Abstract
A neuromechanical approach to control requires understanding how mechanics alters the potential of neural feedback to control body dynamics. Here, we rewrite activation of individual motor units of a behaving animal to mimic the effects of neural feedback without concomitant changes in other muscles. We target a putative control muscle in the cockroach, Blaberus discoidalis (L.), and simultaneously capture limb and body dynamics through high-speed videography and a micro-accelerometer backpack. We test four neuromechanical control hypotheses. We supported the hypothesis that mechanics linearly translates neural feedback into accelerations and rotations during static postural control. However, during running, the same neural feedback produced a nonlinear acceleration control potential restricted to the vertical plane. Using this, we reject the hypothesis from previous work that this muscle acts primarily to absorb energy from the body. The conversion of the control potential is paralleled by nonlinear changes in limb kinematics, supporting the hypothesis that significant mechanical feedback filters the graded neural feedback for running control. Finally, we insert the same neural feedback signal but at different phases in the dynamics. In this context, mechanical feedback enables turning by changing the timing and direction of the accelerations produced by the graded neural feedback.
Keywords: motor control, stimulation, muscle, insect, running, neuromechanics
1. Introduction
A neuromechanical perspective of locomotion emphasizes that both neural and mechanical systems can exert control over body dynamics [1–6]. For slow, precise locomotor behaviours, detailed neurophysiological studies have revealed neural feedback strategies for control with seemingly little dependence on underlying mechanics [7–10]. However, as an organism moves more dynamically, sensory structures are strained and deformed [11,12], processing delays become challenging [3,6,13,14], muscles undergo dynamic oscillations with complex history dependence [1,15–18] and inertial effects increase [3,4]. As a result, organisms moving at high speeds can adopt mechanical control strategies. In these cases, feed-forward neural activation maintains steady-state dynamics, but perturbation responses are mediated through the physics of the system, a process termed mechanical feedback [4,5,19–21]. This dichotomy of control into strictly neural or mechanical strategies belies the fact that both neural and mechanical elements will ultimately contribute to controlling nearly every behaviour. As an integrated neuromechanical perspective of motor control emerges, a central challenge remains as to how the mechanics of a given task shape the potential of neural signals to control movement [4,5,10,15,22–26].
Perturbation studies are an established approach to revealing how an organism's neural and mechanical systems respond to enable stability and manoeuvrability [1,5,26–28]. During steady-state locomotion, an animal delivers neural signals to its musculo-skeletal system, which in turn generates forces and torques on the body's centre of mass (COM; figure 1). Perturbing body dynamics alters the state of the musculo-skeletal system (figure 1, right arrow). Mechanical feedback may mediate this response by, for example, altering a muscle's force via changes in strain without changes in activation. Neural feedback could also mediate this response through changing the activation of muscles. By observing the resulting changes in neuromuscular activation and the body dynamics, we can reveal the patterns of neural feedback caused by the perturbation [1,5,28]. However, it remains difficult to ascribe a causal relationship between a specific change in neural activation and corresponding changes in body dynamics. This is due to two factors. First, many changes typically occur in parallel, so it is hard to isolate their effects. Second, it is difficult to predict how changes in a muscle's activation, or even its work output, are translated through the rest of the musculo-skeletal system to affect body dynamics. While correlating muscle activation changes and dynamics across a variety of perturbations can suggest causal relationships, and careful biomechanical models can offer potential explanations [25,29–31], it is necessary to test these hypotheses with direct experimentation in the intact, behaving animal. This is especially true during dynamic behaviours where it is difficult to predict a muscle's function from anatomy alone or even a careful examination of its in situ physiological properties [16,32,33].
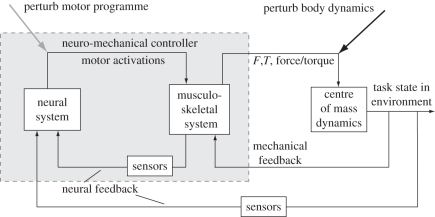
Figure 1.
Neuromechanical control diagram. Control of locomotion arises from the actions of an animal's neural and musculo-skeletal systems. Motor commands activate muscles, which in conjunction with skeletal and connective elements generate forces and torques on the centre of mass (COM) or other targets of control. Here, we consider the mechanical control arising from the musculo-skeletal system separately from the target of control, in this case the dynamics of the COM. This division emphasizes the mechanical control that arises when changes in body state directly stretch, deform, reorient or otherwise alter the state of the musculo-skeletal system without intervening changes in neural activity. This mechanical feedback affects how motor activations are translated into COM dynamics. Neural feedback, whether extero- or proprioceptive, acts through sensors to modify motor signals, typically with significant delays. Perturbation of body dynamics (black arrow) is a classic method for revealing the changes in neural and mechanical state that arise during recovery of steady-state behaviour. Here, we take a complementary approach of directly perturbing motor signals (grey arrow). This reveals the causal responses in body dynamics arising from neural feedback to individual motor units.
To this end, we develop a complement to external perturbation studies and directly test a muscle's potential to control body dynamics when its activation is modulated by neural feedback (figure 1, left arrow). We identify a muscle's control potential, the causal relationship of changes in motor activation to body dynamics, through rewriting precise patterns of muscle action potentials (MAPs) in intact, running animals. It is well known that neural commands and couplings between musculo-skeletal elements are reconfigured for different tasks [1,5,23,24]. Control potentials reveal how these different states condition the translation of specific neural control signals, i.e. varying patterns of muscle activations, into forces and torques on the body. By directly altering a specific muscle's activity, we can separate the effects of multiple, parallel neural feedback pathways.
Cockroaches, Blaberus discoidalis (L.), provide a tractable system for these questions, as muscles are innervated by relatively few motor neurons whose resulting MAPs (or spikes) are clearly distinguishable in electromyogram (EMG) recordings (figure 2a) [5,35–37]. The dynamics of preferred-speed running in cockroaches are well-characterized and are known to contribute significantly to the stability of the body [3,38,39]. However, substantial neural feedback occurs in both stability and navigation tasks [5,13,40]. Finally, isolated muscle experiments have revealed the functional role of a number of cockroach limb muscles during steady-state running [18,32] and their work output over a range of possible stimulus and strain parameters [16] using a morphologically detailed musculo-skeletal model [29].
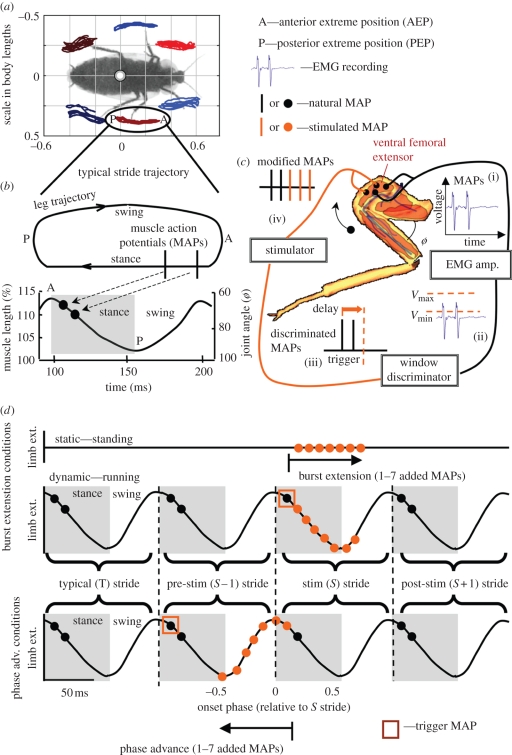
Figure 2.
