Abstract
In this paper, I review the relevance of the niche to biogeography, and what biogeography may tell us about the niche. The niche is defined as the combination of abiotic and biotic conditions where a species can persist. I argue that most biogeographic patterns are created by niche differences over space, and that even ‘geographic barriers’ must have an ecological basis. However, we know little about specific ecological factors underlying most biogeographic patterns. Some evidence supports the importance of abiotic factors, whereas few examples exist of large-scale patterns created by biotic interactions. I also show how incorporating biogeography may offer new perspectives on resource-related niches and species interactions. Several examples demonstrate that even after a major evolutionary radiation within a region, the region can still be invaded by ecologically similar species from another clade, countering the long-standing idea that communities and regions are generally ‘saturated’ with species. I also describe the somewhat paradoxical situation where competition seems to limit trait evolution in a group, but does not prevent co-occurrence of species with similar values for that trait (called here the ‘competition–divergence–co-occurrence conundrum’). In general, the interface of biogeography and ecology could be a major area for research in both fields.
Keywords: biogeography, competition, ecology, evolution, niche
1. Introduction
Ecology and biogeography are two fields with many obvious connections, but the relationship between them has often been troubled [1]. On the one hand, those studying large-scale historical (i.e. phylogenetic) biogeography have tended to ignore ecology entirely, with little or no consideration of the idea that distributions of organisms are influenced by factors such as habitat tolerances or species interactions. This is well-illustrated in the excellent and integrative textbook by Lomolino et al. [2], in which ecology goes largely unmentioned in the chapter on historical biogeography. On the other hand, ecologists have often tended to ignore biogeography (but with some important exceptions; [3,4]). For example, ecologists often do not study how large-scale biogeographic history influences the patterns of diversity and community structure that they study, nor how ecological processes create biogeographic patterns (review in [1]).
One place where these two fields should come together (but often fail to) is in the concept of the niche. Based on Hutchinson's [5] general concept, the niche describes the set of abiotic and biotic conditions where a species can persist [6]. Many ecologists favour a concept of the niche based on resources and species interactions at the local scale (i.e. an Eltonian niche concept; [7]). On the other hand, more biogeographically oriented ecologists often prefer a concept focusing on the environmental conditions determining the large-scale distribution of species (i.e. a Grinnellian niche concept; [7]). I consider these to represent equally valid conceptualizations of different aspects of the general Hutchinsonian niche concept [6], and they clearly have many important intersections (e.g. local-scale biotic interactions may set range limits).
In this paper, I will review what the niche can tell us about biogeography and what biogeography can tell us about the niche. I have previously written about the gulf between biogeography and ecology [1] and how the niche and biogeography may be related [8]. Those papers focused largely on climatic niches, with relatively little emphasis on the potential role of biotic factors and species interactions in creating biogeographic patterns. Here, I will first focus on the importance of the niche to biogeographic patterns, and the factors that set the range limits of species and clades (i.e. the Grinnellian niche). I will then focus on what the combination of biogeography, phylogeny and resource-related traits can tell us about niches, particularly related to species interactions and the Eltonian niche. Note that here and throughout, I refer to biogeographic patterns as those at the scale of species range sizes and larger (e.g. within continents and between continents).
2. Why the niche matters for biogeography
(a). Range limits create biogeographic patterns and ecological niches create range limits
Biogeographic patterns arise primarily through limits on dispersal. By ‘dispersal’, I mean the process by which species expand their ranges, including both the movement of individuals to a new location and their successful establishment there. If there were no limits on dispersal, every species could potentially be distributed everywhere, and spatial patterns of distribution and diversity would be absent or due entirely to chance. But of course, we know that there are indeed large-scale patterns of diversity and distribution (e.g. major biogeographic provinces, more species richness in the tropics [2,9]).
Given this argument, the niche matters for biogeography because the range limits of species (and clades) are set primarily by ecological factors (but see below). By definition, species generally cannot spread outside of their ecological niche, the set of conditions where they can persist (potentially including both abiotic and biotic factors). Therefore, most so-called ‘geographic barriers’ and ‘physical barriers’ (e.g. oceans, rivers and mountains) are simply areas of unsuitable habitat, and are entirely organism-specific (e.g. [1]). For example, oceans limit dispersal for most terrestrial and freshwater species, but not necessarily for marine organisms. Further, many species occur in both freshwater and marine environments, depending on the stage in their life cycle. In terrestrial environments, a river may be a barrier for some organisms, but not others (e.g. especially not for organisms whose niche includes freshwater habitats). Mountains may limit dispersal in some cases, but mountains are generally only barriers when they create zones of unsuitable habitat for the organism in question. Many other barriers to dispersal are more subtle and more clearly related to habitat differences (mesic versus arid environments, forest versus open habitat and rivers versus streams). The arguments above are neither new nor surprising, but the idea that ecology influences biogeography remains surprisingly rare in both fields (e.g. biogeographers often ignore ecology, whereas ecologists often treat ecology and biogeography as competing explanations for patterns of diversity and distribution). One consequence of this niche-based view of biogeography is that biogeography should not be viewed as ‘neutral’ (and then contrasted with factors related to the niche), as is often done in the current ecological literature (for recent reviews see [10,11]).
Of course, simply saying that biogeographic patterns are created by differences in habitat over space does not tell us how these habitat differences limit dispersal. For example, these limits could be set by abiotic factors, biotic factors or some combination. I address these types of factors below. But first, I briefly digress to address whether range limits actually have to be explained by niche differences over space.
(b). Must geographic range limits be explained by the niche?
What sets the range limits of species? If the range of a species is not limited by unsuitable ecological conditions, then one major alternative is that there has simply not been enough time for the species to reach the full geographic extent of the range allowed by the spatial distribution of geographically contiguous and ecologically suitable conditions. This topic has been addressed in some recent studies (e.g. [12,13]) using data from distributions of extant species. For example, Paul et al. [13] quantified for each species the ‘potential range’ (based on species-distribution modelling (SDM) with climatic data), actual range extent (how much of the span of the range is occupied) and actual range occupancy (how many potentially suitable pixels are occupied), and then tested how these variables were related to species age in a genus of Neotropical trees. They found that younger species generally occupy less of their potential range extents than older species, suggesting that species ranges (or parts of their ranges) may sometimes be determined by limited time for dispersal, rather than ecological limits on dispersal. In contrast, Schurr et al. [12] found no evidence that species range sizes increased with their inferred age for South African Proteaceae.
Furthermore, some organisms may be able to disperse across barriers of unsuitable habitat to colonize non-contiguous regions of suitable habitat (e.g. rafting to islands). For these species, range limits may be determined by the combination of habitat differences over space and the ability of the organisms to disperse across unsuitable conditions, rather than the unsuitable conditions alone.
(c). Which ecological factors can set range limits?
A variety of ecological factors may set the range limits of species to create biogeographic patterns. These factors are often categorized as being abiotic versus biotic [2,14]. For terrestrial organisms, abiotic factors that may set range limits include climate (temperature, precipitation), pH and unsuitable aquatic habitats (for a thorough review of these and other factors see [2]). For aquatic organisms, abiotic factors include temperature, salinity and oxygen content. Biotic factors include competition, predation, parasitism and limits on the ranges of prey or mutualistic species. Range limits may also be explained by a combination of abiotic and biotic factors. For example, climate may not set the range limits of a species directly owing to their tolerances to heat, cold or drought, but may instead influence the distribution of an important resource (e.g. insect prey for birds [15]). Finally, the factors that set the range limits of any given species may simply depend on the part of that species' range that is being considered [16]. For example, a single species could have its poleward range limits set by tolerance to cold, its southern limits set by competition and its eastern and western borders set by completely different factors.
Sexton et al. [14] recently reviewed empirical studies addressing the causes of range limits. Of 146 studies that addressed abiotic factors associated with range limits, they found that abiotic factors were supported in 112 (77%), partially supported in 17 (12%; where ‘partial support’ means that abiotic factors were supported for some but not all of the species included in the study), and not supported in 17 (12%). Biotic factors were addressed in 51 studies, and were supported in 31 (61%), partially supported in eight (16%), and not supported in 12 (24%). Among these 51 studies, 26 focused on the effects of competition, 10 on predation, seven on diseases and eight on host distributions; for each biotic factor, the majority of studies supported the factor in question, but for predation, diseases and host distributions, a nearly equal number of studies failed to support that factor. Eight studies examined interactions between abiotic and biotic factors, and these interactions were supported in seven (88%) and not supported in one (12%). Of course, the conclusions that can be drawn from this review may be biased by what factors the authors of the original studies chose to investigate (e.g. investigators may be biased towards investigating factors that they have some a priori reason to believe are important in their system). Nevertheless, there is clearly widespread support for the idea that abiotic factors often set species ranges (either directly or indirectly through interactions with biotic factors) and that competition may be an important biotic factor setting range limits.
(d). Niche conservatism
Species range limits are not simply set by unsuitable abiotic and biotic conditions at their range margins, but also by the failure of individuals to adapt to those unsuitable conditions (e.g. [17–19]). Species can potentially adapt to changing environmental conditions over time (e.g. [20]) and to different sets of co-occurring species (e.g. [21]). Therefore, to explain large-scale biogeographic patterns, we also need to explain why species do not simply adapt to the ecological conditions at the margins of their geographic ranges and continue expanding their ranges. Without such limits, every species could be everywhere, and again there would be few non-random biogeographic patterns. Niche conservatism is simply the idea that species will retain similar ecological traits over time (review in [22]). One important consequence of niche conservatism may be to limit the geographic ranges of species and clades over time [8]. Several studies now support the idea that niche conservatism in climatic distributions may be important in setting range limits and thereby creating biogeographic patterns of distribution and species richness (table 1). However, many studies have also found evidence for rapid shifts in climatic distributions among species (e.g. [27,39]). A major challenge for future studies is to determine what trait (or traits) would allow a given species to expand its range (e.g. physiological tolerance to freezing temperatures), and then uncover the within-species processes that underlie the success or failure of these traits to evolve at the range margins.
Table 1.
