Abstract
Fishes with internalized and endothermic red muscles (i.e. tunas and lamnid sharks) are known for a stiff-bodied form of undulatory swimming, based on unique muscle–tendon architecture that limits lateral undulation to the tail region even though the red muscle is shifted anteriorly. A strong convergence between lamnid sharks and tunas in these features suggests that thunniform swimming might be evolutionarily tied to this specialization of red muscle, but recent observations on the common thresher shark (Alopias vulpinus) do not support this view. Here, we review the fundamental features of the locomotor systems in lamnids and tunas, and present data on in vivo muscle function and swimming mechanics in thresher sharks. These results suggest that the presence of endothermic and internalized red muscles alone in a fish does not predict or constrain the swimming mode to be thunniform and, indeed, that the benefits of this type of muscle may vary greatly as a consequence of body size.
Keywords: endothermy, tuna, lamnid shark, thunniform locomotion, thresher shark, muscle power
1. Undulatory swimming in fishes
Among the most common forms of locomotion in fishes is body undulation. This is powered by the blocks of muscle on each side of the body that typically contain both aerobic (red) and glycolytic (white) fibres (figure 1), which together may comprise 50 per cent or more of the body mass. A sequential contraction of myomeres alternately along both sides of the body generates a propulsive wave of bending that travels backward with increasing amplitude (figure 2). Each portion of the body involved in propagating the wave accelerates some water backwards and sideways, producing a reaction force that has a forward component contributing to thrust. But there is also a lateral component that wastes kinetic energy and tends to make the head recoil sideways [3]. Body undulation also increases viscous drag several fold compared with the same body moving in a rigid posture [3]. In non-thunniform swimmers, muscle contractions along the body are coupled to the adjacent vertebrae and cause local bending; thus, a progressive increase in proportional shortening (or strain) of muscle is needed to create the increased lateral displacement in more posterior regions. The relation between strain amplitude and the amount of bending it causes is dependent on the distance of the muscle fibres from the backbone, i.e. their effective mechanical advantage. In steady swimming, the thin subcutaneous layer of red muscle powers the undulatory motion, and strain amplitudes range from as low as 3 per cent in the anterior up to 8 per cent in the posterior [4]. This is only about half the strain typically achieved in the skeletal muscle of flying and running vertebrates [5]. Since contractile work and power are highly dependent on strain amplitude, we might expect high strains should be used to maximize performance, but this is only seen in extreme turns or fast starts when the body is thrown into much greater curvature than in steady swimming [6].
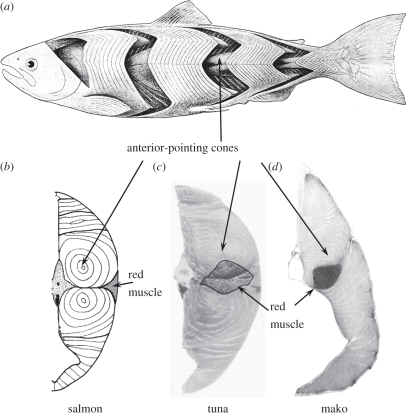
Figure 1.
(a) A diagram of a salmonid with skin removed to show the lateral muscle which contains two types of fibres, anaerobic white muscle and aerobic red muscle (modified from Greene & Greene [1]). White muscle is folded into a series of W-shaped segments called myomeres, one for each vertebra, with lateral posterior-pointing cones and medial anterior-pointing cones. These nested cones are seen as concentric rings in cross section (b). Tunas (c) and lamnid sharks (d) have elongated myomeres, so they have more rings. Red muscle sits just under the skin in non-thunniform fishes (b), but in tunas and lamnids it is found deep in the body, forming a loin at the anterior cones of the myomeres (c,d).
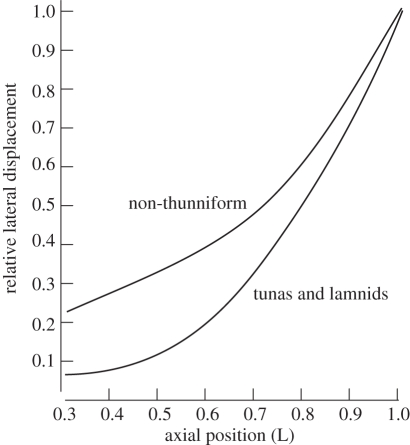
Figure 2.
A generalized plot showing trends in the lateral displacement relative to the body midline axis (normalized to the maximum value at the tail tip) as a function of position along the body (redrawn from Donley et al. [2]). Thunniform swimmers (tuna and lamnid) isolate body movement to the tail region whereas non-thunniforms have higher displacements in mid-body.
This particular design prompts the question: why is muscle shortening limited to such small degrees in routine swimming? One obvious problem is that while lateral motion is needed to generate thrust, it also has negative consequences, as described above. Paradoxically, while higher muscle strains could increase power output, they would also increase the power required to swim because of greater undulation, more drag and kinetic energy losses. The most common compromise seen in non-thunniform swimmers is a bending wave that has modest lateral amplitude over much of the body, not achieving high degrees of muscle shortening and power production. If the thrust production could be focused to the tail so that the muscle located in the main part of the body moved the tail by some linkage mechanism, instead of causing local bending, then muscle strains could be increased even while lateral motion was restricted to the tail, and energy saved. It turns out that tunas and lamnid sharks have done just this as they independently and convergently evolved ‘thunniform’ locomotor systems [2]. These are characterized by an anterior shift in the muscle mass that powers swimming and a concomitant reduction in undulation of the mid-body region (figure 2).