Rewriting the motor code to individual muscles. We tracked individual limb trajectories (a) using tracking methods adapted from Revzen & Guckenheimer [34]. A typical stride (b) is composed of a stance and a swing period during which the putative control muscle, a ventral femoral extensor, undergoes shortening and lengthening, respectively. ‘A’ and ‘P’ indicate the anterior and posterior extreme positions, respectively. EMGs (c(i)) from the ventral femoral extensor were recorded, and spikes detected with a hardware voltage discriminator (c(ii)). We triggered a stimulator after an adjustable delay (c(iii)) and evoked addition muscle action potentials (c(iv)). This approach enables real-time, phase-locked manipulation of muscle activation, which we used to modify muscle activation either by extending the burst of activation (d, top traces) or advancing the phase of activation onset (d, bottom traces). In the first set of conditions, we added one, three, five or seven additional MAPs (orange dots) triggered off of the first spike in the stimulated (S) stride (orange square). We imposed the same patterns of mimicked neural feedback under both static conditions (top) where the animal maintained a stationary posture with no movement of the limbs and during running (middle), where cockroaches ran steadily with typical activation and limb extension (T stride). During phase-advance conditions (bottom), MAP addition was triggered off of the preceding burst of activity (S − 1 stride). In both spike addition and phase-advance experiments, dynamic effects of modified muscle activity persisted into the S + 1 stride. Note that we do not graphically represent the limb kinematics changes that result from modified activity because these depended on each particular condition and are presented in the results (figures 4 and 7).
To identify a muscle's causal contribution to control and its determinants, we must integrate its effects on COM dynamics, limb kinematics and individual muscle work output. In this paper, we consider the control potential of neural feedback on COM dynamics and limb kinematics in two in vivo experiments. In a companion paper [41], we explore the mechanism behind these changes, employing a modified in situ work-loop technique to reveal how the work output of the individual muscle changes under neural feedback.
We test four motor control hypotheses about how neural and mechanical feedback strategies are combined through the function of a ventral femoral extensor muscle. Our first control hypothesis is that an extensor muscle drives COM motion as a linear function of the number of imposed MAPs during static conditions. In our second control hypothesis, we propose that during running, mechanical feedback translates the same increasing number of MAPs into greater additional energy absorption, as has been suggested by previous studies of muscle function [16]. Our third control hypothesis is that the control potential of neural feedback to this muscle should be similarly shaped function across static and dynamic conditions even if the gains change. Finally, we change the phase of MAPs sent to this muscle to test the fourth control hypothesis that this muscle can adopt a turning control potential.
Our first set of experimental conditions addresses control hypotheses 1–3. We test the effects of known patterns of neural feedback in static (standing) and dynamic (running) contexts (figure 2). We focus on the middle leg femoral extensors because of their putative control function [5,16,37,40]. During very slow locomotion, within the bottom 10 per cent of a cockroach's range [5], many dynamically stable gaits do not exist, and cockroaches maintain static stability [39,42]. In these cases, cockroaches negotiating obstacles and responding to increases in load typically increase activity to femoral extensors, and this neural feedback is correlated with increased joint velocity and body pitch [37,40,43]. These results suggest that under static or quasi-static behaviours, the control potential of a femoral extensor should act much as its anatomical designation suggests, performing as a linear limb extensor to drive COM motions (control hypothesis 1).
When cockroaches run rapidly, the role of neural feedback to the femoral extensors is not easily predicted. During perturbation experiments on rough terrain, cockroaches appear to adopt a strategy relying more on mechanical feedback. In the face of moderate obstacles, motor activations from the fast motor neuron do not change [5]. However, neural feedback is layered on top of this feed-forward strategy. When the magnitude of the perturbation increases, activation of these muscles changes significantly, exhibiting additional MAPs that extend the activation burst [5]. While this seems consistent with obstacle traversal, the ventral femoral extensor, denoted 179 in the hind leg and 137 in the middle leg (numbering follows Carbonell [44]), typically functions as a brake during running. It absorbs energy from the limb by developing forces to resist swing in proportion to the amount of activation [16]. Neural feedback may act to increase energy absorption, with ascent over obstacles arising from parallel changes in other muscles (control hypothesis 2). Alternatively, the ventral femoral extensor could have control effects directly contributing to obstacle traversal arising from mechanical feedback transforming the normal energy-absorbing function of the muscle.
We next compare the standing and running control potentials. If the control potential of the ventral femoral extensor is determined primarily by neural feedback altering its activation (control hypothesis 3), then its control potential should be the same in both the static and dynamic contexts. However, if the control potential used during posture control is not sufficient for running stability and manoeuvrability, then the animal may use mechanical feedback to modulate its effect for this locomotor context.
Finally, we leverage our approach of rewriting the neuromuscular activity to explore the control potential of the same muscle under patterns of neural feedback that are hypothesized to be involved in control. The phase of motor activation is known to alter the ventral femoral extensor's work output [16] and has been suggested to control limb stance–swing transitions [37,45]. Changes in the timing of the middle leg's kinematics are one proposed mechanism for how turning is accomplished in legged animals [45]. However, cockroaches employ a large number of different turning strategies and it is difficult to conclude that the presence or absence of a particular change in the timing of neuromuscular activity is sufficient to evoke turning. By adding MAPs ahead of the normal burst, but in the same increasing pattern as before (figure 2d, bottom versus top traces), we can test whether this change in timing can control turning (control hypothesis 4). If changing the onset phase of activity does have a significant turning control potential, then mechanical feedback and the relative timing of neural activation and mechanical strain should affect horizontal and rotational plane body dynamics.
2. Methods
(a). Animals
We used male and non-gravid female adult cockroaches. Overall, 20 cockroaches were used across all experimental conditions (nine for burst extension trials; 11 for phase advancement). Masses were on average 2.50 ± 0.52 g (s.d.). Cockroaches were housed in communal plastic containers at room temperature (22°C) on a 12 L : 12 D cycle and fed dog chow and fruit ad libitum.
(b). In vivo experimental preparation
Manipulation of neuromuscular activity to mimic neural feedback to individual motor units requires stimulating MAPs with precise, repeatable timing matched to any existing activity in the muscle. To obtain steady-state neuromuscular activity, we monitored natural EMG activity via standard bipolar electrode methods [5,40]. Cockroaches were cold anaesthetized and their bodies restrained. Both a recording and a stimulating pair of 50 µm silver wires (California Fine Wire Company, Grover Beach, CA, USA) were inserted along the proximal–distal axis of the ventral femoral extensor to maximize recording and stimulus efficacy. A fifth common reference electrode was placed in the abdomen.
Repeatable imposition of added MAPs required appropriate timing of stimulation within the gait cycle. Cockroaches run with an alternating tripod gait (figure 2a, blue versus red limbs). Femoral extensors shorten during stance phase as the limb extends and coxa–femur (CF) joint angle increases (figure 2b). Steady-state patterns of neuromuscular activation typically included two to three MAPs occurring approximately 10 per cent of stride cycle after the onset of stance [5,16]. To phase lock imposed MAPs to existing steady-state activity, we passed EMGs through a custom hardware voltage discriminator (figure 2c(ii)). A digital one-shot circuit picked off the first discriminated spike in a burst and triggered stimulation of evoked MAPs with controllable delays (figure 2c(iii)). Stimuli were passed through a stimulus isolation unit (Grass-Telefactor) before activating the muscle through the pair of stimulus electrodes (figure 2c(iv)). We used short, 0.5 ms stimuli of 4 V, and a constant interstimulus interval (ISI) of 10 ms, matching the typical interval used during running [5]. Errors owing to digital processing were less than 500 µs because amplification, discrimination and triggering were all implemented in hardware.
