Abstract
Muscles power movement, yet the conceptual link between muscle performance and locomotor performance is poorly developed. Frog jumping provides an ideal system to probe the relationship between muscle capacity and locomotor performance, because a jump is a single discrete event and mechanical power output is a critical determinant of jump distance. We tested the hypothesis that interspecific variation in jump performance could be explained by variability in available muscle power. We used force plate ergometry to measure power produced during jumping in Cuban tree frogs (Osteopilus septentrionalis), leopard frogs (Rana pipiens) and cane toads (Bufo marinus). We also measured peak isotonic power output in isolated plantaris muscles for each species. As expected, jump performance varied widely. Osteopilus septentrionalis developed peak power outputs of 1047.0 ± 119.7 W kg−1 hindlimb muscle mass, about five times that of B. marinus (198.5 ± 54.5 W kg−1). Values for R. pipiens were intermediate (543.9 ± 96.2 W kg−1). These differences in jump power were not matched by differences in available muscle power, which were 312.7 ± 28.9, 321.8 ± 48.5 and 262.8 ± 23.2 W kg−1 muscle mass for O. septentrionalis, R. pipiens and B. marinus, respectively. The lack of correlation between available muscle power and jump power suggests that non-muscular mechanisms (e.g. elastic energy storage) can obscure the link between muscle mechanical performance and locomotor performance.
Keywords: power output, muscle mechanics, jumping performance, anuran
1. Introduction
Animal movement is powered by muscle, yet the relationship between muscle power output and locomotor power output is not necessarily a simple one. For example, consider the question: do animals that produce the most powerful movements have the most powerful muscles? On the one hand, we might expect that just as the rated horsepower of a car's engine gives a reasonable idea of its capacity for speed or acceleration, animals that produce very powerful movements should also have big engines, either in the form of powerful muscles or a large muscle mass. On the other hand, just as a car's performance depends on the effective function of key components beyond the engine (e.g. the effectiveness of power transmission via gears and linkages, the stiffness of the chassis, the traction of the tyres on the road), the structure and function of musculoskeletal components beyond muscles have profound influence on the power developed in movement. In biological systems, of notable importance is the action of elastic mechanisms, which can uncouple muscle power from locomotor power by acting as temporary sinks or sources of mechanical energy.
In this study, we explore the link between muscle and locomotor power in jumping frogs. Frog jumping is a good system for studying the production of power in locomotion for a number of reasons. First, jumping is powered by a single contraction, and there is evidence that for maximal jumps, there is full recruitment of the hindlimb musculature [1]. Second, unlike activities for which the mechanical limit to performance is unclear (e.g. running), the ‘mechanical currency’ for jumping is unambiguous. Jump distance is directly proportional to the mechanical energy developed during the takeoff period, thus the longest jumps will be produced when muscle work is maximized [2]. Third, the properties of frog muscles are well studied and well understood. Finally, there is good evidence that frogs use an elastic mechanism to amplify power output during jumping [2,3]. Thus, comparative studies of jumping power output allow us to explore the extent to which elastic mechanisms might decouple jump performance from muscle performance.
2. Methods
(a). Animals
Three species of anurans were studied: Osteopilus septentrionalis (Cuban tree frogs), Rana pipiens (leopard frogs) and Bufo marinus (cane toads). Average body masses for the animals used for final data collection were 11.9 ± 4.6, 73.1 ± 5.8 and 130.7 ± 47.4 g for O. septentrionalis, R. pipiens and B. marinus, respectively. Animals were acquired from licensed vendors and housed (12 L : 12 D) in small groups with water ad libitum and vitamin-enriched crickets bi-weekly. Osteopilus septentrionalis and B. marinus were provided with a temperature gradient created by commercial heat tape (24–28.8°C). Bufo marinus were kept on a dry wood substrate with moistened moss, while O. septentrionalis were kept in a humid environment (approx. 88% humidity). Rana pipiens were kept in large water basins with a dry platform at room temperature (20°C). All animal procedures were approved by the Brown University Institutional Animal Care and Use Committee.
(b). Jumping trials
A jumping arena was constructed out of 80/20 T-slotted aluminium framing with clear Plexiglas and vinyl curtains for the sides (60 cm width, 180 cm length, 60 cm height). This arena was secured to the top of a large table and built around a small custom-made three-axis force plate. To damp out table vibrations, each table leg of the arena was placed in a bucket of sand. Animals were positioned on the surface of the force plate and verbal cues or gestures were used to encourage them to jump. The two larger species, R. pipiens and B. marinus, were jumped with an added textured surface on the force plate to prevent slipping. Osteopilus septentrionalis did not require a textured surface because of the adhesive nature of their feet. All measurements were taken at a room temperature of 20–23°C.