2. Stiff-bodied swimmers: tunas and lamnid sharks
In addition to endothermy, which enhances muscle power output [7–9], tunas and lamnids have thick bodies, highly tapered to a narrow caudal peduncle with a stiff hydrofoil-like tail fin that generates thrust by forward-directed hydrodynamic lift [10,11]. This design concentrates the locomotor muscle centrally while reducing mass and maximizing lateral motion in the posterior region (figure 2). The loins of red muscle fibres are situated medially, deep in the body, rather than superficially as in other fishes (figure 1). The peak of the red muscle cross-sectional area occurs at about the level of the main dorsal fin, which is the thickest part of the body. This anterior and medial positioning of the aerobic muscle might seem to be disadvantageous for powering continuous swimming because the muscle is far from the tail and close to the bending axis, but instead it turns out to provide substantial benefit; in fact, this anatomical specialization is the basis for the thunniform swimming mode that these two groups employ.
The most important feature of this anatomy is the elongation of the lateral myomeres and their long-reaching posteriorly directed tendinous structures. Details of the muscle and connective tissue anatomy in both groups have largely been worked out by Gemballa and colleagues [4,12]; while specific differences in tendon architecture occur, the convergence to similar function is unmistakeable. In addition to elongation of the myomeres, the deep position of red muscle fibres allows a more anterior positioning of the red muscle, and linkage of the anterior-pointing myoseptal cones to tendons unavailable for superficial fibres, ones that can span large numbers of body segments [4] (figure 3). In tunas, the anterior cone tendons join others in the horizontal septum that are directed obliquely to posterior vertebrae. This is in contrast to the arrangement of superficial red muscle fibres in non-thunniform fishes that insert onto the posterior portion of much shorter lateral tendons (figure 3), and link into oblique tendons with much steeper angles to the vertebrae, thus causing local bending. In lamnid sharks, the internalized red muscle inserts onto the anterior portion of the oblique tendons, which project caudally through the white muscle to the posterior cones and into skin in the region of the peduncle [2,4] (S. Gemballa 2006, unpublished data). These anatomical traits thus explain how red muscle contractions in the mid-body region of thunniform swimmers generate lateral motion in the caudal region rather than locally, essentially uncoupling the red muscle action from the local bending.
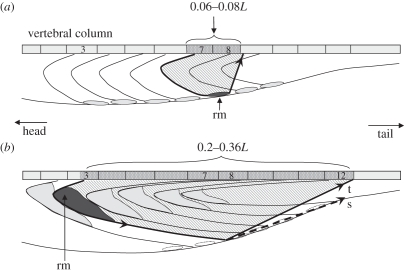
Figure 3.
A conceptual diagram showing how anatomical specializations in tunas and lamnids provide stiff-bodied swimming. Views represent horizontal sections at the level of the vertebral column, looking down into the left side of the body. In non-thunniform fishes, such as trout (a), each myomere extends over only 6–8% of the body length (L). Red muscle (rm) forms thin blocks under the skin and exerts force, via tendons, to the local vertebrae (heavy black arrow). For example, the red muscle associated with the vertebra labelled 7 will contract and bend the joint between 7 and 8. Thus, red muscle contraction is in phase with local bending. The myomeres in thunniform fishes (b) are highly elongated, spanning 20–25% of L, which shifts mass forward and aids in streamlining. Red muscle is located internally, near the vertebrae at the tip of the anterior cone in each myomere, and does not contract at the same time as the adjacent white muscle. Through long tendons, red muscle forces in thunniforms are projected backwards along the fish (heavy black arrow) to the vertebrae in a tuna (t) or the skin in a lamnid (s). Here, for example, the red muscle associated with myomere 7 links forward to vertebra 3 and transmits force and bending posteriorly to vertebra 12.
A result of this uncoupling is that red muscle shortening and local body curvature are not in phase; red muscle shortening lags local curvature (and local white muscle shortening) by 15–20% of a tail beat cycle in mako sharks and tunas [13,14], consistent with these contractions being in phase with lateral motion located much more posteriorly. Consequently, there are significant portions of each contraction cycle where the deep red muscle is still lengthening while the adjacent white muscle is being shortened, and vice versa (figure 4). Thus, a substantial degree of shearing between these muscle layers must occur, permitted by a lubricative sheath surrounding the red muscle such that it can shorten independently of the surrounding white muscle [12,13]. This unique physical feature, shared by tunas and lamnids, has been described as the hallmark of thunniform swimming [16].
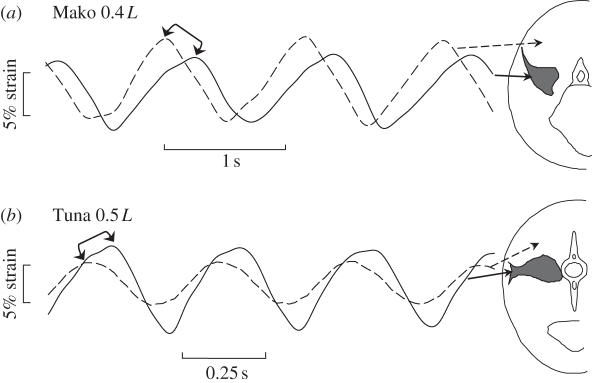
Figure 4.
Red muscle contracts out of phase with the adjacent white muscle in thunniform swimmers; (a) shortfin mako shark (Isurus oxyrinchus), and (b) yellowfin tuna (Thunnus albacares). Cross sections at mid-body of each species indicate the internalized location of red muscles. In the lamnid shark, strain in active deep red muscle is delayed relative to strain in the adjacent inactive white muscle, both measured by sonomicrometry. In the tuna, strain in the deep red muscle (measured by sonomicrometry) occurs later in time than white muscle strain predicted from local body curvature. Bracket arrows indicate a period where the red muscle is still lengthening while the adjacent white muscle is shortening. Data from Donley et al. [13] and Katz et al. [15]. (a) Solid lines, red muscle; dashed lines, white muscle. (b) Solid lines, red muscle; dashed lines, curvature.