(c). Centre of mass dynamics
We capture both kinetics and kinematics of the cockroach's COM during the normal and stimulated strides. We also consider changes over the full multi-stride time course of the perturbation response. To monitor kinetics continuously, we outfitted each cockroach with a custom micro-accelerometer backpack, following previous methods [46]. Each backpack contained a three-axis micro-electromechanical system accelerometer (MMA7260Q Freescale Semiconductor, Inc. Austin, TX, USA) mounted on a small signal-conditioning circuit board and affixed above the animal's COM (first abdominal segment) with cyanoacrylate glue. We placed retroreflective markers on five balsa wood arms projecting from the backpack and tracked them with high-speed videography at 500 fps (AOS Technologies AG, Switzerland). Fitting the five digitized points in a two-dimensional camera view to a geometric model of the arms' configuration enabled three-dimensional reconstruction of the animal's pitch, roll and yaw [46]. We report yaw velocity changes instead of yaw, because the cockroach's heading was not fixed within the arena and angular velocity was more comparable across trials. A complete backpack weighed approximately 600 mg, less than one-quarter the mass of a typical cockroach. Since the majority of a backpack's mass was localized to the region immediately above each cockroach's COM, rotational moments of the animal were not significantly altered in the pitch and yawing directions. The small moment created by the rising arm of the backpack did not produce roll motions outside the range of those reported in previous experiments [5]. Backpacks were calibrated and accelerations were corrected for gravity using the kinematic tracking to define the relative orientation of the backpack with respect to the gravity vector at each point in time [46]. We off-loaded acceleration data from the backpack via an approximately 0.5 m tether of 50 µm wires attached to a data acquisition board (National Instruments, BNC 2090, Austin, TX, USA).
(d). Limb kinematics
We tracked individual limb trajectories using an automated tracking program [34]. We calculated two different phase variables to characterize gait timing (figure 2a,d). Limb phase (ϕL) is defined by the kinematic cycle of the middle left leg, which contained the target muscle. A limb phase of zero was defined as the onset of limb extension or the anterior extreme position (AEP), which is approximately the point of stance initiation [5,45]. Limb phase has been used extensively in the literature [5,7,8,45,47] and is important for determining the relative timing of muscle activity with respect to muscle strain resulting from limb movement [16]. However, limb phase is typically a poor estimate of the actual neuromechanical gait of the animal because trajectories of individual limbs are highly variable [34]. The relative timing of a periodic gait is better captured by extracting a single phase variable from dimensionally reducing the set of all limb and COM positions and velocities in both the fore–aft and lateral directions. We calculated this phase, termed kinematic phase (ϕK), using an instantiation of the phase algorithm from Revzen & Guckenheimer [34]. Kinematic phase was robust to changes in individual limb kinematics. We therefore used kinematic phase rather than limb phase to define individual strides and when analysing changes in whole-body dynamics to imposed neural feedback.
(e). Real-time alteration of motor activation patterns
(i). Extending activation bursts
After implanting electrodes and affixing the accelerometer backpack, we allowed the cockroaches to recover for at least 1 h prior to experimentation. In the first set of animals, we added the same patterns of mimicked neural feedback during static (standing) and dynamic (running) behaviours. In the dynamic context, stimulated MAPs were added following the first naturally occurring MAP, thereby preserving the natural phase of bursting onset (figure 2d, middle trace). We added one, three, five or seven evoked MAPs to each burst of motor activation (figure 2d, orange dots). Since natural bursts contain a variable number of spikes (typically two in our experiments) during high-speed running, we began adding spikes 10 ms after the first naturally occurring spike, effectively overwriting naturally occurring spikes that would have occurred. As a result, the one added MAP condition resulted in a burst of two potentials and was comparable to natural running. We discarded the few trials in which other natural spikes occurred during or immediately following the stimulation burst.
During static control conditions, there was no well-defined periodic motion and the ventral femoral extensor was quiescent in steady state (figure 2d, top trace). We therefore imposed MAPs at arbitrary times, although with a constant 10 ms ISI (figure 2d, orange dots). We confirmed that stimulation evoked a MAP by monitoring our recording electrodes (figure 2c(i)). In our companion paper [41], we find that extra-cellularly evoked MAPs and naturally evoked spikes are similarly efficacious in generating muscle work. We calculated impulses (force × time) on the COM, equivalent to the net change in momentum imparted to the animal through ground reaction forces. During running trials, we reported the change in vertical, fore–aft and lateral impulses with respect to the preceding unperturbed typical (T) stride (figure 2d, middle trace). The stride in which spikes were modified was labelled the S stride and the subsequent stride was labelled S + 1.
(ii). Advancing burst phase
In the second set of animals, we advanced the onset phase of the burst, by adding spikes prior to natural activation. We triggered off the first MAP of the muscle's activity in the stride immediately preceding our target stride (the S − 1 stride; figure 2d, bottom trace) and then added spikes at the beginning of the burst in the subsequent stride (S stride). We again added one, three, five or seven MAPs (figure 2d, orange dots). In this case, we overwrote the first natural MAP in the S stride. Since spike timing required us to estimate the stride period prior to stimulus, the actual phase of the imposed MAPs was variable. We reclassified the conditions based on the actual phase of the onset. We binned runs into groups over phase intervals of 0.125 with phase advance of −1, representing a full stride advance (91 ms). A positive phase indicates that the first MAP occurred in the S stride, while a negative phase indicates that spiking began prior to the onset of this stride (i.e. during the S − 1 stride).
(f). Statistical design and analysis
Overall, we analysed 120 burst extension trials distributed equally across static and dynamic conditions. For these experiments, an animal ran or stood in an open arena during one of the eight stimulus conditions (adding one, three, five or seven MAPs during standing or running). The order of conditions was randomized, but a complete cycle of conditions was tested. Phase-advance experiments were conducted on a larger number of animals (11 vs. 9) and trials (177 vs. 120) in order to achieve adequate filling of phase bins for statistical analysis. In all cases, the experimental design was balanced so that each stimulation condition was represented equally for each animal. A few trials were necessarily discarded owing either to synchronization failure in data acquisition or changes in behaviour (e.g. contact with the arena wall or catastrophic impulses or rotations greater than 3 s.d. from the mean response). However, no more than three trials in each condition were eliminated in this way. During running trials, cockroaches locomoted at a mean speed of 32.4 ± 8.9 cm s−1 (mean ± s.d.) when we extended activation bursts and 37.2 ± 8.0 cm s−1 when we advanced phase. All trials were approximately one-half maximal speed (approx. 66 cm s−1) for running cockroaches [5,48].
Statistics were analysed using JMP (SAS Institute, Inc., Cary, NC, USA) and Matlab (Mathworks, Natick, MA, USA) software. All motor activation conditions in the standing and running contexts were discrete and ordinal. To test for significant differences across conditions, we used ANOVAs and Tukey HSD tests. If each subsequent activation level was significantly different and monotonically increasing or decreasing, we considered relationship graded. To test for differences above baseline at any particular condition, we used paired t-tests between the modified stride (S or S + 1) and its paired typical (T) stride or Welch's t-tests to test whether the responses were significantly different from zero. Data are reported as means ± s.e.m., unless otherwise noted. All results were confirmed with non-parametric Wilcoxon or Kruskal–Wallis tests to ensure that normality assumptions did not impact our conclusions.