Ground reaction forces (GRFs) were measured in three axes. The output of the force plate was amplified and recorded into a PC using a 16-bit data acquisition system (PCMIO-16, National Instruments, TX, USA) operating at 4000 Hz. Force plate data were analysed using Igor Pro software (Wavemetrics; Lake Oswego, OR, USA). On each measurement day, the force plate was calibrated for all three axes. Vertical forces were calibrated with scaled weights while medio-lateral and fore-aft forces were calibrated by applying force measured with a Kistler single-axis force transducer (type 9203, Kistler Instruments, NY, USA).
To get best estimates of maximal jump performance, we first screened individuals for their tendency to jump well in the arena. We chose the best four individuals for each species (of 6–10 screened), and analysed the best five jumps of each individual (from approx. 20 trials). The selection of these trials was made on the basis of the greatest peak GRF. A total of 20 jumps was analysed per species.
The GRF during jumping was measured along three axes. Force profiles were used to determine the duration of takeoff. The beginning of the jump was identified as the time at which force exceeded body weight, while the time at which force fell to zero was the end of the jump. Takeoff time was the difference between the time of the end and the time of the beginning of the jump.
Force plate ergometry was used to determine instantaneous power outputs during jumping [4]. For convenience, power was first calculated separately in the vertical and horizontal planes and then summed. Vertical acceleration was calculated by subtracting body weight from the measured vertical force and dividing by total body mass. Lateral accelerations were calculated by dividing lateral forces by body mass. By taking the first and second time integrals of the accelerations, velocities and the position of the centre of mass were calculated, respectively. These variables were used to calculate instantaneous kinetic and potential energies of the animal's centre of mass. Summed work values were differentiated with respect to time to obtain instantaneous power output.
(c). Muscle mass and tendon dimensions
In order to calculate a conservative average power output per unit hindlimb muscle mass, we assumed that all hindlimb muscles contributed to the jump. All hindlimb muscles were dissected out post-mortem and weighed. Iliosacral muscles were not included. These muscles may contribute to jump power, but they amount to less than 0.5 per cent of body mass [2]. Combined limb muscle mass was divided by body mass to calculate leg muscle mass as a per cent of body mass. Peak muscle-specific power during jumping was calculated by dividing peak power measured in watts per kilogram body mass by the leg muscle mass percentage value.
Tendon dimensions were recorded for isolated plantaris tendons. Tendon length was measured as the distance from the proximal end of the superficial aponeurosis to the tendon insertion. Tendon cross-sectional area was calculated by dividing tendon volume by length, and volume was estimated by dividing the isolated tendon mass by a density of 1.14 g cm−3. This approach is approximate for a tendon such as the plantaris, where morphology varies along the length of the tendon and there is regional variation in cross-sectional area.
(d). In vitro preparation
Measurements of muscle mechanical properties were taken from isolated plantaris longus (PL) muscle. The PL is a large muscle and the primary ankle extensor. Fibre typing studies indicate that its composition is representative of other muscles involved in jumping [5]. The muscle was isolated post-mortem by careful dissection under oxygenated anuran Ringer's solution at room temperature (20°C). The proximal origin of the muscle was left attached to the knee joint and the distal tendon was severed near the ankle joint. The isolated knee joint, including sections of the femur and tibia, was clamped securely to the bottom of the measurement chamber (B. marinus and R. pipiens) or onto a custom-fabricated clamp for O. septentrionalis. A small aluminium clamp attached near the distal muscle–tendon junction was secured to a dual-mode muscle servomotor (Aurora 310B-LR or 300B, Aurora Scientific, Cambridge, MA, USA) with lightweight aircraft cable or a lightweight metal rod. Muscle preparations were maintained in a chamber with a regular exchange of Ringer's solution and a constant supply of oxygen.
Muscle stimulation was achieved in B. marinus and R. pipiens by direct stimulation of the sciatic nerve. The nerve was isolated from the thigh muscles and the thin connective tissue around the nerve was carefully removed. A bipolar electrode nerve cuff, constructed of two silver wires and plastic tubing (7 mm length, 1.5 mm inner diameter), was gently placed around the sciatic nerve just proximal to the plantaris origin on the knee. To stimulate the muscle, wire leads from the nerve cuff were connected to a Grass S48 stimulator (Grass Technologies, West Warwick, RI, USA). The sciatic nerve of O. septentrionalis was not large enough to allow consistent contact with a nerve cuff. Instead, two platinum plates were placed on either side of the isolated muscle and connected to a Grass S48 stimulator to provide field stimulation in the muscle chamber.
