Abstract
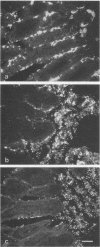
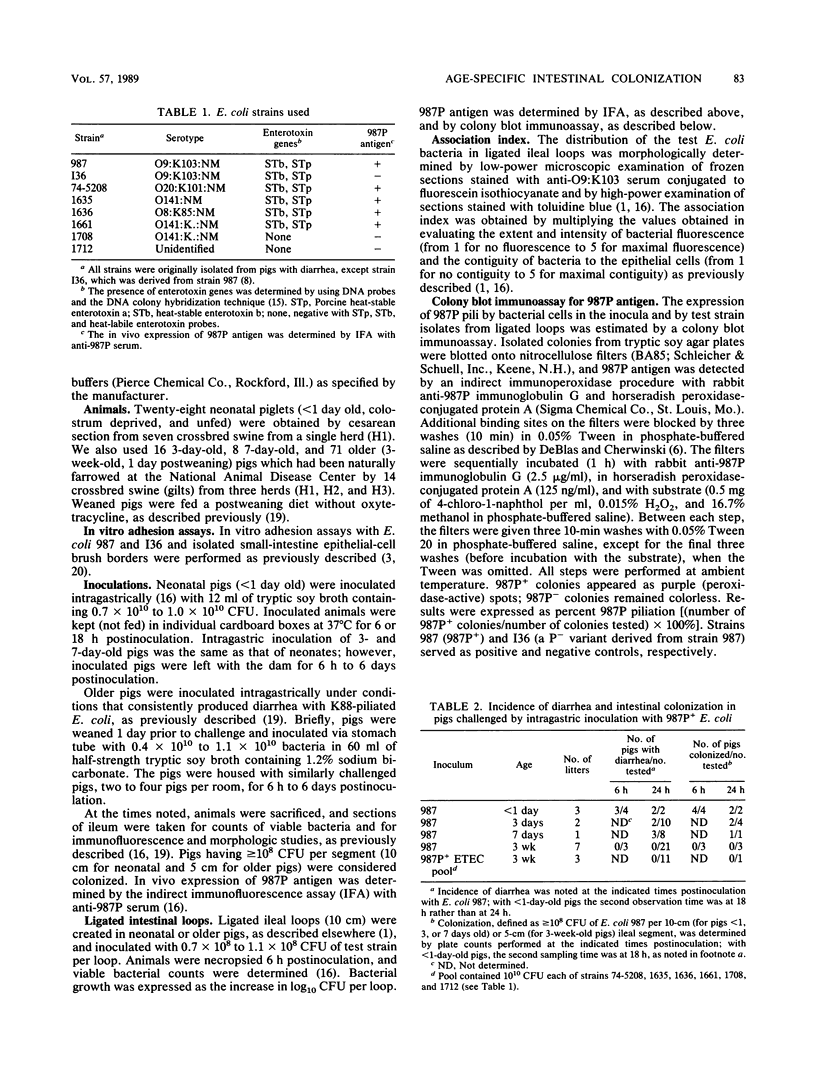
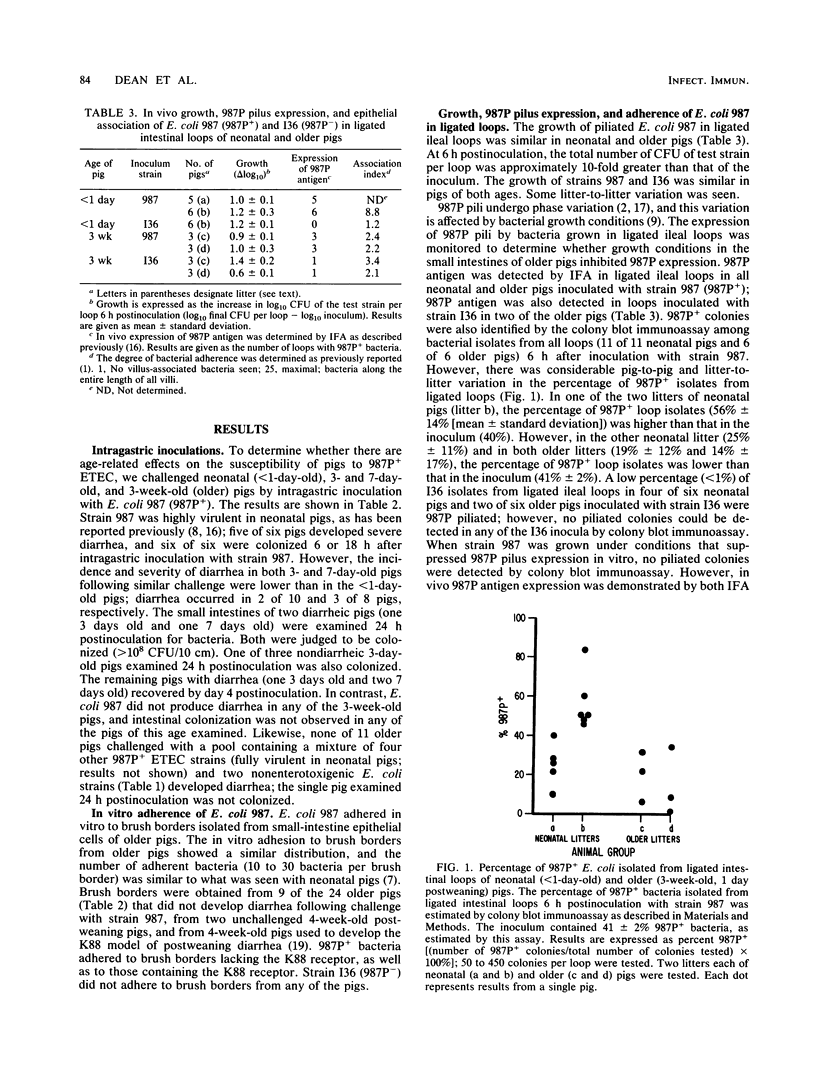
Neonatal (less than 1-day-old), 3- and 7-day old, and older (3-week-old postweaning) pigs were challenged by intragastric inoculation with 987P-piliated (987P+) enterotoxigenic Escherichia coli (ETEC) 987. Neonatal pigs were colonized (i.e., there were greater than or equal to 10(8) CFU of test strain per 10-cm ileal segment) and developed diarrhea. Intestinal colonization and the incidence and severity of diarrhea were lower in 3- and 7-day old pigs than in neonates. Older pigs were not colonized and did not develop diarrhea following oral inoculation with five strains of 987P+ ETEC. Strain 987 (987P+) adhered in vitro to intestinal epithelial cell brush borders isolated from both neonatal (sensitive) and older (resistant) pigs. The in vivo growth and expression of 987P pilus by strain 987 in ligated ileal loops created in neonatal and older pigs were similar. The in vivo adherence of 987P+ ETEC to intestinal epithelium in ligated ileal loops in neonatal and older pigs was compared. In neonatal pigs, most of the bacteria were in layers associated with the villous epithelium. In older pigs, most of the bacteria were associated with mucus-like material in the intestinal lumen. We concluded that swine develop an innate resistance to 987P+ ETEC by 3 weeks of age. This resistance does not appear to be due to an absence of 987P-specific receptors in the intestines of the older pig or to an inability of 987P+ bacteria to grow and express pili in the older pig. We hypothesized that the resistance of older pigs to 987P-mediated disease is due to release of 987P-specific receptors into the intestinal lumen, where these receptors facilitate bacterial clearance rather than bacterial adherence to intestinal epithelium and colonization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertschinger H. U., Moon H. W., Whipp S. C. Association of Escherichia coli with the small intestinal epithelium. I. Comparison of enteropathogenic and nonenteropathogenic porcine strains in pigs. Infect Immun. 1972 Apr;5(4):595–605. doi: 10.1128/iai.5.4.595-605.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- De Blas A. L., Cherwinski H. M. Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies. Anal Biochem. 1983 Aug;133(1):214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. In vitro adhesion of piliated Escherichia coli to small intestinal villous epithelial cells from rabbits and the identification of a soluble 987P pilus receptor-containing fraction. Infect Immun. 1982 Jun;36(3):1192–1198. doi: 10.1128/iai.36.3.1192-1198.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. Location and distribution of a receptor for the 987P pilus of Escherichia coli in small intestines. Infect Immun. 1985 Feb;47(2):345–348. doi: 10.1128/iai.47.2.345-348.