3. Thresher sharks: almost back again
(a). A limited knowledge of swimming biomechanics
Studies of biomechanics of swimming in fishes possessing internalized and regionally endothermic red muscles are relatively few, and restricted to only a few species. This is due in part to the challenges associated with studying them. Tunas and lamnid sharks are technically and logistically difficult to study, and not inexpensive to obtain. But of more consequence and perhaps intrigue is the apparent scarcity of species with these traits. As previously described, the tunas and lamnid sharks have demonstrated a remarkable convergence in anatomy, swimming mechanics and to some extent thermal sensitivity of contraction of their red muscles [2,11,12,17–19]. These shared patterns of functional anatomy in fishes with internalized and regionally endothermic red muscles seem to be relatively singular and invariant, which make them appear convincing. However, it is difficult to draw robust conclusions when comparing few and distantly related species, which leaves us wondering whether each or all of the associations between internalized red muscle and endothermy, thunniform swimming, local phase shifts between contraction of red and white muscles owing to shearing and the relatively high thermal sensitivity of red muscle contraction, are obligatory elements in the convergence with a clear adaptive basis. It also leaves somewhat intractable the compelling question of whether the internalized red muscle imparts primarily a mechanical advantage for thunniform swimming, or a physiological advantage of thermal stability and/or increased temperature for enhanced muscle performance [20]. In this light, we need more and more closely related species to study.
(b). Thresher sharks (family Alopiidae): a unique opportunity
This family includes three species, the common thresher, Alopias vulpinus, the bigeye thresher, Alopias superciliosus, and the pelagic thresher, Alopias pelagicus [21]. Of particular appeal is the existence within this family of a species possessing internalized and regionally endothermic red muscles, the common thresher, contrasting with the other two species that have lateral, subcutaneous and, in all probability, wholly ectothermic red muscles [18,22,23]. Phylogenetic evidence suggests the common thresher, with its internalized and regionally endothermic red muscles, is the ancestral sister taxon to the other two thresher species, and that the common threshers acquired this phenotype independently of the lamnid sharks (discussed in [23]). The existence of a species (the common thresher) that is closely related to the Lamnidae but having acquired the internalized red muscle phenotype independently, and a family, the thresher sharks, that exhibits species both with and without the internalized red muscle phenotype, provides a valuable opportunity to ask questions about the design.
Bernal & Sepulveda [24] recently confirmed speculations of red muscle endothermy in the common thresher shark, as suggested by the presence of a vascular counter-current heat exchanger [22]. A detailed account of red muscle distribution in the three thresher species reveals patterns consistent with those noted in other fishes with either superficial or internalized red muscle [18,23]. Specifically, all three species possess about the same relative amount of red muscles, similar to other sharks studied (2.3–3.0% of body mass), being distributed along the length of the body, including in the tail. Of note, the muscle is more prevalent in the posterior half of the body and is present both epaxially and hypaxially in the bigeye and pelagic threshers, as also observed in most ectothermic bony fishes and elasmobranchs [18,25]. Yet, in the common thresher and lamnid sharks, with internalized red muscles, the greatest cross-sectional area is shifted towards the anterior of the fish, in the region of the dorsal fin, and is exclusively epaxial until past the caudal peduncle. Thus, the association and proposed convergence between possession of internalized red muscles and both endothermy and muscle distribution appear to extend to the common thresher shark.
The tunas and lamnids show clear convergence in swimming biomechanics. As noted, long tendons direct forces from red muscles posteriorly towards the tail; hence, red muscle shortening and local body curvature are not in phase. Shearing between adjacent red and white muscles such that red fibres can shorten independently of the surrounding white muscle has been documented in both groups [12,13]. This design also allows propulsion to be generated primarily at the tail with little body bending towards the anterior, resulting in stiff-bodied, thunniform swimming.
It might then be hypothesized that this mechanism of muscle shearing and force transmission, and the resultant stiff-bodied swimming, is also convergent and a requisite design in fishes with internalized red muscles. In this regard, there are similarities in the contractile function of red muscles that appear to support the convergence of thunniform swimming biomechanics. These include internalized muscles that function at optimal strain for maximal power output during swimming in tunas [16,26]. This has not yet been tested in sharks with internalized muscles and would be very valuable to know. In studies on fishes with superficial red muscles, there are several examples in which strain during swimming clearly does not result in maximal power, but in a few examples it does (reviewed in [27]), allowing us to at least conclude that strain is not universally optimized for power in fishes with superficial muscle, and, thus far, it does appear to be in fishes with internalized red muscles. Further, there is an almost fully consistent observation of a lack of axial variation in contractile kinetics of red muscles in fishes with internalized red muscles, including recent observations of red muscle contraction kinetics in common thresher sharks (figure 5), compared with the usually notable axial variation in fishes with superficial red muscles [9,27–29].
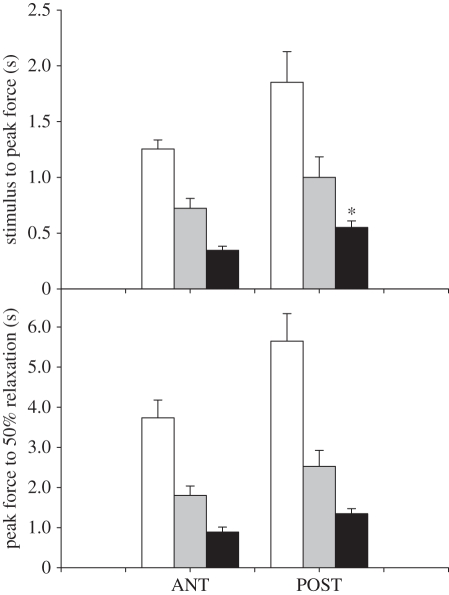
Figure 5.