(e). Muscle property measurements
Servomotor signals were collected using a 16-bit data acquisition system (National Instruments USB-6251). Data were collected at 1000 Hz using Igor Pro software. Supramaximal stimulation voltage was determined by increasing the voltage of isometric twitch contractions until twitch force no longer increased. This stimulation voltage (4–8 V for large nerve cuff preparations, 50–100 V for small platinum plate set ups) was used for all subsequent muscle contractions within a single experiment. Optimum muscle length (L0) was determined from a constructed twitch length–tension curve. The peak length of the twitch length tension curve was used to estimate L0, and all subsequent measurements were taken within ±7% of this length.
A series of tetanic isotonic contractions were used to characterize each muscle's force–velocity curve. Smooth tetanic contractions were attained with a stimulation pulse duration of 0.2 ms at a frequency of 100 pulses s−1. Contractions were obtained over a range of forces and velocities by adjusting the maximum motor force and stimulation train duration (100–400 ms). The servomotor directly measured whole muscle force and length change. Force and velocity values were measured after force reached a plateau. Contraction velocity (mm s−1) was determined by differentiating muscle length measured by the servomotor. Effects of muscle fatigue were minimized by allowing a rest period of at least 5 min between successive tetanic contractions. A force–velocity curve was constructed for each muscle using values taken from nine to 10 isotonic contractions. Isometric contractions at 300 ms train duration were recorded at the beginning and end of the series of isotonic shortening contractions to check for potential changes in maximum isometric force. Maximum isometric force (P0) did not change significantly during any experiment.
We measured muscle fibre length, muscle mass, leg muscle mass and whole body mass to standardize the muscle contractile properties between different muscles. Fibre length was measured from an incision in the mid-coronal plane of the PL. Each species' force–velocity curve was characterized by fitting the data with a rectangular hyperbola [6]. Power was calculated as the product of force and velocity from the acquired data. To determine peak isotonic power, power was plotted against shortening velocity and fit with a second-order polynomial.
(f). Data analysis
To compare jump variables between species, we performed a nested analysis of variance (ANOVA) with species as the effect and individual nested within species. To compare isolated muscle properties, we performed an ANOVA with species as the effect.
3. Results
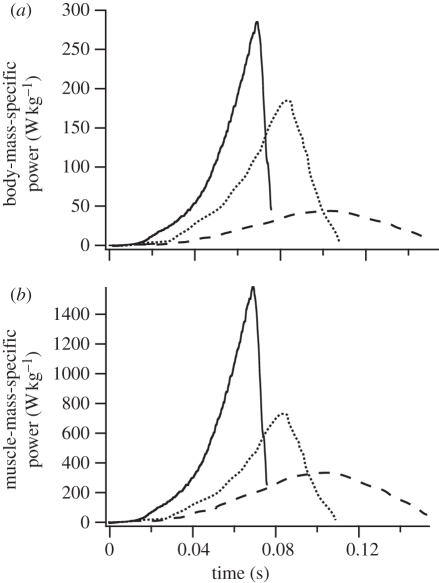
The power developed during jumping varied widely among the study species. Instantaneous power output measurements for representative jumps demonstrate the typical pattern of much greater jump power outputs in Cuban tree frogs when compared with the other two species. Values for leopard frogs were intermediate, and cane toad power outputs were the lowest (figure 1). This pattern was present when power was calculated on either a body-mass-specific or a muscle-mass-specific basis (using the total hindlimb muscle mass as denominator). Thus, differences in total power output during jumping are not explained by differences in the available muscle mass (figure 1b).
Figure 1.
Instantaneous power output for representative jumps from B. marinus (dashed line), R. pipiens (dotted line) and O. septentrionalis (solid line). Osteopilus septentrionalis developed much greater power outputs compared with the other species on both a body-mass-specific basis (a) and when power outputs were normalized to total hindlimb muscle mass (b). Power outputs were measured by force plate ergometry.
