Abstract
Flapping flight places strenuous requirements on the physiological performance of an animal. Bird flight muscles, particularly at smaller body sizes, generally contract at high frequencies and do substantial work in order to produce the aerodynamic power needed to support the animal's weight in the air and to overcome drag. This is in contrast to terrestrial locomotion, which offers mechanisms for minimizing energy losses associated with body movement combined with elastic energy savings to reduce the skeletal muscles' work requirements. Muscles also produce substantial power during swimming, but this is mainly to overcome body drag rather than to support the animal's weight. Here, I review the function and architecture of key flight muscles related to how these muscles contribute to producing the power required for flapping flight, how the muscles are recruited to control wing motion and how they are used in manoeuvring. An emergent property of the primary flight muscles, consistent with their need to produce considerable work by moving the wings through large excursions during each wing stroke, is that the pectoralis and supracoracoideus muscles shorten over a large fraction of their resting fibre length (33–42%). Both muscles are activated while being lengthened or undergoing nearly isometric force development, enhancing the work they perform during subsequent shortening. Two smaller muscles, the triceps and biceps, operate over a smaller range of contractile strains (12–23%), reflecting their role in controlling wing shape through elbow flexion and extension. Remarkably, pigeons adjust their wing stroke plane mainly via changes in whole-body pitch during take-off and landing, relative to level flight, allowing their wing muscles to operate with little change in activation timing, strain magnitude and pattern.
Keywords: bird flight, neuromuscular function, muscle power, fascicle strain
1. Introduction
Birds power flight primarily by large pectoralis muscles that depress the wings at the shoulder. The dominant role and large size of the pectoralis muscle, therefore, enable a critical assessment of how muscle function is tailored to meet the mechanical power requirements of flapping flight over a range of flight conditions. The smaller supracoracoideus muscle of birds, about one-fifth the size of the pectoralis, is the primary wing elevator active during upstroke, particularly at slow to moderate speeds and during hovering (at faster flight speeds, wing elevation is probably produced passively by aerodynamic forces acting on the wings, which remain extended during upstroke to maintain lift through bound circulation [1,2]). Smaller extrinsic and intrinsic wing muscles assist in modulating wing orientation and controlling wing shape. These muscles probably contribute to adjustments of the wing's performance as an aerofoil [3–7] and, thus, may indirectly affect flight power requirements. However, because of their small size, the intrinsic muscles of the wing probably contribute little additional mechanical power for flight.
Prior analyses of muscle–tendon architecture have shown that muscles differ widely in their design for changing length while producing force, but because of their conservative properties for force production and relative fibre strain (ratio of activated length change relative to resting fibre length), skeletal muscles generally perform about the same amount of work in proportion to their mass [8–11]. Longer fibred muscles, such as the avian pectoralis, however, are well suited to producing the larger movements required for moving the wings to produce effective aerodynamic power for weight support and to overcome drag. In addition to having longer fibres, greater operating strains also enhance the range of movement that a muscle generates. Thus, the operating strains of certain flight muscles are expected to be greater than those of muscles that support an animal's weight during terrestrial locomotion [12] that contract over more limited strain ranges, allowing more economical force production. Muscles, having short fibres that attach to a longer tendon such as those found in the legs of terrestrial animals, produce large forces and can recover substantial elastic energy from their tendon and aponeurosis [12–15]. These muscles are best used for movements that require little net shortening or lengthening of the muscle. Consequently, pinnate muscles having these architectural features are commonly found in distal limb regions. The intrinsic wing muscles of birds are commonly short fibred and pinnate, and have long tendons. This enables these muscles to control distal movements of the wing while, at the same time, being small and lightweight. Their function has not been much studied to date, beyond a few comparative functional anatomical descriptions [7,16,17] and assessment of their neuromuscular activity patterns [3,16,17]. Even so, these studies are important because they provide a framework for future studies that seek to assess how the smaller intrinsic wing muscles are used to achieve flight across different conditions, and in birds with differing wing designs and flight styles.
In the context of this earlier work, the functions of the two primary flight muscles of birds, the pectoralis and supracoracoideus, are reviewed here in relation to the mechanical power needed to meet the aerodynamic requirements for flapping flight. The vast majority of morphological and physiological work has largely focused on the pectoralis because of its dominant role in powering avian flight. Consequently, much of the review of avian muscle function will focus on the pectoralis, with particular comparison to its antagonist, the supracoracoideus. Preliminary in vivo analyses of the triceps and biceps muscles, which control wing shape via elbow extension and flexion, are also considered in relation to changes in flight performance required for take-off, landing and manoeuvring flight. Future directions for research to improve our understanding of the neuromuscular control and functional design of avian flight are also identified.
2. Functional anatomy of primary avian flight muscles
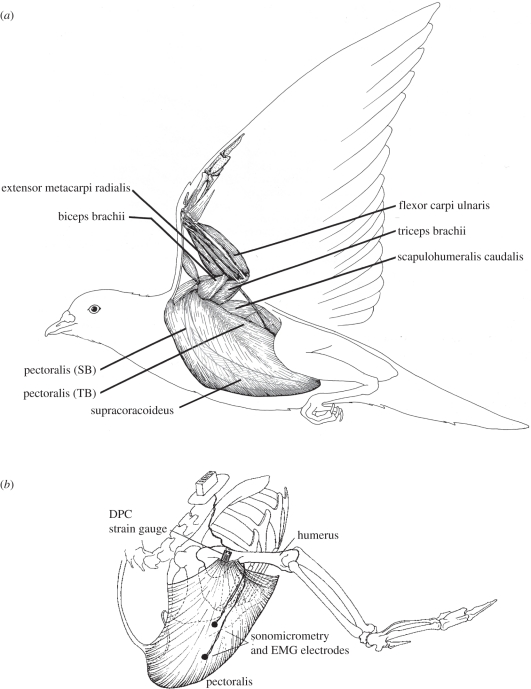
The pectoralis is a large muscle (approx. 8–11% body mass; [15,16]) that attaches to the humerus of the wing at the deltopectoral crest (DPC; figure 1). Its main portion (sternobrachialis, SB) originates from an enlarged sternal keel, with more anterior fibres arising from the furcula, or ‘wishbone’. A much smaller portion (thoracobrachialis, TB) originates dorsally from ribs. The fibres of the TB and the posterior region of the SB insert on an internal aponeurosis that merges with the more anterior SB fibres before attaching to the DPC. In addition to producing mechanical work during downstroke, the pectoralis also pronates the wing. The smaller supracoracoideus lies deep to the pectoralis, also originating from the keel of the sternum, and is about one-fifth of the pectoralis in mass (approx. 2% body mass). By means of its tendon, which inserts and acts dorsally at the shoulder as a pulley, the supracoracoideus elevates and supinates the wing during upstroke [18–21]. Whereas the pectoralis is composed of generally long fibres with modest pinnation (pigeon: 31–67 mm, mean 41 mm), the supracoracoideus is a classic bipinnate muscle with short fibres (pigeon: 16–23 mm, mean 20 mm). It produces elevation and supination of the wing by means of a long tendon that passes dorsally over the shoulder, via the triosseal foramen of the avian pectoral girdle, before attaching to the dorsal surface of the proximal humerus adjacent to the DPC. The pectoralis is composed mainly of fast-oxidative (type IIa) fibres (approx. 85% in pigeons) with a smaller component of fast-glycolytic (type IIb) fibres [20,21]. Fibre-type composition of the supracoracoideus, to my knowledge, has not been examined in pigeons, but in the European starling is composed of a greater fraction (68%) of fast-glycolytic versus fast-oxidative fibres [22]; whereas, in zebra finches, Anna's hummingbirds [23] and Atlantic puffins [24], the supracoracoideus is exclusively composed of fast-oxidative fibres.