3. Results
(a). Burst extension experiments
(i). Postural control potential
Increasing motor activation to the ventral femoral extensor mimicked the effects of graded neural feedback. Adding MAPs during static posture control resulted in linear actuation of the body in all degrees of freedom (figure 3a). Body pitch and roll as well as yaw, lateral and forward velocities increased with the addition of even a single spike (t-tests, all p < 0.005). Body impulses in the vertical, fore–aft and lateral directions also increased (t-tests, all p < 0.01). Each subsequent increase in activation produced a graded increase in every variable up to the maximum level of activation (ANOVA and Tukey HSD test, p < 0.005). While lateral and fore–aft impulses were significantly larger than vertical impulse (paired t-tests, p < 0.0001 and p < 0.02, respectively), the effects were of the same order of magnitude. Some rotations and lateral impulse were negative, which corresponded to motion away from the stimulated limb under our axis conventions. Finally, unlike the accelerations and rotations, velocity responses approached a maximum at the highest level of spiking (HSD tests, p > 0.1). Overall, the muscle propels the body upwards and away from the stimulated leg.
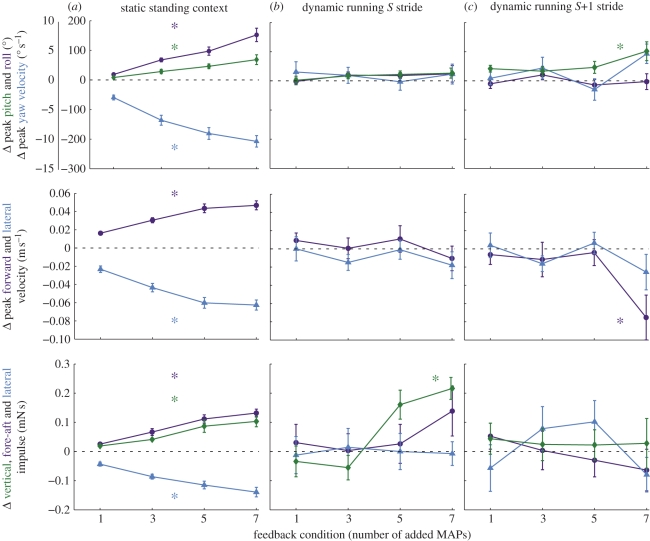
Figure 3.
Control potentials of body dynamics resulting from burst extension in the ventral femoral extensor. The control potential of adding MAPs to the ventral femoral extensor differed significantly between static (a) and dynamic (b,c) conditions. During running, control effects persisted through the S stride (b) into the S + 1 stride (c). We used the change in yaw velocity or angular momentum rather than the net change in yaw, because the latter was highly variable from stride-to-stride. During running, significant effects (asterisks) were all nonlinear, with changes only arising at the highest levels of activation. Green lines (diamonds) represent vertical and pitching motions, blue lines (triangles) are lateral and yaw motions and purple lines (circles) are forward and roll motions. Since all values are changes compared with the steady state, positive and negative values reflect net changes in momentum or rotation in opposite directions. Positive is upwards, forwards and towards the stimulated leg (in lateral and yaw).
(ii). Running control potential
Unlike posture control, the addition of a single spike had no detectable effect during running on any aspect of body dynamics (paired t-tests, all p > 0.05). Even as we increased the effective neural feedback, adding more MAPs, the COM did not rotate or change speed regardless of the spikes added (ANOVA, p > 0.1; figure 3b). In the S + 1 stride (figure 3c), the COM did show an increase in pitch and decrease in forward velocity compared with the normal stride, but only in the most extreme condition with seven added MAPs (Welch's t-test, p < 0.05). There were no shifts in lateral velocity, nor yaw or roll in the S + 1 running stride (ANOVA, p > 0.05).
Increasing activation also had very different effects on COM accelerations compared with standing. There was no change in lateral or fore–aft impulses during either the S or S + 1 stride under any spike addition conditions (ANOVAs, p > 0.1; figure 3b). However, there was significant control of vertical impulse, which increased during the S stride (ANOVA, p < 0.05). Despite this, the effect of increasing activation did not show the same linear relationship as during static trials. Rather, the addition of one or three MAPs was not statistically different from a typical stride (Tukey HSD and Welch's t-test, p > 0.05), but when five or seven MAPs were added, the vertical impulse was increased above those of normal strides (Tukey HSD and Welch's t-tests, p < 0.001). Indeed, the mean difference between the three and five added MAP conditions (0.216 mN s) was 2.5 times the difference between one and seven added MAPs during static control (0.084 mN s). Further, there was no difference between five and seven added MAPs in the running trials (Tukey HSD, p > 0.1), indicating that the greatest control effect of spike addition occurred between three and five added spikes. The resulting control relationship between increasing activation and COM vertical impulse was nonlinear and indicates a discrete transition: below five added spikes, there is no significant control potential, but above that level, neural feedback to this muscle would recruit additional vertical acceleration greater than any of its capabilities during standing control. Vertical impulse during the S + 1 stride was not altered compared with normal strides (ANOVA, p > 0.1; figure 3b).
(iii). Effects of spike addition on limb kinematics
Simulating the effects of neural feedback during running altered limb kinematics in a nonlinear fashion (figure 4). The stimulated middle leg extended further, increasing the posterior extreme position (PEP) with five or more added MAPs (paired t-tests, p < 0.001; figure 4). However, adding one or three MAPs did not change extension (paired t-test, p > 0.05; figure 4, top plots), nor did increased activation above five MAPs provide further change (Welch's t-test, p > 0.1). Duty factor, the portion of the stride spent in stance (figure 2b), increased with the addition of five or seven MAPs (paired t-tests, p < 0.001), but not in the one or three spike cases (paired t-tests, p > 0.05).
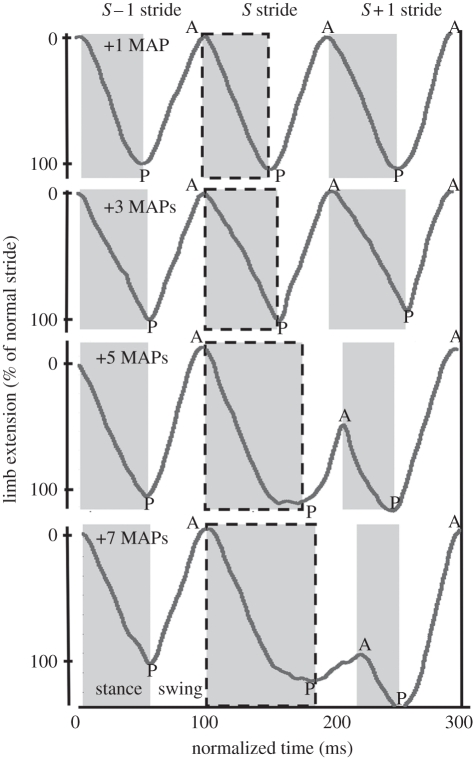
Figure 4.
Limb kinematic response. Individual limbs were tracked using high-speed videography (figure 2a) [34] and the resulting limb extensions for the stimulated middle left leg were compared across spike addition conditions. Limb kinematics changed nonlinearly in a manner parallel to vertical impulse response during running (figure 3b). Extension during stance was significantly increased when five or seven spikes were added during the S stride, causing a posterior shift in the PEP (‘P’) and a truncated swing period both in distance and time (all p < 0.01). The stance phase of the S + 1 stride under these conditions was correspondingly shortened and shifted posterior before returning to an AEP (‘A’) indistinguishable from the typical stride. Notably, the relative timing on the end of the S + 1 stride was constant across all conditions, indicating that the underlying clock-like activation of the alternating tripod gait was not significantly altered.
