Abstract
Despite continual fluctuations in walking surface properties, humans and animals smoothly transition between terrains in their natural surroundings. Walking transitions have the potential to influence dynamic balance in both the anterior–posterior and medial–lateral directions, thereby increasing fall risk and decreasing mobility. The goal of the current manuscript is to provide a review of the literature that pertains to the topic of surface slope transitions between level and hill surfaces, as well as report the recent findings of two experiments that focus on the neuromuscular strategies of surface slope transitions. Our results indicate that in anticipation of a change in surface slope, neuromuscular patterns during level walking prior to a hill are significantly different from the patterns during level walking without the future change in surface. Typically, the changes in muscle activity were due to co-contraction of opposing muscle groups and these changes correspond to modifications in head pitch. In addition, further experiments revealed that the neck proprioceptors may be an initial source of feedback for upcoming surface slope transitions. Together, these results illustrate that in order to safely traverse varying surfaces, transitions strides are functionally distinct from either level walking or hill walking independently.
Keywords: locomotion, gait, uphill, downhill, proprioception, vestibular
1. Experiment 1: Introduction
Despite continual fluctuations in walking surface properties, humans and animals smoothly transition between terrains in their natural surroundings. A walking transition is the process of moving from an initial condition to a final condition where there is a mandatory change in joint kinematics and muscle activity between conditions. Examples of these walking transitions include modifications to surface slope, navigation of stairs, alterations in stiffness and changes in direction. Walking transitions have the potential to influence dynamic balance in both the anterior–posterior and medial–lateral directions. In fact, previous research has documented walking transitions as the primary cause of falls and a reason for decreased mobility [1–3]. Therefore, understanding the neuromuscular strategies during walking transitions will provide critical information for the fields of rehabilitation, prosthetics and robotics.
The goal of the current manuscript is to provide a review of the literature that pertains to the topic of surface slope transitions (level surface to hill surface) and report the recent findings of two experiments that focus on the neuromuscular strategies of these transitions. First, we recount pertinent past results related to hill walking independently, transitions onto an uphill slope, and the temporal–spatial patterns during both uphill and downhill transitions. Second, we present the findings from Experiment 1, which is a focus on muscle activity and head pitch angle during the anticipation as well as the transition to a sloped surface in adult humans. Third, we briefly summarize past results on how individual sensory systems potentially aid in surface slope transitions. Fourth and finally, we present the findings from Experiment 2, which is an evaluation of the contribution of neck proprioreceptors as well as the vestibular system during hill walking in decerebrate cats. Although our two experimental models differ in obvious and numerous ways, we aim to provide a link between the two protocols that enables us to gain insight into how the sensory systems potentially influence transitions.
The transition from level to hill walking involves a reorientation of the body with respect to gravity. Muscle activity can be altered by this change in position based upon information about orientation of the body with respect to the head from neck proprioceptors and orientation of the head with respect to gravity from vestibular apparatus [4,5]. Thus, both of the current experiments involve evaluating the pitch of the head in order to gain an understanding of how these sensory systems may influence the neuromuscular patterns during surface slope transitions. For the adult human experiments, we wanted to answer the following questions: (i) How many steps prior to an uphill or downhill slope do muscle activity patterns change in anticipation of the new surface? (ii) Do the modifications in muscle activity parallel the timing of modifications to head pitch? For the decerebrate cat experiments, we wanted to answer the following questions: (i) How does head pitch influence uphill and downhill walking? (ii) How does the vestibular system affect muscle activity patterns during hill walking? In short, it is plausible that humans and animals adopt a distinct neuromuscular strategy during the transition strides between a level and a hill surface.
Uphill and downhill walking both require modifications to the typical gait parameters of level walking, such as kinematics, kinetics and EMG. Sun et al. [6] investigated the influence of surface slope on the gait kinematics of 2400 people walking on naturally varying hills in the environment. They found that walking speed is significantly slower during uphill walking when compared with downhill walking [6]. However, not as intuitive, this decreased speed was the product of a significant decrease in step frequency and a slight increase in step length. By contrast, during downhill walking, the main effect was a shorter step length as a means to counteract the reduction in friction. In laboratory settings, similar kinematic trends have been recorded with the addition of joint kinematics. For instance, compared with level walking, during uphill walking, flexion of the hip, knee and ankle joints are greater at heel strike, and extension of these joints is greater at midstance. These kinematic alterations are necessary in order to raise the limb for foot clearance and heel strike. During downhill walking, flexion of the hip is less during late swing and early stance while the flexion of the knee is greater during stance [7,8].
The kinematic data illustrate modifications to body segment configurations, which indicate that both ground reaction forces and joint moments are also altered due to a change in surface slope [9]. Compared with level walking, during both uphill and downhill walking, the normal component of the ground reaction force is similar in shape with differing peak values while the parallel component demonstrates specific, significant differences dependent upon grade. During uphill walking the braking force is less and the propulsive force is greater, whereas during downhill walking the braking force is greater and the propulsive force is less [8,10]. In terms of joint moment kinetics, during uphill walking the hip extensor moment is greater and the flexor moment is delayed. Also, as the uphill slope increases, both the knee extensor and ankle extensor moments are greater than level walking. Directly opposite, during downhill walking the hip extensor moment is delayed, while knee extensor and ankle extensor moments are less [7,11].
Based upon the identifiable kinematic and kinetic patterns during hill walking, EMG data also demonstrate unique patterns [12]. Compared with level walking, during uphill walking, mean activity during stance is greater in each lower leg muscle group with the exception of ankle flexors. During downhill walking, mean activity during stance is greater in hip flexor, knee extensor and ankle flexor muscle groups but less in the ankle extensor muscles. Similarly, compared with level walking, the timing of muscle activity bursts is dependent upon slope. During uphill walking, with the exception of ankle flexors, burst duration is shorter. While during downhill walking, the muscles associated with hip flexion, knee extension and ankle extension demonstrate longer burst duration [13].
Similar EMG patterns during hill walking have been documented in cats [14,15]. Smith and her co-workers [16–19] described the activation patterns in hind limb musculature during a variety of tasks, including walking on slopes. During uphill walking, activation of the ankle extensors and knee extensors is greater due to the higher propulsive force requirements. During downhill walking, the orientation of the limb axis with the gravity vector results in an imposed extensor moment at the hip. This external force requires a change in the balance of required muscle moments such that the activation of the knee flexors is less, and of the hip flexors is greater during stance compared with the conditions for level walking.
Earhart & Bastian [20] and Prentice et al. [21] conducted investigations on the transition between level and sloped surfaces. Earhart & Bastian [20] examined the strategies of taking a single step on an inclined wedge. Each participant walked on a level surface, stepped on an incline wedge with one foot, and continued walking on an elevated level surface. Interestingly, participants used two different strategies dependent upon the slope of the wedge. For the 10° wedge trials, peak hip flexion occurred during mid-swing and peak ankle flexion occurred during late stance, whereas for the 20° and 30° trials, these peak values occurred during heel strike and mid-stance. These individual strategies corresponded to the muscle activity data. As the wedge inclination increased, hip and knee flexor muscles were activated close to heel strike, and ankle flexor activity was prolonged after heel strike. At an intermediate incline of 15°, the participants inconsistently switched between the two strategies. In summary, the neuromechanical and neuromuscular systems were coordinated to provide efficient adaptations for numerous scenarios [20].
Prentice et al. [21] also investigated the transition from a level to an incline surface, but the surface was a ramp. They collected walking kinematic data during the approach to the ramp and during the initial step on the ramp for 3°, 6°, 9° and 12° conditions. Both limb and trunk motions were modified in a scaled fashion in order to navigate surface slope transitions. They observed that participants anticipate the transition by modifying their trunk orientation and leg swing trajectory prior to the first step on the sloped surfaces. Subsequent to the transition, the swing limb response consisted of two specific stages. During the first step, the swing leg trajectory was exaggerated for the present slope. While during the second step, the trajectory was specific for the present slope. So the first step ensured foot clearance while the second step tailored the trajectory for the new surface slope [21].
Past studies differentiate between gait strategies by evaluating temporal–spatial parameters, such as speed, step length, stance time and step width [22–26]. For instance, in an effort to improve anterior–posterior balance, adults walk with a slower speed, shorter step length and greater stance time [26,27]. In order to enhance medial–lateral balance, adults walk with a wider step width by maintaining a larger distance between the heels or toes [26,28,29]. Together, changes in the mean magnitude of these parameters illustrate that individuals modify their gait patterns as a precaution to a future decrease in balance. Moreover, changes in stride-to-stride variability of speed, step length and stance time illustrate that individuals modify their patterns as a reaction to a current decrease in balance [26]. Thus, when comparing temporal–spatial gait strategies between strides of level, hill and transition walking, it is important to examine both mean differences as well as variability differences.
We recently analysed modifications in temporal–spatial parameters during hill walking transitions [30]. We hypothesized that in comparison with level walking, the transition strides (toe-off on level to toe-off on hill), from a level surface to an uphill surface (L-UP) or to a downhill surface (L-DN), would indicate the adoption of a distinct gait strategy with a larger base of support (BOS). Twenty men and 20 women completed level and hill trials on a walkway with a 15° portable ramp apparatus. We collected data during the transition strides between level and hill surfaces as well as the level and hill surfaces independently.