Figure 1.
(a) Anatomical organization of avian wing musculature (adapted from Dial [3]), showing key muscles that have been studied, and (b) showing the general sites used to record pectoralis force via deltopectoral crest (DPC) bone strain, pectoralis fascicle strain and neuromuscular activation (EMG).
3. In vivo assessment of avian muscle function during flight
Because of its focal insertion on the ventral surface of the DPC in pigeons (figure 1b), doves, cockatiels, budgerigars, magpies and certain other species of birds, forces produced by the pectoralis can be estimated directly by means of strains recorded using a strain gauge bonded to the dorsal surface of the DPC (in several avian species, the pectoralis also inserts along the ventral proximal shaft of the humerus, preventing this approach). Details for exposing and attaching metal foil strain gauges to obtain strain-calibrated in vivo recordings of pectoralis force are described elsewhere [25,26]. Although some uncertainty exists in the calibration of DPC strain to pectoralis muscle force [27], such recordings provide a reliable and temporally detailed recording of time-varying muscle force. Other methods for obtaining muscle force and estimates of mechanical power output for bird flight also have their limitations [28,29]. A similar skeletal-strain-based approach to extract the time-varying force transmitted by the supracoracoideus muscle via the muscle's tendinous insertion on to the proximal dorsal shaft of the humerus has also been used [30].
In combination with DPC strain-force recordings of the pectoralis and the supracoracoideus, in vivo measurements of muscle fascicle strain are obtained in localized muscle sites by means of sonomicrometry, a technique based on measurements of the propagation of sound pulses within the muscle to determine length changes [31]. Because the sonomicrometry transducers lie adjacent to muscle fascicle bundles, they provide a measure of fascicle strain rather than muscle fibre strain per se. Nevertheless, the two measures are likely to be quite similar. In the large pectoralis, sonometric measurements obtained from multiple sites (anterior and posterior SB and TB) in pigeons showed similar fascicle strain levels in the larger SB portion of the muscle, but smaller strains in the most posterior SB and TB portions of the muscle [32]. By averaging the sonomicrometry data for fascicle strain across recording sites (weighted by the estimated fraction of muscle mass that each site represents) or by relying on a single recording site within the muscle and assuming the site is representative for the muscle as a whole, the total work of the muscle can be assessed based on the muscle's length change. Muscle work is therefore determined by fascicle strain multiplied by average fascicle length, in relation to the time-varying force the muscle produces. The product of muscle fascicle length change and force is visualized as a work loop over the course of a wingbeat, or muscle contraction, cycle. The timing of muscle activation is recorded simultaneously using fine-wire electromyography (EMG) electrodes inserted into and anchored adjacent to those fascicles for which a sonometric evaluation of strain is recorded [31]. The EMG provides a measure of the timing of muscle activation and relative motor recruitment in relation to muscle force and length change. In total, the force, strain and neuromuscular activation recorded from the muscle serve to describe the temporal dynamics of the muscle's contractile performance across a range of flight conditions.
4. Functional analysis of pectoralis and supracoracoideus muscles during flight
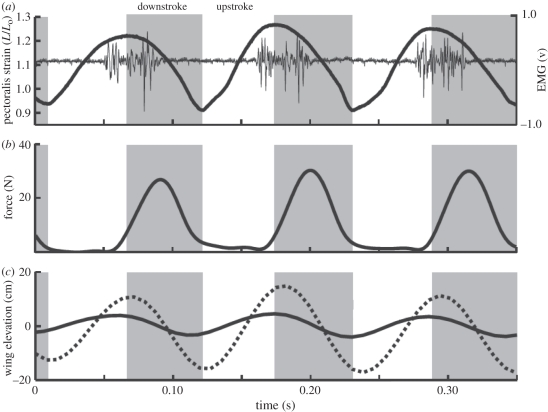
The pectoralis muscle is activated to contract late in the upstroke, prior to wing reversal (figure 2a). Force development follows soon after the start of activation (approx. 2–8 ms in pigeons and cockatiels) and peaks early in the downstroke, continuing until the end of the downstroke. The pectoralis undergoes a slight stretch or remains nearly isometric (depending on the species and flight condition studied), as force develops late in the upstroke and through wing reversal to begin the downstroke (figures 2 and 3). By developing force while nearly isometric or being briefly stretched, the rate of force rise and the magnitude of peak force are appreciably enhanced owing to force–velocity effects [35,36]. As a result, the work that the pectoralis performs is substantially increased while the muscle shortens during the remainder of the downstroke. Deactivation of the pectoralis occurs early in the downstroke, almost coincident with the timing of peak force generation. This allows the muscle to relax to near zero force prior to being stretched passively in the upstroke. Importantly, this reduces the antagonistic (‘negative’) work required of the supracoracoideus to elevate the wing. The timing of pectoralis deactivation relative to its continuing force production points to the problematic nature of inferring muscle force production based on EMG recordings alone.
Figure 2.
Representative in vivo recordings of pectoralis fascicle strain, neuromuscular activation (EMG) and force for three wingbeats in a cockatiel flying at 7 m s−1 in a wind tunnel. Solid line, wrist; dotted line, wingtip. Adapted from Hedrick et al. [33].