Adding five or seven MAPs continued to alter limb kinematics through the swing phase of the stimulated (S) stride and into the S + 1 stride (figure 4). Holding stance for longer in the S stride results in a truncated stride, where the leg begins to swing later than normal and is therefore returned to stance before it can regain its normal anterior position. The S stride swing was shortened in both time (paired t-tests of five and seven added MAP strides vs. normal strides, p = 0.04, p = 0.006) and distance (paired t-tests, p < 0.004, p < 0.001). As a result, the AEP of the limb was shifted posteriorly (paired t-test, p < 0.001; figure 4, bottom plots). During the subsequent S + 1 stance period, the limb extended for a shorter time and distance than during a normal stride (paired t-tests, all p < 0.05), but ended its retraction in an even more extended PEP than during the S stride (p < 0.001; figure 4, bottom plot, letter P). Following this shortened stance, there was an increase in limb swing time and distance (paired t-tests, p < 0.005), returning the limb to an AEP at the end of the S + 1 stride that was comparable (five added MAPs, p = 0.5) or even significantly anterior to (seven added MAPs, p < 0.004) the AEP at the beginning of the S stride. None of the above kinematic changes were significant during the addition of one or three MAPs (paired t-tests, all p > 0.1).
(b). Phase advancement experiments
(i). Control potential
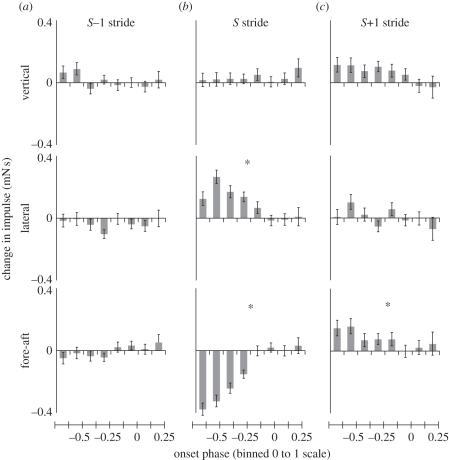
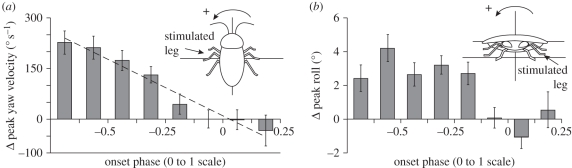
Unlike the burst extension experiments, adding MAPs to advance the phase of activation had no detectable effect on vertical impulse (ANOVA, p > 0.1; figure 5b). Instead, the animal began to turn. Adding spikes in this context now controlled several horizontal plane and rotational variables that were unchanged during the running burst extension trials. During the S stride, the fore–aft impulse was significantly reduced (ANOVA, p < 0.0001), while there was a significant increase in lateral impulse (ANOVA, p < 0.0001). However, the lateral impulse was in the direction of the stimulated limb rather than away from it (figure 5b). As the cockroach's COM slowed and accelerated towards the stimulated leg, its rotational momentum increased as it was rotated into the turn (ANOVA, p < 0.0001; figure 6a). At the same time, the body rolled away from the stimulated leg towards the outside of the turn (ANOVA, p < 0.0001; figure 6b). Yaw velocity showed a strong raded relationship with phase advance (ANOVA, Tukey HSD, p < 0.001; figure 6a). In all other significant variables, the response was nonlinear, with significant effects arising quickly once phase was sufficiently advanced. There were no detectable differences until phase was advanced beyond −0.125 (Welch's t-tests, all p > 0.1), significant changes arose as phase was advanced from −0.125 to −0.375 (ANOVA, p < 0.05) and further phase change did not significantly alter dynamics (all p > 0.1; figure 5b). The one exception was further reduction of forward impulse during the most phase-advanced trials (Welch's t-test, p < 0.01).
Figure 5.
COM impulse control potentials during phase advancement of activation. No significant changes in COM dynamics occurred during the (a) S − 1 stride, whereas the response in the (b) S and (c) S + 1 strides was restricted to the horizontal plane. Here, a positive lateral impulse towards the stimulated leg indicated a net change in momentum to the left. All effects were nonlinear, being significant only when phase was advanced one-eighth of the way back into the S − 1 stride (phase = −0.125). Asterisks indicate statistical significance of the ANOVA.
Figure 6.
Rotational control potential during phase advancement. (a) Cockroaches underwent significant transient increases in yaw velocity and (b) roll in response to phase-advanced activity in the targeted ventral femoral extensor. Notably, the yaw response was again in the direction of the stimulated limb (left) as was the case for lateral impulse (figure 5). Roll, however, was away from the stimulated leg.
In the S + 1 stride, the animal did not continue to accelerate laterally (ANOVA, p > 0.1; figure 5c, middle), but fore–aft impulse was increased, compensating for the slowing of the COM in the S stride. This change was again nonlinear, only arising when phase was advanced to at least −0.125 (ANOVA, p < 0.0001; t-test, p < 0.05; figure 5c, bottom). There were no significant effects in the S − 1 stride (figure 5a).
Finally, as a function of our experimental protocol, the number of added MAPs necessarily increased as phase was advanced. We disambiguated these two variables by taking advantage of strides with similar phases of activation but different numbers of spikes. These groups of strides did not show significant effects of spike number separate from phase differences (t-tests and Kruskal–Wallis tests for eight phase bins and for each COM variable; all p > 0.1). Additionally, repeating our analyses using number of spikes instead of phase of activation did not change our statistical results.
(ii). Limb kinematics
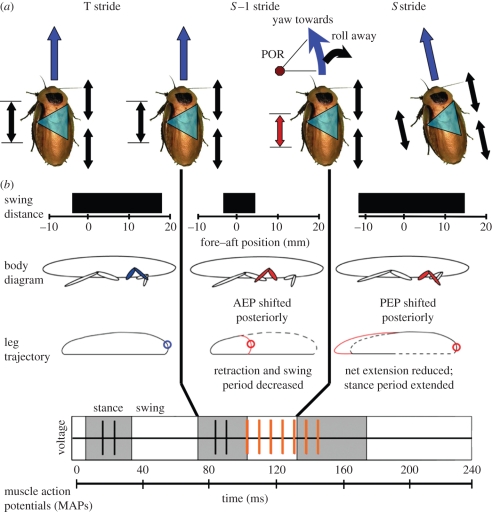
Limb kinematics again changed in a nonlinear manner at the same phases as the changes in body dynamics (figure 7a,b). Given sufficient phase change, the limb began to touch down early at the end of the S − 1 stride (ANOVA, p < 0.0001; figure 7b). As a result, the overall stride period of the S − 1 stride was shortened (ANOVA, p < 0.0001), reducing its duty factor (ANOVA, p < 0.0001) and prematurely terminating protraction (swing). This moved the limb's touch-down position posteriorly at the start of the S stride (ANOVA, p < 0.001). Because the S stride began in a more extended position, the overall extension during stance was also shorter, although the time in stance for the stride actually increased (ANOVA, p < 0.0001). The swing phase at the end of the S stride was increased (ANOVA, p < 0.0001) similar to the S + 1 swing phase in the burst extension experiments, returning the limb to its normal position in the gait cycle. The anterior touch-down position at the end of the S stride was still posterior to normal (ANOVA, p < 0.01) and correction continued into the S + 1 stride. By the end of stance, the S + 1 stride was indistinguishable from a normal stride (ANOVA, p > 0.1). All of the above differences were again only significant when phase was advanced beyond −0.125.
Figure 7.
Initiation of turning as a result of muscle phase advance. Upon phase advance of the ventral femoral extensor's activation, the cockroach underwent a COM dynamic response that was consistent with initiating a turn to the left (a). Roll away from the virtual point of rotation (POR) was consistent with inertial effects as the cockroach accelerated through the turn. S − 1 stride swing phase was significantly shortened (b), which shifted the subsequent AEP posterior to normal positioning. The shortened swing of the S − 1 stride and the stance period of the S stride together constituted a truncated stride that was shifted posterior to the average stride. While limb extension was shortened during the S stance period, the amount of time in stance was actually increased. Since the muscle was shortening during this phase, the change in limb kinematics may allow it to do greater positive work than in a normal stride. In the leg trajectory plots, dashed lines indicate the normal trajectory and the blue circle indicates the normal AEP. Red lines and red circles indicate the swing trajectory and AEPs in the modified strides. In the companion paper [41], we consider how the concomitant changes in strain and muscle activation can alter muscle work output.