Selected examples suggesting that niche conservatism in climatic tolerances contributes to large-scale biogeographic patterns, listed taxonomically. Various approaches were used in these studies. For example, some used SDM to identify climatic factors likely responsible for setting range limits and then showed that these climatic factors were strongly concordant with the phylogeny or otherwise strongly conserved (e.g. [23–25]). One used a novel simulation-based approach to generate species richness patterns under different levels of niche conservatism [26]. These represent only a sampling of studies and approaches that have been used to address niche conservatism and evolution with climatic and geographic data (e.g. [27–33]).
| biogeographic pattern | reference |
|---|---|
| global-scale distribution of plant clades | [34] |
| high mid-elevation species richness in Asian fish | [35] |
| high tropical species richness and distribution of major clades in hylid treefrogs | [23] |
| high temperate richness in some hylid treefrogs | [36] |
| high mid-elevation species richness in North American plethodontid salamanders | [25] |
| high temperate richness in some colubrid snakes | [37] |
| distribution of clades and latitudinal patterns of community structure in North American emydid turtles | [24] |
| species richness patterns in South American birds | [26] |
| global species richness patterns across mammals | [38] |
(e). Evidence for abiotic versus biotic factors in creating large-scale biogeographic patterns
(i). The case for abiotic factors
Given that there are many different types of abiotic and biotic factors that could set species range limits, which of them might be most important in creating large-scale biogeographic patterns? Note that by ‘large-scale biogeographic patterns’ I mean patterns that involve significant proportions of one or more continents (i.e. the size of the geographic range of a species or clade). There is considerable indirect evidence for the widespread importance of climate and other abiotic factors. This evidence includes many smaller scale studies of species range limits ([14]; see above), and the turnover in flora and fauna at the edges of many biogeographic provinces, especially where these edges are within rather than between landmasses. Such edges include those between the Nearctic and Neotropical realms, the Palaearctic and Oriental realms, and the Palaearctic and Ethiopian regions [2,9]. Similarly, there is extensive turnover in flora and fauna at different elevations, and these elevational differences are also thought to be related to climate [2,40]. However, in many cases, it is uncertain whether range limits are set directly by tolerances to climatic conditions, or by secondary factors that are themselves influenced by climate.
Recent studies using SDM (also known as niche modelling) also suggest that climatic tolerances may be responsible for creating many large-scale patterns of distribution and diversity (table 1). Here, I present a more detailed example from my research where climatic variation seems to set range limits across multiple species in a clade, contributing to a large-scale pattern of biogeography and species richness.
This example involves hylid treefrogs in the New World, at the interface between the Nearctic and Neotropical zoogeographic realms [9]. Hylids have an ancient origin in the tropics, recent dispersal of some clades to the temperate zone and restricted dispersal of other tropical clades into temperate areas (i.e. North America), all of which help explain low hylid richness in temperate regions and high richness in tropical regions [23]. Analyses of the northern range limits of four tropical treefrog clades in northeastern Mexico suggest that these clades fail to extend their ranges further north into temperate regions because they are unable to tolerate the higher temperature seasonality (i.e. cooler winters) north of their current ranges [23]. Species-distribution models using temperature seasonality alone accurately predict the northern range limits of these species. For example, for five of six species, a model based on temperature seasonality alone correctly predicts their absence in 100 per cent of localities to the immediate north of the range of each species, localities where hylids are known to be present but the species in question are apparently absent. For the sixth species, this model correctly predicts 86 per cent of these ‘absence’ localities. Furthermore, hylid species from temperate North America have lower critical thermal minima than the tropical hylid species that have been tested [41], supporting the idea that range expansion into temperate regions requires physiological adaptations that these tropical species lack. The northern limits of these hylid clades in northeastern Mexico are broadly similar to those of many other tropical clades in this region, including caecilian amphibians, corytophanid and iguanid lizards, and boine snakes [42] as well as atelid monkeys, ramphastid and tinamid birds (toucans and tinamous), and Amazona parrots [43]. Importantly, this broad concordance across ecologically diverse clades suggests that these limits are not set by pathogens, predators or competitors, and might be set by physiological tolerances instead. Furthermore, these tropical treefrog clades occur hundreds of kilometres south of the southern range limits of the temperate treefrog lineages, suggesting that competition with temperate treefrogs does not set their northern range limits (i.e. competition might be a plausible hypothesis if tropical and temperate treefrog species had abutting geographic ranges, but not if they are geographically distant from each other). Dramatic differences in body size between species of these tropical treefrog clades also argues against them sharing similar sets of competitors, predators or prey that would cause them to have similar northern range limits. Although these patterns together implicate climatic tolerances in setting the range limits of these clades, further work is still needed to better determine the specific mechanisms by which their distributions are limited.
There is also evidence for the general importance of climate on species ranges from two types of human impacts. The first is invasive species. Broad-scale analyses of vertebrate invasive species and their latitudinal distributions suggest that invasive species tend to successfully invade regions with climates similar to their native ranges (e.g. [8,44]), as do more detailed analyses using SDMs (e.g. [45,46]). In general, these patterns of distribution in invasive species offer support for the importance of abiotic climatic factors in setting range limits, because the exotic species are presumably removed from the set of species that they would interact with in their native ranges. In contrast, some studies suggest that invasive species may have novel climatic distributions in their introduced ranges relative to their native ranges (e.g. [47–50]), but these studies are generally interpreted as evidence that climatic tolerances of the invasive species changed, rather than their ranges being unaffected by climate (although alternative explanations are also possible).
Second, there is evidence that many species are presently shifting their geographical and elevational ranges owing to climate change (e.g. [51,52]). This pattern implies that their previous range limits were set by their climatic tolerances, although it does not rule out biotic factors completely.
(ii). The case for biotic factors
Interspecific interactions can also be important in the distributions of organisms. However, many examples involve small-scale patterns of distribution, and it is not clear that the same processes would explain large-scale biogeographic patterns in these same systems. For example, Connell's [53] classic work on the barnacle (Chthamalus stellatus) in the intertidal zone in Scotland shows how the distribution of this species is explained by a combination of biotic (predation and competition) and abiotic factors (desiccation). However, the pattern of distribution in question is a vertical distribution of a few metres on an island coastline, and it is not clear if these same factors would explain the large-scale biogeographic distribution of this species (or the clade in which it is embedded).
There are many other examples where biotic factors influence smaller scale distribution patterns (e.g. different habitats within a region) but not necessarily larger patterns (e.g. presence in one region versus another). For example, competition may influence the vertical distribution of species on mountains (e.g.[54,55]). Species assemblages of damselfly larvae (Enallagma) in lakes differ depending on whether fish or dragonfly larvae are the top predator (e.g. [56,57]). Here, predation creates a mosaic of different habitats for damselflies within a region, but not a large-scale biogeographic pattern.
Anderson et al. [58] proposed a promising methodology to test for the effects of competitive exclusion on the distributions of pairs of closely related species, by combining SDM with analyses of geographic overlap. To support competitive exclusion, one species should be absent in areas that are climatically suitable for both species, near where their ranges abut. They applied this approach to two species of South American pocket mice and found evidence for competitive exclusion, but this involved a somewhat limited geographic area (i.e. five localities).
(iii). Where are the large-scale biogeographic patterns created by interspecies interactions?
At present, I am unaware of any large-scale biogeographic patterns that are created by competitive interactions or by biotic interactions in general. I do not claim that such patterns do not occur, just that there is a paucity of good examples. There are at least two obvious (not mutually exclusive) causes that may explain this. First, such patterns are truly rare. Second, those who study large-scale biogeography do not generally consider competition (or other biotic factors) as a potential explanation for the patterns that they study, especially given that they have tended not to consider ecological factors at all.
If we were looking for large-scale biogeographic patterns created by competition or other biotic factors, what sort of pattern might we expect to see? Following from Anderson et al. [58], one might expect to see two clades with abutting geographic distributions, where the range of each clade is climatically suitable for the other (but especially for the species in each clade that are geographically adjacent to each other). However, such a pattern might also arise simply owing to limited dispersal among the species in both clades. This pattern could be made more compelling if there was extensive geographic range overlap among species within clades (indicating considerable dispersal), but abutting distributions between clades (indicating that dispersal is limited by species interactions). Furthermore, evidence of competitive release could also lend support (e.g. one species or clade expands its range in the absence of the other). Of course, any hypothesis of competition or other species interactions from biogeographic patterns would be greatly strengthened by direct evidence of these interactions from local-scale experimental or observational studies.
Here, I draw attention to one type of geographic pattern that might merit further scrutiny as an outcome of species interactions. This is the pattern of disjunct distribution within a clade, where different lineages (i) occur in distinct, large-scale regions, (ii) are separated from each other by a clade of potential competitors, and (iii) the intervening regions separating the two clades are climatically suitable (such that the disjunct clades are not merely separated by unsuitable environment [58]).
For example, the plethodontid salamander genus Hydromantes occurs both in western North American and southern Europe [59]. In North America, the closely related genus Plethodon does not occur sympatrically with Hydromantes, but is the most diverse group of salamanders to the north and east of its range (i.e. separating European and North American Hydromantes). Furthermore, Hydromantes in western North America occur from relatively low elevations (300 m; Hydromantes shastae) to greater than 3500 m (Hydromantes platycephalus; [59]), strongly suggesting that their distributions are not limited by a narrow range of climatic tolerances. Clearly, Hydromantes must have been distributed from western North America to Europe in the past. Presently, these two clades within Hydromantes appear as islands separated by a sea of potential competitors. Indeed, six of the eight European species literally occur on an island (Sardinia). It is not obvious why Hydromantes might be competitively inferior to other salamanders (if they are). Interestingly, Hydromantes species do share a highly derived feeding system, which allows them to project their tongues out of their bodies for long-distance prey capture [60], but might be less efficient at close range.
A similar example involves the two subgenera of slender salamanders (Batrachoseps) in western North America, where one subgenus (Plethiopsis) has species in both the southern Sierra Nevada mountains and in northern Oregon, whereas intervening regions (i.e. the northern Sierra Nevada) are inhabited by species of the other subgenus (Batrachoseps) which is more diverse and widely distributed (e.g. [61]). The subgenera are broadly overlapping in their climatic distributions overall (based on data from [39] for 1861 localities from 20 described species), but also show some differentiation in species mean values for some climatic variables (i.e. Plethiopsis species occur in environments that are, on average, colder in winter (Bio6, Bio11) and with more precipitation during the driest portions of the year (Bio 14, Bio17)). Overall, this pattern of disjunct distribution in Plethiopsis species separated by Batrachoseps may reflect the effects of competition, or may also reflect somewhat different climatic distributions of the two clades, some combination of these two factors, or other factors entirely. Of course, these examples are highly speculative (especially without direct evidence for the role of competition) and are in need of detailed analysis. They are included here only to illustrate one type of pattern that might result from competitive exclusion at large biogeographic scales.