We evaluated speed, step length and stance time as well as heel and toe step width during double support. In addition, we calculated the total area between both feet as a measure of BOS. Finally, we evaluated the variability of speed, step length and stance time by calculating the standard deviation between trials of each condition for each participant. This measure was used to evaluate within-subject, trial-to-trial, variability as opposed to subject-to-subject variability, which is represented for each variable as standard deviation.
Our results illustrated that healthy, young adults did adopt a distinct gait strategy different from both level and hill walking during all of the transition strides (table 1 and figure 1). During the L-DN transition, speed, step length and stance time were significantly less than level walking while variability, step width and BOS were all greater. During the L-UP transition, speed was less than level walking, and step length variability and BOS were both greater. In sum, we accepted our hypothesis that transition strides were unique compared with both level and hill walking strides.
Table 1.
Temporal–spatial parameters for the transition and hill walking conditions. All data are represented as the mean raw values of each trial averaged for all 40 participants, mean (standard deviation). Italicized values represent level walking. Bold values represent a statistically significant difference when compared with level walking, p < 0.05 (modified from Gottschall et al. [30]).
| dependent variable | transition stride |
||||
|---|---|---|---|---|---|
| L-DN | DN | level | UP | L-UP | |
| (a) speed (m s−1) | 1.25 (0.17) | 1.33 (0.19) | 1.39 (0.12) | 1.25 (0.13) | 1.36 (0.16) |
| step length (cm) | 66.9 (12.9) | 65.1 (9.5) | 73.0 (5.5) | 70.6 (5.7) | 79.6 (8.5) |
| stance time (ms) | 612 (17) | 622 (30) | 631 (20) | 648 (15) | 633 (22) |
| (b) speed variability (m s−1) | 0.1 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.03 (0.02) |
| step length variability (cm) | 3.2 (1.1) | 1.9 (0.0) | 1.8 (0.8) | 3.1 (1.2) | 2.5 (1.4) |
| stance time variability (ms) | 14 (6) | 13 (7) | 11 (5) | 12 (8) | 13 (5) |
| (c) step width heel (cm) | 11.9 (2.9) | 10.7 (2.9) | 10.2 (2.9) | 10.2 (3.8) | 11.2 (3.7) |
| step width toe (cm) | 8.9 (5.3) | 8.8 (5.9) | 6.9 (4.8) | 5.8 (4.7) | 6.1 (4.5) |
| BOS area (cm2) | 416.2 (145.8) | 384.7 (163.4) | 364.5 (132.2) | 421.7 (153.0) | 427.7 (160.9) |
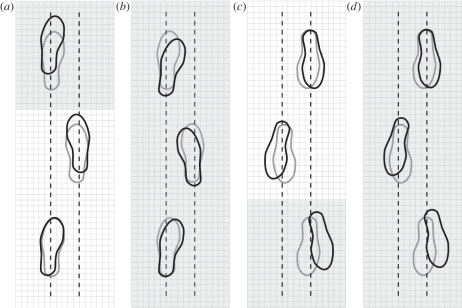
Figure 1.
Illustration of level walking strides (grey feet) and non-level strides (black feet) for (a) level surface to an uphill surface (L-UP), (b) uphill surface only (UP), (c) level surface to a downhill surface (L-DN) and (d) downhill surface (DN) only. Shaded regions represent the hill surface and non-shaded regions represent the level surface. The foot placement locations represent actual changes in step length as well as step width at the heel and toe. Each small box is equal to a 3 × 3 cm square (modified from Gottschall et al. [30]).
To begin, during the L-DN transition speed was significantly slower due to a shorter step length than level walking, while during the L-UP transition step length was significantly longer. Chen et al. [31] observed that clearing an obstacle of minimal height resulted in a 10 per cent greater step length and this length increased more as the obstacle height increased [31]. These results correspond closely to the 11 per cent longer step length during the L-UP condition because of the increase in toe clearance owing to the edge of the ramp.
Step length variability was also significantly greater during L-DN and L-UP compared with level walking (table 1). Chen et al. [31] reported that step length variability was greater when stepping over an obstacle and Maki [26] concluded that this increase was an indication of increased fall risk. Therefore, it is probable that participants perceived these strides as an obstacle and they attempted various strategies to maintain anterior–posterior balance during the transition.
Step width at the heel and toe exhibited significant changes in multiple conditions. Step width at the heel was wider during L-DN as participants adopted an increased BOS to improve medial–lateral balance. It is possible that there are metabolic determinants to these step width modifications. Donelan et al. [32] manipulated step width while measuring metabolic cost. They concluded that walking adults prefer a step width that minimized metabolic cost while maintaining balance. So it appears that during L-DN, the participants prioritized the maintenance of balance over the minimization of metabolic cost.
By contrast, toe step width was significantly smaller during both the L-UP and UP conditions. It is possible that this modification is a strategy used to improve propulsion. Erdemir & Piazza [33] support this idea and stated that internal or external rotation of the foot has the potential to modify the force-generating capacity of the ankle extensors. Previous research has evaluated the effects of toe position and speed. For example, Ho et al. [34] discovered a correlation between narrow toe position and faster walking speed in children. It appears that the participants prioritized propulsion over medial–lateral balance during these conditions, as the BOS was smaller than during level walking.
Experiment 1 investigates two questions: How many steps prior to an uphill or downhill slope do muscle activity patterns change in anticipation of the new surface? Do the modifications in muscle activity parallel the timing of modifications to head pitch?
Our primary rationale for Experiment 1 is that evaluating the temporal–spatial muscular patterns and head pitch angles during the anticipation strides will indicate the required time course and potential sensory mechanisms necessary for appropriate transitions. According to our previous results, we hypothesized that in comparison with level walking, speed would be slower and variability would be greater during the downhill anticipation strides while step length variability would be greater during the uphill anticipation strides. In addition, due to the mandatory modifications in muscle activity between level and hill walking, we hypothesized that the anticipation strides would be significantly different from both level and hill walking independently.
2. Experiment 1: Methods
Ten men and 10 women (age = 21.7 ± 2.2 yr, height = 1.72 ± 0.1 m, mass = 70.0 ± 13.2 kg, mean ± s.d.) completed the protocol. Prior to involvement, each participant confirmed that they had no muscular, joint or neurological disorders that would prevent them from recreational walking for 30 min. All of these healthy students gave written informed consent that followed the guidelines of The Pennsylvania State University Human Research Committee.
Each participant completed a standing trial and a series of randomly assigned walking conditions on the level and hill surfaces. All of the walking trials were completed at a self-selected velocity along a 25 m walkway. We used a custom-built portable apparatus composed of a 2.4 m ramp inclined at 15° continuous with a 4.8 m plateau. The minimum total walking distance was 14.2 m.
A complete dataset was comprised of the successful completion of five level walking trials and 40 hill walking trials. Due to the limited collection volume of the motion analysis system, we shifted the apparatus to collect the appropriate stride. We collected five walking trials during the two hill only strides, downhill ramp (DN), and uphill ramp (UP); two transition strides, level plateau to downhill ramp (L-DN) and level floor to uphill ramp (L-UP); and also as two anticipation strides six and four steps away from both the uphill (6-UP, 4-UP) and downhill (6-DN, 4-DN) ramp. A trial was defined as successful if a full stride of the left leg from toe-off to toe-off was captured in the collection volume. A transition stride was defined to include the left toe-off and right foot contact on the original surface and the left foot contact (step 1) and right foot contact (step 2) on the transition surface. A stride was defined in this way in order to evaluate step length and step width during the first double support after the transition. Participants were instructed to begin walking with the left or right leg at the start of each trial to adjust for changes in stride characteristics between conditions.
We collected kinematic data with a six-camera, passive marker three-dimensional photogrametry system (Motion Analysis Corporation, Santa Rosa, CA, USA). The calibration residual was less than 0.5 mm in a capture volume of approximately 2 × 2 × 2 m. Prior to data collection, we placed retroreflective markers bilaterally on the temporal fossa, mastoid process, medial clavicle, anterior superior iliac spine, lateral femoral epicondyle, lateral malleolus, posterior calcaneous, fifth metatarsal and first metatarsal. We also placed a single marker on the sacral crest and seventh cervical spine. For the current study, we measured the head pitch angle between the head and trunk as two segments: the head segment between the temporal fossa and mastoid process and the trunk segment between the seventh cervical spine and the medial clavical. We collected the marker data at 100 Hz and post-processed the data with EVaRT software (v. 3.21, Motion Analysis Corporation). A purpose-written Matlab program (v. R2006b, The Mathworks, Natick, MA, USA) was used for subsequent data processing that included a low-pass filter for the marker trajectories at 7 Hz (fourth-order, dual-pass, Butterworth).
We measured EMG signals using a telemetered amplifier system (Bortec Octopus AMT-8, Calgary, AB, Canada). Prior to electrode placement, we prepared the skin with fine sandpaper and rubbing alcohol. We placed bipolar, silver–silver chloride, surface electrodes (Vermed, A10 041, Bellows Falls, VT, USA; 1 × 1.5 cm rectangles) over four muscles (lateral gastrocnemius (LA), tibialis anterior (TA), rectus femoris (RF) and biceps femoris (BF)) of the left leg according to the recommendations by Cram & Kasman [35]. We verified that the cross talk between muscles was negligible with a series of contractions suggested by Winter et al. [36] and Cram & Kasman [35].