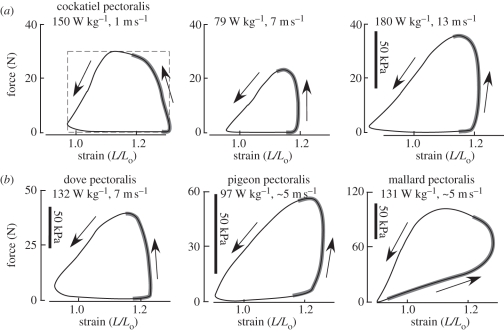
Figure 3.
Representative in vivo work-loop patterns produced by the (a) pectoralis of cockatiels (Nymphicus hollandicus) at three different flight speeds (adapted from Hedrick et al. [33]), and (b) the pectoralis of three other species: ring-neck doves (Streptopelia risoria), pigeons (Columba livia) and mallard ducks (Anas platyrynchos) (adapted from Tobalske et al. [27], Biewener et al. [31] and Williamson et al. [34]). The force produced by the muscle is plotted against its fascicle strain (L/Lo, where Lo is the muscle's resting length: strain = 1.0). In the first panel of (a), the dashed rectangle denotes the maximum work that the muscle could produce for its maximum force and strain; the realized work of the muscle is 68% of its theoretical maximum. The strain range for all muscles is the same (0.9–1.3, or 40% range of muscle length change), but force ranges differ in (b) owing to the different-sized muscles. The bold grey portion of each work loop represents the period of neuromuscular activation measured by EMG. Arrows denote the direction of force and fascicle length changes.
For those species studied [27,33,34], the in vivo force–length work behaviour of the pectoralis is generally similar across a range of flight speeds and conditions (figure 3). As noted above, activation of the pectoralis in these species occurs late in upstroke, as the muscle is being lengthened (this is most extreme in the mallard, figure 3b) or is nearly isometric, allowing the muscle to develop force rapidly for a given level of activation. In contrast to classical expectations for the operating fascicle strain of a muscle (approx. 10–15% of resting length based on isometric force–length properties [35,36]), the pectoralis of these species undergoes strains of 32–40% during different flight conditions (take-off, ascending and descending flight and changes in speed during level flight), stretching 20–30% beyond the muscle's resting length (measured when the wings are folded against the bird's body on the perch), and shortening 8–12% less than the resting length. This large strain excursion underlies the ability of the pectoralis to perform substantial work during the downstroke of each contraction cycle. Forces produced by the pigeon pectoralis were found to vary about 40 per cent across flight conditions, ranging from take-off and ascending flight to landing and descending flight [26]. Forces produced by the cockatiel pectoralis during level flight across speeds ranging from 1 to 14 m s−1 in a wind tunnel were found to vary by 65 per cent [33]. These forces are estimated to be less than 40–60% of the peak isometric force that the muscle can generate [26], reflecting in part the rapid shortening that the muscle undergoes to produce work. In cockatiels, doves and pigeons, the pectoralis achieves 58–73% of the maximum theoretical work output possible for the observed force and active strain range [30,33] (figure 3a).
Not surprisingly, the supracoracoideus of pigeons exhibits mirror-like force, length and activation timing patterns relative to the pectoralis [30] (figure 4). As the main upstroke muscle, the supracoracoideus is activated late in downstroke just prior to wing reversal. The muscle develops force rapidly while being nearly isometric, reaching peak force very early in the upstroke. The early onset of force development by the supracoracoideus probably reflects its role in decelerating and re-accelerating the wing during the downstroke–upstroke transition, as well as its role in wing supination [19]. Estimates of the elastic energy storage within the supracoracoideus tendon (51 ± 62 mJ during level and 88 ± 85 mJ during ascending flight) are consistent with this role, given that the magnitude of inertial kinetic energy exceeds the amount of elastic energy stored and returned by the supracoracoideus tendon [30]. The additional inertial power of the wing's motion is probably transformed into useful aerodynamic power mainly in the downstroke, as has been traditionally assumed [37]. The rapid supination of the wing produced by the supracoracoideus is important for achieving a short-duration upstroke, with the potential for positive lift generation in birds with wing-tip reversal flight kinematics [38] or for minimizing unwanted negative lift. It also maximizes the duration of downstroke lift production and was probably an important feature in the evolution of an active flapping flight stroke [19]. Rapid supination of the wing to initiate upstroke in rufous hummingbirds [39] is key to this species' ability to generate positive upstroke lift, which has been estimated to be 25–33% of their total lift production [40]. In pigeons, the amount of force produced antagonistically between the two muscles was estimated to be small [30]. During slow level flight, the negative work of the pigeon pectoralis just prior to the end of the upstroke is about 18 per cent of the positive work the muscle performs during the downstroke. This may well reflect a role in absorbing inertial energy of the wing as it is decelerated late in upstroke [30]. By comparison, negative work of the pigeon supracoracoideus is 14 per cent of the positive work that the muscle performs and occurs late in downstroke to decelerate the wing at this time.
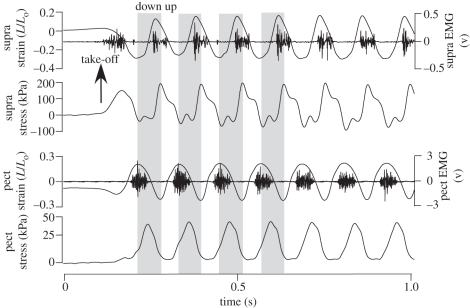
Figure 4.
Representative recordings of the pigeon supracoracoideus (wing elevation) fascicle strain, EMG and force, and pectoralis (wing depression) fascicle strain, EMG and force recorded during take-off from an elevated perch platform and level free flight at approximately 4.5 m s−1 (seven wingbeats are shown). Grey panels represent the downstroke for the initial four wingbeat cycles, with the upstroke on a white background (adapted from Tobalske & Biewener [30]).
The short fibres of the bipinnate supracoracoideus muscle require them to operate over large strains, similar to those of the pectoralis. Supracoracoideus fascicle strains range from 33 to 40 per cent of the muscle's resting length during descending, ascending and level flight [30]. The supracoracoideus fascicles also undergo a smaller degree of stretch relative to their rest length (6–12% across flight conditions) compared with their net shortening strain (−27% for all flight conditions). This pattern of fascicle length change relative to resting length is opposite to the pattern of strain observed within pectoralis fascicles, which lengthen by 20–30% of their resting length before shortening to approximately 10 per cent less than rest at the end of downstroke (figure 4). Interestingly, the modulation of muscle strain in the supracoracoideus reflects mainly differences in the degree of wing depression (stretching the supracoracoideus and its tendon) that occur at the end of downstroke across the three flight conditions that were studied. Because of its relatively small size, the pigeon supracoracoideus generates 1.6 times the mass-specific muscle power output of the pectoralis. This reflects the much greater operating stresses (force normalized to physiological cross-sectional area) of the supracoracoideus, which ranged from 85 to 125 kPa for descending versus ascending flight, compared with stresses of 50–58 kPa in the pectoralis across the same flight condition [30], and 57–76 kPa in an earlier study of the pigeon pectoralis when corrected for the muscle's estimated myofibrillar area [26].