4. Discussion
Perturbing neuromuscular activity reveals the potential for neural feedback to a specific muscle to control the body (figure 1). Similar patterns of neural feedback can have different consequences during static and dynamic behaviours. In a standing context, graded increases in activation cause the ventral femoral extensor to act like a linear actuator. In a running context, neural feedback is filtered through mechanics to nonlinearly enhance vertical impulse while having no effect on horizontal plane dynamics. Finally, the control potential of this muscle under phase advancement is to generate rotational movements and lateral acceleration, without vertical plane effects. Phase modulation can lead to turning, but does so in a direction towards the stimulated leg and the response is nonlinear. These results support the hypotheses that as behaviours become more dynamic, neural feedback operates to switch the overall dynamic state of the system, such as recruiting a brake-like muscle to accelerate the body, but does not provide precisely graded control even when neuromuscular activity changes linearly. The method of directly altering activity in individual motor units complements external perturbations in a classical system identification approach, enabling functional characterization of different feedback pathways while preserving the dynamics of the intact biological system.
(a). Control potential under static conditions
Simulating neural feedback during static, standing control supports the idea that translation of neural activation of the ventral femoral extensor results in predictable graded actuation consistent with the muscle's anatomical designation (i.e. femoral extension). The addition of spikes led to a graded control potential in the dynamic variables considered (figure 3; hypothesis 1). These results are consistent with static muscle twitch response and musculo-skeletal models which show that stress develops approximately linearly through six added MAPs, before levelling off as the muscle begins to attain tetanus [16,29,33]. Here, neural feedback seems to primarily determine muscle function, and mechanics linearly translates spikes into impulses.
(b). Control potential during running—burst extension
With the same neural feedback signals in a dynamic context, the control potential of the ventral femoral extensor is neither to absorb energy and decelerate the body nor to act as a linear motor as in the static conditions. We reject both alternative hypotheses predicted from prior experimentation, simulation and modelling (hypothesis 2). Rather, we show a novel control potential for the ventral femoral extensor. Extending the motor activation burst produced a significant increase in COM vertical impulse followed by a pitching rotation in the subsequent stride (figure 3). However, this effect arose only above a threshold of sufficient motor activation. The control potential of this muscle in the full context of the freely behaving animal is to produce accelerations that are nonlinear (figure 3). We reject that this muscle's function in control can be predicted from changes in its motor activation alone (hypothesis 3). Instead, biomechanical context plays a role in shaping the effects of neural feedback.
(c). Context dependency in a muscle's control potential
Interestingly, several variables that had been modulated in the static standing context were no longer changed in the dynamic running context. The lack of change in these variables during running is not simply because of a greater amount of variation obscuring our results. Rather, we would probably detect an effect if it was present because the order of magnitude of the change owing to increasing activation in the static behaviour is at least as great as the variation during running. We conclude that the transformation of neural feedback into dynamics (figure 1) is changing between the two behavioural contexts. Further, the nonlinear control of vertical impulse and pitch indicate that the dynamic control response is readily detectable when there is a functional shift in the muscle between the three and five added MAP conditions.
We reject a uniform control potential for the ventral femoral extensor (control hypothesis 3) and support a significant mechanical role in generating the running control potential. The nervous system must exert its effects through the existing dynamics [5,23], and here we have discovered that this neuromechanical coupling can reveal unexpected control functions even at the level of individual muscles. During slow locomotion, simulations of neural feedback acting through a set of coordination rules have had success in capturing locomotion in quasi-static regimes, including walking, gap crossing and foothold searching [8,10,47,49,50]. For posture control tasks, the linear control potentials of the ventral femoral extensor (figure 3a) support the simple transfer functions in these models. Other femoral extensors active during walking also show a linear correlation between activation and joint velocity [37,40]. However, during dynamic running conditions, neural feedback may not act to precisely prescribe the complete dynamic response necessary to accomplish the task (e.g. obstacle traversal), but rather may act on top of stable mechanical gaits to return the animal to its region of mechanical stability when particularly challenging circumstances require neural control. This idea is consistent with models showing that neural feedback controllers for quasi-static locomotion can actually lead to instability at higher speeds, whereas models that instead enforce patterns of favourable dynamics are more successful [4,6].
Low-dimensional, template models of the running cockroach can give insight into the response to imposed muscular activation. Lateral leg spring dynamics [38,39] may account for the lack of a significant change in roll or yaw. Activation on one side of a tripod of legs could be effectively compressing a virtual horizontal plane spring [39], whose dynamics subsequently channel the perturbation into the vertical plane. Additionally, the nonlinear response we observe is consistent with bifurcation properties of these models, whereby dynamical systems suddenly adopt markedly different behaviour when an input parameter passes a critical value [3]. This type of discrete transition has been shown to occur even in very simple models of legged running with model parameters matched to cockroach dynamics [3,39]. The nervous system may simplify its control task by relying on rapid mechanical feedback, allowing neural feedback to act with greater delays or adjusting the animal's gait from stride-to-stride rather than within stride [5].
(d). Control potential of running—phase advance
One strength of our approach is to reveal the control potential of patterns of neural feedback that have not yet been observed. In our second set of experiments, we considered the effects of adding MAPs in the same behavioural context of running, but altering a different parameter of the neural code. We supported the hypothesis that advancing the phase of activation of the ventral extensor muscle would produce COM dynamic responses consistent with the onset of turning (hypothesis 4). The increased lateral acceleration (figure 5, middle plot) and yaw rotational velocity (figure 6a) were both in the direction of the stimulated muscle, indicating that phase advancing the left leg actually could initiate a turn to the left (figure 6a). These changes are similar to those observed in natural turning [45].
(e). Mechanisms for change in control potential
The filtering of neural feedback through mechanics to generate the running control potentials could arise at several stages of the translation process from neuromuscular activity to COM dynamics. These include the level of individual muscle work output, the transition from muscle work output to limb dynamics or from limb forces to body dynamics. During burst extension, limb kinematics changed in a nonlinear manner (figure 4). Given sufficient increases in motor activation, the extension of stance and the corresponding reduction of swing resulted in an S stride with a larger duty factor (figure 4, dashed boxes). Muscle work output is intimately coupled to limb kinematics and depends on activation, strain, velocity and length of the shortening cycle [16,33]. Changes in strain on the muscle could alter its work output in ways not predicted by previous work loops [16] that showed only increased energy absorption. Full et al. [16] considered changes in limb extension and numbers of added MAPs, but our in vivo manipulation of motor activation suggests that duty factor is the critical parameter that enables the functional change in the muscle. There is precedence for this functional shift in vertebrates. When running guinea fowl encounter an unexpected drop, joint work output can change to dissipate the excess energy or translate it to changes in forward momentum [51].
Novel mechanisms may underlie the turning dynamics brought on by phase advancement (figure 7b). When phase was advanced, a shortened swing phase during the S − 1 phase preceded the longer S stride stance period and changed its timing relative to the rest of the gait cycle (figure 7b, middle column). The body rolling away from the stimulated leg side is similar to the inertial effects that occur when a mass is accelerated around a curve (figures 6b and 7). Whether this response is purely inertial or arises from ground reaction forces producing an active moment in the roll direction will require force plate analyses that are a logical extension beyond the scope of this paper. Interestingly, the dynamic responses could arise from either greater work output from the stimulated leg acting with a concomitant shift in centre of pressure behind the COM, or through a reduction or absorption of energy in this limb causing a net impulse change to the left. Here, we find further evidence supporting both hypotheses. The increase in roll going around the turn could arise from increasing left-side ground reaction forces, but the reduction in the fore–aft impulse during both the S and S + 1 strides is consistent with energy absorption. In our companion paper [41], we will test the capability of this muscle to absorb or produce energy using the in vivo kinematics to specify the relevant strain conditions for the muscle.