To analyse the EMG data, we created linear envelopes by first high-pass filtering the data at 20 Hz, full wave rectifying, then low-pass filtering at 5 Hz [37]. Each stride was further divided into seven time segments. Swing phase was then categorized into: (i) initial swing from 0 to 34 per cent of swing phase, (ii) mid-swing from 34 to 60 per cent of swing phase, (iii) terminal swing from 60 to 100 per cent of swing phase. Stance phase was categorized into: (iv) initial stance from 0 to 20 per cent of stance phase, (v) mid-stance from 20 to 57 per cent of stance phase, (vi) late stance from 57 to 81 per cent of stance phase, and (vii) terminal stance from 81 to 100 per cent of stance phase. For each participant, we normalized the mean EMG amplitude (mEMG) for each experimental condition based on the mEMG for the level walking condition.
All data were analysed across transition conditions using a repeated measures design (ANOVA). Where appropriate, we performed Newman–Keuls post hoc tests to analyse the differences between conditions and reported all values as mean ± standard deviation. Significance was defined as p < 0.05.
3. Experiment 1: Results
The temporal–spatial results indicate that healthy young adults begin to transition as early as six steps prior to the future hill. The anticipatory adjustments were particularly evident during the downhill conditions. In comparison with level walking, speed was significantly slower, stance time variability was less and toe step width was wider (table 2, all values, p < 0.05). These details illustrate that the participants consistently walked cautiously in approach of the downhill slope. The adjustments were different during the uphill conditions where stance time variability was greater and the BOS was greater. Another interesting change was a significant increase in step length four steps from the ramp. It is possible that four steps prior to the ramp are crucial in term of assuring an optimal distance for a safe transition step.
Table 2.
Temporal–spatial parameters for the two anticipation walking conditions. (a) speed, step length and stance time; (b) variability calculated as the between-trial standard deviation of each participant for speed, step length variability and stance time; (c) step width at the heel and toe, and base of support area. All data are represented as the mean raw values of each trial averaged for all 20 participants, mean (standard deviation). Italicized values represent level walking. Bold values represent a statistically significant difference when compared with level walking (p < 0.05).
| dependent variable | anticipation stride |
||||
|---|---|---|---|---|---|
| 6-DN | 4-DN | level | 4-UP | 6-UP | |
| (a) speed (m s−1) | 1.38 (0.17) | 1.29 (0.17) | 1.42 (0.11) | 1.41 (0.19) | 1.39 (0.18) |
| step length (cm) | 74.6 (3.7) | 73.9 (5.1) | 73.5 (3.9) | 77.9 (6.8) | 72.3 (4.8) |
| stance time (ms) | 611 (24) | 626 (29) | 622 (17) | 621 (15) | 630 (19) |
| (b) speed variability (m s−1) | 0.04 (0.02) | 0.04 (0.01) | 0.04 (0.01) | 0.03 (0.01) | 0.02 (0.02) |
| step length variability (cm) | 1.2 (1.1) | 1.8 (0.0) | 2.1 (0.8) | 2.1 (1.4) | 1.7 (1.2) |
| stance time variability (ms) | 6 (6) | 6 (7) | 10 (5) | 14 (5) | 16 (8) |
| (c) step width heel (cm) | 10.4 (3.0) | 9.0 (1.1) | 9.6 (2.5) | 9.7 (4.1) | 10.2 (4.2) |
| step width toe (cm) | 9.9 (3.9) | 9.7 (3.5) | 7.3 (2.5) | 6.9 (5.7) | 7.7 (3.9) |
| BOS area (cm2) | 371.2 (156.4) | 341.1 (161.0) | 369.3 (129.1) | 476.3 (118.3) | 487.5 (109.7) |
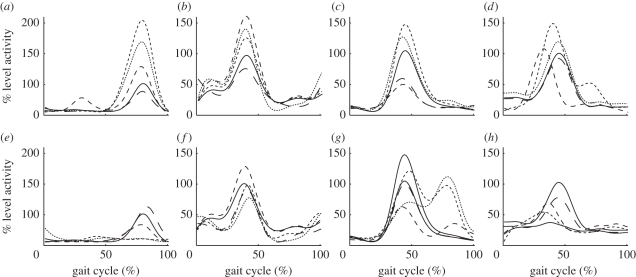
With minimal kinematic changes, the original assumption would be that net joint moments would not be significantly different, however it is possible that the same net moments could be produced with greater co-contraction, resulting in greater joint stiffness and perturbation responses. The muscle activity data indicate that the anticipation strides as well as the transitions strides are distinct from both level and hill walking (figure 2). For the uphill conditions, LG activity was significantly greater than level walking during stance of 4-UP, L-UP and UP with the highest activity during L-UP (figure 2a, all values p < 0.01). For the downhill conditions, LG activity was significantly less during L-DN and DN (figure 2e, all values p < 0.05). Similarly, TA activity was also greater for the uphill conditions than level walking during 4-UP, L-UP and UP during terminal swing and early stance while the only difference during the downhill conditions was a greater activity during 4-DN during terminal swing (figure 2b,f; all values p < 0.05). For the RF, activity was significantly greater for terminal swing and early stance of the uphill conditions L-UP and UP, and less during 6-UP and 4-UP (figure 2c, all values p < 0.05). By contrast, there was an additional burst of activity for the RF during mid- to late stance during L-DN and DN (figure 2g, all values p < 0.01). Finally, for the BF, activity was significantly greater during L-UP and UP during initial stance and significantly earlier during 4-UP while the only significant difference during the downhill conditions was during DN (figure 2d,h, all values p < 0.05).
Figure 2.
Muscle activity mean envelope of all 20 participants for the (a,e) lateral gastrocnemius (LG), (b,f) tibialis anterior (TA), (c,g) rectus femoris (RF) and (d,h) biceps femoris (BF) during level walking (L, solid lines), six steps before the slope (6-UP/DN, long dashed lines), four steps before the slope (4-UP/DN, medium dashed lines), transition onto the slope (L-UP/DN, short dashed lines), and the stride on the slope (UP/DN, dotted lines) for the (a–d) uphill and (e–h) downhill conditions.
Figure 3.
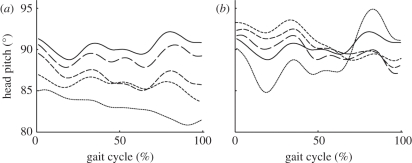
Mean head pitch angle of all 20 participants during level walking (L, solid lines), six steps before the slope (6-UP/DN, long dashed lines), four steps before the slope (4-UP/DN, medium dashed lines), transition onto the slope (L-UP/DN, short dashed lines), and the stride on the slope (UP/DN, dotted lines) for the (a) uphill and (b) downhill conditions.
Similar to the muscle activity data, the mean head pitch angles demonstrate that the anticipation and transition strides are significantly different from level walking (figure 3). The mean pitch angle during level walking was 91.1° while the mean pitch angle during uphill walking was 83.7° (figure 3a). Overall, during the uphill anticipation strides, the mean head pitch was less than level walking. To add, as the participants approached the slope, the head pitch angle decreased and reached a minimum as the participants completed a full stride on the ramp (p < 0.05). On the other hand, the mean angle during downhill walking was 87.1° but at the beginning of the stride the angle was 90.1° and increased to 92.4° (figure 3b). When the participants completed a full stride on the ramp in the downhill direction, the head pitch angle was significantly greater than level walking (p < 0.05). Overall, it appears that these modifications to head pitch stabilize the head with respect to ground or the gravity vector, thereby maintaining a constant input to the otolith organs.
4. Experiment 1: Discussion
Together, our muscle activity and head pitch data indicate that the neck proprioceptors as well as the vestibular system may aid in the adaptation of transition strides. Beginning with the initiation of walking, the participants could see the ramp apparatus. During the uphill condition, head pitch was less than level walking beginning four steps prior to the ramp. This modification in head pitch corresponded to greater co-activation of the LG and TA in preparation for the future increase in propulsive demands. Head pitch continued to decrease throughout the transition as well as during uphill walking. Similarly, during the downhill conditions, head pitch was also less during the anticipation and transition strides but was greater during downhill walking. So head pitch is potentially used as a means to modify gaze during the preparation for the hill during level walking as well as to stabilize the vestibular system during hill walking.
Another example of modifications in neuromuscular strategies with a known perturbation is anticipated tripping. Pijnappels et al. [38] investigated the temporal–spatial patterns of young adults during walking after they had been warned about the possibility of a trip. Compared with the control, level walking, there were no differences in walking speed, step length or stance time, but step width and minimum foot clearance were slightly, yet significantly greater. In a subsequent study, Pijnappels et al. [39] collected EMG data during walking on level ground, immediately after a trip, and several trials after a trip occurred. Similar to the kinematic results, they recorded small but statistically significant changes compared with the level condition. Semitendinosus (ST), RF, vastus lateralis (VL) and TA activity were greatest during the trials immediately after a trip, illustrating a cautious strategy with co-contraction. Both the current study and these past studies demonstrate how level walking in unique scenarios can elicit cautious neuromuscular strategies despite the fact that the participants are, in fact, walking on level ground.