5. Comparative data for avian pectoralis power output versus speed
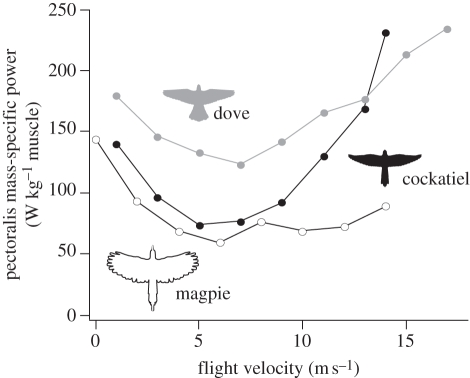
Because the pectoralis is the dominant avian flight muscle (in pigeons, the pectoralis represents 60% of total wing muscle mass, A. A. Biewener 2010, unpublished data), the muscle's power output can be used to assess how whole-body power output and, indirectly, aerodynamic power output vary as a function of flight condition and speed in a bird. Measurements of pectoralis mechanical power output and wingbeat frequency have been published for black-billed magpies (Pica pica), cockatiels (Nymphicus hollandicus) and ringed-neck doves (Streptopelia risoria) across a range of flight speeds while flying level and steady in a wind tunnel [27,41] (figure 5). Except for magpies, the other two species showed a U-shaped power versus flight speed curve, generally consistent with aerodynamic theory. This reflects high induced power costs at slow flight speeds and hovering that decease as speed increases, and high profile and parasite power costs (owing to increasing wing and body drag) at higher flight speeds. The absence of an observed increase in pectoralis muscle power at higher flight speeds in magpies may reflect either an inability of this species, with its lower aspect ratio and less pointed wings, to achieve sufficient thrust in order to overcome the profile and parasite drag costs it incurs at higher flight speeds limiting the top speed that it can achieve [27], or that the birds were unwilling to fly at faster speeds in the wind tunnel. Although the wind tunnel used to study the magpies was smaller (50% less in cross-dimensions of the working section) than that used to study the cockatiels and doves, artefacts such as a possible ground or wall effect [42] were not judged by the authors to be the basis for the magpies' lower power cost at faster flight speeds. In the two other species (cockatiels and doves), pectoralis muscle power output at the fastest flight speeds exceeded that produced when the birds were nearly hovering (figure 5). Thus, although pectoralis power output was high as expected during 1 m s−1 flight in the magpies, it remains unclear why the muscle's power output did not reach or exceed this level at faster flight speeds.
Figure 5.
Comparative flight power curves for three avian species, showing changes in pectoralis mass-specific muscle power (determined from calibrated DPC-strain-force and fascicle strain recordings) versus flight speed in a wind tunnel (adapted from Tobalske et al. [27]).
Given that other muscles are involved in flapping flight and do mechanical work, it is certainly the case that the total muscle mechanical power requirement for flight is greater than estimates based on the pectoralis alone. In the study of pigeons, for which pectoralis and supracoracoideus muscle power output were both determined [30], inclusion of supracoracoideus power output increases the total power output of flight by nearly 25 per cent. Pectoralis power output across flight modes was 3.2 times greater than that of the supracoracoideus but less than the nearly fivefold difference in muscle mass. Together, these two muscles represent 71 per cent (A. A. Biewener 2010, unpublished data) of the total fight muscle mass of a pigeon. If the remaining smaller extrinsic and intrinsic wing muscles perform the same relative mass-specific work, this would suggest a total power requirement that may be nearly 40 per cent greater than that determined for the pectoralis alone.
Aerodynamic models for estimating the power requirements of the flight of birds at different speeds [43–45] are commonly used to infer ecological strategies for maximizing a bird's flight range or minimizing the metabolic power requirement for flight as a function of time [46]. Although measurements of pectoralis muscle mechanical power output are consistent with the general change in power versus flight speed (being highest at slow and fast speeds, with a minimum at an intermediate flight speed), the absolute magnitude of the power cost for flapping flight across species and speeds remains uncertain. Arguments for one approach and/or method being superior to another remain unconvincing. This is due to assumptions and simplifications that quasi-steady aerodynamic theory makes to estimate flight power requirements, and uncertainties in the calibration of pectoralis force and assessment of regional fascicle strain profiles from localized fascicle recordings on the experimental side. More recent attempts to estimate muscle power output based on isolated work-loop muscle measurements in relation to EMG recordings made during flight [28,29] also have their limitations. These include estimating muscle recruitment from relative EMG magnitude across flight speeds to adjust the maximally stimulated muscle power measurements derived from in vitro work experiments. Such an approach necessarily determines the change in flight power requirements based on changes in recorded EMG intensity. It also results in lower estimates of flight muscle power requirements of cockatiels (minimum power cost = approx. 40 W kg−1 at 7 m s−1) compared with those (74–79 W kg−1 at 5–7 m s−1) obtained using DPC-based force measurements [27,33]. Additional studies that refine the use of these approaches, or use other methods [47], will improve our ability to quantify the absolute power costs of flapping flight for particular species operating across various flight conditions. Consistent with the in vitro muscle work and EMG intensity results that ascribe change in muscle power output across flight speed owing to changes in EMG intensity [28,29], results based on in vivo fascicle strain, EMG and DPC-strain-calibrated force recordings [27,33] also showed EMG intensity to be highly correlated with muscle force (R2 = 0.92). In the latter studies, changes in EMG intensity accounted for 65 per cent of the modulation of muscle power, with changes in fascicle strain amplitude accounting for 25 per cent and changes in wingbeat frequency only 10 per cent of the modulation in muscle power [27,33].
