor cell lines would provide zero HLA-A, -B and -DR mismatched (i.e. HLA compatible) tissue for around 17 and 11 per cent of the UK population, respectively. As few as three iPSC lines derived from blood group O individuals bearing the three most common and highly conserved extended HLA haplotypes would have the potential to provide zero HLA-A, -B and -DR mismatched tissue for around one-third of the UK population.
Table 1.
Identification of potential homozygous HLA induced pluripotent stem cell donors.
| conserved homozygous HLA haplotype | no. potential HLA allele matched donors on BMDW registry | number of HLA matchesa | cumulative number of HLA matchesb |
|---|---|---|---|
| A*01:01, B*08:01, C*07:01, DRB1*03:01, DQB1*02:01 | greater than 20 000 | 1741 (17%) | 1741 (17%) |
| A*02:01, B*44:01, C*05:01, DRB1*04:01, DQB1*03:02 | greater than 2500 | 1074 (11%) | 2750 (27%) |
| A*03:01, B*07:02, C*07:02, DRB1*15:01, DQB1*06:02 | greater than 7000 | 874 (9%) | 3540 (35%) |
aNumber of zero HLA-A, -B and -DR mismatched individuals in a cohort of 10 000 consecutive organ donors reported to the UK NHS Blood and Transplant (NHSBT) (1991–2003), considered representative of HLA types in the UK population.
bCumulative number of zero HLA-A, -B and -DR mismatched individuals in a cohort of 10 000 consecutive organ donors reported to NHSBT (1991–2003), after correction for heterozygous individuals that are matched with more than one donor.
An important condition of volunteer unrelated HSC donation is that the identity of the donor and recipient is withheld from each other (and their respective medical centres), and that the generous act of stem cell donation is entirely altruistic. In the case of both living and deceased organ donation for transplantation, national legislation, professional societies and ethical bodies worldwide require donation to be altruistic and free from coercion. The question arises as to whether volunteer stem cell donors identified for inclusion in a prospective stem cell bank should also be altruistic or whether donors can be financially recompensed, and if so, what is the monetary value of donating a small sample of bodily tissue. Moreover, if tissue donation were to remain altruistic, to what extent should commercial companies and individuals profit from this gift by volunteer tissue donors in their opportunity to help tens of thousands of people? Undoubtedly, the commercial benefits of private enterprise and investment lead to innovation, but unfettered commercialism may restrict the potential benefits of regenerative medicine to only those individuals that are able to pay. An alternative option is that national and international stem cell banks may be developed as a public utility run by a charity or public sector with open access for bona fide accredited medical centres.
In summary, the application of regenerative medicine and stem cell banking using somatic tissue derived from pluripotent stem cell lines has great potential for treating many human diseases and for exploring both their genetic aetiology and opportunities for drug development. However, many questions need to be addressed concerning the practicality of offering affordable and durable treatment on such a large scale. Questions about the efficacy of using autologous and allogeneic hESCs and iPSCs, and the potential for the immune response to destroy transplanted allogeneic (HLA mismatched) stem cell-derived somatic tissue, need to be addressed to guide the future design and utility of national and international stem cell banks. This information will enable stakeholders (patients, public, clinicians, scientists, politicians and ethicists) to debate approaches for maximizing the benefit of this valuable future resource.
Acknowledgements
C.J.T., E.M.B. and J.A.B. were supported by the NIHR Cambridge Biomedical Research Center.