Time from stimulus to peak force (upper panel) and time from peak force to 50% relaxation (lower panel) during isometric twitches of red muscles from anterior (ANT) and posterior (POST) axial locations in common thresher sharks at different temperatures. As reported for other sharks, both ectothermic and endothermic, there is a slowing of the twitch kinetics with decreasing temperature, but little or no difference in twitch kinetics between muscles from anterior (40% fork length) and posterior (60% fork length) locations. *Different from ANT, p < 0.05. (J. Donley, C. Sepulveda, S. Aalbers, D. McGillivray, D. Syme & D. Bernal 2009, unpublished data). White bars, 8°C; grey bars, 16°C; black bars, 24°C.
Yet the convergence in design appears to become ambiguous when the mechanics of swimming are considered. The common thresher shark proves a particularly interesting species in which to test this hypothesis, as it possesses internalized red muscles yet also possesses an extremely heterocercal caudal fin with an upper lobe that is as long as the body [30]. The tail is used for propulsion, but also for feeding where prey are struck by the end of the tail when alongside the shark [28]. The need for this dexterity, as well as the remarkable length of the tail and the resultant lack of rigidity, would seem to preclude the stiff-bodied locomotion noted in other fishes with internalized red muscles. Thus, to better understand the functional morphology and physiology of internalized red muscles, it is important to know whether red muscle shears white muscle in common thresher sharks, and whether the fish rely on a thunniform mode of swimming in spite of appearances that they would not.
(c). Muscle function in swimming threshers
Owing to extreme logistical challenges, measures of muscle strain during swimming in large sharks such as common threshers have not been attempted until recently. Based at the Pfleger Institute for Environmental Research in Oceanside, California, Bernal et al. [29] captured common thresher sharks off the coast by angling. While still in the open ocean, they implanted sonomicrometry crystals into red and overlying white muscles at adjacent sites along the length of the shark. Using 15 m cables to attach the crystals to a sonomicrometry system onboard the research vessel, the fish could be released to swim alongside the vessel, being only coaxed to remain within the limits of the cables. Sequences of tail beats were obtained from 10 fish, ranging from 155 to 190 cm fork length, which excludes the elongate tail, and 61–103 kg mass.
Shortening of white muscles was observed to be out of phase with the adjacent red muscle, similar to observations in tunas and mako sharks. Yet only in about half of the fish observed did white muscle shortening precede that in the adjacent red muscle. In the other fish, shortening in the red muscle led that in the white muscle. Further, the magnitude of the phase shift varied considerably between fish. In some fish, the phase shift was very small, to the extent it could be considered absent. In other fish, it approached 180°, such that the peaks of strain in the red and white muscles were fully out of phase. Of note, the phase shift remained quite constant within individual fish at the location of the recordings. Bernal et al. [29] conclude that the more undulatory swimming mode of common thresher sharks (perhaps subcarangiform) might explain the variability in both the magnitude and polarity (red leading white or vice versa) of the phase shift between strain in red and white muscles across sharks. For example, if the forces exerted by red muscles of the common thresher shark are distributed along the caudal region of the shark, rather than focused to an elongate hypaxial lateral tendon that directs forces at or near the base of the caudal fin as observed in mako sharks [12], then we might expect not only a more undulatory mode of swimming, but variability in the phase shift that is dependent on the specific location of the sampling site and also on the degree of undulation (i.e. number of wavelengths along the shark's body) (figure 6). The degree of undulation may be influenced by relative swimming speed and shark size, and could probably be adjusted to suit circumstances (e.g. by altering recruitment patterns of the red muscle). Hence, a large degree of variability in the phase shift might be expected between fish, but not within a fish unless they changed swim speed or the relative extent of undulation of their body while swimming. A detailed analysis of the musculotendinous architecture of the common thresher shark is required to further understand these associations.
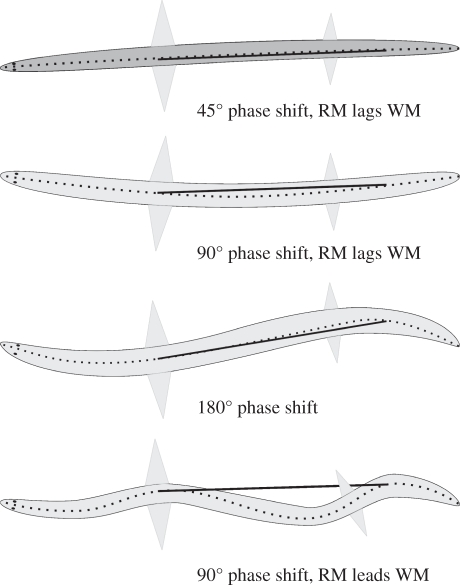
Figure 6.
Hypothetical effects of relative degree of body curvature on the phase relationship between strain of internalized red muscles (RM) and overlying white muscles (WM), assuming the red muscle is able to shorten independently of the overlying white muscle and the white muscle shortens in phase with local body curvature. Dashed line represents midline of the fish. Solid line represents the line of action of the internalized red muscle whose functional origin and insertion, in this example, span 50% of the fish's length. The upper panel shows the phase relationship as observed in the thunniform mako shark and yellowfin tuna, where strain of the red muscle lags that of the adjacent white muscle by about 40° of a strain cycle. Subsequent panels show increasing degrees of body curvature and increasing phase lags between red and white muscle shortening, whereby in the third panel strain in the red and white muscles are 180° out of phase, and in the bottom panel strain of the red muscle leads rather than lags that of the adjacent white muscle.