Using measurements of DPC-strain-calibrated pectoralis force and fascicle strain to determine in vivo pectoralis power output, the comparative power curves for the different species studied to date suggest that wing loading, as well as wing and tail shape, is probably an important determinant of a species' relative muscle power cost. Doves have the highest wing loading (36 N m−2) of the species studied to date [2] and correspondingly have the highest relative flight power cost over a broad range of speeds (figure 5). Magpies have the lowest aspect ratio wings (5.0; versus budgerigars: 7.3, cockatiels: 7.0 and doves: 5.7) and rounded wingtips, which probably helps to lower their muscle mass-specific power requirements but may also limit the fastest speeds they can achieve.
At present, it would be imprudent to place heavy reliance on the accuracy of experimental or theoretical modelling results to specify precisely whether a species has a minimum power cost at a particular flight speed, given the uncertainty and limitations to the resolution and accuracy of currently available approaches used to estimate flight power costs. For example, whereas oxygen consumption data for cockatiels [48,49] indicate a minimum metabolic power cost at 10 m s−1, measurements of pectoralis muscle power data suggest a minimum in the range of 5–7 m s−1 [27,29]. Combining the metabolic power results for cockatiels with their mechanical muscle power results [49] indicates that muscle efficiency increases with flight speed, ranging from 6.9 to 11.2 per cent based on the muscle power data of Morris & Askew [29], or from 12.2 to 28.3 per cent based on the DPC-pectoralis force and fascicle strain recordings of Tobalske et al. [27].
Differences in muscle efficiency are likely given that the shortening velocity of the pectoralis muscle fascicles varies with flight speed. For cockatiels [27,33], fascicle shortening velocities ranged from 5.19 to 6.73 muscle lengths per second across flight speeds from 1 to 13 m s−1. The range of efficiencies derived from in vitro muscle measurements adjusted for EMG intensity [29] are low compared with those expected for vertebrate skeletal muscle, which range from 20 to 28 per cent at optimal shortening velocities [50]. It seems surprising that the evolution of flight muscle function in cockatiels and other birds would be constrained to substantially lower efficiencies. Although wingbeat frequency varies only slightly across flight speeds (10% in cockatiels), the magnitude of pectoralis fascicle strain changes in a shallow U-shaped pattern, paralleling changes in pectoralis force [29], which results in the overall muscle power versus speed relationship that is observed for cockatiels (figure 5). Although fascicle strain rate varies with flight speed, the generally uniform contractile properties of the pectoralis across a range of flight speeds [27,29] (figure 3) reflect the strikingly uniform fibre-type characteristics of the avian pectoralis [21–23]. This is in contrast to the much larger change in fascicle shortening velocity with running speed that occurs in the leg muscles of terrestrial animals [51–54].
6. Muscle function in relation to the control of take-off, landing and manoeuvring flight
Whereas the pectoralis and supracoracoideus are mainly responsible for producing the mechanical power required for sustained flapping flight in birds, it is unclear whether the activity of these large flight muscles is modulated to achieve manoeuvring flight behaviours, or whether the smaller wing extrinsic and intrinsic muscles are recruited to adjust wing orientation and wing shape. Past work based on three-dimensional kinematics, muscle force and EMG recordings suggest two possibilities. In pigeons [6,55], left and right pectoralis muscles appear to exhibit differential timing of force development and magnitude, with downstroke of the outside wing phase advanced relative to the inside wing of a turn. In rose-breasted galahs [5], little difference in the kinematic timing of downstroke or pectoralis EMG activity was noted during 90° turns. Instead, there was evidence of differential activation of the left and right biceps muscles, with the inside biceps showing stronger activity, indicative of increased elbow flexion and reduction of inside wing span. In both sets of studies, however, more detailed kinematics of wing shape and motion during these manoeuvres was not available given the limited resolution of the motion-analysis systems used at the time. Future work will benefit from improved kinematic resolution during turning flight, combined with further study of left wing versus right wing muscle contractile asymmetry.
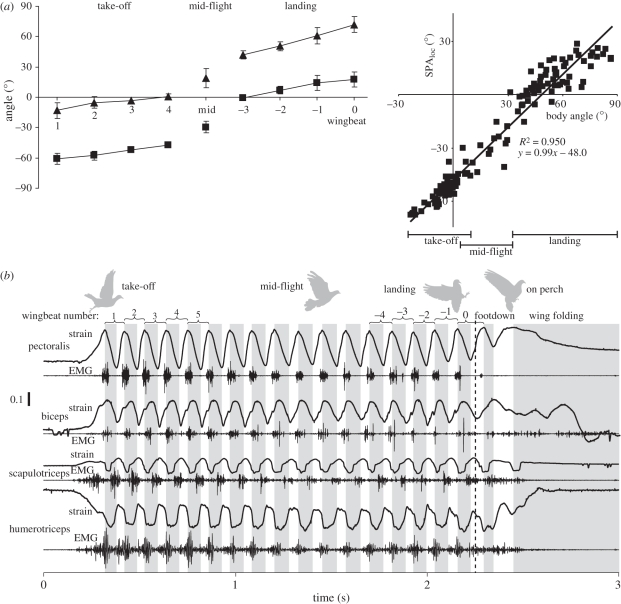
In studies of pigeons taking-off from an elevated perch platform, flying level and landing on a similar perch, measurements of wing, body and tail kinematics reveal little change in wing or tail movements relative to the bird's body [56]. Instead, most of the changes in global orientations of the tail, wing and wing stroke plane, which determine the aerodynamic properties of the bird's flight stroke, are achieved by changes in body pitch (figure 6a). During take-off, pigeons pitch forward (head down) inclining their stroke plane to a more vertical orientation to provide increased thrust for acceleration after the take-off jump from the perch. During landing, the pigeon pitches back (head up), changing its stroke plane to a more horizontal orientation to help decelerate as it lands. Changes in the global stroke plane angle during take-off and landing are significantly greater and less, respectively, than observed during level flight.
Figure 6.
(a) Changes in wing stroke plane (SPAloc) and body pitch angle (in global space) of a pigeon during successive wingbeats of take-off, mid-level flight and landing (adapted from Berg & Biewener [56]). The strong correlation of wing stroke plane angle versus body angle is shown to the right. Filled triangles, body angles; filled squares, SPAloc. (b) Representative in vivo recordings of muscle strain and activation (EMG) of extrinsic and intrinsic wing muscles of a pigeon during take-off, level (approx. 4.5 m s−1) and landing flight corresponding to a similar sequence shown in (a) above (adapted from Berg [57]).