Our results suggest that head pitch influences the gain of the lower limb musculature and aids in the transition between level and hill surfaces. Individuals with increased neck tone and decreased neck mobility may suffer additional challenges during walking on non-level surfaces. For instance, individuals with Parkinson's disease rotate the head and the trunk simultaneously during walking and turning [40–42]. To add, Franzen et al. [43] correlated neck tone and walking performance on six functional tests. For individuals with Parkinson's disease, greater neck tone corresponded to greater variability and therefore balance was compromised. An inability to freely modulate the position of the head independent of the trunk may reduce the mobility in Parkinson's disease patients as well as individuals with reduced control of the head position.
5. Experiment 2: Introduction
The visual system typically dominates the alternative sensory systems during walking when exploring the environment [44,45]. Vision allows walking adults to anticipate necessary changes in gait based upon the location of the new surface [46]. Warren et al. [47] added to the principle by concluding that optic flow during movement is a key component during walking in complex environments. The critical time period for this anticipatory modulation of the walking pattern appears to occur at least two steps ahead of the obstacle or perturbation [48]. This is an appropriate time frame for the amendment of the swing leg trajectory in order to maintain dynamic balance [49].
Numerous studies have demonstrated that the vestibular system also aids in the maintenance of balance as well as directional tuning during walking [50]. Fukuda [51] discovered that when individuals with vestibular neuritis closed their eyes while stepping in place, the body would rotate in the direction of the deficit [51]. The significance of vestibular tone apparently decreases with either increasing step frequency or increasing speed [52–54]. Dietz and co-workers attributed this decline in vestibular input to spinal cord mechanisms [55,56]. Thus, the vestibular system may be an additional factor in the maintenance of dynamic balance especially in moderate speed walking scenarios that involve a change in head pitch.
Head pitch, defined as the angle between the head and trunk segments, contributes both to the feedback of various sensory systems as well as stability of the head in space. In short, head pitch assists in dynamic balance during locomotion in addition to providing compensation of trunk motion [57]. For example, the position of the head will influence the direction of vision, the activity of the otolith organs and semicircular canals, and stimulation of the neck proprioreceptors. Cromwell et al. [58] documented the sagittal plane kinematics of the head, neck and trunk during locomotion. They demonstrated that head pitch had the greatest variability of the three segments and that the neck was typically extended in opposition to the flexed trunk. During level walking, the extended neck causes a flexed head position in order to maintain the orientation of the head within the BOS. In addition, the flexed head aids in gaze stabilization and vestibular function. Pozzo et al. [59] estimated that this combination of head flexion, neck extension and trunk flexion optimizes the feedback from the horizontal semicircular canals and otolith organs.
Several investigators have examined similar locomotor paradigms and provided justification regarding these neural mechanisms for slope transitions. Recently, af Klint et al. [60] concluded that both cutaneous and proprioceptive afferent fibres provide appropriate within-step information as participants walked over an uneven support surface [50]. More specifically, numerous studies have concluded that stretch reflexes in the lower limbs are a primary source of rapid feedback by applying perturbations to the ankle [61,62] and hip [63]. Earlier, Roberts [64] demonstrated that neck proprioceptors as well as the vestibular apparatus initiate modifications in muscle activity by pitching the surface or pitching both the surface and the head. Hence, it is realistic to suggest that limb and neck proprioceptors, as well as the vestibular afferents contribute to the initiation of appropriate muscular patterns during surface slope transitions.
Our past data on decerebrate cats demonstrated that the position of the head aids in the generation of these smooth transitions [65]. We manipulated head pitch during level walking and the EMG results resembled previous research on muscle patterns of intact cats during hill walking. For instance, muscle activity during level walking with a head pitch nose down mimicked uphill walking. Muscle activity during level walking with a head pitch nose up mimicked downhill walking. These alterations in muscle activity occurred within one step of altering the head pitch but dissipated as level walking continued. In summary, the time course of muscle activity patterns due to modifications in head pitch is immediate and transitory. We therefore concluded that, since body orientation had not changed, the vestibular apparatus and neck proprioceptor afferent signals eventually cancelled each other. In other words, the transient effect was presumably an immediate response triggered by the neck afferents, which was then cancelled by a delayed, yet prolonged, signal from the vestibular system.
Experiment 2 investigates two questions: How does head pitch influence uphill and downhill walking? How does the vestibular system affect muscle activity patterns during hill walking?
Our primary rationale for the second experiment is that evaluating the time course of modifications to treadmill slope and head pitch on muscle activity patterns will indicate the sequence of effects from the individual sensory systems. According to our previous results, we hypothesized that when we pitched the treadmill without manipulating head position, the appropriate muscle activity for hill walking would be delayed but prolonged. In addition, our secondary rationale is that evaluating the muscle activity of decerebrate walking cats while head pitch is manipulated following a bilateral labyrinthectomy will indicate if the neck proprioceptors can independently regulate the appropriate walking patterns. Since the putative body orientation signal is composed of both neck and vestibular afferent information, we hypothesized that when we remove the vestibular apparatus, the neck proprioceptors could initiate the immediate but transient appropriate muscle activity similar to hill walking.
6. Experiment 2: Methods
Six decerebrate cats walked at 0.7 m s−1 on a treadmill positioned level, 50 per cent uphill or 50 per cent downhill, with the head pitch either parallel to the treadmill, 50 per cent pitch nose down or 50 per cent pitch nose up. We collected EMG data from five hindlimb muscles. At the conclusion of these initial experiments, we successfully performed a bilateral labyrinthectomy on three of the animals and subsequently collected additional walking data. The Emory University Institutional Animal Care and Use Committee approved all the procedures used in this study.
For each experiment, we started by anaesthetizing the cat with isoflurane and inserting EMG electrodes. The electrodes were 38 Gauge braided and coated, stainless steel wire with approximately 1 mm of the Teflon insulation removed. We tied the wires in staggered fashion to prevent contact of the de-insulated ends, and inserted the wires into each muscle using a needle and the long end of the suture. We implanted these wires in select hindlimb muscles: iliopsosas (ILIO; hip flexor), anterior BF (aBF; hip extensor), anterior semimembranosus (aSM; hip extensor), ST (hip extensor and knee flexor) and VL (knee extensor). The EMG electrodes were connected to the custom preamplification stage using miniature gold connectors.
Following EMG electrode insertion, the cat was positioned over a treadmill in natural stance with the head fixed in a sterotaxic frame and hip height controlled with a clamp attached to the base of the tail. Next, we performed a premammillary decerebration [65]. The brainstem was transected rostral to the superior colliculus, at approximately a 45° angle, in order to preserve the mammillary bodies and subthalamic nucleus. All brain matter rostral to the transection was removed. Following the gradual reduction of anaesthesia until discontinuation, lasting approximately an hour, we imposed a rhythmic pattern by turning on the treadmill. In order to manipulate head pitch, the stereotaxic frame was mounted on a pivot located at the base of the skull at the atlanto-occipital joint. In order to manipulate treadmill pitch, the treadmill frame was mounted at the appropriate angle with a custom built wooden lift secured to the frame with c-clamps.
After the initial head pitch and treadmill hill protocol, we performed a bilateral labyrinthectomy on each of the animals. We temporarily removed the head of the animal from the stereotaxic frame and manually held the head at the base of the skull. Next, we drilled a first hole into the external auditory meatus at a 90° angle followed by a second hole into the cranium tympanic bulla beginning approximately half a centimetre inferior at a 45° angle superiorly and medially. Following the experiment termination, we verified that performing a dissection destroyed the vestibular apparatus.
We processed the EMG data in two stages. First, we determined when in a stride the muscles were active, and second, we quantified the magnitude of activation. After collection at a rate of 2000 Hz, the data were band-pass filtered to retain frequencies between 10 and 500 Hz. A Matlab program was created to determine the difference between the baseline and periods of muscle activity. We defined a period as active if the EMG activity exceeded a threshold of two standard deviations above the baseline mean for at least 50 ms. For the amplitude analysis of the active periods, we full-wave rectified the band-pass-filtered signals and calculated the mEMG.
We analysed EMG data, separated into swing and stance activity, across all conditions using a 7-level one-way repeated measures analysis of variance (ANOVA). Additionally, we performed a Newman–Keuls post hoc test to analyse the differences between each experimental condition. Significance was defined as p < 0.05.