References
- 1.Diabetes UK. See http://www.diabetes.org.uk
- 2.Parkinson's Disease Society. See http://www.parkinsons.org.uk
- 3.Le Blanc K., et al. 2008. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371, 1579–1586 10.1016/S0140-6736(08)60690-X (doi:10.1016/S0140-6736(08)60690-X) [DOI] [PubMed] [Google Scholar]
- 4.Tyndall A., Gratwohl A. 2009. Adult stem cell transplantation in autoimmune disease. Curr. Opin. Hematol. 16, 285–291 10.1097/MOH.0b013e32832aacb3 (doi:10.1097/MOH.0b013e32832aacb3) [DOI] [PubMed] [Google Scholar]
- 5.Eisenstein M. 2010. IPSCs: one cell to rule them all? Nat. Methods 7, 81–85 10.1038/nmeth0110-81 (doi:10.1038/nmeth0110-81) [DOI] [Google Scholar]
- 6.Zinkernagel R. M., Doherty P. C. 1979. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv. Immunol. 27, 51–177 10.1016/S0065-2776(08)60262-X (doi:10.1016/S0065-2776(08)60262-X) [DOI] [PubMed] [Google Scholar]
- 7.Clausen H., Hakomori S. 1989. ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang 56, 1–20 10.1111/j.1423-0410.1989.tb03040.x (doi:10.1111/j.1423-0410.1989.tb03040.x) [DOI] [PubMed] [Google Scholar]
- 8.Ito N., Hirota T. 1992. Histochemical and cytochemical localisation of blood group antigens. Prog. Histochem. Cytochem. 25, 1–85 [DOI] [PubMed] [Google Scholar]
- 9.Springer G. F., Horton R. E. 1969. Blood group isoantibody stimulation in man by feeding blood group-active bacteria. J. Clin. Invest. 48, 1280–1291 10.1172/JCI106094 (doi:10.1172/JCI106094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul L. C., Baldwin W. M. 1987. Humoral rejection mechanisms and ABO incompatibility in renal transplantation. Transplant Proc. 19, 4463–4467 [PubMed] [Google Scholar]
- 11.Cooper D. K. 1990. Clinical survey of heart transplantation between ABO blood group incompatible recipients and donors. J. Heart Transplant. 9, 376–381 [PubMed] [Google Scholar]
- 12.Molne J., Bjorquist P., Andersson K., Diswall M., Jeppsson A., Strokan V., Rydberg L., Breimer M. E. 2008. Blood group ABO antigen expression in human embryonic stem cells and in differentiated hepatocyte- and cardiomyocyte-like cells. Transplantation 86, 1407–1413 10.1097/TP.0b013e31818a6805 (doi:10.1097/TP.0b013e31818a6805) [DOI] [PubMed] [Google Scholar]
- 13.Candinas D., Gunson B. K., Nightingale P., Hubscher S., McMaster P., Neuberger J. M. 1995. Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet 346, 1117–1121 10.1016/S0140-6736(95)91797-7 (doi:10.1016/S0140-6736(95)91797-7) [DOI] [PubMed] [Google Scholar]
- 14.Gratwohl A., Döhler B., Stern M., Opelz G. 2008. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. Lancet 372, 49–53 10.1016/S0140-6736(08)60992-7 (doi:10.1016/S0140-6736(08)60992-7) [DOI] [PubMed] [Google Scholar]
- 15.Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. 1987. Structure of the human class I histocompatibility antigen, HLA-A2. Nature 329, 506–512 10.1038/329506a0 (doi:10.1038/329506a0) [DOI] [PubMed] [Google Scholar]
- 16.Bradley J. A., Bolton E. M., Pedersen R. A. 2002. Stem cell medicine encounters the immune system. Nat. Rev. Immunol. 2, 859–871 10.1038/nri934 (doi:10.1038/nri934) [DOI] [PubMed] [Google Scholar]
- 17.Fischer-Lindahl K., Wilson D. B. 1977. Histocompatibility antigen-activated cytotoxic T lymphocytes. I. Estimates of the absolute frequency of killer cells generated in vitro. J. Exp. Med. 145, 500–507 10.1084/jem.145.3.500 (doi:10.1084/jem.145.3.500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skinner M. A., Marbrook J. 1976. An estimation of the frequency of precursor cells which generate cytotoxic lymphocytes. J. Exp. Med. 143, 1562–1567 10.1084/jem.143.6.1562 (doi:10.1084/jem.143.6.1562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor A. L., Negus S. L., Negus M., Bolton E. M., Bradley J. A., Pettigrew G. J. 2007. Pathways of helper CD4 T cell allorecognition in generating alloantibody and CD8 T cell alloimmunity. Transplantation 83, 931–937 10.1097/01.tp.0000257960.07783.e3 (doi:10.1097/01.tp.0000257960.07783.e3) [DOI] [PubMed] [Google Scholar]