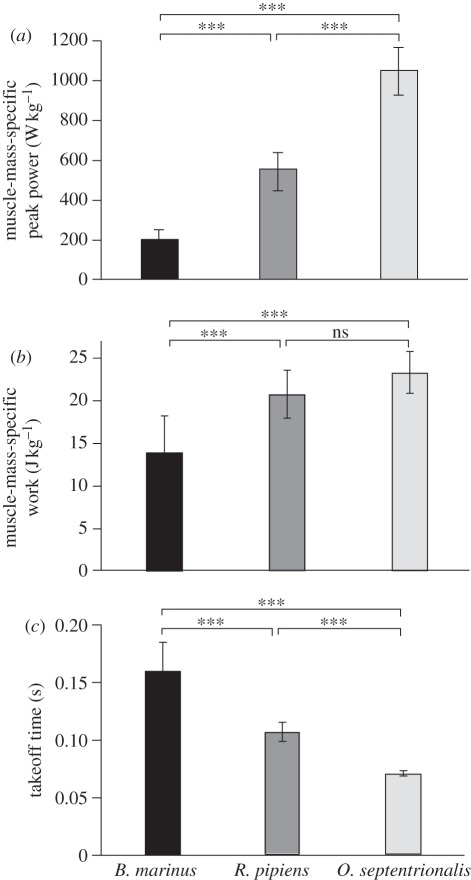
Summary statistics for maximal jumps demonstrate that the jump power developed per unit muscle mass varies among species (figure 2a). The peak power developed per unit muscle mass for the best jumps elicited averaged 1047.0 ± 119.7 W kg−1 for O. septentrionalis, while B. marinus produced peak powers of 198.5 ± 54.5 W kg−1 during jumping. Power is work divided by time, and the high power outputs in O. septentrionalis were explained in part by greater work performed (figure 2b), and in part by a shorter time of takeoff (figure 2c). The shorter jump times in O. septentrionalis were associated with faster accelerations and much higher GRFs. Peak GRFs were 9.1±0.5, 6.1 ± 0.5 and 1.4 ± 0.9 body weights (BW) in O. septentrionalis, R. pipiens and B. marinus, respectively. Work developed per unit muscle mass during a jump was significantly lower in B. marinus (13.9 ± 4.3 J kg−1 muscle) when compared with O. septentrionalis (23.3 ± 2.4 J kg−1). The value measured for muscle work output in R. pipiens during a jump (20.8 ± 2.8 J kg−1) was intermediate between that of the other two species but not significantly different from O. septentrionalis.
Figure 2.
Peak instantaneous power output, measured per unit hindlimb muscle mass, varied among species, with the highest powers developed by O. septentrionalis and the lowest by B. marinus (a). These differences were explained in part by differences in the total work performed (b) and in part by differences in the duration of takeoff (c). Asterisks denote significant differences (p < 0.05) between pairs indicated.
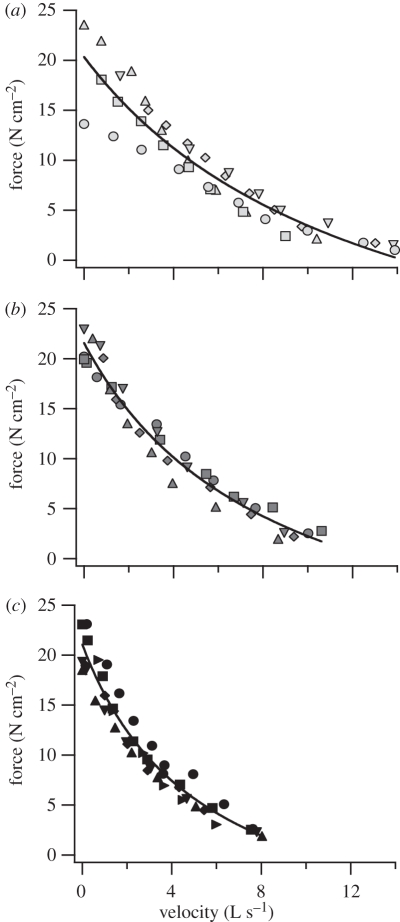
Isotonic contractile properties revealed some differences among the three species' muscles (figure 3). Osteopilus septentrionalis plantaris had a higher maximum unloaded shortening velocity (Vmax) when compared with that of B. marinus (table 1, p = 0.02). Both O. septentrionalis (p = 0.009) and R. pipiens (p = 0.02) plantaris muscles produced greater peak isotonic power output than B. marinus. There was no difference in Vmax or peak power output between O. septentrionalis and R. pipiens plantaris. There was no significant difference in peak isometric force (Po) among the species. To estimate the peak muscle power output available per unit body mass, mass-specific power output of the plantaris muscle was multiplied by the proportion of body weight contributed by the hindlimb muscle mass. Because of a relatively large hindlimb muscle mass, R. pipiens' total capacity for power production was the highest of the three species (table 1). Bufo marinus' total capacity for power production was the lowest of the three species.
Figure 3.
Isotonic force–velocity curves for m. plantaris longus for (a) O. septentrionalis, (b) R. pipiens and (c) B. marinus. Data for different individuals are denoted with different symbols and are fitted with a rectangular hyperbola [6].
Table 1.