Patterns of clades replacing each other over time and space in the fossil record might also offer evidence for the effects of biotic interactions on large-scale biogeographic patterns. As one potential example, Rosenzweig & McCord [62] discussed how an older group (straight-necked turtles; Amphichelydia) was replaced by younger clades (turtles with neck flexion; Cryptodira, Pleurodira) over time across continents. They called this general process ‘incumbent replacement’. However, these authors hypothesized that this pattern of geographic replacement was created by differential rates of speciation and extinction in each clade owing to the presence of a key adaptation in the younger clades (neck flexion), rather than through impacts of one clade on another.
(iv). Evidence against the role of species interactions in large-scale biogeography
Two types of patterns also offer evidence against the general importance of species interactions in shaping large-scale biogeographic patterns. Specifically, both biotic interchanges and invasive species suggest that regions remain open to invasion over time, despite the presence of a resident biota. Vermeij [63] reviewed biotic interchanges during the Neogene, and suggested that in many (but not all) natural interchanges, there were no invasion-related extinctions and that these interchanges often increased species richness in each region. These cases included the marine transequatorial, trans-Arctic and trans-Pacific interchanges.
Second, the many studies of invasive species may also have some relevance to the question of whether species interactions can drive large-scale patterns of biogeography. For example, studies of vertebrate extinctions on islands suggest that competition from exotic species was not the sole cause of extinction for any species, and was only rarely a potentially contributing cause (less than 10% of cases; [64]). In contrast, few native plant species on islands have been lost, and the addition of exotic species has primarily increased species richness, typically doubling plant richness on most islands [64]. Further, there is little evidence to suggest that islands have become saturated with native or exotic plant species over time. These results suggest the possibility that species interactions, or at least competition, may not drive large-scale biogeographic patterns in plants.
Of course, indirect human impacts also provide evidence for the possible effects of species interactions on distribution and diversity patterns. For example, the majority of vertebrate extinctions in the past 500 years have been related to predation by exotic species on islands (either alone or in combination with other factors; [64]). This suggests the potential for natural predators to drive species extinct, either locally or globally, although island vertebrates might also be unusually vulnerable relative to mainland species.
Similarly, the apparent extinctions of dozens of Neotropical montane frog species associated with chytrid fungus infections may illustrate how pathogens can create large-scale biogeographic patterns (e.g. [65]). For example, among the 85 species of the bufonid genus Atelopus, 72 are either extinct or critically endangered owing largely to chytrid infection (e.g. most have not been seen in more than 10 years; [66]). It should be noted that although these chytrid-related declines are generally considered to have an anthropogenic cause (e.g. owing to human spread and/or climate change), the specific role that humans have played is still unresolved (e.g. [67]).
(v). Niche pre-emption: integrating abiotic and biotic factors over time
In the preceding sections, I have discussed biotic and abiotic factors as competing explanations for biogeographic patterns. But biotic and abiotic factors might also be integrated by niche pre-emption over macroevolutionary time scales (i.e. a species or clade fails to expand into a new abiotic niche because it is already occupied by another species or clade, not because it is intrinsically incapable of evolving to occupy that niche). For example, species of a low-elevation clade might lack physiological tolerances to survive climatic conditions at high elevations, whereas species of a high-elevation clade might be unable to tolerate conditions at lower elevations. In this case, the distributions of the two clades would appear to be explained by abiotic factors alone and not by biotic factors. But if one of these clades entered a region where the other clade was absent, its elevational range might expand over macroevolutionary time scales to include a set of species collectively spanning all elevations (e.g. through evolution of new species that are adapted to tolerate these relatively novel climatic conditions). In other words, over short time scales the limited elevational distribution of a clade might be explained by physiological tolerances, but over longer time scales this limited elevational distribution may be explained by the lack of ecological opportunity owing to species interactions (niche pre-emption).
Such a pattern seems to have occurred in tropical plethodontid salamanders [39]. Many salamander clades occur in broad-scale sympatry in Middle America (Mexico to Panama), but tend to occur at either lower elevations (e.g. Oedipina) or higher elevations (e.g. Pseudoeurycea). However, a single, relatively young clade has radiated in South America (Bolitoglossa subgenus Eladinea), where it occurs from sea level to greater than 3000 m, and shows dramatically accelerated rates of climatic-niche evolution and species diversification [39]. Analyses across all plethodontid clades show that rates of niche evolution are generally higher in those clades that show less regional-scale geographic overlap with other clades, supporting the idea that niche pre-emption may be important in limiting climatic niche distributions over time (i.e. niche conservatism), and suggest that abiotic and biotic factors may be integrated over time to drive biogeographic patterns.
3. What biogeography can tell us about the eltonian niche and species interactions
Up to this point, I have focused on the importance of considering the niche in analyses of large-scale biogeographic patterns. Now, I turn to the question of what large-scale biogeographic analyses can tell us about the niche, and shift my emphasis here from the Grinnellian niche to the Eltonian niche. I suggest that analyses incorporating large-scale, historical (phylogenetic) biogeography may offer useful new perspectives on questions relating to species interactions and the Eltonian niche.
As one example, I focus here on the question of whether communities can become saturated with ecologically similar species over time. In some ways, this is a fundamental question in ecology, closely tied to the ideas of competitive exclusion and limiting similarity (e.g. [16,68]). If communities within a region have become saturated with species over evolutionary time (e.g. given tens of millions of years), then it should not be possible for ecologically similar species to invade the region and local communities within it. Large-scale biogeography can offer insights into these invasions, especially when coupled with time-calibrated phylogenies and data on traits related to resource utilization. Below, I describe several case studies to demonstrate this point.
These examples are drawn largely from research on amphibians and reptiles (but mostly treefrogs) and will illustrate two themes. First, that regions (and local communities) can be invaded by species that have traits similar to those found among the resident species in the regional species pool or community. Second, even in cases where some evidence suggests that competition and species interactions are important in driving and constraining the evolution of niche-related traits, competition does not prevent sympatry (co-occurrence) of species with similar trait values on that same axis. I then revisit these themes (and related issues) after describing these examples. But I issue the caveat that these examples do not necessarily represent an unbiased analysis.
(a). Case 1: treefrog invasions in lowland Middle America
The first case involves hylid treefrog invasions in Middle American lowlands [69,70]. In Middle America (Mexico to Panama), approximately 80 per cent of the approximately 160 hylid species in the region are descended from the first clade (Hylini) to invade Middle America from South America (where hylids originated), approximately 60–80 million years ago (Mya). This clade radiated extensively in terms of species numbers, habitats (lowland to highland), microhabitats (including species that breed exclusively in streams, ponds and arboreal sites, respectively) and body sizes (approx. 20 mm snout–vent length to greater than 100 mm). Within a given locality, microhabitat and body size may be the most important resource-related traits for hylids, given that microhabitat determines where they live and breed and body size effectively determines what they will eat (most seem to be generalist insectivores where body size determines prey size but not prey type (e.g. [71,72]). Thus, the radiation of Hylini would appear to have led to occupation (if not saturation) of the major resource-related niches within the region. Yet, the lowlands of Middle America (less than 1000 m) have been invaded more recently by most of the major clades of South American hylids (e.g. the genera Dendropsophus, Hypsiboas, Scinax and Trachycephalus). Throughout the lowlands of Middle America, ponds may be inhabited by ecologically similar species from both the Middle American and South American lineages, and with little obvious evidence of competitive exclusion. For example, in eastern Mexico, one can find two very similar species with nearly identical body sizes calling together in microsympatry, one from the Middle American clade (Tlalocohyla picta) and one from a recent South American invasion (Dendropsophus microcephalus; [70]; J. J. Wiens 2005, personal observation). In many localities, species from recently invading South American clades may outnumber those of the resident Middle American clade [69,70].
(b). Case 2: evolution and sympatry of treefrog body sizes
My collaborators and I have also investigated how the large-scale biogeography and sympatry of clades influences the rate of body-size evolution in hylid treefrogs [73]. There are eight major clades of treefrogs, six of which occur in sympatry throughout most of South America. Another clade (Pelodryadinae) occurs in Australia (including adjacent islands), and the eighth (Hylini) occurs in Middle America, North America, Europe and Asia. Diverse tropical communities tend to have similar ranges of body sizes (with maximum snout–vent length of males ranging from approximately 20 to 100 mm [71]). In Australia and Middle America, this range can be encompassed by a single clade. In South America, the other six clades tend to occupy somewhat smaller portions of this range (e.g. species of the Dendropsophus, Pseudis and Scinax clades tend to be smaller, and species of Cophomantini, Lophiohylini and Phyllomedusinae tend to be larger). An analysis of rates of phenotypic evolution using Brownie [74] shows significantly higher rates of body-size evolution in the primarily allopatric clades, suggesting that sympatry slows the rate of body-size evolution in the other six clades. Given this pattern, one might expect that communities become ‘filled’ or saturated with species of a given body size, such that no more can evolve or occur together. Nevertheless, many communities contain multiple species with similar size. For example, one of the most diverse sites in South America (Santa Cecilia, Ecuador [75]) contains seven species of Dendropsophus with very similar maximum male body sizes (from approximately 20 to 25 mm). Thus, competitive interactions seem to slow the rate of body-size evolution in sympatric clades in South America (which might suggest the saturation of these communities with treefrogs of a given size), yet competitive interactions do not prevent the co-occurrence of many species with similar values for this trait in the same locality.