The uniform motion of the pigeon's wings relative to its body during take-off, level and landing flight suggests that the control of wing and body movement across these key phases of flight relies on subtle shifts in aerodynamic and inertial forces produced by the tail and wings relative to the body to control body pitch. The pitch moment of inertia of a bird, though greater than its roll moment of inertia, is still quite small. As a result, slight shifts in the orientation of net aerodynamic force produce the observed pitch acceleration. In pigeons, the shift in the direction of net aerodynamic force need only be approximately 8 mm relative to its centre of mass to produce the observed pitch moment [56]. Consistent with this, no significant differences were observed in the neuromuscular activation (EMG) or contractile strain behaviour of the wing muscles examined (figure 6b) [57]. This result suggests that the control of body orientation and wing motion relative to the body does not require substantial changes in flight muscle activation and contractile function. Instead, the highly manoeuvrable bodies of many birds (low pitch, roll and yaw moments of inertia) enable them to achieve changes in body and wing orientation that allow rapid sharp turning, or to shift from take-off to landing flight, with subtle changes in neuromuscular function that are likely to prove challenging to identify.
7. Discussion and summary
Muscle function in bird flight depends on the production of substantial mechanical work performed at a high rate. Although skeletal muscles generally have a similar capacity for generating mass-specific work, the avian pectoralis is well suited to performing work with large length excursions. This is a prerequisite for powering flight because the wings must move through a large excursion during downstroke to produce effective aerodynamic lift. The pectoralis achieves this by having relatively long fascicles that shorten over a large fraction (up to 42%) of their length. The timing of muscle activation late in upstroke also allows the pectoralis to rapidly develop force under nearly isometric or stretching conditions. This elevates the work that the muscle performs as it shortens (figure 3).
Because of its large size and principal role in producing aerodynamic lift, the contractile function of the avian pectoralis provides a valuable index for the power requirements of flight based on measurements of its force production, contractile strain and neuromuscular activation. This is in contrast to the multiple muscle groups in the limbs of running animals that contribute to muscle power for movement. Nevertheless, a functional examination of the broader suite of wing muscles is needed in order to understand how flight movements, particularly those during manoeuvring, are controlled. Although much smaller wing muscles may not contribute significantly to the mechanical power underlying flight, by adjusting the orientation and shape of the wing, they can alter the wing's aerodynamic properties and, thus, influence how aerodynamic forces and power are shifted between the wings for manoeuvring.
An unexpected result is that shifts in body, tail and wing movement during take-off, level and landing flight of pigeons are achieved mainly by changes in whole-body pitch, rather than by changes in wing or tail motion relative to the body itself. The degree to which turning flight is achieved by left versus right asymmetries of smaller wing muscles, acting to ‘steer’ the bird around a turn, as opposed to modulation of the larger power-producing pectoralis and/or supracoracoideus muscles remains unclear. Evidence exists that both sets of muscles may contribute to the necessary aerodynamic asymmetries that result in a turning manoeuvre. The low moments of inertia and highly manoeuvrable bodies of birds mean that left versus right asymmetries in turning flight, or fore-aft asymmetries in aerodynamic force production during take-off and landing flight, are likely to be small and challenging to identify.
Future studies will benefit from improved imaging that will allow detailed changes in wing shape, orientation and movement to be quantified and related to the timing and magnitude of muscle activation, and where possible, changes in muscle length, force and work. These measurements become increasingly difficult for smaller muscles, located more distally in the wing. Force measurements, in particular, are difficult to obtain for most muscles, hampering the ability to assess muscle force and work output in relation to manoeuvring flight. In the case where muscles are too small, or forces cannot be recorded directly, in vitro or in situ measurements of muscle force [29] can play an important role for assessing the muscle's contractile properties and role(s) in flight. The remarkable ability of birds to fly over a range of speeds while often manoeuvring through complex environments makes understanding the neuromuscular and aerodynamic features of these flight behaviours of considerable interest to physiologists, biomechanists and aeronautical engineers.
Similarly, the aerodynamic and metabolic power requirements for flight are of considerable interest to avian and evolutionary ecologists interested in the strategies that birds use to forage and migrate to ensure a successful life history. For this reason, additional free flight data on bird metabolism, characteristic flight speeds and behaviour need to be linked to additional experimental assessments of flight energy metabolism and musculoskeletal function. While quasi-static aerodynamic models can provide a rough estimate of flight costs, the importance of non-steady aerodynamic effects on flight power costs is now well recognized and cannot be ignored. Thus, additional modelling and experimental studies that seek to yield improved measurements of muscle function and aerodynamic power output are needed.
Acknowledgements
The author thanks his many past students, postdocs and collaborators for the fun and exciting work shared to understand bird muscle function in relation to flight performance. He also thanks Mr Pedro Ramirez for his expert and devoted help in caring for the birds. Much of the author's work has been funded by the National Science Foundation, most recently by IOS-0744056.
Footnotes
One contribution of 15 to a Theme Issue ‘Integration of muscle function for producing and controlling movement’.