Species comparison of muscle properties from in vitro preparations. P0 is the maximum muscle force produced during in vitro preparation; Vmax is the maximum muscle shortening velocity (means ± s.e.m.).
| species | P0 (N cm−2) | Vmax (l s−1) | muscle-mass-specific peak power (W kg−1) | % leg muscle | body-mass-specific peak power (W kg−1) |
|---|---|---|---|---|---|
| O. septentrionalis (n = 5) | 21.4 ± 2.0 | 13.5 ± 1.1 | 312.7 ± 28.9 | 18 | 56.3 ± 2.3 |
| R. pipiens (n = 5) | 22.7 ± 1.2 | 12.4 ± 0.9 | 321.8 ± 48.5 | 25 | 80.4 ± 5.4 |
| B. marinus (n = 6) | 20.2 ± 1.3 | 10.4 ± 0.3 | 262.8 ± 23.2 | 13 | 34.2 ± 1.1 |
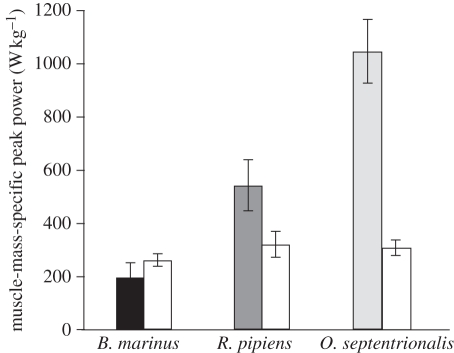
Large differences in peak instantaneous power developed during a jump were not explained by differences in power-generating capacity of the hindlimb musculature (figure 4 and table 1). Peak power output during a jump, measured per unit hindlimb muscle mass, was approximately 500 per cent greater in O. septentrionalis when compared with B. marinus, while the mean peak muscle power output in these species differed by only 20 per cent (figure 4).
Figure 4.
Peak power output per unit muscle mass developed during a jump (shaded bars) compared with peak power output per unit muscle mass developed in vitro (white bars). Differences in power developed during jumping were not explained by differences in the power-producing capacity of the hindlimb muscles.
4. Discussion
The results of this study demonstrate that within frogs, jumping ability is not determined by available muscle power. Measured power outputs during jumping were approximately fivefold greater in the tree frog (O. septentrionalis) than in the toad (B. marinus). Power outputs during jumping for the ranid species were intermediate. Neither the amount of muscle, nor the power capacity per gram of muscle, could explain the very high power outputs of O. septentrionalis. Osteopilus septentrionalis generated power outputs during jumping that were five times greater than that of B. marinus, while the power-generating capacity of its hindlimb musculature was only 20 per cent more than that of B. marinus. Osteopilus septentrionalis produced about twice the power of R. pipiens during jumping, yet the power-producing capacity of these two species' muscles was the same.
The present results indicate that factors other than the power-producing capability of the hindlimb musculature are important in determining jumping ability in anurans. The discrepancy between muscle power and peak power output during a jump may be explained by the important role of the storage and recovery of muscle work in elastic structures. The observation that peak power output during a jump exceeds the available muscle power in R. pipiens and O. septentrionalis is evidence that elastic mechanisms serve to amplify muscle power output by decoupling muscle power production from the application of mechanical power to the body. Supramaximal power outputs during jumping were first presented as evidence of an elastic power-amplifying mechanism in a comparative study of several species of frogs [3]. Since then, evidence for elastic power amplification has been observed in other frog species [7–10], as well as in jumping mammals [11] and birds [12]. In frogs, it is hypothesized that muscle work is stored in elastic structures early in the jump, or before the beginning of the jump, and then released rapidly during the second half of the takeoff phase [2,13]. Sonomicrometer measurements of muscle length change support the idea of elastic pre-storage, as muscle shortening occurs before body movement [13,14]. No evidence has yet been found for an anatomical catch that allows the pre-storage of elastic energy, but it has been hypothesized that an inertial catch mechanism may be mediated by a variable mechanical advantage to facilitate energy storage and recovery [3,13].
Our estimates of both the power produced by the hindlimb musculature as well as the power available from the hindlimb musculature require some assumptions. First, we included the entire hindlimb musculature in our estimates of the mass available to produce power. If only a subset of hindlimb muscles are important in jumping, as some studies suggest [5,15], then our value overestimates the actual muscle mass involved in developing the power observed during jumping. Such an overestimate in active hindlimb muscle mass would lead to an underestimate of the power actually developed by active muscles, and an underestimate of power amplification. We also assume that the power-producing capacity of the plantaris muscle is representative of other muscles involved in jumping. Fibre typing indicates that it is similar in myosin heavy chain composition to other muscles involved in jumping, while several hindlimb muscles have myosin isoform profiles that suggest lower power outputs than plantaris [5]. Thus, the PL power values used here may overestimate the power available from the entire hindlimb musculature. This would tend to lead to an underestimate of the degree of muscle power amplification during jumping.