(c). Case 3: evolution, biogeography and sympatry of the treefrog ecomorph
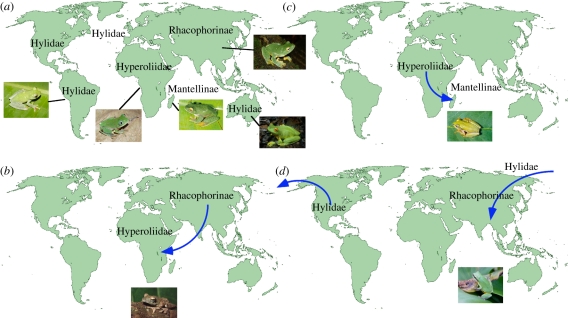
Every major tropical and temperate region has one or more frog species belonging to a ‘treefrog ecomorph’, characterized most obviously by enlarged toe pads [42]. Many regions are dominated by a single, separate evolutionary origin of the treefrog ecomorph (figure 1a). For example, Africa is dominated by hyperoliids, Asia by rhacophorine ranids and Madagascar by mantelline ranids. Hylid treefrogs dominate South America, but have also spread to Australia, Middle America, North America, Europe and Asia. These patterns suggest that selection may favour evolution of the treefrog ecomorph in regions where it is not already present, but may not favour multiple origins of this ecomorph in sympatry in the same region (but see below). However, consideration of the large-scale biogeography of these clades suggests that the radiation of these lineages in each region does not prevent invasion of these regions by treefrogs of other lineages from other regions.
Figure 1.
Combining historical biogeography and trait evolution reveals a lack of saturation in regional species pools in treefrogs. (a) The dominant clade of treefrogs (in terms of number of species) in major regions around the world is shown, along with representative species of these clades. Hylids are the dominant clade in the New World (Hypsiboas rufitelus shown here), Australia (Litoria xanthomera shown), and Europe, hyperoliids in Africa (Leptopelis notatus shown), rhacophorine ranids in Asia (Rhacophorus kio shown) and mantelline ranids on Madagascar (Boophis albilabris shown). (b) Despite the radiation of hyperoliid treefrogs in Africa, the region has been invaded more recently by rhacophorine ranids (genus Chiromantis) from Asia (Chiromantis rufescens shown). (c) Despite the radiation of mantelline treefrogs in Madagascar, the island has nevertheless been invaded more recently by hyperoliid treefrogs (Heterixalus) from Africa (Heterixalus boettgeri shown here). (d) Despite the radiation of rhacophorine treefrogs in Asia, the region has been more recently invaded by New World treefrogs (genus Hyla; Hyla chinensis shown). Photo credits: Hypsiboas rufitelus, Hyla chinensis (J. J. Wiens), Litoria xanthomera (J.-M. Hero), Leptopelis notatus (A. Schiotz), Rhacophorus kio (D. Edmonds), Boophis albilabris, Heterixalus boettgeri (M. Vences), Chiromantis rufescens (T. Leenders). Map outlines are from www.free-world-maps.com.
For example, in Africa (figure 1b), a large number of hyperoliid species (approx. 214 species [59]) have been evolving there for approximately 80 Myr (stem age [76]; note that I use estimated stem versus crown-group ages depending on which are available and comparable in a given case, but both ages should generally be strongly correlated with each other). However, despite this impressive radiation of hyperoliid treefrogs, the region has recently been invaded by rhacophorine treefrogs from Asia (Chiromantis; approximately 20–30 Mya; [77]), which have diversified into four species and dispersed widely across Africa.
Similarly, in Madagascar (figure 1c), the mantelline ranids have diversified into approximately 191 species [59], including a large genus of the treefrog ecomorph (Boophis) with 72 species (although the number of origins of treefrog ecomorphs in other genera is uncertain). Boophis has a stem-group age of approximately 50–55 Myr [77,78]. Yet, Madagascar has also been invaded by a hyperoliid treefrog (Heterixalus), which appears to have colonized Madagascar approximately 15 Mya [78] and has diversified into 11 species on Madagascar [59] and may occur syntopically with Boophis [79].
In Asia (figure 1d), despite the large radiation of rhacophorine treefrogs (approx. 210 species [59]), the region has nevertheless been invaded by hylid treefrogs (genus Hyla) from North and Middle America. Asian Hyla have spread from Japan and Korea to Thailand and India, and diversified into approximately 10 species [59], which are broadly sympatric with rhacophorines. The rhacophorines are approximately 68 Myr old (crown-age; [77]) whereas Asian hylids are approximately 18–24 Myr old (crown-age [23]).
In South America, the dominant group of treefrogs is the family Hylidae (more than 460 species; [23]). However, there are also two other major clades of the treefrog ecomorph (Centrolenidae, 150 species; Hemiphractidae, 95 species [59]). These three clades are divergent ecologically: South American hylids are predominantly and ancestrally lowland pond breeders [69], centrolenids are predominantly highland stream breeders [42] and hemiphractids are either direct developers or highland pond breeders [80]. All three clades may occur sympatrically at some sites (e.g. [75]).
Also, there has been a major radiation of stream-dwelling, montane hylids in Middle America [70,81]. This is a region where centrolenids are present but not diverse (with 14 species [59]), and may have arrived only in the last 3 million years [82]. Indeed, only three of the 14 centrolenid species are endemic to Middle America, whereas the others have ranges that include South America [59]. In contrast there has been only a small radiation of stream-dwelling hylids in the Andes (Hyloscirtus, approximately 30 species [59]), where centrolenids predominate. Centrolenids and hylids are broadly sympatric in both Middle and South America, both regionally and along individual streams ([59]; J. J. Wiens 2005, personal observation), again suggesting that these habitats are not saturated with species of either clade.
In summary, the global-scale biogeography of the treefrog ecomorph suggests numerous cases where this ecomorph radiates in a given region. However, despite radiations that have occurred over tens of millions of years and generated dozens of species, each region can still be colonized by another clade of the same ecomorph. These patterns strongly suggest that these regions are not saturated with species of the treefrog ecomorph.
Finally, I note that many of these regions contain other frogs that are partially or fully arboreal, but lack the obvious morphological and ecological similarity to the treefrog ecomorphs discussed here (e.g. some microhylids, ranine ranids and terraranans [71]). However, the presence of additional arboreal lineages in sympatry only reinforces the idea that treefrog radiations do not prevent the co-occurrence of other arboreal lineages in a region.
(d). Case 4: snakelike lizards
Moving away from treefrogs, snakelike lizard ecomorphs offer another example of how biogeography may illuminate the role of competition in divergence and coexistence. Snakelike lizards have evolved literally dozens of times across the phylogenetic history of squamates [83]. These snakelike lizards consist of two general ecomorphs: a short-tailed burrowing ecomorph and a long-tailed surface-dwelling ecomorph. Both ecomorphs are present in most major biogeographic regions. Most origins of snakelike lizards are of the short-tailed burrowing ecomorph, and this large number of origins may be related to the restricted geographical range of most of the origins, and their appearance in different geographical areas within a region. However, resolving this question will require more fine-scaled phylogenies of the family Scincidae, in which the majority of origins of this ecomorph have occurred.
By contrast, there have been five origins of the long-tailed ecomorph, giving a somewhat clearer picture for this morph. These origins exhibit a biogeographic pattern that is largely consistent with the idea that the origin of one morph in a region restricts subsequent origins of that same ecomorph in the same region. There has been a single origin of the morph (the genus Ophisaurus) that has spread between North America, Middle America, Europe, North Africa and Asia, and no other members of this morph occur in this region. Similarly, there has been a single origin in South America (Ophiodes) and another in Australia (Pygopodidae), regions where Ophisaurus are absent. In Africa, there have been two separate origins of the long-tailed morph, one in the cordylid genus Chaemasaura and one in the gerrhosaurid genus Tetradactylus [83]. These two clades overlap extensively now, although two of the three Chaemasaura have a somewhat more northerly distribution than most Tetradactylus, and may have arisen in allopatry [84]. Interestingly, these two genera differ remarkably in body size (Chaemasaura are about twice as long as Tetradactylus [85]), which may reduce potential competition between them.
The interesting pattern offered by the snakelike lizards is that, despite the limited number of evolutionary origins among regions, it clear that there can be extensive sympatry between members of this ecomorph within a given region. For example, range maps suggest that four species of Ophisaurus may occur in sympatry in the southeastern United States [86], three species of Ophiodes may occur in sympatry in Uruguay [87], and as many as eight species of surface-dwelling pygopodids may co-occur in Western Australia [88]. Thus, even if competition may constrain the same ecomorph from evolving multiple times in the same region in most regions around the world, competition does not prevent the build-up of many species of the same ecomorph in sympatry within a region (at least at the broad scale).
(e). Summary and synthesis
The examples here are intended to illustrate the idea that historical biogeographic studies may offer an exciting and largely untapped database for ecological questions relating to the Eltonian niche. Such analyses may be particularly fruitful when historical biogeography is combined with a time-calibrated phylogeny, data on niche-related traits and information on the composition of local communities. Specifically, studying the natural invasion of different regions by different clades of closely related or ecologically similar species over time may reveal the results of thousands of natural, long-term experiments relevant to species interactions and the Eltonian niche.
The examples presented here all offer variations on a similar major theme: that (in these cases) regions and communities do not ‘fill up’ or become saturated with species, even after tens of millions of years. In many of these examples, a lineage that first successfully invades a region diversifies and seems to occupy the relevant niche space. However, despite these radiations, the regions (and their local communities) can still be invaded by ecologically similar species. These patterns suggest that the regions and communities in question are not saturated with species (i.e. they can still be invaded). Saturation is a pervasive but controversial issue in ecology (e.g. [16,68]), related to the concepts of limiting similarity and competitive exclusion. A very similar idea in the recent literature is that there are resource-related ‘ecological limits’ on how many species can exist in a region or clade over time (e.g. [89]).
Other lines of evidence may also support the hypothesis that communities do not become saturated, but somewhat less directly. These include (i) the tendency of invasive species to increase richness within a region rather than driving resident species to extinction through competition (see above [64]), (ii) correlations of local richness with the size of the regional species pool (e.g. [3,90]), (iii) positive relationships between local richness and the amount of time that a region has been occupied by a clade (e.g. [73,91,92]), and (iv) increasing local richness over time in the fossil record (review in [93]). In contrast, the line of evidence that has recently been considered the strongest support for ecological limits (a lack of relationship between the ages of named higher taxa and their richness [89]) may simply reflect a tendency for higher taxa of the same rank to be of similar age within a given clade [93]. Further, the age–richness relationship can be absent even in groups where palaeontological evidence shows strongly increasing local richness over time (i.e. angiosperms [93]). This is clearly a topic in need of further study.