There was no clear relationship between fish size and either the magnitude or polarity of the phase shift in the common thresher sharks studied [29]. However, the fish all swam with a tail beat frequency of about 0.5 Hz and varied in fork length by only about 20 per cent, which might make any trends between size/speed and phase shift difficult to detect. Further, as each individual fish may adopt a different degree of body undulation while swimming, and because the magnitude/polarity of the phase shift may be influenced by more than just fish size, a more detailed analysis will be required to understand the basis of the variability in phase shift.
All told, we still note that all fishes with internalized red muscles show consistently some degree of shearing between red and overlying white muscles [13–15,29]. Conversely, phase shifts between the strain trajectory of adjacent red and white muscles have not been observed in species with superficial red muscles, which do not exhibit thunniform swimming (e.g. [31,32]) nor possess a lubricative sheath that would permit shearing. However, the undulatory swimming mode in the common thresher shark leads us to conclude that an anteriorly located power plant of red muscle, specifically driving caudal-fin based and a resultant stiff-bodied mode of swimming, is not an obligatory attribute of fishes with internalized red muscles. Given the relatively few species of fish that possess internalized red muscles, it would seem unfitting to consider the common thresher shark an exception to a rule. In this sense, we have come back from a stance of considering that fishes possessing internalized red muscles adopt thunniform swimming as a necessary element in convergence of design, back to the likelihood that while internalized red muscles clearly impacts the mechanics of swimming (shearing of red and white muscles), it does not in itself predict or constrain the swimming mode to be thunniform. Of particular relevance to this observation, the alleged benefits of economy and performance gained by thunniform swimming cannot be the sole adaptive basis of internalized red muscles (for further discussion, see [2,17,20]). An alternative, that regional endothermy and associated gains in performance (power output) might be the selective advantage, has likewise been questioned [20]. There is more to learn.
(d). Consequences of internalized red muscle on performance
While we cannot yet resolve the evolutionary basis of internalized red muscles, new data on the thermal sensitivity of muscle contraction with which to compare the regionally endothermic tunas, lamnid and thresher sharks with some ectothermic species will allow us to pursue it further. To date, all fishes studied with internalized red muscles show some degree of regional endothermy, with members of the lamnids appearing to elevate red muscle temperature most, tunas also being very competent and the common thresher shark perhaps the most modest in this regard [33,34]. Might these patterns hold clues to the evolutionary origins of internalized red muscles, or at least the consequences of regional endothermy on red muscle performance? Salmon sharks (Lamna ditropis) move to the North Pacific Ocean to pursue salmon [35], where they are challenged by fast-moving prey and performance-suppressing cold water. Shortfin mako sharks (Isurus oxyrinchus) live in relatively warm temperate and tropical waters, preferring water temperatures of 17–22°C and rarely being observed in water cooler than this except perhaps for occasional forays into deeper water [36]. Likewise, tunas tend to be tropical and subtropical in distribution, but tagging studies reveal that Atlantic bluefin tuna make deep dives and often move considerable distances such that they commonly encounter water temperatures of 10°C [37,38]. The three thresher species overlap in thermal niche [39], but common threshers with red muscle endothermy have the widest latitudinal distribution [40] and bigeye threshers with cranial endothermy [41] inhabit the deepest water [41,42], such that the two species showing some form of endothermy encounter the coolest water [23]. Thus, we see some associations between both thermal habitat and apparent endothermic capacity of these fishes.
The well-established literature on thermal sensitivity and acclimation of contractile properties of red muscles in fishes suggests we might expect patterns related to habitat and muscle temperature. Of particular consequences are the effects of temperature on kinetics of contraction and power output. Acutely cooled muscles demonstrate increased twitch durations, reduced power output and reduced cycling frequencies at which they produce maximal power (see [27] for a review). While regionally endothermic red muscles of sharks and tunas do not violate these patterns, they do appear to display different sensitivities to cooling based on the extent of endothermy exhibited by a particular species. Rates of isometric force contraction/relaxation in regionally endothermic red muscles of mako sharks show a higher Q10 than those for superficial red muscle of ectothermic leopard sharks [9]. Regionally endothermic red muscle from mako sharks have a much higher optimal cycle frequency for power output and produce relatively more power at warm temperatures than do the ectothermic red muscles of leopard sharks, and conversely the red muscles from leopard sharks produce more power than mako in the cold [9]. Like mako sharks, the regionally endothermic red muscles of yellowfin tunas do relatively better in the warm than the red muscles from the closely related but ectothermic bonito [7]. Also similar to the regionally endothermic muscle from mako sharks, highly endothermic red muscles from salmon sharks perform very well at temperatures as high as 30°C and perhaps higher [8], temperatures that appear to inhibit red muscles of ectothermic leopard sharks [9] and bonito [7]. Yet the red muscles of salmon sharks that do so well in the warm are functionally incapacitated when allowed to cool to even 20°C. It has been speculated that regional endothermy of the red muscle allows enhanced performance (power) in fishes that inhabit cold environments, yet comparisons of performance by the warm-adapted endothermic muscle and cool-adapted ectothermic muscle operating at their respective normal body temperatures have not been entirely convincing that this advantage is notable [20].