7. Experiment 2: Results
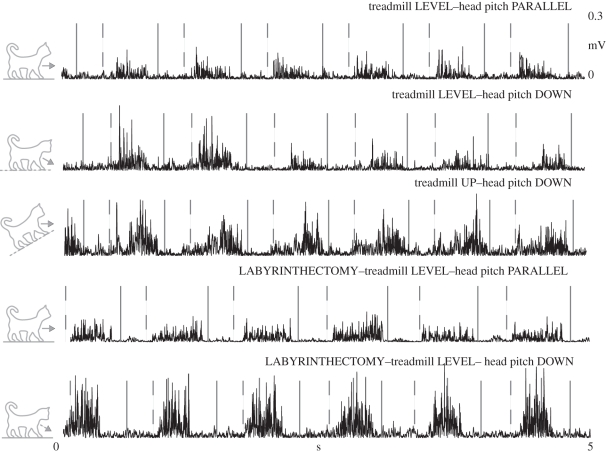
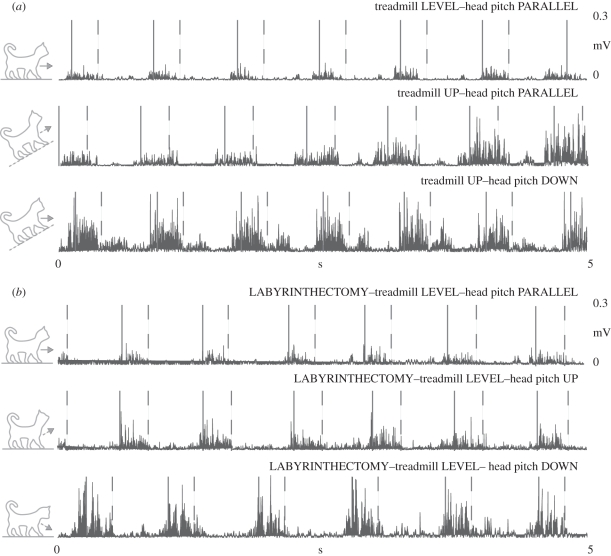
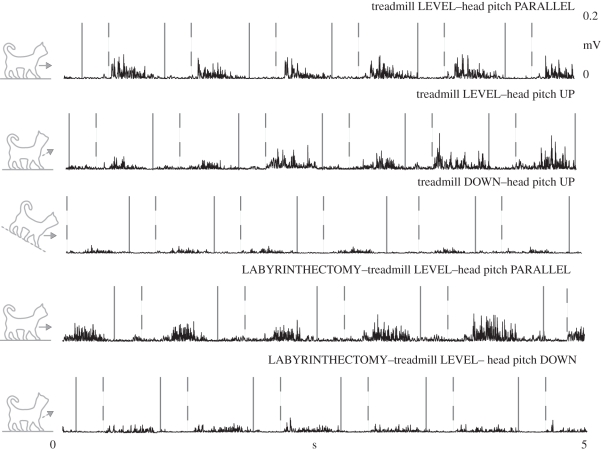
As we hypothesized, during uphill and downhill walking with the head pitch parallel, the muscle activity did not initially correspond with the previous reports of hill walking (table 3 and figures 4 and 5). However, after approximately three steps, the muscle activity began to resemble typical slope traces. Identical to our previous study, our level data immediately after we manipulated head pitch were also equivalent to the research on hill walking (figures 4–7). Subsequent to the labyrinthectomy, our level data for decerebrate walking cats immediately after we manipulated head pitch were surprisingly equivalent to the past slope data for intact cats (figures 5–7).
Figure 6.
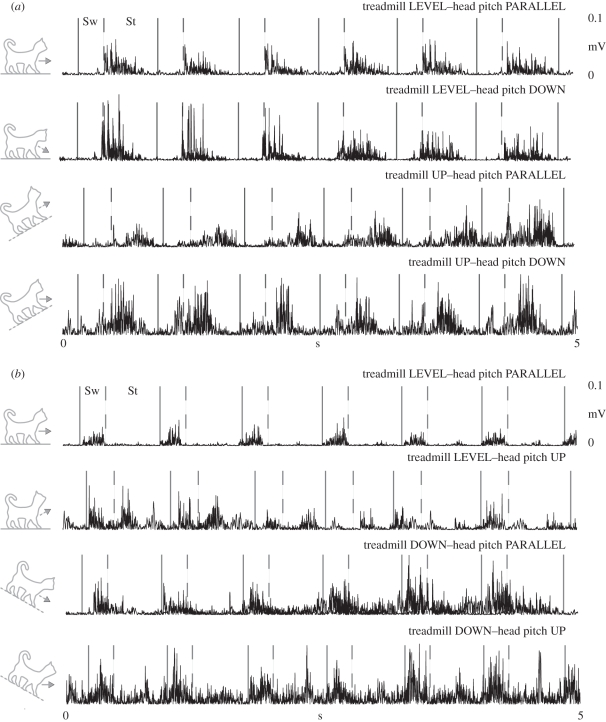
Typical EMG records from a representative vastus lateralis (VL) muscle before and after a labyrinthectomy. The area following the solid line and prior to the dashed line is swing (Sw), while the area following the dashed line is stance (St).
Table 3.
Values are mean + standard deviation for the active periods of the first step of three conditions, with three trials for each animal. Bold values represent a statistically significant difference when compared with level walking with the head pitch parallel, p < 0.05. Each column label is an indication of the treadmill pitch followed by the head pitch. For example, up-dn denotes when the treadmill was pitch up and the head was pitch down.
| muscle | transition condition |
|||||
|---|---|---|---|---|---|---|
| up-dn | post level-dn | level-dn | level-up | post level-up | down-up | |
| aSM | 258.95 (21) | 268.47 (37) | 272.97 (12) | 5.94 (5) | 25.63 (23) | 52.58 (18) |
| ILIO—swing | 198.52 (13) | 142.78 (23) | 140.96 (16) | 93.97 (9) | 101.27 (24) | 98.56 (13) |
| ILIO—stance | 65.84 (5) | 72.58 (33) | 64.28 (13) | 850.19 (132) | 752.65 (204) | 923.41 (98) |
| ST—swing | 265.32 (22) | 258.91 (47) | 232.30 (31) | 67.69 (12) | 65.29 (24) | 65.58 (15) |
| ST—stance | 140.25 (10) | 135.51 (21) | 129.78 (9) | 93.61 (16) | 83.57 (17) | 81.52 (12) |
| VL | 242.23 (22) | 196.62 (26) | 212.65 (18) | 65.82 (16) | 98.65 (34) | 78.25 (23) |
| aBF | 142.59 (12) | 137.84 (27) | 145.45 (15) | 6.21 (4) | 36.95 (13) | 48.99 (5) |
Figure 4.
Typical electromyography (EMG) records from a representative (a) anterior semimembranosus (aSM) and (b) iliopsosas (ILIO) muscle. The area following the solid line and prior to the dashed line is swing (Sw), while the area following the dashed line is stance (St).
Figure 5.
Typical EMG records from a representative semitendinosus (ST) muscle (a) before and (b) after a labyrinthectomy. The area following the solid line and prior to the dashed line is swing (Sw), while the area following the dashed line is stance (St).
Figure 7.
Typical EMG records from a representative BF muscle before and after a labyrinthectomy. The area following the solid line and prior to the dashed line is swing (Sw), while the area following the dashed line is stance (St).
The aSM, typically active during stance, demonstrated the transient effect of head pitch and the delayed effect of treadmill pitch during uphill conditions (table 3 and figure 4a). For instance, specific to the transient effect of head pitch, aSM muscle activity during level walking with a 50 per cent head pitch down immediately mimicked uphill walking for two steps. But after these initial steps, the elevated stance activity decreased back to a level pattern. Specific to the delayed effect of treadmill pitch, aSM muscle activity during uphill walking with the head parallel did not resemble typical uphill walking patterns until at least the third step. On the other hand, aSM activity during uphill walking with a 50 per cent head pitch down mimicked uphill walking for the entire trial. Similarly, ILIO, aBF, ST and VL all demonstrated appropriate uphill walking patterns immediately after the treadmill was sloped with the head pitch down (table 3).
Likewise, the ILIO elicited the transient effect of head pitch and delayed effect of treadmill pitch during downhill conditions (table 3 and figure 4b). For example, ILIO muscle activity during level walking with a 50 per cent head pitch up immediately mimicked downhill walking patterns for two steps, which included stance phase activity unique to the decline. Again, these patterns were not permanent, the stance phase activity returning to quiescence after less than 2 s after the head pitch. During downhill walking with head pitch parallel, the ILIO muscle activity did not resemble typical downhill walking patterns until at least the third step. Equivalent to the uphill results of aSM, ILIO activity during downhill walking with the 50 per cent head pitch up mimicked downhill walking for the entire trial. Likewise, aBF, ST, aSM and VL all demonstrated appropriate downhill walking patterns immediately after the treadmill was sloped with the head pitch up (table 3).
Finally, the labrinthectomy protocol did not influence the immediate effect of our data during level walking with (figures 5–7). The ST, VL and aBF demonstrated the similar transient effects of head pitch and the delayed response of treadmill pitch. In detail, muscle activity during level walking with the head parallel did not differ between the pre- and post-labrinthectomy trials with the exception of small increases in background activity. Interestingly, the transient effect of the head pitch protocol was actually extended following the labrinthectomy, as the magnitude of the muscle activity did not return to level values for five to seven steps.
8. Experiment 2: Discussion
The mechanisms by which these changes in muscular activity occur are unknown, but it seems likely that some combination of the various sources of sensory feedback would influence the resulting patterns. Since the transition from level to hill walking involves a reorientation of the body with respect to gravity, signals representing body orientation could contribute to the altered motor patterns. This signal can, in principle, be derived from information about orientation of the body with respect to the head from neck proprioceptors and orientation of the head with respect to gravity from the vestibular apparatus. Integration of these two signals during walking transitions can provide an estimate of body orientation with respect to the gravitational vector [5].