A second major theme in these examples is that competition may be important in limiting the evolution of resource-related traits, but not in limiting the co-occurrence of species with similar values for these traits. I call this the competition–divergence–co-occurrence conundrum. The strongest evidence for this pattern comes from the analysis of body-size evolution and biogeography in hylid treefrogs [73]. In hylids, the two biogeographically isolated clades have faster rates of size evolution than the six sympatric clades in South America, even though many species with similar body size may co-occur in local communities. Thus, species interactions seem to limit the rate of body-size evolution, but they do not prevent co-occurrence of species with similar size. In general, this competition–divergence–co-occurrence conundrum combines two common observations in evolutionary ecology that together seem somewhat paradoxical: that ecological opportunity (i.e. ‘open niches’ or absence of ecologically similar species) seems to promote trait divergence and radiation (e.g. [94,95]), but that many ecologically similar, closely related species often occur in sympatry (e.g. Dendroica warblers; [96]). However, it specifically refers to the case when the evolution of a given trait (related to resource usage) appears to be constrained by sympatry of clades and yet sympatric species share similar values for that same trait.
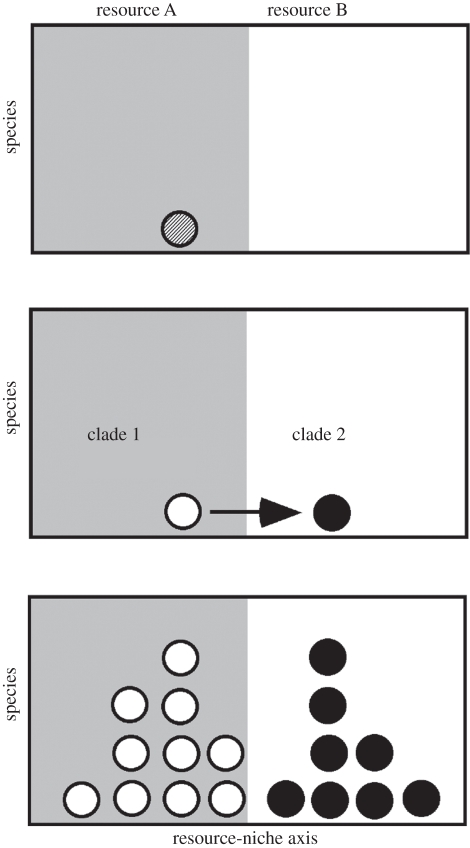
How is this possible? One potential scenario is as follows (figure 2). Selection may initially favour invasion of new (resource-related) niches most strongly when those niches are effectively empty (e.g. when a lineage first invades a new island or region [94]). Once a resource-related niche is ‘occupied’ by evolution of a specialist on that resource (or by dispersal of a specialist into the region or community), the selective advantage for evolving to use that same resource again may decrease, leading to slower rates of trait evolution. Thus, a resource-related niche may become occupied from an evolutionary standpoint when a single species uses it. However, this does not necessarily mean that this niche is saturated to the point that no other species can use this resource at all. In fact, some ecological theory suggests that greater ecological similarity between a pair of species may actually make it more difficult for one to competitively exclude the other over finite time scales (e.g. [97,98]). Thus, it may be relatively easy for a species to invade a community or region where the resident species have similar resource-related traits (where the invading species come from a separate origin of the trait in a different region, or from in situ speciation within the region). Overall, this speculation suggests one potential scenario that may explain how competition can limit the evolutionary origins of a trait but still allow the accumulation of many species in sympatry sharing that trait (figure 2). However, explicit modelling is clearly needed, and the key assumption to test is that it is easier to invade an occupied niche through dispersal of similar species into the community rather than through repeated evolution of the same trait in sympatry.
Figure 2.
Simple hypothetical example illustrating the diversification of a group in a region over time along a resource-niche axis (which can be divided into two major resource types, A and B). Circles represent the mean position of each species on the niche axis, open circles indicate species of clade 1, filled circles indicate species of clade 2 and the circle with lines indicates the common ancestor of these clades. (a) The biogeographic region is first colonized by a species having a given mean position on the niche axis and using resource A. (b) Given that resources along most of the niche axis are initially not used, there is a rapid splitting into two species (the ancestors of clades 1 and 2) that specialize on different resources and occupy different positions along the niche axis. (c) Once both resources are occupied (i.e. at least one species uses each), there are no further evolutionary transitions between the two resources between the two clades (i.e. competition limits further major trait changes). Nevertheless, species continue to accumulate in each clade, with species in each clade using the same major resource type. The co-occurrence of species sharing the same resource type may be facilitated by the ecological similarity of these species (competitive exclusion takes a very long time when the species are ecologically similar), or by ecological divergence on other resource-niche axes.
Of course, it should be pointed out that species in a given group that are ecologically similar for one resource-related trait can potentially diverge along other niche axes, and thus occur in sympatry without competitive exclusion. However, such a scenario leaves open the question of why sympatry should limit the rate of trait evolution in the same group (i.e. suggesting the influence of competition). Again, the challenge is to reconcile the observation of limited trait evolution owing to sympatry of clades with the sympatry of multiple species sharing similar values for that same trait.
In addition to treefrog body sizes [73], the case studies involving global treefrog ecomorphs and snakelike lizard ecomorphs may offer similar examples of competitively constrained evolution without local saturation. In these examples, an ecomorph tends to evolve only once in a region (suggesting competitive constraints on further evolution of this ecomorph after a trait has evolved), but many similar species of the ecomorph may evolve in situ and live in sympatry (as in the lizards) and the region can still be invaded by other lineages of the same ecomorph (as in treefrogs). However, in these two examples, it is not so clear that competition prevents the same ecomorph from arising in the same region. For example, there is no statistical test for competitive limits on the number of trait origins within a region, and there is some contradictory evidence (i.e. repeated origins of the treefrog ecomorph in South America and of the same lizard ecomorph in Africa, although there are also major ecological differences associated with these repeated origins in these cases). Nevertheless, even in these two cases, it seems that the resource-related niches in question (e.g. for arboreal frogs) become initially occupied by evolution or dispersal, but do not become filled. Other systems also offer cases where sympatric clades do not seem to constrain each other's evolution at all, such that regionally sympatric clades undergo parallel patterns of evolution and similar species from different clades occur in sympatry (e.g. [99]).
Overall, these examples suggest some interesting implications. First, they may help explain the paucity of cases where competition creates large-scale biogeographic patterns. Specifically, these examples (figure 1) suggest that competition need not prevent the large-scale geographic overlap of clades of ecologically similar species. Also, the number of species in a community or region may not depend heavily on the details of the distribution of traits among species (e.g. for the treefrogs, tropical communities around the world typically contain a similar range of body sizes among species, but there can be many sympatric species with similar body sizes [71]). If true, this may explain why patterns of local richness can be strongly correlated with factors like regional richness (e.g. [90]) or by time (e.g. [73,91]), and why detailed information on the traits of species in the local species pool may not always be necessary to strongly predict local richness.
These case studies also suggest many areas for future research. First, how does invasion of an ‘occupied region’ influence rates of diversification and trait evolution for the invading lineage (e.g. [69,95]), relative to the residents of the region and relative to the close relatives of the invaders in their native land? Also, which lineages are able to invade? Do the invading lineages have particular ecological traits relative to the native fauna? Are they able to invade all of the communities within the region, or only a subset, and what factors predict which communities they can invade? Is it possible that the lineages that have successfully become established in the region are only a fraction of those that have arrived there? Of course, when considering living taxa, we generally only know which lineages were successfully able to invade the region, and little or nothing about those that were unsuccessful. While extinction can certainly be a problem, it may not be in every case. For example, the observation that ecologically similar species from different origins (in situ evolution versus dispersal) can occur in sympatry stands regardless of what other lineages may have gone extinct. There is also a need for theoretical work to explore the issue of niche occupation (by evolution) relative to niche filling (saturation). In fact, there is relatively little theory that relates ecological processes to biogeographic and macroevolutionary patterns (but for a relevant example without biogeography see McPeek [100]).
Finally, it may be worthwhile to examine the possible advantages of considering historical biogeography for studies of resource-related niches and species interactions, relative to other approaches. As one example, the field of ‘community phylogenetics’ uses the relatedness of species in communities to make inferences about ecological processes (e.g. [101–103]). However, this approach typically does not use historical biogeography directly, and so does not consider which lineages with which traits invaded a region at what time. Incorporating historical biogeography might offer a more direct assessment of ‘community assembly’ than patterns of relatedness alone.
4. ‘Niche versus neutral’ perspectives in ecology
In the paired contribution to this symposium, Chase & Myers [10] provide an excellent review of the schism between ‘niche’ and ‘neutral’ perspectives in ecology, a major debate in the recent literature (see also Weiher et al. [11]). They also emphasize a methodological approach for disentangling the relative roles of niche and neutral perspectives that involves testing for changes in species composition and diversity along environmental gradients (versus null models). This seems like a very useful approach for testing whether species distributions are random with respect to the environmental variable(s) tested. However, I would caution against making too strict a dichotomy between the niche and ‘regional and biogeographic processes’. Many large-scale, regional biogeographic patterns also appear to be determined (directly or indirectly) by environmental gradients (e.g. climate), such as the latitudinal diversity gradient (e.g. [104,105]). In fact, these biogeographic patterns may also be strongly related to the niche and niche conservatism (table 1; [22]). Similarly, showing that richness varies non-randomly along an environmental gradient does not mean that evolutionary and biogeographic processes (like speciation and abiotic and biotic limits on colonization) are unimportant in creating this pattern, even if this gradient is within a single region. Quite the opposite, the processes of speciation and dispersal (along with extinction) are the only processes that directly change species numbers [1,3,106]. Thus, these are the processes that directly create diversity gradients in the first place (and the relationships between diversity and abiotic and biotic variables), regardless of spatial scale (e.g. [73,90,93]). The approach advocated by Chase & Myers [10] should be useful for identifying diversity and distribution patterns that are related to the included environmental variables, but some caution may be warranted when considering which ‘neutral processes’ can actually be ruled out. The results may also depend heavily on what environmental variables are considered (a major issue for biogeographic approaches as well, such as SDM).
5. Conclusions and prospects
A consideration of first principles suggests that biogeographic patterns are created by spatial variation in habitat and species niches (and niche conservatism). Given this, I would argue that we know little about the origins of large-scale biogeographic patterns, because historical biogeographers have generally tended to ignore the ecological underpinnings of these patterns.