(e). The influence of fish size on muscle function
Perhaps not surprisingly but somewhat novel in our thinking about how fish muscles behave, fish size might be an important factor influencing how we interpret the thermal sensitivity of contraction kinetics. Comparison of twitch kinetics from muscle of similar-sized yellowfin and skipjack tuna confirms the expectation that slower tail-beat frequencies during swimming in yellowfin tuna are accompanied by red muscles with slower contraction kinetics, both the twitch and cycle frequency for maximal power production [16,26]. Said another way, fishes that swim more slowly have muscles that are slower, in spite of being warm. Likewise, relatively large sharks such as the salmon and common thresher (body mass 60–160 kg and near 200 cm fork lengths of those specimens studied) exhibit substantially slower twitches than smaller sharks at similar temperatures, double or triple the duration of those in smaller mako sharks (about 100 cm fork length, mass not indicated) [8,9] (figure 5). Very large sharks with regionally endothermic red muscles (e.g. salmon and common thresher) have what can only be described as agonizingly slow twitches at cooler temperatures, exceeding several seconds at about 10°C and near 2 s even at 15°C. Such slow kinetics seems to prohibit these muscles from powering swimming at even modestly cold temperatures. Part of the explanation probably lies in these muscles being adapted to function well in warm temperatures, as experienced in these regionally endothermic fishes, and by extension not performing well in the cold. Yet part of the answer also probably lies in these animals simply being very large and beating their tails at very slow frequencies in the first place, and whose red muscles thus must remain relatively warm as their tails simply would not function to generate thrust if they moved more slowly.
Emerging relationships suggest that fish size may be a critical element tempering expectations about how contraction kinetics and power should respond to temperature, expectations based on previous studies from smaller, ectothermic fishes. The cycle frequency at which power is maximal is a physiologically relevant predictor of thermal sensitivity of contractile performance, and it is generally expected that cooler temperatures will reduce the cycle frequency at which power is maximized. Yet the leopard shark, with ectothermic red muscles, appears relatively insensitive to temperature in this regard (figure 7), as might be beneficial for a fish that routinely experiences cool temperatures. By contrast, red muscles of relatively small mako sharks, which are notably regionally endothermic, show pronounced sensitivity to temperature, with the cycle frequency for maximal power increasing about 10-fold over a 13°C temperature range. While such patterns are not surprising qualitatively, whereby cold-adapted muscles perform well over a broad range of cooler temperatures while warm-adapted muscles tend to perform poorly in the cold, the remarkable insensitivity of one phenotype to temperature and the extreme sensitivity of another are not readily predictable. Further, if very large sharks are incorporated into this analysis, the expected pattern is not supported even qualitatively. Red muscles of common thresher sharks with modest endothermy and from salmon sharks with marked endothermy appear almost insensitive to the effects of temperature on cycle (tail-beat) frequency for maximal power (figure 7), at least in a physiological context.
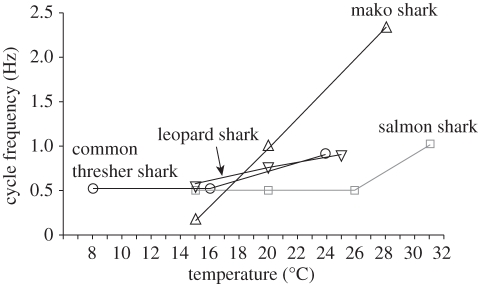
Figure 7.
Cycle (tail-beat) frequency at which the red muscle produces maximal power at different temperatures for different shark species. Ectothermic red muscles from the relatively small (about 100 cm fork length) leopard shark (black inverted triangles) are not particularly sensitive to temperature, while regionally endothermic red muscle from relatively small (also about 100 cm fork length) mako sharks (black upright triangles) show a pronounced sensitivity to temperature of the optimal cycle frequency for power output. Relatively large specimens (about 200 cm fork length) of salmon shark (grey squares) and common thresher sharks (black circles), both with regionally endothermic red muscle, show a very small impact of temperature on optimal cycle frequency for power output. (Data from Bernal et al. [8], Donley et al. [9] and J. Donley, C. Sepulveda, S. Aalbers, D. McGillivray, D. Syme, D. Bernal 2009, unpublished data.)
This is not to say that power itself is not highly temperature sensitive in these highly endothermic species, where it has been argued that the red muscles of salmon sharks are mammal-like in producing substantial power only at temperatures above 20°C [8]. Rather, the contraction kinetics of these muscles from very large sharks is such that they only function at slow cycle frequencies in the first place. Cooling them down does result in a slowing of the optimal cycle frequency for power, but a drop from about 1 to 0.5 Hz or perhaps slightly slower does not seem remarkable and is probably not physiologically relevant. A fish the size of a salmon or thresher shark beating its tail once every 4 s will not be an effective pelagic predator. It is proposed then, that very large fishes with regionally endothermic red muscles may not show the same sorts of benefits of warmth on tail-beat frequencies, nor the apparent constraints of cooling that have been touted for smaller fishes, simply because very large fishes possess muscles that are very slow to start with. Changing from very slow to even slower in the cold is not particularly noteworthy nor conducive to swimming, and significant gains in speed with warming are likewise not realized given the constraints of size. Thus, perhaps smaller sharks including juvenile and young lamnids with fast yet still notably endothermic red muscles experience the benefits of warming on swim speed but the detriments of cooling as well. Large lamnids and threshers possess red muscles that are slow owing to size regardless of temperature, and function well only when warm.
Acknowledgements
The authors thank NSERC and NSF for financial support. In addition, DAS thanks Diego Bernal, Jeanine Donley, Chugey Sepulveda, and the staff at Pfleger Institute of Environmental Research for collaboration and field support.
Footnotes
One contribution of 15 to a Theme Issue ‘Integration of muscle function for producing and controlling movement’.