- 20.Simeonovic C. J., Bowen K. M., Kotlarski I., Lafferty K. J. 1980. Modulation of tissue immunogenicity by organ culture. Comparison of adult islets and fetal pancreas. Transplantation 30, 174–179 10.1097/00007890-198009000-00004 (doi:10.1097/00007890-198009000-00004) [DOI] [PubMed] [Google Scholar]
- 21.Lechler R. I., Batchelor J. R. 1982. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J. Exp. Med. 155, 31–41 10.1084/jem.155.1.31 (doi:10.1084/jem.155.1.31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drukker M., Katz G., Urbach A., Schuldiner M., Markel G., Itskovitz-Eldor J., Reubinoff B., Mandelboim O., Benvenisty N. 2002. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl Acad. Sci. USA 99, 9864–9869 10.1073/pnas.142298299 (doi:10.1073/pnas.142298299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L., et al. 2004. Human embryonic stem cells possess immune-privileged properties. Stem Cells 22, 448–456 10.1634/stemcells.22-4-448 (doi:10.1634/stemcells.22-4-448) [DOI] [PubMed] [Google Scholar]
- 24.Drukker M., Katchman H., Katz G., Even-Tov Friedman S., Shezen E., Hornstein E., Mandelboim O., Reisner Y., Benvenisty N. 2006. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells 24, 221–229 10.1634/stemcells.2005-0188 (doi:10.1634/stemcells.2005-0188) [DOI] [PubMed] [Google Scholar]
- 25.Kofidis T., deBruin J. L., Tanaka M., Zwierzchoniewska M., Weissman I., Fedoseyeva E., Haverich A., Robbins R. C. 2005. They are not stealthy in the heart: embryonic stem cells trigger cell infiltration, humoral and T-lymphocyte-based host immune response. Eur. J. Cardiothorac. Surg. 28, 461–466 10.1016/j.ejcts.2005.03.049 (doi:10.1016/j.ejcts.2005.03.049) [DOI] [PubMed] [Google Scholar]
- 26.Swijnenburg R. J., et al. 2005. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation 112, I166–I172 10.1161/CIRCULATIONAHA.104.525824 (doi:10.1161/CIRCULATIONAHA.104.525824) [DOI] [PubMed] [Google Scholar]
- 27.Robertson N. J., Brook F. A., Gardner R. L., Cobbold S. P., Waldmann H., Fairchild P. J. 2007. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc. Natl Acad. Sci. USA 104, 20 920–20 925 10.1073/pnas.0710265105 (doi:10.1073/pnas.0710265105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medzhitov R., Janeway C. A. J. 1997. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91, 295–298 10.1016/S0092-8674(00)80412-2 (doi:10.1016/S0092-8674(00)80412-2) [DOI] [PubMed] [Google Scholar]
- 29.Bryceson Y. T., Long E. O. 2008. Line of attack: NK cell specificity and integration of signals. Curr. Opin. Immunol. 20, 344–352 10.1016/j.coi.2008.03.005 (doi:10.1016/j.coi.2008.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruggeri L., Aversa F., Martelli M. F., Velardi A. 2006. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol. Rev. 214, 202–218 10.1111/j.1600-065X.2006.00455.x (doi:10.1111/j.1600-065X.2006.00455.x) [DOI] [PubMed] [Google Scholar]
- 31.Stinchcombe J. C., Griffiths G. M. 2007. Secretory mechanisms in cell-mediated cytotoxicity. Annu. Rev. Cell Dev. Biol. 23, 495–517 10.1146/annurev.cellbio.23.090506.123521 (doi:10.1146/annurev.cellbio.23.090506.123521) [DOI] [PubMed] [Google Scholar]
- 32.Suarez-Alvarez B., et al. 2010. Epigenetic mechanisms regulate MHC and antigen processing molecules in human embryonic and induced pluripotent stem cells. PLoS ONE 5, e10192. 10.1371/journal.pone.0010192 (doi:10.1371/journal.pone.0010192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakayama T., Tabar V., Rodriguez I., Perry A. C., Studer L., Mombaerts P. 2001. Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science 292, 740–743 10.1126/science.1059399 (doi:10.1126/science.1059399) [DOI] [PubMed] [Google Scholar]