At a smaller scale, the literature on species range limits suggests that abiotic factors are important in a large number of studies, but studies that consider biotic factors typically support their role as well. Little is known about how ecological niches create larger-scale patterns across multiple species within a clade. Nevertheless, there is some evidence for the importance of climatic factors. The role of biotic factors in shaping large-scale biogeographic patterns remains largely unexplored, and new methods for testing for the effects of biotic interactions at larger spatial scales are needed. Integration between biotic and abiotic factors over time may also be important in creating large-scale patterns over long time scales, and there is some evidence that niche pre-emption may prevent species from evolving into a given climatic niche.
Conversely, I have argued that large-scale biogeography may offer useful insights on species interactions and the Eltonian niche. By combining information from historical biogeography, time-calibrated phylogenies, and resource-related traits, I show several examples in which species are able to invade regions (or communities) where ecologically similar species are already present. This pattern suggests that communities are not necessarily saturated with species over time in these cases, even after a clade has been present in a region for tens of millions of years and has diversified into a hundred or more species. However, these selected examples do not represent an unbiased analysis, and there may be many other cases where lineages cannot invade. Nevertheless, these examples suggest that saturation is not universal.
I have also described evidence that suggests that competition can limit trait evolution but not co-occurrence of species having similar values for that same trait (e.g. [73]). I call this somewhat paradoxical scenario the competition–divergence–co-occurrence conundrum. This scenario contrasts the common observations that sympatry between clades seems to limit ecological radiation (e.g. ecological opportunity promotes adaptive radiation [94]), but that ecologically similar species often occur in sympatry (e.g. MacArthur's warblers [96]). Theoretical work is needed to reconcile these seemingly conflicting patterns, and more empirical work is needed to test their generality.
Many other questions regarding the Eltonian niche might be addressed by combining historical biogeography and trait evolution with time-calibrated phylogenies. This combination can reveal when traits are added to a region and how they come to be in local communities (e.g. [69]). Thus, historical biogeography may help infer (for example) the order of trait assembly in community, and the assembly of food webs over time. Biogeographic information on the timing of colonization of regions may also help explain species richness of local communities (e.g. [73,91]). Interestingly, this usage of phylogenies in community ecology is actually quite different from the burgeoning field of community phylogenetics, which typically focuses on using the relatedness of species in local communities to infer competition or habitat filtering (e.g. [101–103]). New approaches are needed that can incorporate insights from historical biogeography into the study of community assembly (e.g. [69]).
Acknowledgements
I thank D. Jenkins and R. Ricklefs for inviting me to participate in this symposium and the International Biogeographic Society and National Science Foundation for providing financial support for travel to the meeting to present this paper. For helpful comments on the manuscript, I thank J. Chase, D. Jenkins and R. Ricklefs. For permission to use their frog images (figure 1), I thank D. Edmonds, J.-M. Hero, T. Leenders, A. Schiotz and M. Vences.
References
- 1.Wiens J. J., Donoghue M. J. 2004. Historical biogeography, ecology, and species richness. Trends Ecol. Evol. 19, 639–644 10.1016/j.tree.2004.09.011 (doi:10.1016/j.tree.2004.09.011) [DOI] [PubMed] [Google Scholar]
- 2.Lomolino M. V., Riddle B. R., Brown J. H. 2006. Biogeography, 3rd edn Sunderland, MA: Sinauer Associates [Google Scholar]
- 3.Ricklefs R. E. 1987. Community diversity: relative roles of local and regional processes. Science 235, 167–171 10.1126/science.235.4785.167 (doi:10.1126/science.235.4785.167) [DOI] [PubMed] [Google Scholar]
- 4.Ricklefs R. E., Schluter D. (eds) 1993. Species diversity in ecological communities: historical and geographical perspectives. Chicago, IL: University of Chicago Press [Google Scholar]
- 5.Hutchinson G. E. 1957. Concluding remarks. Cold Spring Harbor Symp. 22, 415–427 [Google Scholar]
- 6.Holt R. D. 2009. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc. Natl Acad. Sci. USA 106, 19 659–19 665 10.1073/pnas.0905137106 (doi:10.1073/pnas.0905137106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soberon J. 2007. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123 10.1111/j.1461-0248.2007.01107.x (doi:10.1111/j.1461-0248.2007.01107.x) [DOI] [PubMed] [Google Scholar]
- 8.Wiens J. J., Graham C. H. 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Ann. Rev. Ecol. Evol. Syst. 36, 519–539 10.1146/annurev.ecolsys.36.102803.095431 (doi:10.1146/annurev.ecolsys.36.102803.095431) [DOI] [Google Scholar]
- 9.Wallace A. R. 1876. The geographical distribution of animals. London, UK: MacMillan [Google Scholar]
- 10.Chase J. M., Myers J. A. 2011. Disentangling the importance of ecological niches from stochastic processes across scales. Phil. Trans. R. Soc. B 366, 2351–2363 10.1098/rstb.2011.0063 (doi:10.1098/rstb.2011.0063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiher E., Freund D., Bunton T., Stefanski A., Lee T., Bentivenga S. 2011. Advances, challenges, and a developing synthesis of ecological community assembly theory. Phil. Trans. R. Soc. B 366, 2403–2413 10.1098/rstb.2011.0056 (doi:10.1098/rstb.2011.0056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schurr F. M., Midgley G. F., Rebelo A. G., Reeves G., Poschlod P., Higgins S. I. 2007. Colonization and persistence ability explain the extent to which plant species fill their potential range. Global. Ecol. Biogeogr. 16, 449–459 10.1111/j.1466-8238.2006.00293.x (doi:10.1111/j.1466-8238.2006.00293.x) [DOI] [Google Scholar]
- 13.Paul J. R., Morton C., Taylor C. M., Tonsor S. J. 2009. Evolutionary time for dispersal limits the extent but not the occupancy of species' potential ranges in the tropical plant genus Psychotria (Rubiaceae). Am. Nat. 173, 188–199 10.1086/595762 (doi:10.1086/595762) [DOI] [PubMed] [Google Scholar]
- 14.Sexton J. P., McIntyre P. J., Angert A. L., Rice K. J. 2009. The evolution and ecology of geographic range limits. Ann. Rev. Ecol. Evol. Syst. 40, 415–436 10.1146/annurev.ecolsys.110308.120317 (doi:10.1146/annurev.ecolsys.110308.120317) [DOI] [Google Scholar]
- 15.Gross S. J., Price T. D. 2000. Determinants of the northern and southern range limits of a warbler. J. Biogeogr. 27, 869–878 10.1046/j.1365-2699.2000.00440.x (doi:10.1046/j.1365-2699.2000.00440.x) [DOI] [Google Scholar]
- 16.MacArthur R. H. 1972. Geographic ecology. New York, NY: Harper and Row [Google Scholar]
- 17.Kirkpatrick M., Barton N. H. 1997. Evolution of a species' range. Am. Nat. 150, 1–23 10.1086/286054 (doi:10.1086/286054) [DOI] [PubMed] [Google Scholar]
- 18.Gaston K. J. 2003. The structure and dynamics of geographic ranges. Oxford, UK: Oxford University Press [Google Scholar]
- 19.Gaston K. J. 2009. Geographic range limits: achieving synthesis. Proc. R. Soc. B 276, 1395–1406 10.1098/rspb.2008.1480 (doi:10.1098/rspb.2008.1480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis M. B., Shaw R. G. 2001. Range shifts and adaptive responses to Quaternary climate change. Science 292, 673–679 10.1126/science.292.5517.673 (doi:10.1126/science.292.5517.673) [DOI] [PubMed] [Google Scholar]
- 21.Thompson J. N. 2005. The geographic mosaic of coevolution. Chicago, IL: University of Chicago Press [Google Scholar]
- 22.Wiens J. J., et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324 10.1111/j.1461-0248.2010.01515.x (doi:10.1111/j.1461-0248.2010.01515.x) [DOI] [PubMed] [Google Scholar]
- 23.Wiens J. J., Graham C. H., Moen D. S., Smith S. A., Reeder T. W. 2006. Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: treefrog trees unearth the roots of high tropical diversity. Am. Nat. 168, 579–596 10.1086/507882 (doi:10.1086/507882) [DOI] [PubMed] [Google Scholar]
- 24.Stephens P. R., Wiens J. J. 2009. Bridging the gap between historical biogeography and community ecology: niche conservatism and community structure in emydid turtles. Mol. Ecol. 18, 4664–4679 10.1111/j.1365-294X.2009.04378.x (doi:10.1111/j.1365-294X.2009.04378.x) [DOI] [PubMed] [Google Scholar]
- 25.Kozak K. H., Wiens J. J. 2010. Niche conservatism drives elevational diversity patterns in Appalachian salamanders. Am. Nat. 176, 40–54 10.1086/653031 (doi:10.1086/653031) [DOI] [PubMed] [Google Scholar]