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. Purification and characterization of a receptor for the 987P pilus of Escherichia coli. Infect Immun. 1985 Jan;47(1):98–105. doi: 10.1128/iai.47.1.98-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Nagy B., Moon H. W. Colonization of porcine small intestine by Escherichia coli: colonization and adhesion factors of pig enteropathogens that lack K88. J Infect Dis. 1977 Apr;135(4):531–539. doi: 10.1093/infdis/135.4.531. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E., Richter P. Escherichia coli 987P pilus: purification and partial characterization. J Bacteriol. 1981 May;146(2):784–789. doi: 10.1128/jb.146.2.784-789.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Moseley S. L., Huq I., Alim A. R., So M., Samadpour-Motalebi M., Falkow S. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J Infect Dis. 1980 Dec;142(6):892–898. doi: 10.1093/infdis/142.6.892. [DOI] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E. Colonization of porcine intestine by enterotoxigenic Escherichia coli: selection of piliated forms in vivo, adhesion of piliated forms to epithelial cells in vitro, and incidence of a pilus antigen among porcine enteropathogenic E. coli. Infect Immun. 1977 Apr;16(1):344–352. doi: 10.1128/iai.16.1.344-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy B., Moon H. W., Isaacson R. E. Colonization of porcine small intestine by Escherichia coli: ileal colonization and adhesion by pig enteropathogens that lack K88 antigen and by some acapsular mutants. Infect Immun. 1976 Apr;13(4):1214–1220. doi: 10.1128/iai.13.4.1214-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels P. L., Moon H. W., Schneider R. A. Development of resistance with host age to adhesion of K99+ Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1980 Apr;28(1):298–300. doi: 10.1128/iai.28.1.298-300.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento J. I., Casey T. A., Moon H. W. Postweaning diarrhea in swine: experimental model of enterotoxigenic Escherichia coli infection. Am J Vet Res. 1988 Jul;49(7):1154–1159. [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The influence of plasmid-determined and other characteristics of enteropathogenic Escherichia coli on their ability to proliferate in the alimentary tracts of piglets, calves and lambs. J Med Microbiol. 1978 Nov;11(4):471–492. doi: 10.1099/00222615-11-4-471. [DOI] [PubMed] [Google Scholar]
- Söderlind O., Thafvelin B., Möllby R. Virulence factors in Escherichia coli strains isolated from Swedish piglets with diarrhea. J Clin Microbiol. 1988 May;26(5):879–884. doi: 10.1128/jcm.26.5.879-884.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. A., Francis D. H. Fimbriae and enterotoxins associated with Escherichia coli serogroups isolated from pigs with colibacillosis. Am J Vet Res. 1986 Feb;47(2):213–217. [PubMed] [Google Scholar]