In terms of timing, muscle activity patterns due to modifications in head pitch are immediate and transitory. On the contrary, muscle activity patterns due to modifications in treadmill pitch are delayed and prolonged. For instance, during uphill and downhill walking, when the head pitch was parallel to the treadmill muscle activity patterns did not immediately resemble the optimal intact cat hill walking patterns. To add, following the labyrinthectomy, it appears that the neck proprioceptors independently initiated modifications to muscle activity patterns appropriate for hill walking. In detail, following removal of the vestibular organs, altering head pitch during decerebrate cat level walking causes significant muscle activity pattern changes that resemble intact cat hill walking muscle activity patterns.
Our results demonstrated that vestibular information directly contributes to the prolonged regulation of central pattern generation, but our results did not demonstrate whether cutaneous receptors or limb proprioceptors contribute to pattern generation. These afferents could certainly provide information about body orientation, which is known to influence central pattern generation. In fact, contribution from cutaneous and limb afferents is probable since uphill and downhill walking alters both the direction and the magnitude of limb forces [66], and head pitch could transmit a change in force to the limbs. However, our data indicate that neck and vestibular afferent information is the primary source of sensory information in this context. First, the positions of the limbs were unaltered during our head pitch or hill conditions, so feedback regarding body position from the limbs was not modified. Second, the delayed effect of head pitch corresponded to the response to treadmill slope and the delayed effect was eliminated by the labyrinthectomy. Thus, it is unlikely that cutaneous and limb afferents are responsible for the changes in muscle activity during our experimental conditions. But, in natural surroundings, forces and joint angles would probably change for the different tasks, and sensory information from the limbs would thereby govern pattern generation as well.
Our data illustrate the effect of neck proprioceptors on muscle activity during walking, which is similar to early findings during postural tasks. Magnus & de Kleijn [67] collected muscle activity data on standing cats. They performed a bilateral labyrinthectomy and manipulated head pitch to examine the independent influence of the neck proprioceptors. A head down orientation triggered increased muscle activity in the hind limbs. By contrast, a head up orientation triggered increased activity in the forelimbs [67]. Together our data demonstrate that the neck proprioceptors can modulate muscle activity patterns in the limbs without vestibular organ feedback. In other words, these afferents are potentially regulating the central pattern generator.
With respect to our conclusion regarding the neck proprioceptors, we do not dismiss the functional importance of the vestibular afferents and recognize the constraints of our protocol. One interpretation is that this delay in the vestibular response is due to the fact that the experimenter imposed the head movements. In voluntary head movements, associated motor commands to the brainstem could result in more complete cancellation of the two signals. Alternatively, the advanced neck afferent signal could provide an initial priming of the change in motor pattern. The summation of these two signals solves the problem identified by Holst [68] and Holst & Mittelstaedt [69]. Since voluntary head movements would result in equivalent changes in both signals, the subtracted value representing body orientation would remain constant for that particular slope and direction. These interactions were further quantified by work of Boyle & Pompeiano [70,71] and Ezure & Wilson [72]. Also, the interaction of the neck and vestibular afferents could, in principle, provide an estimate of body orientation in space to the spinal cord or the cerebellum [4,5,73]. We suggest that this estimate of body orientation reaches the spinal central pattern generators and modulates activity in the appropriate way, perhaps through the pattern formation network of the spinal central pattern generator [74]. If, as is often the case, the animal maintains its head level with respect to gravity, then body orientation is coded by feedback only from neck proprioceptors.
The present experiments are relevant to human health for several reasons. First, recent studies have demonstrated similar results between mechanical demands and EMG patterns in humans [75,76]. Little is known about the mechanisms that mediate appropriate adaptation of muscular activation patterns during these tasks. We recognize that the strategies used by bipedal animals might be somewhat different from those used by quadrupeds. However, both humans and animals use similar sources of sensory information, and defining these sources and the manner in which they are integrated is a major goal of this project. Second, elderly individuals have difficulty navigating changes in slope and surface. Our data provide evidence that the decline of balance could be a result of proprioceptive afferent deterioration. Third, one of the promising current approaches to the treatment of spinal cord injury is to take advantage of the intrinsic motor capacities of the spinal cord, including the central pattern generator, using spinal cord stimulation. In order to make muscle activity patterns appropriate to the slope, it is important to understand how spinal circuits use sensory cues to adapt to different surfaces. The present studies demonstrate that sensory information is required to appropriately regulate the central pattern generator, and provide a basis for designing engineered control systems for this purpose.
9. Summary
Humans and animals adopt a distinct neuromuscular strategy during the transition between a level and hill surface. Healthy young adults adjust their neuromuscular patterns at least four steps prior to transitioning onto a hill surface. Typically, the changes in muscle activity were due to co-contraction of opposing muscle groups and these changes correspond to modifications in head pitch. During all of the anticipation strides, as well as during uphill walking, head pitch was less than level walking, but during downhill walking head pitch was greater. As for our animal experiments, head pitch significantly influenced the muscle activity patterns of the decerebrate cats. In addition, it appears that the neck proprioceptors may be an initial source of feedback for upcoming surface slope transitions. Together, these results illustrate that transition strides are functionally distinct from either level walking or hill walking independently. In the future, we aim to explore if these transition strategies found in the laboratory are used in the real world.
Acknowledgements
This work was supported by National Institutes of Health Grants NS054542 (JSG Postdoctoral NRSA) and NS20855 (TRN R01). We thank Riley Sheehan and Keith Stern of the Pennsylvania State University Neuromotion Laboratory as well as Claire Honeycutt, Kyla Ross and Victoria Stahl of the Emory University Neurophysiology Laboratory for valuable help with data collection. In addition, we thank Young-Hui Chang for providing helpful suggestions on an earlier draft of the manuscript.
Footnotes
One contribution of 15 to a Theme Issue ‘Integration of muscle function for producing and controlling movement’.
References
- 1.Ashley M. J., Gryfe C. I., Amies A. 1977. A longitudinal study of falls in an elderly population II. Some circumstances of falling. Age Ageing 6, 211–220 10.1093/ageing/6.4.211 (doi:10.1093/ageing/6.4.211) [DOI] [PubMed] [Google Scholar]
- 2.Tinetti M. E., Doucette J., Claus E., Marottoli R. 1995. Risk factors for serious injury during falls by older persons in the community. J. Am. Geriatr. Soc. 43, 1214–1221 [DOI] [PubMed] [Google Scholar]
- 3.Polcyn A. F., Lipsitz L. A., Kerrigan D. C., Collins J. J. 1998. Age-related changes in the initiation of gait: degradation of central mechanisms for momentum generation. Arch. Phys. Med. Rehabil. 79, 1582–1589 10.1016/S0003-9993(98)90425-7 (doi:10.1016/S0003-9993(98)90425-7) [DOI] [PubMed] [Google Scholar]
- 4.Suzuki I., Timerick S. J., Wilson V. J. 1985. Body position with respect to the head or body position in space is coded by lumbar interneurons. J. Neurophysiol. 54, 123–133 [DOI] [PubMed] [Google Scholar]