- 26.Rangel T. F. L. V. B., Diniz-Filho J. A. F., Colwell R. K. 2007. Species richness and evolutionary niche dynamics: a spatial pattern-oriented simulation experiment. Am. Nat. 170, 602–616 10.1086/521315 (doi:10.1086/521315) [DOI] [PubMed] [Google Scholar]
- 27.Evans M. E. K., Smith S. A., Flynn R., Donoghue M. J. 2009. Climate, niche evolution, and diversification of the ‘bird-cage’ evening primroses (Oenothera, sections Anogra and Kleinia). Am. Nat. 173, 225–240 10.1086/595757 (doi:10.1086/595757) [DOI] [PubMed] [Google Scholar]
- 28.Peterson A. T., Soberon J., Sanchez-Cordero V. 1999. Conservatism of ecological niches in evolutionary time. Science 285, 1265–1267 10.1126/science.285.5431.1265 (doi:10.1126/science.285.5431.1265) [DOI] [PubMed] [Google Scholar]
- 29.Kozak K. H., Wiens J. J. 2006. Does niche conservatism drive speciation? A case study in North American salamanders. Evolution 60, 2604–2621 [PubMed] [Google Scholar]
- 30.Warren D. L., Glor R. E., Turelli M. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883 10.1111/j.1558-5646.2008.00482.x (doi:10.1111/j.1558-5646.2008.00482.x) [DOI] [PubMed] [Google Scholar]
- 31.Cooper N., Jetz W., Freckleton R. P. 2010. Phylogenetic comparative approaches for studying niche conservatism. J. Evol. Biol. 23, 2529–2539 10.1111/j.1420-9101.2010.02144.x (doi:10.1111/j.1420-9101.2010.02144.x) [DOI] [PubMed] [Google Scholar]
- 32.Hua X., Wiens J. J. 2010. Latitudinal variation in speciation mechanisms in frogs. Evolution 64, 429–443 10.1111/j.1558-5646.2009.00836.x (doi:10.1111/j.1558-5646.2009.00836.x) [DOI] [PubMed] [Google Scholar]
- 33.Smith S. A., Donoghue M. J. 2010. Combining historical biogeography with niche modeling in the Caprifolium clade of Lonicera (Caprifoliaceae, Dipsacales). Syst. Biol. 590, 322–341 10.1093/sysbio/syq011 (doi:10.1093/sysbio/syq011) [DOI] [PubMed] [Google Scholar]
- 34.Crisp M. D., et al. 2009. Phylogenetic biome conservatism on a global scale. Nature 458, 754–756 10.1038/nature07764 (doi:10.1038/nature07764) [DOI] [PubMed] [Google Scholar]
- 35.Li J., He Q., Hua X., Zho J., Xu H., Chen J., Fu C. 2009. Climate and history explain the species richness peak at midelevation for Schizothorax fishes (Cypriniformes: Cyprinidae) distributed in the Tibetan Plateau and its adjacent regions. Glob. Ecol. Biogeogr. 18, 264–272 10.1111/j.1466-8238.2008.00430.x (doi:10.1111/j.1466-8238.2008.00430.x) [DOI] [Google Scholar]
- 36.Smith S. A., Stephens P. R., Wiens J. J. 2005. Replicate patterns of species richness, historical biogeography, and phylogeny in Holarctic treefrogs. Evolution 59, 2433–2450 [PubMed] [Google Scholar]
- 37.Pyron R. A., Burbrink F. T. 2009. Can the tropical conservatism hypothesis explain temperate species richness patterns? An inverse latitudinal biodiversity gradient in the New World snake tribe Lampropeltini. Glob. Ecol. Biogeogr. 18, 406–415 10.1111/j.1466-8238.2009.00462.x (doi:10.1111/j.1466-8238.2009.00462.x) [DOI] [Google Scholar]
- 38.Buckley L. B., et al. 2010. Phylogeny, niche conservatism, and the latitudinal diversity gradient in mammals. Proc. R. Soc. B 277, 2131–2138 10.1098/rspb.2010.0179 (doi:10.1098/rspb.2010.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozak K. H., Wiens J. J. 2010. Accelerated rates of climatic-niche evolution underlie rapid species diversification. Ecol. Lett. 13, 1378–1389 10.1111/j.1461-0248.2010.01530.x (doi:10.1111/j.1461-0248.2010.01530.x) [DOI] [PubMed] [Google Scholar]
- 40.Merriam C. H. 1894. Laws of temperature control of the geographic distribution of terrestrial animals and plants. Natl Geogr. 6, 229–238 [Google Scholar]
- 41.John-Alder H. B., Morin P. J., Lawler S. P. 1988. Thermal physiology, phenology, and distribution of tree frogs. Am. Nat. 132, 506–520 10.1086/284868 (doi:10.1086/284868) [DOI] [Google Scholar]
- 42.Vitt L. J., Caldwell J. P. 2009. Herpetology: an introductory biology of reptiles and amphibians, 3rd edn. San Diego, CA: Academic Press [Google Scholar]
- 43.NatureServe 2004. InfoNatura: birds, mammals, and amphibians of Latin America, version 4.1. Arlington, VA: NatureServe; See http://www.natureserve.org/infonatura [Google Scholar]
- 44.Sax D. F. 2001. Latitudinal gradients and geographic ranges of exotic species: implications for biogeography. J. Biogeogr. 28, 139–150 10.1046/j.1365-2699.2001.00536.x (doi:10.1046/j.1365-2699.2001.00536.x) [DOI] [Google Scholar]
- 45.Peterson A. T., Vieglais D. A. 2001. Predicting species invasions using ecological niche modeling: new approaches from bioinformatics attack a pressing problem. Bioscience 51, 363–371 10.1641/0006-3568(2001)051[0363:PSIUEN]2.0.CO;2 (doi:10.1641/0006-3568(2001)051[0363:PSIUEN]2.0.CO;2) [DOI] [Google Scholar]
- 46.Peterson A. T. 2003. Predicting the geography of species invasions via ecological niche modeling. Q. Rev. Biol 78, 419–433 10.1086/378926 (doi:10.1086/378926) [DOI] [PubMed] [Google Scholar]
- 47.Broennimann O., Treier U. A., Muller-Scharer H., Thuiller W., Peterson A. T., Guisan A. 2007. Evidence of climatic niche shift during biological invasion. Ecol. Lett. 10, 701–709 10.1111/j.1461-0248.2007.01060.x (doi:10.1111/j.1461-0248.2007.01060.x) [DOI] [PubMed] [Google Scholar]
- 48.Beaumont L. J., Gallagher R. V., Thuiller W., Downey P. O., Leishman M. R., Hughes L. 2009. Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers. Distrib. 15, 409–420 10.1111/j.1472-4642.2008.00547.x (doi:10.1111/j.1472-4642.2008.00547.x) [DOI] [Google Scholar]
- 49.Rodder D., Lotters S. 2009. Niche shift versus niche conservatism? Climatic characteristics of the native and invasive ranges of the Mediterranean house gecko (Hemidactylus turcicus). Glob. Ecol. Biogeogr. 18, 674–687 10.1111/j.1466-8238.2009.00477.x (doi:10.1111/j.1466-8238.2009.00477.x) [DOI] [Google Scholar]
- 50.Medley K. A. 2010. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Global Ecol. Biogeogr. 19, 122–133 10.1111/j.1466-8238.2009.00497.x (doi:10.1111/j.1466-8238.2009.00497.x) [DOI] [Google Scholar]
- 51.Parmesan C., Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 10.1038/nature01286 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 52.Tingley M. W., Monahan W. B., Beissinger S. R., Moritz C. 2009. Birds track their Grinnellian niche through a century of climate change. Proc. Natl Acad. Sci. USA 106, 19 637–19 643 10.1073/pnas.0901562106 (doi:10.1073/pnas.0901562106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Connell J. H. 1961. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42, 710–723 10.2307/1933500 (doi:10.2307/1933500) [DOI] [Google Scholar]
- 54.Brown J. H. 1971. Mechanisms of competitive exclusion between two species of chipmunks (Eutamis). Ecology 52, 306–311 [Google Scholar]
- 55.Diamond J. M. 1975. Assembly of species communities. In Ecology and evolution of communities (eds Cody M. L., Diamond J. M.), pp. 342–444 Cambridge, MA: Harvard University Press [Google Scholar]
- 56.McPeek M. A. 1990. Determination of species composition in the Enallagma damselfly assemblages of permanent lakes. Ecology 71, 83–98 10.2307/1940249 (doi:10.2307/1940249) [DOI] [Google Scholar]
- 57.McPeek M. A. 1998. The consequences of changing the top predator in a food web: a comparative experimental approach. Ecol. Monogr. 68, 1–23 [Google Scholar]
- 58.Anderson R. P., Peterson A. T., Gómez-Laverde M. 2002. Using niche-based GIS modeling to test geographic predictions of competitive exclusion and competitive release in South American pocket mice. Oikos 98, 3–16 10.1034/j.1600-0706.2002.t01-1-980116.x (doi:10.1034/j.1600-0706.2002.t01-1-980116.x) [DOI] [Google Scholar]
- 59.AmphibiaWeb 2011. Information on amphibian biology and conservation. Berkeley, CA: AmphibiaWeb; See http://amphibiaweb.org/ (accessed January 2011) [Google Scholar]
- 60.Deban S. M., Wake D. B., Roth G. 1997. Salamander with a ballistic tongue. Nature 389, 27–28 10.1038/37898 (doi:10.1038/37898)9288962 [DOI] [Google Scholar]
- 61.Jockusch E. L., Yanev K. P., Wake D. B. 2002. Molecular phylogenetics and speciation in a complex of cryptic salamander species (Plethodontidae: Batrachoseps). Biol. J. Linn. Soc. 76, 361–391 10.1111/j.1095-8312.2002.tb01703.x (doi:10.1111/j.1095-8312.2002.tb01703.x) [DOI] [Google Scholar]
- 62.Rosenzweig M. L., McCord R. D. 1991. Incumbent replacement: evidence for long-term evolutionary progress. Paleobiology 17, 202–213 [Google Scholar]
- 63.Vermeij G. J. 1991. When biotas meet: understanding biotic interchange. Science 253, 1099–1104 10.1126/science.253.5024.1099 (doi:10.1126/science.253.5024.1099) [DOI] [PubMed] [Google Scholar]
- 64.Sax D. F., Gaines S. D. 2008. Species invasions and extinction: the future of native biodiversity on islands. Proc. Natl Acad. Sci. USA 105, 114 900–114 907 10.1073/pnas.0802290105 (doi:10.1073/pnas.0802290105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skerratt L. F., Berger L., Speare R., Cashins S., McDonald K. R., Phillott A. D., Hines H. B., Kenyon N. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4, 125–134 10.1007/s10393-007-0093-5 (doi:10.1007/s10393-007-0093-5) [DOI] [Google Scholar]
- 66.IUCN 2010. IUCN red list of threatened species. Version 2010.4. See http://www.iucnredlist.org (accessed 16 January 2011)