(f). Neuromechanical context
Despite changes in timing during the S stride, the S + 1 stride acted to re-synchronize the alternating tripod such that future strides were indistinguishable from normal. This suggests that there has been no change to the clock-like timing of the alternating tripod gait [5,34], as would be predicted from models of interleg coordination of a slowly locomoting insect [6,8,49,52]. Instead, our results are consistent with a central pattern generator (CPG) acting to establish steady-state running dynamics and neural feedback impinging on these activation patterns below the level where timing occurs [4,6,34]. This concurs with results of invertebrates running on mesh substrates where careful coordinated stepping is not used, and distributed mechanical contact via leg spines enables a steady, clock-like gait [21].
Overall, we support the hypothesis that mechanics of the individual muscle and limb filters neural feedback because the kinematic effects followed a nonlinear response to our linear increase in muscle activation. Still, both mechanical and neural feedback acting subsequent to our stimulation could contribute significantly to the changes in body dynamics. These responses are still causally related to our manipulation even if information is flowing around the control loop and hence are part of the realized control potential (figure 1). Neural feedback is unlikely to be affecting the control potential during the stride immediately following changes in motor activation because we did not observe subsequent changes in EMGs, and the overall clock-like timing of the gait cycle did not change [5,34]. Additionally, neural delays are quite significant and the 90 ms stride period typical of these experiments gives little time for typical (approx. 100–150 ms) [7,53] or even ultra-fast reflexes (approx. 10–40 ms) [54,55] to develop force and affect a change in body dynamics.
During phase advance, neural feedback from other components of the system could be shaping the control potential in the S + 1 stride, generating the pitching moment, lateral and fore–aft changes (figure 3). In this light, it is important to consider that control potentials are revealing the effects of only one component signal in the broader motor programme. Showing how these specific causal relationships are combined, perhaps nonlinearly, into synergies [24,56,57] or motor primitives [22,58], or how they remain flexible, distinct elements [57,59] will test how specific muscles contribute to a control strategy.
Unlike neural feedback, mechanical feedback can act instantaneously as the passive properties of other muscle and skeletal components modulate the transmission of force or work to the COM [4,5,21,60]. Therefore, preserving the coupling of the ventral femoral extensor to other physical components of the system could be critical for revealing the control potential realized in the actual biological system. While researchers have tried to separate the acceleration contributions of individual muscles in a modelling context [30,61], small changes in the models can produce highly variable induced accelerations on the body even when only considering a quasi-static control task [62]. Here, we take advantage of the biological context of the intact, behaving animal to directly test this muscle's contribution. In the companion paper [41], we take the next steps in unravelling the mechanisms by which changes in muscle work output generate these diverse control potentials.
Acknowledgements
We would like to thank Shai Revzen, Jean-Michel Mongeau, Justin Seipel, Lena Ting, Alex Vaughan, Jenny McGuire, Tom Libby and the Berkeley Biomechanics group for their insights during this project. Funding was generously provided by a Fannie and John Hertz Foundation Fellowship to S.S. and NSF Frontiers in Integrative Biological Research Grant 0425878 to R.J.F.
Footnotes
One contribution of 15 to a Theme Issue ‘Integration of muscle function for producing and controlling movement’.
References
- 1.Biewener A. A., Daley M. A. 2007. Unsteady locomotion: integrating muscle function with whole body dynamics and neuromuscular control. J. Exp. Biol. 210, 2949–2960 10.1242/jeb.005801 (doi:10.1242/jeb.005801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickinson M. H., Farley C. T., Full R. J., Koehl M. A. R., Kram R., Lehman S. 2000. How animals move: an integrative view. Science 288, 100–106 10.1126/science.288.5463.100 (doi:10.1126/science.288.5463.100) [DOI] [PubMed] [Google Scholar]
- 3.Holmes P., Full R. J., Koditschek D., Guckenheimer J. 2006. The dynamics of legged locomotion: models, analyses, and challenges. SIAM Rev. 48, 207–304 10.1137/S0036144504445133 (doi:10.1137/S0036144504445133) [DOI] [Google Scholar]
- 4.Koditschek D. E., Full R. J., Buehler M. 2004. Mechanical aspects of legged locomotion control. Arthropod Struct. Dev. 33, 251–272 10.1016/j.asd.2004.06.003 (doi:10.1016/j.asd.2004.06.003) [DOI] [PubMed] [Google Scholar]
- 5.Sponberg S., Full R. J. 2008. Neuromechanical response of musculo-skeletal structures in cockroaches during rapid running on rough terrain. J. Exp. Biol. 211, 433–446 10.1242/jeb.012385 (doi:10.1242/jeb.012385) [DOI] [PubMed] [Google Scholar]
- 6.Klavins E., Komsuoglu H., Full R. J., Koditschek D. 2002. The role of reflexes versus central pattern generators in dynamical legged locomotion. In Neurotechnology for biomimetic robots, pp. 351–382 Cambridge, MA: MIT Press [Google Scholar]
- 7.Buschges A. 2005. Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J. Neurophysiol. 93, 1127–1135 10.1152/jn.00615.2004 (doi:10.1152/jn.00615.2004) [DOI] [PubMed] [Google Scholar]
- 8.Cruse H., Durr V., Schmitz J. 2007. Insect walking is based on a decentralized architecture revealing a simple and robust controller. Phil. Trans. R. Soc. A 365, 221–250 10.1098/rsta.2006.1913 (doi:10.1098/rsta.2006.1913) [DOI] [PubMed] [Google Scholar]
- 9.Grillner S. 2006. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52, 751–766 10.1016/j.neuron.2006.11.008 (doi:10.1016/j.neuron.2006.11.008) [DOI] [PubMed] [Google Scholar]
- 10.Pearson K., Ekeberg O., Buschges A. 2006. Assessing sensory function in locomotor systems using neuro-mechanical simulations. Trends Neurosci. 29, 625–631 10.1016/j.tins.2006.08.007 (doi:10.1016/j.tins.2006.08.007) [DOI] [PubMed] [Google Scholar]
- 11.Combes S. A., Daniel T. L. 2003. Into thin air: contributions of aerodynamic and inertial-elastic forces to wing bending in the hawkmoth Manduca sexta. J. Exp. Biol. 206, 2999–3006 10.1242/jeb.00502 (doi:10.1242/jeb.00502) [DOI] [PubMed] [Google Scholar]
- 12.Sane S. P., Dieudonne A., Willis M. A., Daniel T. L. 2007. Antennal mechanosensors mediate flight control in moths. Science 315, 863–866 10.1126/science.1133598 (doi:10.1126/science.1133598) [DOI] [PubMed] [Google Scholar]