- 5.Manzoni D., Andre P., Pompeiano O. 2004. Proprioceptive neck influences modify the information about tilt direction coded by the cerebellar anterior vermis. Acta Otolaryngol. 124, 475–480 10.1080/00016480410016595 (doi:10.1080/00016480410016595) [DOI] [PubMed] [Google Scholar]
- 6.Sun J., Walters M., Svensson N., Lloyd D. 1996. The influence of surface slope on human gait characteristics: a study of urban pedestrians walking on an inclined surface. Ergonomics 39, 677–692 10.1080/00140139608964489 (doi:10.1080/00140139608964489) [DOI] [PubMed] [Google Scholar]
- 7.Kuster M., Sakurai S., Wood G. A. 1995. Kinematic and kinetic comparison of downhill and level walking. Clin. Biomech. (Bristol, Avon) 10, 79–84 10.1016/0268-0033(95)92043-L (doi:10.1016/0268-0033(95)92043-L) [DOI] [PubMed] [Google Scholar]
- 8.Lay A. N., Hass C. J., Gregor R. J. 2006. The effects of sloped surfaces on locomotion: a kinematic and kinetic analysis. J. Biomech. 39, 1621–1628 10.1016/j.jbiomech.2005.05.005 (doi:10.1016/j.jbiomech.2005.05.005) [DOI] [PubMed] [Google Scholar]
- 9.Lange G. W., Hintermeister R. A., Schlegel T., Dillman C. J., Steadman J. R. 1996. Electromyographic and kinematic analysis of graded treadmill walking and the implications for knee rehabilitation. J. Orthop. Sports Phys. Ther. 23, 294–301 [DOI] [PubMed] [Google Scholar]
- 10.Cham R., Redfern M. S. 2002. Changes in gait when anticipating slippery floors. Gait Posture 15, 159–171 10.1016/S0966-6362(01)00150-3 (doi:10.1016/S0966-6362(01)00150-3) [DOI] [PubMed] [Google Scholar]
- 11.Redfern M. S., Moore P. L., Yarsky C. M. 1997. The influence of flooring on standing balance among older persons. Hum. Factors 39, 445–455 10.1518/001872097778827043 (doi:10.1518/001872097778827043) [DOI] [PubMed] [Google Scholar]
- 12.Leroux A., Fung J., Barbeau H. 1999. Adaptation of the walking pattern to uphill walking in normal and spinal-cord injured subjects. Exp. Brain Res. 126, 359–368 10.1007/s002210050743 (doi:10.1007/s002210050743) [DOI] [PubMed] [Google Scholar]
- 13.Lay A. N., Hass C. J., Nichols T. R., Gregor R. J. 2007. The effects of sloped surfaces on locomotion: an electromyographic analysis. J. Biomech. 40, 1276–1285 10.1016/j.jbiomech.2006.05.023 (doi:10.1016/j.jbiomech.2006.05.023) [DOI] [PubMed] [Google Scholar]
- 14.Fowler E. G., Gregor R. J., Hodgson J. A., Roy R. R. 1993. Relationship between ankle muscle and joint kinetics during the stance phase of locomotion in the cat. J. Biomech. 26, 465–483 10.1016/0021-9290(93)90010-C (doi:10.1016/0021-9290(93)90010-C) [DOI] [PubMed] [Google Scholar]
- 15.Gregor R., Smith J., Smith D., Oliver A., Prilutsky B. 2001. Hindlimb kinetics and neural control during slope walking in the cat: unexpected findings. J. Appl. Biomech. 17, 277–286 [Google Scholar]
- 16.Carlson-Kuhta P., Trank T. V., Smith J. L. 1998. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J. Neurophysiol. 79, 1687–1701 [DOI] [PubMed] [Google Scholar]
- 17.Smith J. L., Carlson-Kuhta P., Trank T. V. 1998. Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J. Neurophysiol. 79, 1702–1716 [DOI] [PubMed] [Google Scholar]
- 18.Trank T. V., Chen C., Smith J. L. 1996. Forms of forward quadrupedal locomotion. I. A comparison of posture, hindlimb kinematics, and motor patterns for normal and crouched walking. J. Neurophysiol. 76, 2316–2326 [DOI] [PubMed] [Google Scholar]
- 19.Zernicke R. F., Smith J. L. 1996. Biomechanical insights into neural control of movement. In Handbook of physiology section 12: exercise: regulation and integration of multiple systems (eds Rowell L. B., Shepherd J. T.), pp. 293–332 New York, NY: Oxford [Google Scholar]
- 20.Earhart G. M., Bastian A. J. 2000. Form switching during human locomotion: traversing wedges in a single step. J. Neurophysiol. 84, 605–615 [DOI] [PubMed] [Google Scholar]
- 21.Prentice S. D., Hasler E. N., Groves J. J., Frank J. S. 2004. Locomotor adaptations for changes in the slope of the walking surface. Gait Posture 20, 255–265 10.1016/j.gaitpost.2003.09.006 (doi:10.1016/j.gaitpost.2003.09.006) [DOI] [PubMed] [Google Scholar]
- 22.Myers A. M., Powell L. E., Maki B. E., Holliday P. J., Brawley L. R., Sherk W. 1996. Psychological indicators of balance confidence: relationship to actual and perceived abilities. J. Gerontol. A Biol. Sci. Med. Sci. 51, M37–M43 [DOI] [PubMed] [Google Scholar]
- 23.Delbaere K., Crombez G., van Haastregt J. C., Vlaeyen J. W. 2009. Falls and catastrophic thoughts about falls predict mobility restriction in community-dwelling older people: a structural equation modelling approach. Aging Ment. Health 13, 587–592 10.1080/13607860902774444 (doi:10.1080/13607860902774444) [DOI] [PubMed] [Google Scholar]
- 24.Giladi N., Herman T., Reider G. I. I., Gurevich T., Hausdorff J. M. 2005. Clinical characteristics of elderly patients with a cautious gait of unknown origin. J. Neurol. 252, 300–306 10.1007/s00415-005-0641-2 (doi:10.1007/s00415-005-0641-2) [DOI] [PubMed] [Google Scholar]
- 25.Balash Y., Hadar-Frumer M., Herman T., Peretz C., Giladi N., Hausdorff J. M. 2007. The effects of reducing fear of falling on locomotion in older adults with a higher level gait disorder. J. Neural Transm. 114, 1309–1314 10.1007/s00702-007-0771-z (doi:10.1007/s00702-007-0771-z) [DOI] [PubMed] [Google Scholar]
- 26.Maki B. E. 1997. Gait changes in older adults: predictors of falls or indicators of fear. J. Am. Geriatr. Soc. 45, 313–320 [DOI] [PubMed] [Google Scholar]
- 27.Eils E., Behrens S., Mers O., Thorwesten L., Volker K., Rosenbaum D. 2004. Reduced plantar sensation causes a cautious walking pattern. Gait Posture 20, 54–60 10.1016/S0966-6362(03)00095-X (doi:10.1016/S0966-6362(03)00095-X) [DOI] [PubMed] [Google Scholar]
- 28.Menant J. C., Steele J. R., Menz H. B., Munro B. J., Lord S. R. 2009. Effects of walking surfaces and footwear on temporo-spatial gait parameters in young and older people. Gait Posture 29, 392–397 10.1016/j.gaitpost.2008.10.057 (doi:10.1016/j.gaitpost.2008.10.057) [DOI] [PubMed] [Google Scholar]
- 29.Schrager M. A., Kelly V. E., Price R., Ferrucci L., Shumway-Cook A. 2008. The effects of age on medio-lateral stability during normal and narrow base walking. Gait Posture 28, 466–471 10.1016/j.gaitpost.2008.02.009 (doi:10.1016/j.gaitpost.2008.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottschall J. G., Okorokov D. Y., Okita N., Stern K. A. In press. Walking strategies during the transition between level and hill surfaces. J. Appl. Biomech. [DOI] [PubMed] [Google Scholar]
- 31.Chen H. C., Ashton-Miller J. A., Alexander N. B., Schultz A. B. 1991. Stepping over obstacles: gait patterns of healthy young and old adults. J. Gerontol. 46, M196–M203 [DOI] [PubMed] [Google Scholar]
- 32.Donelan J. M., Shipman D. W., Kram R., Kuo A. D. 2004. Mechanical and metabolic requirements for active lateral stabilization in human walking. J. Biomech. 37, 827–835 10.1016/j.jbiomech.2003.06.002 (doi:10.1016/j.jbiomech.2003.06.002) [DOI] [PubMed] [Google Scholar]
- 33.Erdemir A., Piazza S. J. 2002. Rotational foot placement specifies the lever arm of the ground reaction force during the push-off phase of walking initiation. Gait Posture 15, 212–219 10.1016/S0966-6362(01)00192-8 (doi:10.1016/S0966-6362(01)00192-8) [DOI] [PubMed] [Google Scholar]
- 34.Ho C. S., Lin C. J., Chou Y. L., Su F. C., Lin S. C. 2000. Foot progression angle and ankle joint complex in preschool children. Clin. Biomech. (Bristol, Avon) 15, 271–277 10.1016/S0268-0033(99)00068-6 (doi:10.1016/S0268-0033(99)00068-6) [DOI] [PubMed] [Google Scholar]
- 35.Cram J. R., Kasman G. S. 1998. Electrode placement (eds Cram J. R., Kasman G. S., Holz J.), Gaithersburg: Aspen [Google Scholar]
- 36.Winter D. A., Fuglevand A. J., Archer S. E. 1994. Crosstalk in surface elctromyography: theoretical and practical estimates. J. Electromyogr. Kinesiol 4, 15–26 10.1016/1050-6411(94)90023-X (doi:10.1016/1050-6411(94)90023-X) [DOI] [PubMed] [Google Scholar]
- 37.Winter D. A. 1991. The biomechanics and motor control of human gait: normal, elderly, and pathological. Waterloo, Ontario, Canada: Waterloo Biomachanics [Google Scholar]
- 38.Pijnappels M., Bobbert M. F., van Dieen J. H. 2001. Changes in walking pattern caused by the possibility of a tripping reaction. Gait Posture 14, 11–18 10.1016/S0966-6362(01)00110-2 (doi:10.1016/S0966-6362(01)00110-2) [DOI] [PubMed] [Google Scholar]