- 67.Lips K. R., Diffendorfer J., Mendelson J., Sears M. 2008. Riding the wave: climate change, emerging infectious disease and amphibian declines. PLoS Biol. 6, e72. 10.1371/journal.pbio.0060072 (doi:10.1371/journal.pbio.0060072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loreau M. 2000. Are communities saturated? On the relationship between α, β and γ diversity. Ecol. Lett. 3, 73–76 10.1046/j.1461-0248.2000.00127.x (doi:10.1046/j.1461-0248.2000.00127.x) [DOI] [Google Scholar]
- 69.Moen D. S., Smith S. A., Wiens J. J. 2009. Community assembly through evolutionary diversification and dispersal in Middle American treefrogs. Evolution 63, 3228–3247 10.1111/j.1558-5646.2009.00810.x (doi:10.1111/j.1558-5646.2009.00810.x) [DOI] [PubMed] [Google Scholar]
- 70.Duellman W. E. 2001. The hylid frogs of Middle America, 2nd edn. Lawrence, KS: Society for the Study of Amphibians and Reptiles [Google Scholar]
- 71.Moen D. S., Wiens J. J. 2009. Phylogenetic evidence for competitively-driven divergence: body-size evolution in Caribbean treefrogs (Hylidae: Osteopilus). Evolution 63, 195–214 10.1111/j.1558-5646.2008.00538.x (doi:10.1111/j.1558-5646.2008.00538.x) [DOI] [PubMed] [Google Scholar]
- 72.Duellman W. E. 2005. Cusco Amazonico: the lives of amphibians and reptiles in an Amazonian rainforest. Ithaca, NY: Comstock Publishing Associates [Google Scholar]
- 73.Wiens J. J., Pyron R. A., Moen D. S. 2011. Phylogenetic origins of local-scale diversity patterns and the origins of Amazonian megadiversity. Ecol. Lett. 10.1111/j.1461-0248.2011.01625.x (doi:10.1111/j.1461-0248.2011.01625.x) [DOI] [PubMed] [Google Scholar]
- 74.O'Meara B. C., Ane C., Sanderson M. J., Wainwright P. C. 2006. Testing for different rates of continuous trait evolution using likelihood. Evolution 60, 922–933 [PubMed] [Google Scholar]
- 75.Duellman W. E. 1978. The biology of an equatorial herpetofauna in Amazonian Ecuador. Misc. Publ. Mus. Nat. Hist. Univ. Kansas 65, 1–352 [Google Scholar]
- 76.Roelants K., Gower D. J., Wilkinson M., Loader S. P., Biju S. D., Guillaume K., Bossuyt F. 2007. Patterns of diversification in the history of modern amphibians. Proc. Natl Acad. Sci. USA 104, 887–892 10.1073/pnas.0608378104 (doi:10.1073/pnas.0608378104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiens J. J., Sukumaran J., Pyron R. A., Brown R. M. 2009. Evolutionary and biogeographic origins of high tropical diversity in Old World frogs (Ranidae). Evolution 63, 1217–1231 10.1111/j.1558-5646.2009.00610.x (doi:10.1111/j.1558-5646.2009.00610.x) [DOI] [PubMed] [Google Scholar]
- 78.Vences M., Vieites D. R., Glaw F., Brinkmann H., Kosuch J., Veith M., Meyer A. 2003. Multiple overseas dispersal in amphibians. Proc. R. Soc. B 270, 2435–2442 10.1098/rspb.2003.2516 (doi:10.1098/rspb.2003.2516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glaw F., Vences M. 2007. Field guide to the amphibians and reptiles of Madagascar, 3rd edn. Köln, Germany: Vences and Glaw Verlag [Google Scholar]
- 80.Wiens J. J., Kuczynski C., Duellman W. E., Reeder T. W. 2007. Loss and re-evolution of complex life cycles in marsupial frogs: can ancestral trait reconstruction mislead? Evolution 61, 1886–1899 10.1111/j.1558-5646.2007.00159.x (doi:10.1111/j.1558-5646.2007.00159.x) [DOI] [PubMed] [Google Scholar]
- 81.Smith S. A., Nieto Montes de Oca A., Reeder T. W., Wiens J. J. 2007. A phylogenetic perspective on elevational species richness patterns in Middle American treefrogs: why so few species in lowland tropical rainforests? Evolution 61, 1188–1207 10.1111/j.1558-5646.2007.00085.x (doi:10.1111/j.1558-5646.2007.00085.x) [DOI] [PubMed] [Google Scholar]
- 82.Guayasamin J. M., Castroviejo-Fisher S., Ayarzagüena J., Trueb L., Vilà C. 2008. Phylogenetic relationships of glassfrogs (Centrolenidae) based on mitochondrial and nuclear genes. Mol. Phylogenet. Evol. 48, 574–595 10.1016/j.ympev.2008.04.012 (doi:10.1016/j.ympev.2008.04.012) [DOI] [PubMed] [Google Scholar]
- 83.Wiens J. J., Brandley M. C., Reeder T. W. 2006. Why does a trait evolve multiple times within a clade? Repeated evolution of snake-like body form in squamate reptiles. Evolution 60, 123–141 [PubMed] [Google Scholar]
- 84.Uetz P. 2011. TIGR Reptile Database. See http://www.reptile-database.org/ (accessed January 2011).
- 85.Branch B. 1998. Field guide to the snakes and other reptiles of southern Africa. Sanibel Island, FL: Ralph Curtis Books [Google Scholar]
- 86.Conant R., Collins J. T. 1991. A field guide to reptiles and amphibians of Eastern and Central North America, 3rd edn. Boston, MA: Houghton-Mifflin [Google Scholar]
- 87.Achaval F., Olmos A. 1997. Anfibios y reptiles de Uruguay. Montevideo, Uruguay: Barreiro y Ramos S.A [Google Scholar]
- 88.Cogger H. G. 1992. Reptiles and amphibians of Australia, 5th edn Hollywood, FL: Ralph Curtis Books [Google Scholar]
- 89.Rabosky D. L. 2009. Ecological limits and diversification rate: alternative paradigms to explain the variation in species richness among clades and regions. Ecol. Lett. 12, 735–743 10.1111/j.1461-0248.2009.01333.x (doi:10.1111/j.1461-0248.2009.01333.x) [DOI] [PubMed] [Google Scholar]
- 90.Harrison S., Cornell H. V. 2008. Toward a better understanding of regional causes of local species richness. Ecol. Lett. 11, 969–979 10.1111/j.1461-0248.2008.01210.x (doi:10.1111/j.1461-0248.2008.01210.x) [DOI] [PubMed] [Google Scholar]
- 91.Stephens P. R., Wiens J. J. 2003. Explaining species richness from continents to communities: the time-for-speciation effect in emydid turtles. Am. Nat. 161, 112–128 10.1086/345091 (doi:10.1086/345091) [DOI] [PubMed] [Google Scholar]
- 92.Stevens R. D. 2006. Historical processes enhance patterns of diversity along latitudinal gradients. Proc. R. Soc. B 273, 2283–2289 10.1098/rspb.2006.3596 (doi:10.1098/rspb.2006.3596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wiens J. J. 2011. The causes of species richness patterns across space, time, and clades and the role of ‘ecological limits’. Q. Rev. Biol. [DOI] [PubMed] [Google Scholar]
- 94.Schluter D. 2000. The ecology of adaptive radiations. Oxford, UK: Oxford University Press [Google Scholar]
- 95.Mahler D. L., Revell L. J., Glor R. E., Losos J. B. 2010. Ecological opportunity and the rate of morphological evolution in the diversification of Greater Antillean anoles. Evolution 64, 2731–2745 10.1111/j.1558-5646.2010.01026.x (doi:10.1111/j.1558-5646.2010.01026.x) [DOI] [PubMed] [Google Scholar]
- 96.MacArthur R. H. 1958. Population ecology of some warblers of northeastern coniferous forests. Ecology 39, 599–619 10.2307/1931600 (doi:10.2307/1931600) [DOI] [Google Scholar]
- 97.Leibold M. A., McPeek M. A. 2006. Coexistence of the niche and neutral perspectives in community ecology. Ecology 87, 1399–1410 10.1890/0012-9658(2006)87[1399:COTNAN]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[1399:COTNAN]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 98.Scheffer M., van Nes E. H. 2006. Self-organized similarity, the evolutionary emergence of groups of similar species. Proc. Natl Acad. Sci. USA 103, 6230–6235 10.1073/pnas.0508024103 (doi:10.1073/pnas.0508024103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kozak K. H., Mendyk R. W., Wiens J. J. 2009. Can parallel diversification occur in sympatry? Repeated patterns of body-size evolution in co-existing clades of North American salamanders. Evolution 63, 1769–1784 10.1111/j.1558-5646.2009.00680.x (doi:10.1111/j.1558-5646.2009.00680.x) [DOI] [PubMed] [Google Scholar]
- 100.McPeek M. A. 2008. The ecological dynamics of clade diversification and community assembly. Am. Nat. 172, E270–E284 10.1086/593137 (doi:10.1086/593137) [DOI] [PubMed] [Google Scholar]
- 101.Webb C. O., Ackerly D. D., McPeek M. A., Donoghue M. J. 2002. Phylogenies and community ecology. Ann. Rev. Ecol. Evol. Syst. 33, 475–505 10.1146/annurev.ecolsys.33.010802.150448 (doi:10.1146/annurev.ecolsys.33.010802.150448) [DOI] [Google Scholar]
- 102.Cavender-Bares J., Ackerly D. D., Baum D. A., Bazzaz F. A. 2004. Phylogenetic overdispersion in Floridian oak communities. Am. Nat. 163, 823–843 10.1086/386375 (doi:10.1086/386375) [DOI] [PubMed] [Google Scholar]
- 103.Cavender-Bares J., Kozak K. H., Fine P. V. A., Kembel S. W. 2009. The merging of community ecology and phylogenetic biology. Ecol. Lett. 12, 693–715 10.1111/j.1461-0248.2009.01314.x (doi:10.1111/j.1461-0248.2009.01314.x) [DOI] [PubMed] [Google Scholar]
- 104.Willig M. R., Kaufman D. M., Stevens R. D. 2003. Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Ann. Rev. Ecol. Evol. Syst. 34, 273–309 10.1146/annurev.ecolsys.34.012103.144032 (doi:10.1146/annurev.ecolsys.34.012103.144032) [DOI] [Google Scholar]
- 105.Mittelbach G. G., et al. 2007. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 10.1111/j.1461-0248.2007.01020.x (doi:10.1111/j.1461-0248.2007.01020.x) [DOI] [PubMed] [Google Scholar]
- 106.Ricklefs R. E. 2004. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 7, 1–15 10.1046/j.1461-0248.2003.00554.x (doi:10.1046/j.1461-0248.2003.00554.x) [DOI] [Google Scholar]