References
- 1.Greene C. W., Greene C. H. 1913. The skeletal musculature of the king salmon. Bull. US Fish. 33, 21–59 [Google Scholar]
- 2.Donley J. M., Sepulveda C. A., Konstantinidis P., Gemballa S., Shadwick R. E. 2004. Convergent evolution in mechanical design of lamnid sharks and tunas. Nature 429, 61–65 10.1038/nature02435 (doi:10.1038/nature02435) [DOI] [PubMed] [Google Scholar]
- 3.Webb P. W. 1975. Hydrodynamics and energetics of fish propulsion. Bull. Fish. Res. Board Can. 190, 1–59 [Google Scholar]
- 4.Shadwick R. E., Gemballa S. 2006. Structure, kinematics, and muscle dynamics in undulatory swimming. In Fish biomechanics (eds Shadwick R. E., Lauder G. V.), pp. 241–280 New York, NY: Academic Press [Google Scholar]
- 5.Biewener A. A. 2003. Animal locomotion, p. 281 Oxford, UK: Oxford University Press [Google Scholar]
- 6.Wakeling J. M. Fast-start mechanics. In Fish biomechanics (eds Shadwick R. E., Lauder G. V.), pp. 333–368 New York, NY: Academic Press [Google Scholar]
- 7.Altringham J. D., Block B. A. 1997. Why do tuna maintain elevated slow muscle temperatures? Power output of muscle isolated from endothermic and ectothermic fish. J. Exp. Biol. 200, 2617–2627 [DOI] [PubMed] [Google Scholar]
- 8.Bernal D., Donley J. M., Shadwick R. E., Syme D. A. 2005. Mammal-like muscles power swimming in a cold-water shark. Nature 437, 1349–1352 10.1038/nature04007 (doi:10.1038/nature04007) [DOI] [PubMed] [Google Scholar]
- 9.Donley J. M., Shadwick R. E., Sepulveda C. A., Syme D. A. 2007. Thermal dependence of contractile properties of the aerobic locomotor muscle in the leopard shark and shortfin mako shark. J. Exp. Biol. 210, 1194–1203 10.1242/jeb.02730 (doi:10.1242/jeb.02730) [DOI] [PubMed] [Google Scholar]
- 10.Chopra M. G. 1974. Hydromechanics of lunate tail swimming propulsion. J. Fluid Mech. 64, 375–391 10.1017/S002211207400245X (doi:10.1017/S002211207400245X) [DOI] [Google Scholar]
- 11.Shadwick R. E. 2005. How tunas and lamnid sharks swim: an evolutionary convergence. Am. Sci 93, 524–531 [Google Scholar]
- 12.Gemballa S., Konstantinidis P., Donley J. M., Sepulveda C., Shadwick R. E. 2006. Evolution of high-performance swimming in sharks: transformations of the musculotendinous system from subcarangiform to thunniform swimmers. J. Morph. 267, 277–293 10.1002/jmor.10412 (doi:10.1002/jmor.10412) [DOI] [PubMed] [Google Scholar]
- 13.Donley J. M., Sepulveda C. A., Konstantinidis P., Gemballa S., Shadwick R. E. 2005. Patterns of red muscle strain/activation and body kinematics during steady swimming in a lamnid shark, the shortfin mako (Isurus oxyrinchus). J. Exp. Biol. 208, 2377–2387 10.1242/jeb.01618 (doi:10.1242/jeb.01618) [DOI] [PubMed] [Google Scholar]
- 14.Shadwick R. E., Katz S. L., Korsmeyer K. E., Knower T., Covell J. W. 1999. Muscle dynamics in skipjack tuna: timing of red muscle shortening in relation to activation and body curvature during steady swimming. J. Exp. Biol. 202, 2139–2150 [DOI] [PubMed] [Google Scholar]
- 15.Katz S. L., Syme D. A., Shadwick R. E. 2001. Enhanced power in yellowfin tuna. Nature 410, 770–771 10.1038/35071170 (doi:10.1038/35071170) [DOI] [PubMed] [Google Scholar]
- 16.Shadwick R. E., Syme D. A. 2008. Thunniform swimming: muscle dynamics and mechanical power production of aerobic fibres in yellowfin tuna (Thunnus albacares). J. Exp. Biol. 211, 1603–1611 10.1242/jeb.013250 (doi:10.1242/jeb.013250) [DOI] [PubMed] [Google Scholar]
- 17.Bernal D., Dickson K. A., Shadwick R. E., Graham J. B. 2001. Analysis of the evolutionary convergence for high performance swimming in lamnid sharks and tunas. Comp. Biochem. Physiol. 129A, 695–726 [DOI] [PubMed] [Google Scholar]
- 18.Bernal D., Sepulveda C., Mathieu-Costello O., Graham J. B. 2003. Comparative studies of high performance swimming in sharks I. Red muscle morphometrics, vascularization and ultrastructure. J. Exp. Biol. 206, 2831–2843 10.1242/jeb.00481 (doi:10.1242/jeb.00481) [DOI] [PubMed] [Google Scholar]
- 19.Graham J. B., Koehrn F. J., Dickson K. A. 1983. Distribution and relative proportions of red muscle in scombrid fishes: consequences of body size and relationships to locomotion and endothermy. Can. J. Zool. 61, 2087–2096 10.1139/z83-274 (doi:10.1139/z83-274) [DOI] [Google Scholar]
- 20.Katz S. L. 2002. Design of heterothermic muscle in fish. J. Exp. Biol. 205, 2251–2266 [DOI] [PubMed] [Google Scholar]
- 21.Gruber S. H., Compagno L. J. V. 1981. Taxonomic status and biology of the bigeye thresher, Alopias superciliosus (Lowe, 1839). Fish. Bull. US Nat. Mar. Fish. Serv. 79, 617–640 [Google Scholar]
- 22.Bone Q., Chubb A. D. 1983. The retinal system of the locomotor muscle in the thresher shark. J. Mar. Biol. Assoc. UK 63, 239–241 10.1017/S0025315400049924 (doi:10.1017/S0025315400049924) [DOI] [Google Scholar]