- 39.Pijnappels M., Bobbert M. F., van Dieen J. H. 2006. EMG modulation in anticipation of a possible trip during walking in young and older adults. J. Electromyogr. Kinesiol. 16, 137–143 10.1016/j.jelekin.2005.06.011 (doi:10.1016/j.jelekin.2005.06.011) [DOI] [PubMed] [Google Scholar]
- 40.Crenna P., Carpinella I., Rabuffetti M., Calabrese E., Mazzoleni P., Nemni R., Ferrarin M. 2007. The association between impaired turning and normal straight walking in Parkinson's disease. Gait Posture 26, 172–178 10.1016/j.gaitpost.2007.04.010 (doi:10.1016/j.gaitpost.2007.04.010) [DOI] [PubMed] [Google Scholar]
- 41.Mesure S., Azulay J. P., Pouget J., Amblard B. 1999. Strategies of segmental stabilization during gait in Parkinson's disease. Exp. Brain Res. 129, 573–581 10.1007/s002210050927 (doi:10.1007/s002210050927) [DOI] [PubMed] [Google Scholar]
- 42.Vaugoyeau M., Viallet F., Aurenty R., Assaiante C., Mesure S., Massion J. 2006. Axial rotation in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 77, 815–821 10.1136/jnnp.2004.050666 (doi:10.1136/jnnp.2004.050666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franzen E., Paquette C., Gurfinkel V. S., Cordo P. J., Nutt J. G., Horak F. B. 2009. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson's disease. Exp. Neurol. 219, 430–438 10.1016/j.expneurol.2009.06.013 (doi:10.1016/j.expneurol.2009.06.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaffe G. J., Alvarado J. A., Juster R. P. 1986. Age-related changes of the normal visual field. Arch. Ophthalmol. 104, 1021–1025 [DOI] [PubMed] [Google Scholar]
- 45.Haas A., Flammer J., Schneider U. 1986. Influence of age on the visual fields of normal subjects. Am. J. Ophthalmol. 101, 199–203 [DOI] [PubMed] [Google Scholar]
- 46.Rossignol S. 1996. Visuomotor regulation of locomotion. Can. J. Physiol. Pharmacol. 74, 418–425 10.1139/cjpp-74-4-418 (doi:10.1139/cjpp-74-4-418) [DOI] [PubMed] [Google Scholar]
- 47.Warren W. H., Jr, Kay B. A., Zosh W. D., Duchon A. P., Sahuc S. 2001. Optic flow is used to control human walking. Nat. Neurosci. 4, 213–216 10.1038/84054 (doi:10.1038/84054) [DOI] [PubMed] [Google Scholar]
- 48.Patla A. E., Vickers J. N. 2003. How far ahead do we look when required to step on specific locations in the travel path during locomotion? Exp. Brain Res. 148, 133–138 10.1007/s00221-002-1246-y (doi:10.1007/s00221-002-1246-y) [DOI] [PubMed] [Google Scholar]
- 49.Patla A. E., Vickers J. N. 1997. Where and when do we look as we approach and step over an obstacle in the travel path? Neuroreport 8, 3661–3665 10.1097/00001756-199712010-00002 (doi:10.1097/00001756-199712010-00002) [DOI] [PubMed] [Google Scholar]
- 50.Fitzpatrick R. C., Wardman D. L., Taylor J. L. 1999. Effects of galvanic vestibular stimulation during human walking. J. Physiol. 517, 931–939 10.1111/j.1469-7793.1999.0931s.x (doi:10.1111/j.1469-7793.1999.0931s.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukuda T. 1959. The stepping test: two phases of the labyrinthine reflex. Acta Otolaryngol. 50, 95–108 10.3109/00016485909129172 (doi:10.3109/00016485909129172) [DOI] [PubMed] [Google Scholar]
- 52.Brandt T., Dieterich M. 1999. The vestibular cortex. Its locations, functions, and disorders. Ann. N Y Acad. Sci. 871, 293–312 10.1111/j.1749-6632.1999.tb09193.x (doi:10.1111/j.1749-6632.1999.tb09193.x) [DOI] [PubMed] [Google Scholar]
- 53.Jahn K., Strupp M., Schneider E., Dieterich M., Brandt T. 2000. Differential effects of vestibular stimulation on walking and running. Neuroreport 11, 1745–1748 10.1097/00001756-200006050-00029 (doi:10.1097/00001756-200006050-00029) [DOI] [PubMed] [Google Scholar]
- 54.Marques B., Colombo G., Muller R., Dursteler M. R., Dietz V., Straumann D. 2007. Influence of vestibular and visual stimulation on split-belt walking. Exp. Brain Res. 183, 457–463 10.1007/s00221-007-1063-4 (doi:10.1007/s00221-007-1063-4) [DOI] [PubMed] [Google Scholar]
- 55.Horstmann G. A., Dietz V. 1988. The contribution of vestibular input to the stabilization of human posture: a new experimental approach. Neurosci. Lett. 95, 179–184 10.1016/0304-3940(88)90653-2 (doi:10.1016/0304-3940(88)90653-2) [DOI] [PubMed] [Google Scholar]
- 56.Horak F., Shupert C., Dietz V., Horstmann G., Black F. O. 1992. Vestibular-somatosensory interaction in rapid responses to head perturbations. Ann. N Y Acad. Sci. 656, 854–856 10.1111/j.1749-6632.1992.tb25274.x (doi:10.1111/j.1749-6632.1992.tb25274.x) [DOI] [PubMed] [Google Scholar]
- 57.Keshner E. A., Cromwell R. L., Rovai G., Peterson B. W. 1992. Mechanisms of head stabilization during random rotations in the pitch plane. Ann. N Y Acad. Sci. 656, 937–939 10.1111/j.1749-6632.1992.tb25300.x (doi:10.1111/j.1749-6632.1992.tb25300.x) [DOI] [PubMed] [Google Scholar]
- 58.Cromwell R. L., Aadland-Monahan T. K., Nelson A. T., Stern-Sylvestre S. M., Seder B. 2001. Sagittal plane analysis of head, neck, and trunk kinematics and electromyographic activity during locomotion. J. Orthop. Sports Phys. Ther. 31, 255–262 [DOI] [PubMed] [Google Scholar]
- 59.Pozzo T., Berthoz A., Lefort L. 1990. Head stabilization during various locomotor tasks in humans. I. Normal subjects. Exp. Brain Res. 82, 97–106 10.1007/BF00230842 (doi:10.1007/BF00230842) [DOI] [PubMed] [Google Scholar]
- 60.af Klint R., Nielsen J. B., Cole J., Sinkjaer T., Grey M. J. 2008. Within-step modulation of leg muscle activity by afferent feedback in human walking. J. Physiol. 586, 4643–4648 10.1113/jphysiol.2008.155002 (doi:10.1113/jphysiol.2008.155002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang J. F., Fung J., Edamura M., Blunt R., Stein R. B., Barbeau H. 1991. H-reflex modulation during walking in spastic paretic subjects. Can. J. Neurol. Sci. 18, 443–452 [DOI] [PubMed] [Google Scholar]
- 62.Sinkjaer T., Andersen J. B., Larsen B. 1996. Soleus stretch reflex modulation during gait in humans. J. Neurophysiol. 76, 1112–1120 [DOI] [PubMed] [Google Scholar]
- 63.Dietz V., Quintern J., Berger W. 1984. Cerebral evoked potentials associated with the compensatory reactions following stance and gait perturbation. Neurosci. Lett. 50, 181–186 10.1016/0304-3940(84)90483-X (doi:10.1016/0304-3940(84)90483-X) [DOI] [PubMed] [Google Scholar]
- 64.Roberts T. 1967. Neurophysiology of postural mechanisms. New York: Plenum Press [Google Scholar]
- 65.Gottschall J. S., Nichols T. R. 2007. Head pitch affects muscle activity in the decerebrate cat hindlimb during walking. Exp. Brain Res. 182, 131–135 10.1007/s00221-007-1084-z (doi:10.1007/s00221-007-1084-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gregor R. J., Smith J. L., Smith D. W., Oliver A., Prilutsikii B. I. 2001. Hindlimb kinetics and neural control during slope walking in the cat: unexpected findings. J. Appl. Biomech. 17, 277–286 [Google Scholar]
- 67.Magnus R., de Kleijn A. 1912. Die Abhängigkeit des Tonus der Extremitätenmuskeln von der Kopfstellung. Pflugers Arch. 145, 455–548(doi:10.1007/BF01681127) [Google Scholar]
- 68.Holst E. 1969. The behavioral physiology of animals and man. Miami, FL: University of Miami Press [Google Scholar]
- 69.Holst E., Mittelstaedt H. 1950. Das reafferenzprincip. Naturwiss 37, 464–476 10.1007/BF00622503 (doi:10.1007/BF00622503) [DOI] [Google Scholar]
- 70.Boyle R., Pompeiano O. 1981. Relation between cell size and response characteristics of vestibulospinal neurons to labyrinth and neck inputs. J. Neurosci. 1, 1052–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boyle R., Pompeiano O. 1981. Convergence and interaction of neck and macular vestibular inputs on vestibulospinal neurons. J. Neurophysiol. 45, 852–868 [DOI] [PubMed] [Google Scholar]
- 72.Ezure K., Wilson V. J. 1984. Interaction of tonic neck and vestibular reflexes in the forelimb of the decerebrate cat. Exp. Brain Res. 54, 289–292 10.1007/BF00236229 (doi:10.1007/BF00236229) [DOI] [PubMed] [Google Scholar]
- 73.Manzoni D., Pompeiano O., Andre P. 1998. Neck influences on the spatial properties of vestibulospinal reflexes in decerebrate cats: role of the cerebellar anterior vermis. J. Vestib. Res. 8, 283–297 10.1016/S0957-4271(97)00077-3 (doi:10.1016/S0957-4271(97)00077-3) [DOI] [PubMed] [Google Scholar]
- 74.Lafreniere-Roula M., McCrea D. A. 2005. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J. Neurophysiol. 94, 1120–1132 10.1152/jn.00216.2005 (doi:10.1152/jn.00216.2005) [DOI] [PubMed] [Google Scholar]
- 75.Lay A. N. 2005. Neuromuscular coordination during slope walking. PhD thesis, Georgia Institute of Technology, Atlanta, GA, USA [Google Scholar]
- 76.Rose J., Gamble J. G. 1994. Human walking. Baltimore, MD: Williams & Wilkin [Google Scholar]