References
- 1.Rayner J. V. M. 1988. Form and function in avian flight. In Current ornithology, vol. 5 (ed. Johnston R. F.), pp. 1–66 New York, NY: Plenum Press [Google Scholar]
- 2.Tobalske B. W., Hedrick T. L., Biewener A. A. 2003. Wing kinematics of avian flight across speeds. J. Avian Biol. 34, 177–184 10.1034/j.1600-048X.2003.03006.x (doi:10.1034/j.1600-048X.2003.03006.x) [DOI] [Google Scholar]
- 3.Dial K. P. 1992. Activity patterns of the wing muscles of the pigeon (Columba livia) during different modes of flight. J. Exp. Zool. 262, 357–373 10.1002/jez.1402620402 (doi:10.1002/jez.1402620402) [DOI] [Google Scholar]
- 4.Dial K. P., Gatesy S. M. 1993. Neuromuscular control and kinematics of the wings and tail during maneuvering flight. Am. Zool. 33, 5 [Google Scholar]
- 5.Hedrick T. L., Usherwood J. R., Biewener A. A. 2007. Low speed maneuvering flight of the rose-breasted cockatoo (Eolophus roseicapillus). II: Inertial and aerodynamic reorientation. J. Exp. Biol. 210, 1912–1924 10.1242/jeb.002063 (doi:10.1242/jeb.002063) [DOI] [PubMed] [Google Scholar]
- 6.Warrick D. R., Dial K. P. 1998. Kinematic, aerodynamic and anatomical mechanisms in the slow, maneuvering flight of pigeons. J. Exp. Biol. 201, 655–672 [DOI] [PubMed] [Google Scholar]
- 7.Vazquez R. J. 1995. Functional anatomy of the pigeon hand (Columba livia): a muscle stimulation study. J. Morphol. 226, 33–45 10.1002/jmor.1052260104 (doi:10.1002/jmor.1052260104) [DOI] [PubMed] [Google Scholar]
- 8.Alexander R. M. 1992. The work that muscles can do. Nature 357, 360–361 10.1038/357360a0 (doi:10.1038/357360a0) [DOI] [PubMed] [Google Scholar]
- 9.Alexander R. M. 2003. Principles of animal locomotion. Princeton, NJ: Princeton University Press [Google Scholar]
- 10.Biewener A. A. 2003. Animal locomotion. Oxford, UK: Oxford University Press [Google Scholar]
- 11.Marsh R. L. 1999. How muscles deal with real-world loads: the influence of length trajectory on muscle performance. J. Exp. Biol. 202, 3377–3385 [DOI] [PubMed] [Google Scholar]
- 12.Biewener A. A. 1998. Muscle function in vivo: the design of muscles used as springs versus muscles used to generate mechanical power. Am. Zool. 38, 703–717 [Google Scholar]
- 13.Alexander R. M. 1988. Elastic mechanisms in animal movement. Cambridge, UK: Cambridge University Press [Google Scholar]
- 14.Biewener A. A., Roberts T. J. 2000. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exer. Sport. Sci. Rev. 28, 99–107 [PubMed] [Google Scholar]
- 15.Roberts T. J., Marsh R. L., Weyand P. G., Taylor C. R. 1997. Muscular force in running turkeys: the economy of minimizing work. Science 275, 1113–1115 10.1126/science.275.5303.1113 (doi:10.1126/science.275.5303.1113) [DOI] [PubMed] [Google Scholar]
- 16.George J. C., Berger A. J. 1966. Avian myology. New York, NY: Academic Press [Google Scholar]
- 17.Raikow R. 1985. Locomotor system. In Form and function in birds (eds King A. S., McLelland J.), pp. 57–147 London, UK: Academic Press [Google Scholar]
- 18.Poore S. O., Ashcroft A., Sanchez-Haiman A., Goslow G. E., Jr 1997. The contractile properties of the m. supracoracoideus in the pigeon and starling: a case for long-axis rotation of the humerus. J. Exp. Biol. 200, 2987–3002 [DOI] [PubMed] [Google Scholar]
- 19.Poore S. O., Sanchez-Haiman A., Goslow G. E. J. 1997. Wing upstroke and the evolution of flapping flight. Nature 387, 799–802 10.1038/42930 (doi:10.1038/42930) [DOI] [Google Scholar]
- 20.Kaplan S. R., Goslow G. E., Jr 1989. Neuromuscular organization of the pectoralis (pars thoracicus) of the pigeon (Columba livia): implications for motor control. Anat. Rec. 224, 426–430 10.1002/ar.1092240311 (doi:10.1002/ar.1092240311) [DOI] [PubMed] [Google Scholar]
- 21.Rosser B. W. C., George J. C. 1986. The avian pectoralis: histochemical characterization and distribution of muscle fiber types. Can. J. Zool. 64, 1174–1185 10.1139/z86-176 (doi:10.1139/z86-176) [DOI] [Google Scholar]
- 22.Goslow G. E. J., Wilson D., Poore S. O. 2000. Neuromuscular correlates to the evolution of flapping flight in birds. Brain Behav. Evol. 55, 85–99 10.1159/000006644 (doi:10.1159/000006644) [DOI] [PubMed] [Google Scholar]
- 23.Welch K. C. J., Altshuler D. L. 2009. Fiber type homogeneity of the flight musculature in small birds. Comp. Biochem. Physiol. B 152, 324–331 10.1016/j.cbpb.2008.12.013 (doi:10.1016/j.cbpb.2008.12.013) [DOI] [PubMed] [Google Scholar]
- 24.Kovacs C. E., Meyers R. A. 2000. Anatomy and histochemistry of flight muscles in a wing-propelled diving bird, the Atlantic puffin Fratercula arctica. J. Morphol. 244, 109–125 (doi:10.1002/(SICI)1097-4687(200005)244:2<109::AID-JMOR2>3.0.CO;2-0) [DOI] [PubMed] [Google Scholar]
- 25.Biewener A. A., Dial K. P., Goslow G. E., Jr 1992. Pectoralis muscle force and power output during flight in the starling. J. Exp. Biol. 164, 1–18 10.1016/0022-0981(92)90132-T (doi:10.1016/0022-0981(92)90132-T) [DOI] [Google Scholar]
- 26.Dial K. P., Biewener A. A. 1993. Pectoralis muscle force and power output during different modes of flight in pigeons (Columba livia). J. Exp. Biol. 176, 31–54 [Google Scholar]
- 27.Tobalske B. W., Hedrick T. L., Dial K. P., Biewener A. A. 2003. Comparative power curves in bird flight. Nature 421, 363–366 10.1038/nature01284 (doi:10.1038/nature01284) [DOI] [PubMed] [Google Scholar]
- 28.Morris C. R., Askew G. N. 2010. The mechanical power output of the pectoralis muscle of cockatiel (Nymphicus hollandicus): the in vivo muscle length trajectory and activity patterns and their implications for power modulation. J. Exp. Biol. 213, 2770–2780 10.1242/jeb.035691 (doi:10.1242/jeb.035691) [DOI] [PubMed] [Google Scholar]
- 29.Morris C. R., Askew G. N. 2010. Comparison between mechanical power requirements of flight estimated using an aerodynamic model and in vitro muscle performance in the cockatiel (Nymphicus hollandicus). J. Exp. Biol. 213, 2781–2787 10.1242/jeb.035709 (doi:10.1242/jeb.035709) [DOI] [PubMed] [Google Scholar]