The observation that Cuban tree frogs develop power outputs during a jump that are more than three times their muscles' capacity, while cane toads develop power outputs that are less than their available muscle power, would seem to indicate that tree frogs have a much better developed system for amplifying power output. Rana pipiens appear to be able to amplify power, though not by as much as Cuban tree frogs. It is not clear why these differences exist. Cane toads would jump farther if they developed the same jump power as Cuban tree frogs, so why have they not developed the same kind of power-amplifying systems? Differences in locomotor mode among these species may provide some of the explanation. Cane toads are hoppers, meaning they jump repeatedly, often for long distances [16]. Cuban tree frogs do not undergo long-distance migrations and use jumping primarily as a single-shot locomotor event. It is possible that anatomical or physiological specializations for extreme power amplification might represent trade-offs in effective or efficient cyclic hopping, or that selection for long-distance jumping has been relaxed in toads that rely to some extent on toxic secretions to deter predators.
Differences in power output in the three study species might also be explained in part by size-dependent variation in the ability to amplify muscle power output. The cane toads in our study were an order of magnitude larger than the Cuban tree frogs (average body mass 130.7 g for B. marinus versus 11.8 g for O. septentrionalis). Mathematical models indicate that smaller animals should be capable of greater power amplification [17]; thus differences in power amplification among species in this study may be explained in part by body size.
Although we did not comprehensively investigate the underlying anatomical basis for the differences in power amplification among species, measurements of the plantaris tendon reveal some differences that may be important for tendon energy storage and recovery. The plantaris muscle has the longest tendon in the hindlimb, and presumably is an important site of elastic energy storage. The length of the tendon measured as a multiple of fibre length varied among species, with O. septentrionalis having the longest and B. marinus the shortest (tendon length/fibre length = 5.2, 6.5 and 8.8 for B. marinus, R. pipiens and O. septentrionalis). A useful metric of a muscle's ability to load a tendon is the ratio of muscle cross-sectional area to tendon cross-sectional area. For a given elastic modulus, an increase in muscle cross-sectional area or a decrease in tendon area will lead to greater tendon strains; thus a greater ratio of muscle area to tendon area should be associated with a greater capacity for elastic energy storage. The muscle area/tendon area ratio varied among the study species, with the lowest value for B. marinus (muscle area/tendon area = 65), and higher values for R. pipiens (muscle area/tendon area = 55) and O. septentrionalis (muscle area/tendon area = 38). Taken together, these tendon measurements suggest that the higher power amplification in R. pipiens and O. septentrionalis is associated with tendons that are relatively longer and thinner, and therefore tend to store more elastic energy when loaded by muscle force production. It is also possible that differences in anatomical features that allow the effective loading and release of this elastic energy storage lead to variation in power amplification. Anatomical ‘catches’ have not yet been identified in frogs, but an inertial catch mechanism may be an important feature of power amplification by elastic energy storage and release [13].
Ultimately, jump distance is determined by the product of average jump power and takeoff time, which amounts to the total work done during a jump. The lower power outputs in B. marinus during jumping were associated with lower total jump work. However, the duration of a jump was much longer in toads than tree frogs; thus, differences in total work output were not as great as differences in peak and average power outputs.
Several comparative studies have examined morphological correlates of jumping performance in frogs (reviewed in [2,18]). Relative leg length, for example, has been identified as an important determinant of jump performance both in comparative studies and theoretical models [17,19]. The present study is not comprehensive enough to draw conclusions about correlations between morphology and performance. However, the observation of widely varying jumping ability in animals with similar muscle power output supports the idea that morphological features are important for jump performance. Furthermore, these data indicate that animals with morphological features generally associated with low jump power output, such as the toads, do not necessarily have proportionally low-power muscles.
(a). The link between muscle power and locomotor power
The link between muscle power and locomotor power has been explored for a number of locomotor systems. The questions that have been addressed generally fall into two categories: (i) is the power developed by the muscular apparatus at any given point in time equal to the power propelling the body, and (ii) can all of the available muscle power be harnessed during maximal effort locomotion? Below, we address these questions in turn, with examples (though not an exhaustive review) from the literature.
The present study provides examples of locomotion for which there is a mismatch between muscle power and locomotor power at any given point in time. The very high power outputs from O. septentrionalis and R. pipiens exceed the available muscle power output and indicate that elastic mechanisms act to amplify muscle power output. This means that locomotor power exceeds muscle power late in the jump, as stored elastic energy is released, while before the jump or early in the jump locomotor power is less than muscle power, because muscles are contracting to load energy into elastic elements. Other power-amplification systems, as well as systems for which elastic mechanisms play a significant role, also feature a mismatch between instantaneous muscle power and locomotor power. Examples include running accelerations in turkeys [20], jumping in humans [21] and limb retraction in horses [22].
Differences between instantaneous muscle power and locomotor power might also result from a failure to effectively transmit muscle power to the motion of the body or the environment (e.g. fluid motion in swimming). The mechanical efficiency, or the ratio of power out to power in, could vary if there were significant frictional losses, at joints for example, or co-contraction of muscle antagonists. The former are generally thought to be relatively small, but recent work has suggested that losses of energy to the dissipative motion of body soft tissues may represent a significant energy sink during terrestrial movement [23]. The relative amount of energy lost to co-contraction in typical movements is difficult to determine and remains an open question.