- 23.Sepulveda C. A., Wegner N. C., Bernal D., Graham J. B. 2005. The red muscle morphology of the thresher sharks (family Alopiidae). J. Exp. Biol. 208, 4255–4261 10.1242/jeb.01898 (doi:10.1242/jeb.01898) [DOI] [PubMed] [Google Scholar]
- 24.Bernal D., Sepulveda C. A. 2005. Evidence for temperature elevation in the aerobic swimming musculature of the common thresher shark, Alopias vulpinus. Copeia 2005, 163–168 10.1643/CP-04-180R1 (doi:10.1643/CP-04-180R1) [DOI] [Google Scholar]
- 25.Donley J. M., Shadwick R. E. 2003. Steady swimming muscle dynamics in the leopard shark Triakis semifasciata. J. Exp. Biol. 206, 1117–1126 10.1242/jeb.00206 (doi:10.1242/jeb.00206) [DOI] [PubMed] [Google Scholar]
- 26.Syme D. A., Shadwick R. E. 2002. Effects of longitudinal body position and swimming speed on mechanical power of deep red muscle from skipjack tuna (Katsuwonus pelamis). J. Exp. Biol. 205, 189–200 [DOI] [PubMed] [Google Scholar]
- 27.Syme D. A. 2006. Functional properties of skeletal muscle. In Fish biomechanics (eds Shadwick R. E., Lauder G. V.), pp. 179–240 New York, NY: Academic Press [Google Scholar]
- 28.Aalbers S. A., Bernal D., Sepulveda C. A. 2010. The functional role of the caudal fin in the feeding ecology of the common thresher shark Alopias vulpinus. J. Fish Biol. 76, 1863–1868 10.1111/j.1095-8649.2010.02616.x (doi:10.1111/j.1095-8649.2010.02616.x) [DOI] [PubMed] [Google Scholar]
- 29.Bernal D., Donley J. M., McGillivray D. G., Aalbers S. A., Syme D. A., Sepulveda C. 2010. Function of the medial red muscle during sustained swimming in common thresher sharks: contrast and convergence with thunniform swimmers. Comp. Biochem. Physiol. 155A, 454–463 10.1016/j.cbpa.2010.01.005 (doi:10.1016/j.cbpa.2010.01.005) [DOI] [PubMed] [Google Scholar]
- 30.Compagno L. J. V. 1984. Sharks of the world. Part I. Hexanchiformes to Lamniformes. FAO Fish. Synop. 125, 1–249 [Google Scholar]
- 31.Shadwick R. E., Steffensen J. F., Katz S. L., Knower T. 1998. Muscle dynamics in fish during steady swimming. Am. Zool. 38, 755–770 10.1093/icb/38.4.755 (doi:10.1093/icb/38.4.755) [DOI] [Google Scholar]
- 32.Katz S. L., Shadwick R. E., Rapoport H. S. 1999. Muscle strain histories in swimming milkfish in steady and sprinting gaits. J. Exp. Biol. 202, 529–541 [DOI] [PubMed] [Google Scholar]
- 33.Carey F. G., Casey J. G., Pratt H. L., Urquhart D., McCosker J. E. 1985. Temperature, heat production and heat exchange in lamnid sharks. Mem. South. Calif. Acad. Sci. 9, 92–108 [Google Scholar]
- 34.Carey F. G., Teal J. M. 1966. Heat conservation in tuna fish muscle. Proc. Natl Acad. Sci. USA 56, 1463–1469 10.1073/pnas.56.5.1464 (doi:10.1073/pnas.56.5.1464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng K. C., Castilho P. C., Morrissette J. M., Landeira-Fernandez A. M., Holts D. B., Schallert R. J., Goldman K. J., Block B. A. 2005. Satellite tagging and cardiac physiology reveal niche expansion in salmon sharks. Science 310, 104–106 10.1126/science.1114616 (doi:10.1126/science.1114616) [DOI] [PubMed] [Google Scholar]
- 36.Casey J. G., Kohler N. E. 1992. Tagging studies on the Shortfin Mako Shark (Isurus oxyrinchus) in the Western North Atlantic. Aust. J. Mar. Freshw. Res. 43, 45–60 10.1071/MF9920045 (doi:10.1071/MF9920045) [DOI] [Google Scholar]
- 37.Block B. A., et al. 2001. Migratory movements, depth preferences, and thermal biology of Atlantic Bluefin Tuna. Science 293, 1310–1314 10.1126/science.1061197 (doi:10.1126/science.1061197) [DOI] [PubMed] [Google Scholar]
- 38.Stokesbury M. J. W., Teo S. L. H., Seitz A., O'Dor R. K., Block B. A. 2004. Movement of Atlantic bluefin tuna (Thunnus thynnus) as determined by satellite tagging experiments initiated off New England. Can. J. Fish. Aquat. Sci. 61, 1976–1987 10.1139/f04-130 (doi:10.1139/f04-130) [DOI] [Google Scholar]
- 39.Hanan D. A., Holts D. B., Coan A. L., Jr 1993. The California drift gill net fishery for sharks and swordfish, 1981–82 through 1990–91. Fish. Bull. 175, 89–95 [Google Scholar]
- 40.Compagno L. J. V. 2001. Sharks of the World. Bullhead, Mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes). FAO Species Catalog. 2, 78–88 [Google Scholar]
- 41.Weng K. C., Block B. A. 2004. Diel vertical migration of the bigeye thresher shark (Alopias superciliosus), a species possessing orbital retia mirabilia. Fish. Bull. 102, 221–229 [Google Scholar]
- 42.Nakano H., Matsunaga H., Okamoto H., Okazaki M. 2003. Acoustic tracking of bigeye thresher shark Alopias superciliosus in the Eastern Pacific Ocean. Mar. Ecol. Prog. Ser. 265, 255–261 10.3354/meps265255 (doi:10.3354/meps265255) [DOI] [Google Scholar]