- 30.Tobalske B. W., Biewener A. A. 2008. Contractile properties of the pigeon supracoracoideus during different modes of flight. J. Exp. Biol. 211, 170–179 10.1242/jeb.007476 (doi:10.1242/jeb.007476) [DOI] [PubMed] [Google Scholar]
- 31.Biewener A. A., Corning W. R., Tobalske B. T. 1998. In vivo pectoralis muscle force–length behavior during level flight in pigeons (Columba livia). J. Exp. Biol. 201, 3293–3307 [DOI] [PubMed] [Google Scholar]
- 32.Soman A., Hedrick T. L., Biewener A. A. 2005. Regional patterns of pectoralis fascicle strain in the pigeon Columba livia during level flight. J. Exp. Biol. 208, 771–786 10.1242/jeb.01432 (doi:10.1242/jeb.01432) [DOI] [PubMed] [Google Scholar]
- 33.Hedrick T. L., Tobalske B. W., Biewener A. A. 2003. How cockatiels (Nymphicus hollandicus) modulate pectoralis power output across flight speeds. J. Exp. Biol. 206, 1363–1378 10.1242/jeb.00272 (doi:10.1242/jeb.00272) [DOI] [PubMed] [Google Scholar]
- 34.Williamson M. R., Dial K. P., Biewener A. A. 2001. Pectoralis muscle performance during ascending and slow level flight in mallards (Anas platyrynchos). J. Exp. Biol. 204, 495–507 [DOI] [PubMed] [Google Scholar]
- 35.Lieber R. L. 1992. Skeletal muscle structure and function. Baltimore, MD: Williams and Wilkins [Google Scholar]
- 36.McMahon T. A. 1984. Muscles, reflexes, and locomotion. Princeton, NJ: Princeton University Press [Google Scholar]
- 37.Pennycuick C. J., Hedenström A., Rosén M. 2000. Horizontal flight of a swallow (Hirundo rustica) observed in a wind tunnel, with a new method for directly measuring mechanical power. J. Exp. Biol. 203, 1755–1765 [DOI] [PubMed] [Google Scholar]
- 38.Tobalske B., Dial K. P. 1996. Flight kinematics of black-billed magpies and pigeons over a wide range of speeds. J. Exp. Biol. 199, 263–280 [DOI] [PubMed] [Google Scholar]
- 39.Tobalske B. W., Warrick D. R., Clark C. J., Powers D. R., Hedrick T. L., Hyder G. A., Biewener A. A. 2007. Three-dimensional kinematics of hummingbird flight. J. Exp. Biol. 210, 2368–2382 10.1242/jeb.005686 (doi:10.1242/jeb.005686) [DOI] [PubMed] [Google Scholar]
- 40.Warrick D. R., Tobalske B. W., Powers D. R. 2005. Aerodynamics of the hovering hummingbird. Nature 435, 1094–1097 10.1038/nature03647 (doi:10.1038/nature03647) [DOI] [PubMed] [Google Scholar]
- 41.Dial K. P., Biewener A. A., Tobalske B. W., Warrick D. R. 1997. Direct assessment of mechanical power output of a bird in flight. Nature 390, 67–70 10.1038/36330 (doi:10.1038/36330) [DOI] [Google Scholar]
- 42.Rayner J. M. V. 1991. On the aerodynamics of animal flight in ground effect. Phil. Trans. R. Soc. Lond. B 334, 119–128 10.1098/rstb.1991.0101 (doi:10.1098/rstb.1991.0101) [DOI] [Google Scholar]
- 43.Pennycuick C. J. 1968. Power requirements for horizontal flight in the pigeon Columba livia. J. Exp. Biol. 49, 527–555 [Google Scholar]
- 44.Pennycuick C. J. 1989. Bird flight performance. A practical calculation manual. Oxford, UK: Oxford University Press [Google Scholar]
- 45.Rayner J. V. M. 1999. Estimating power curves of flying vertebrates. J. Exp. Biol. 202, 3449–3461 [DOI] [PubMed] [Google Scholar]
- 46.Hedenström A. 2008. Adaptations to migration in birds: behavioural strategies, morphology and scaling effects. Phil. Trans. R. Soc. B 363, 287–299 10.1098/rstb.2007.2140 (doi:10.1098/rstb.2007.2140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Usherwood J. R., Hedrick T. L., McGowan C. P., Biewener A. A. 2005. Dynamic pressure maps for wings and tails of pigeons in slow, flapping flight, and their energetic implications. J. Exp. Biol. 208, 355–369 10.1242/jeb.01359 (doi:10.1242/jeb.01359) [DOI] [PubMed] [Google Scholar]
- 48.Bundle M. W., Hansen K. S., Dial K. P. 2007. Does the metabolic rate–flight speed relationship vary among geometrically similar birds of different mass? J. Exp. Biol. 210, 1075–1083 10.1242/jeb.02727 (doi:10.1242/jeb.02727) [DOI] [PubMed] [Google Scholar]
- 49.Morris C. R., Nelson F. E., Askew G. N. 2010. The metabolic power requirements of flight and estimations of flight muscle efficiency in the cockatiel (Nymphicus hollandicus). J. Exp. Biol. 213, 2788–2796 10.1242/jeb.035717 (doi:10.1242/jeb.035717) [DOI] [PubMed] [Google Scholar]
- 50.Woledge R. C., Curtin N. A., Homsher E. 1985. Energetic aspects of muscle contraction. London, UK: Academic Press; [PubMed] [Google Scholar]
- 51.Daley M. A., Biewener A. A. 2003. Muscle force–length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J. Exp. Biol. 206, 2941–2958 10.1242/jeb.00503 (doi:10.1242/jeb.00503) [DOI] [PubMed] [Google Scholar]
- 52.Gabaldón A. M., Nelson F. E., Roberts T. J. 2004. Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. J. Exp. Biol. 207, 2277–2288 10.1242/jeb.01006 (doi:10.1242/jeb.01006) [DOI] [PubMed] [Google Scholar]
- 53.Gillis G. B., Biewener A. A. 2001. Hindlimb muscle function in relation to speed and gait: in vivo strain and activation in a hip and knee extensor of the rat (Rattus norvegicus). J. Exp. Biol. 204, 2717–2731 [DOI] [PubMed] [Google Scholar]
- 54.Gillis G. B., Flynn J. P., McGuigan P., Biewener A. A. 2005. Patterns of strain and activation in the thigh muscles of goats across gaits during level locomotion. J. Exp. Biol. 208, 4599–4611 10.1242/jeb.01940 (doi:10.1242/jeb.01940) [DOI] [PubMed] [Google Scholar]
- 55.Warrick D. R., Dial K. P., Biewener A. A. 1998. Asymmetrical force production in the maneuvering flight of pigeons. Auk 115, 916–928 [Google Scholar]
- 56.Berg A. M., Biewener A. A. 2010. Wing and body kinematics of takeoff and landing flight in the pigeon (Columba livia). J. Exp. Biol. 213, 1651–1658 10.1242/jeb.038109 (doi:10.1242/jeb.038109) [DOI] [PubMed] [Google Scholar]
- 57.Berg A. M. 2010. Kinematics, aerodynamics, and neuromuscular function of avian flight: takeoff and landing, ascent and descent. PhD thesis, Harvard University, Cambridge, MA [Google Scholar]