The question of whether or not animals can harvest all available muscle power during maximal effort locomotion has been addressed in several locomotor systems, and the results are mixed. Data from sprinting lizards indicate that the total locomotor power output during a maximum speed sprint on the level is only about 25 per cent of the power developed during an uphill sprint [24]. This observation strongly suggests that during maximum-speed running only a fraction of available muscular power is used, presumably because another mechanical variable, such as peak force production, limits speed [25]. In terrestrial accelerations there is a greater demand for mechanical power to increase the kinetic energy of the body, and a study combining isolated muscle power measurements with measurements of locomotor power in accelerating lizards found a close agreement between peak locomotor power and available muscle power [26]. Askew et al. [27] also found relatively good agreement between maximum in vitro power of the pectoralis muscle and maximum in vivo power during takeoff flights in quail. A match between in vivo and in vitro power output during swimming has also been observed for scallops [28] and fish [29]. Taken together, these studies suggest that for activities that require high cyclical power output, such as swimming and flying, performance may be tightly coupled to the power capacity of locomotor muscles. However, data from the present study as well as others suggest that for activities for which power amplification is effective, such as single-shot ballistic movements, the link between muscle power and locomotor performance may be weak.
Acknowledgements
We thank the organizers, Tim Higham and Andy Biewener, for inviting us to participate in this symposium. We also thank Henry Astley for help with experiments. This study was supported by grants from the NSF (IOS0642428 to T.J.R.) and NIH (F32AR054246 to E.A. and AR055295 to T.J.R.).
Footnotes
One contribution of 15 to a Theme Issue ‘Integration of muscle function for producing and controlling movement’.
References
- 1.Lutz G. J., Rome L. C. 1994. Built for jumping: the design of the frog muscular system. Science 263, 370–372 10.1126/science.8278808 (doi:10.1126/science.8278808) [DOI] [PubMed] [Google Scholar]
- 2.Marsh R. L. 1994. Advances in veterinary science and comparative medicine (ed. Jones J. H.), pp. 51–111 New York, NY: Academic Press [Google Scholar]
- 3.Marsh R. L., John-Alder J. B. 1994. Jumping performance of hylid frogs measured with high-speed cine film. J. Exp. Biol. 188, 131–141 [DOI] [PubMed] [Google Scholar]
- 4.Cavagna G. A. 1975. Force plates as ergometers. J. Appl. Physiol. 39, 174–179 [DOI] [PubMed] [Google Scholar]
- 5.Lutz G. J., Bremner S., Lajevardi N., Lieber R. L., Rome L. C. 1998. Quantitative analysis of muscle fibre type and myosin heavy chain distribution in the frog hindlimb: implications for locomotory design. J. Muscle Res. Cell Motil. 19, 717–731 10.1023/A:1005466432372 (doi:10.1023/A:1005466432372) [DOI] [PubMed] [Google Scholar]
- 6.Hill A. V. 1938. The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. B 126, 136–195 10.1098/rspb.1938.0050 (doi:10.1098/rspb.1938.0050) [DOI] [Google Scholar]
- 7.James R. S., Wilson R. S. 2008. Explosive jumping: extreme morphological and physiological specializations of Australian rocket frogs (Litoria nasuta). Physiol. Biochem. Zool. 81, 176–185 10.1086/525290 (doi:10.1086/525290) [DOI] [PubMed] [Google Scholar]
- 8.Navas C. A., James R. S., Wakeling J. M., Kemp K. M., Johnston I. A. 1999. An integrative study of the temperature dependence of whole animal and muscle performance during jumping and swimming in the frog Rana temporaria. J. Comp. Physiol. B 169, 588–596 10.1007/s003600050259 (doi:10.1007/s003600050259) [DOI] [PubMed] [Google Scholar]
- 9.Peplowski M. M., Marsh R. L. 1997. Work and power output in the hindlimb muscles of Cuban tree frogs Osteopilus septentrionalis during jumping. J. Exp. Biol. 200, 2861–2870 [DOI] [PubMed] [Google Scholar]
- 10.Wilson R. S., Franklin C. E., James R. S. 2000. Allometric scaling relationships of jumping performance in the striped marsh frog Limnodynastes peronii. J. Exp. Biol. 203, 1937–1946 [DOI] [PubMed] [Google Scholar]