- 34.Fairchild P. J. 2010. The challenge of immunogenicity in the quest for induced pluripotency. Nat. Rev. Immunol. 10, 868–875 10.1038/nri2878 (doi:10.1038/nri2878) [DOI] [PubMed] [Google Scholar]
- 35.Pilat N., Wekerle T. 2010. Transplantation tolerance through mixed chimerism. Nat. Rev. Nephrol. 6, 594–605 10.1038/nrneph.2010.110 (doi:10.1038/nrneph.2010.110) [DOI] [PubMed] [Google Scholar]
- 36.Opelz G., Döhler B. 2007. Effect of HLA compatibility on kidney graft survival: comparative analysis of two decades. Transplantation 84, 137–143 10.1097/01.tp.0000269725.74189.b9 (doi:10.1097/01.tp.0000269725.74189.b9) [DOI] [PubMed] [Google Scholar]
- 37.Kurtzberg J., et al. 2008. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood 112, 4318–4327 10.1182/blood-2007-06-098020 (doi:10.1182/blood-2007-06-098020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor C. J., Bolton E. M., Pocock S., Sharples L. D., Pedersen R. A., Bradley J. A. 2005. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet 366, 2019–2025 10.1016/S0140-6736(05)67813-0 (doi:10.1016/S0140-6736(05)67813-0) [DOI] [PubMed] [Google Scholar]
- 39.Rao M. S., Auerbach J. M. 2006. Estimating human embryonic stem-cell numbers. Lancet 367, 650. 10.1016/S0140-6736(06)68261-5 (doi:10.1016/S0140-6736(06)68261-5) [DOI] [PubMed] [Google Scholar]
- 40.Lin G., et al. 2009. HLA-matching potential of an established human embryonic stem cell bank in China. Cell Stem Cell 5, 461–465 10.1016/j.stem.2009.10.009 (doi:10.1016/j.stem.2009.10.009) [DOI] [PubMed] [Google Scholar]
- 41.Nakatsuji N., Nakajima F., Tokunaga K. 2008. HLA-haplotype banking and iPS cells. Nat. Biotechnol. 26, 739–740 10.1038/nbt0708-739 (doi:10.1038/nbt0708-739) [DOI] [PubMed] [Google Scholar]
- 42.Lee J. E., Kang M. S., Park M. H., Shim S. H., Yoon T. K., Chung H. M., Lee D. R. 2010. Evaluation of 28 human embryonic stem cell lines for use as unrelated donors in stem cell therapy: implications of HLA and ABO genotypes. Cell Transplant 19, 1383–1395 10.3727/096368910X513991 (doi:10.3727/096368910X513991) [DOI] [PubMed] [Google Scholar]
- 43.Cibelli J. B., et al. 2002. Parthenogenetic stem cells in nonhuman primates. Science 295, 819. 10.1126/science.1065637 (doi:10.1126/science.1065637) [DOI] [PubMed] [Google Scholar]
- 44.Lin H., Lei J., Wininger D., Nguyen M.-T., Khanna R., Hartmann C., Yan W.-L., Huang S. C. 2003. Multilineage potential of homozygous stem cells derived from metaphase II oocytes. Stem Cells 21, 152–161 10.1634/stemcells.21-2-152 (doi:10.1634/stemcells.21-2-152) [DOI] [PubMed] [Google Scholar]
- 45.Vrana K. E., et al. 2003. Nonhuman primate parthenogenetic stem cells. Proc. Natl Acad. Sci. USA 100(Suppl. 1), 11 911–11 916 10.1073/pnas.2034195100 (doi:10.1073/pnas.2034195100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Revazova E. S., Turovets N. A., Kochetkova O. D., Agapova L. S., Sebastian J. L., Pryzhkova M. V., Smolnikova V. I., Kuzmichev L. N., Janus J. D. 2008. HLA homozygous stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 10, 11–24 10.1089/clo.2007.0063 (doi:10.1089/clo.2007.0063) [DOI] [PubMed] [Google Scholar]
- 47.Lin G., OuYang Q., Zhou X., Gu Y., Yuan D., Li W., Liu G., Liu T., Lu G. 2007. A highly homozygous and parthenogenetic human embryonic stem cell line derived from a one-pronuclear oocyte following in vitro fertilization procedure. Cell Res. 17, 999–1007 10.1038/cr.2007.97 (doi:10.1038/cr.2007.97) [DOI] [PubMed] [Google Scholar]
- 48.Allen N. D., Barton S. C., Hilton K., Norris M. L., Surani M. A. 1994. A functional analysis of imprinting in parthenogenetic embryonic stem cells. Development 120, 1473–1482 [DOI] [PubMed] [Google Scholar]
- 49.Szabó P., Mann J. R. 1994. Expression and methylation of imprinted genes during in vitro differentiation of mouse parthenogenetic and androgenetic embryonic stem cell lines. Development 120, 1651–1660 [DOI] [PubMed] [Google Scholar]