- 11.Aerts P. 1997. Vertical jumping in Galago senegalensis: the quest for an obligate mechanical power amplifier. Phil. Trans. R. Soc. Lond. B 353, 1607–1620 10.1098/rstb.1998.0313 (doi:10.1098/rstb.1998.0313) [DOI] [Google Scholar]
- 12.Henry H. T., Ellerby D. J., Marsh R. L. 2005. Performance of guinea fowl Numida meleagris during jumping requires storage and release of elastic energy. J. Exp. Biol. 208, 3293. 10.1242/jeb.01764 (doi:10.1242/jeb.01764) [DOI] [PubMed] [Google Scholar]
- 13.Roberts T. J., Marsh R. L. 2003. Probing the limits to muscle-powered accelerations: lessons from jumping bullfrogs. J. Exp. Biol. 206, 2567–2580 10.1242/jeb.00452 (doi:10.1242/jeb.00452) [DOI] [PubMed] [Google Scholar]
- 14.Azizi E., Roberts T. J. 2010. Muscle performance during frog jumping: influence of elasticity on muscle operating lengths. Proc. R. Soc. B 277, 1523–1530 10.1098/rspb.2009.2051 (doi:10.1098/rspb.2009.2051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calow L. J., Alexander R. M. 1973. A mechanical analysis of a hind leg of a frog. J. Zool. Lond. 171, 293–321 10.1111/j.1469-7998.1973.tb05341.x (doi:10.1111/j.1469-7998.1973.tb05341.x) [DOI] [Google Scholar]
- 16.Phillips B. L., Brown G. P., Webb J. K., Shine R. 2006. Invasion and the evolution of speed in toads. Nature 439, 803. 10.1038/439803a (doi:10.1038/439803a) [DOI] [PubMed] [Google Scholar]
- 17.Alexander R. M. 1995. Leg design and jumping technique for humans, other vertebrates and insects. Phil. Trans. R. Soc. Lond. B 347, 235–248 10.1098/rstb.1995.0024 (doi:10.1098/rstb.1995.0024) [DOI] [PubMed] [Google Scholar]
- 18.James R. S., Navas C. A., Herrel A. 2007. How important are skeletal muscle mechanics in setting limits on jumping performance? J. Exp. Biol. 210, 923–933 10.1242/jeb.02731 (doi:10.1242/jeb.02731) [DOI] [PubMed] [Google Scholar]
- 19.Zug G. R. 1978. Anuran locomotion—structure and function. 2: Jumping performance of semiaquatic, terrestrial, and arboreal frogs. Smith. Contrib. Zool. 276, 1–31 [Google Scholar]
- 20.Roberts T. J., Scales J. A. 2002. Mechanical power output during running accelerations in wild turkeys. J. Exp. Biol. 205, 1485–1494 [DOI] [PubMed] [Google Scholar]
- 21.Bobbert M. F. 2001. Dependence of human squat jump performance on the series elastic compliance of the triceps surae: a simulation study. J. Exp. Biol. 204, 533–542 [DOI] [PubMed] [Google Scholar]
- 22.Wilson A. M., Watson J. C., Lichtwark G. A. 2003. Biomechanics: a catapult action for rapid limb protraction. Nature 421, 35–36 10.1038/421035a (doi:10.1038/421035a) [DOI] [PubMed] [Google Scholar]
- 23.Devita P., Janshen L., Rider P., Solnik S., Hortobágyi T. 2008. Muscle work is biased toward energy generation over dissipation in non-level running. J. Biomech. 41, 3354–3359 10.1016/j.jbiomech.2008.09.024 (doi:10.1016/j.jbiomech.2008.09.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farley C. T. 1997. Maximum speed and mechanical power output in lizards. J. Exp. Biol. 200, 2189–2195 [DOI] [PubMed] [Google Scholar]
- 25.Weyand P. G., Sternlight D. B., Bellizzi M. J., Wright S. 2000. Faster top running speeds are achieved with greater ground forces not more rapid leg movements. J. Appl. Physiol. 89, 1991–1999 [DOI] [PubMed] [Google Scholar]
- 26.Curtin N. A., Woledge R. C., Aerts P. 2005. Muscle directly meets the vast power demands in agile lizards. Proc. R. Soc. B 272, 581–584 10.1098/rspb.2004.2982 (doi:10.1098/rspb.2004.2982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Askew G. N., Marsh R. L., Ellington C. P. 2001. The mechanical power output of the flight muscles of blue-breasted quail (Coturnix chinensis) during take-off. J. Exp. Biol. 204, 3601–3619 [DOI] [PubMed] [Google Scholar]
- 28.Marsh R. L., Olson J. M., Guzik S. K. 1992. Mechanical performance of scallop adductor muscle during swimming. Nature 357, 411–413 10.1038/357411a0 (doi:10.1038/357411a0) [DOI] [PubMed] [Google Scholar]
- 29.Wakeling J. M., Johnston I. A. 1998. Muscle power output limits fast-start performance in fish. J. Exp. Biol. 201, 1505–1526 [DOI] [PubMed] [Google Scholar]