- 13.Lee J., Sponberg S. N., Loh O. Y., Lamperski A. G., Full R. J., Cowan N. J. 2008. Templates and anchors for antenna-based wall following in cockroaches and robots. IEEE Trans. Robot. 24, 130–143 10.1109/TRO.2007.914847 (doi:10.1109/TRO.2007.914847) [DOI] [Google Scholar]
- 14.Venkadesan M., Guckenheimer J., Valero-Cuevas F. J. 2007. Manipulating the edge of instability. J. Biomech. 40, 1653–1661 10.1016/j.jbiomech.2007.01.022 (doi:10.1016/j.jbiomech.2007.01.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniel T. L., Tu M. S. 1999. Animal movement, mechanical tuning and coupled systems. J Exp. Biol. 202, 3415–3421 [DOI] [PubMed] [Google Scholar]
- 16.Full R. J., Stokes D. R., Ahn A. N., Josephson R. K. 1998. Energy absorption during running by leg muscles in a cockroach. J. Exp. Biol. 201, 997–1012 [DOI] [PubMed] [Google Scholar]
- 17.Josephson R. K. 1985. Mechanical power output from striated-muscle during cyclic contraction. J. Exp. Biol. 114, 493–512 [Google Scholar]
- 18.Ahn A. N., Full R. J. 2002. A motor and a brake: two leg extensor muscles acting at the same joint manage energy differently in a running insect. J. Exp. Biol. 205, 379–389 [DOI] [PubMed] [Google Scholar]
- 19.Jindrich D. L., Full R. J. 2002. Dynamic stabilization of rapid hexapedal locomotion. J. Exp. Biol. 205, 2803–2823 [DOI] [PubMed] [Google Scholar]
- 20.Kubow T. M., Full R. J. 1999. The role of the mechanical system in control: a hypothesis of self-stabilization in hexapedal runners. Phil. Trans. R. Soc. Lond. B 354, 849–861 10.1098/rstb.1999.0437 (doi:10.1098/rstb.1999.0437) [DOI] [Google Scholar]
- 21.Spagna J. C., Goldman D. I., Lin P. C., Koditschek D. E., Full R. J. 2007. Distributed mechanical feedback in arthropods and robots simplifies control of rapid running on challenging terrain. Bioinspir. Biomim. 2, 9–18 10.1088/1748-3182/2/1/002 (doi:10.1088/1748-3182/2/1/002) [DOI] [PubMed] [Google Scholar]
- 22.Bizzi E., Cheung V. C. K., d'Avella A., Saltiel P., Tresch M. 2008. Combining modules for movement. Brain Res. Rev. 57, 125–133 10.1016/j.brainresrev.2007.08.004 (doi:10.1016/j.brainresrev.2007.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiel H., Ting L., Ekeberg O., Hartmann M. 2009. The brain in its body: motor control and sensing in a biomechanical context. J. Neurosci. 29, 12 807–12 814 10.1523/JNEUROSCI.3338-09.2009 (doi:10.1523/JNEUROSCI.3338-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ting L. H. 2007. Dimensional reduction in sensorimotor systems: a framework for understanding muscle coordination of posture. In Computational neuroscience: theoretical insights into brain function (eds Cisek P., Drew T., Kalaska J. F.), pp. 301–325 Amsterdam, The Netherlands: Elsevier; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valero-Cuevas F. J., Hoffmann H., Kurse M. U., Kutch J. J., Theodorou E. A. 2009. Computational models for neuromuscular function. IEEE Rev. Biomed. Eng. 2, 110–135 10.1109/RBME.2009.2034981 (doi:10.1109/RBME.2009.2034981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowan N., Fortune E. 2007. The critical role of locomotion mechanics in decoding sensory systems. J. Neurosci. 27, 1123–1128 10.1523/JNEUROSCI.4198-06.2007 (doi:10.1523/JNEUROSCI.4198-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ristroph L., Bergou A., Ristroph G. 2010. Discovering the flight autostabilizer of fruit flies by inducing aerial stumbles. Proc. Natl Acad. Sci. USA 107, 4820–4824 10.1073/pnas.1000615107 (doi:10.1073/pnas.1000615107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Full R. J., Kubow T., Schmitt J., Holmes P., Koditschek D. 2002. Quantifying dynamic stability and maneuverability in legged locomotion. Integr. Comp. Biol. 42, 149–157 10.1093/icb/42.1.149 (doi:10.1093/icb/42.1.149) [DOI] [PubMed] [Google Scholar]
- 29.Full R. J., Ahn A. N. 1995. Static forces and moments generated in the insect leg: comparison of a three-dimensional musculoskeletal computer model with experimental measurements. J. Exp. Biol. 198, 1285–1298 [DOI] [PubMed] [Google Scholar]
- 30.Zajac F. E., Neptune R. R., Kautz S. A. 2002. Biomechanics and muscle coordination of human walking—part I: Introduction to concepts, power transfer, dynamics and simulations. Gait Posture 16, 215–232 10.1016/S0966-6362(02)00068-1 (doi:10.1016/S0966-6362(02)00068-1) [DOI] [PubMed] [Google Scholar]
- 31.Delp S. L., Loan J. P. 2000. A computational framework for simulating and analyzing human and animal movement. Comput. Sci. Eng. 2, 46–55 10.1109/5992.877394 (doi:10.1109/5992.877394) [DOI] [Google Scholar]
- 32.Ahn A. N., Meijer K., Full R. J. 2006. In situ muscle power differs without varying in vitro mechanical properties in two insect leg muscles innervated by the same motor neuron. J. Exp. Biol. 209, 3370–3382 10.1242/jeb.02392 (doi:10.1242/jeb.02392) [DOI] [PubMed] [Google Scholar]
- 33.Josephson R. K. 1999. Dissecting muscle power output. J. Exp. Biol. 202, 3369–3375 [DOI] [PubMed] [Google Scholar]
- 34.Revzen S., Guckenheimer J. M. 2008. Estimating the phase of synchronized oscillators. Phys. Rev. E. 78, 051907. 10.1103/PhysRevE.78.051907 (doi:10.1103/PhysRevE.78.051907) [DOI] [PubMed] [Google Scholar]
- 35.Pearson K. G., Iles J. F. 1970. Discharge patterns of coxal levator and depressor motoneurones of cockroach, Periplaneta americana. J. Exp. Biol. 52, 139–165 [DOI] [PubMed] [Google Scholar]
- 36.Pipa R. I., Cook E. F. 1959. Studies on the Hexapod nervous system. I. The peripheral distribution of the thoracic nerves of the adult cockroach, Periplaneta americana. Ann. Entomol. Soc. Am. 52, 695–710 [Google Scholar]
- 37.Watson J. T., Ritzmann R. E. 1998. Leg kinematics and muscle activity during treadmill running in the cockroach, Blaberus discoidalis: II. Fast running. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 182, 23–33 10.1007/s003590050154 (doi:10.1007/s003590050154) [DOI] [PubMed] [Google Scholar]
- 38.Seipel J., Holmes P. 2006. Three-dimensional translational dynamics and stability of multi-legged runners. Int. J. Rob. Res. 25, 889–902 10.1177/0278364906069045 (doi:10.1177/0278364906069045) [DOI] [Google Scholar]
- 39.Schmitt J., Garcia M., Razo R. C., Holmes P., Full R. J. 2002. Dynamics and stability of legged locomotion in the horizontal plane: a test case using insects. Biol. Cybern. 86, 343–353 10.1007/s00422-001-0300-3 (doi:10.1007/s00422-001-0300-3) [DOI] [PubMed] [Google Scholar]
- 40.Watson J. T., Ritzmann R. E. 1998. Leg kinematics and muscle activity during treadmill running in the cockroach, Blaberus discoidalis: I. Slow running. J. Comp. Physiol. A Sens. Neural Behav. Physiol. 182, 11–22 10.1007/s003590050153 (doi:10.1007/s003590050153) [DOI] [PubMed] [Google Scholar]
- 41.Sponberg S., Libby T., Mullens C. H., Full R. J. 2011. Shifts in a single muscle's control potential of body dynamics are determined by mechanical feedback. Phil. Trans. R. Soc B 366, 1606–1620 10.1098/rstb.2010.0368 (doi:10.1098/rstb.2010.0368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ting L. H., Blickhan R., Full R. J. 1994. Dynamic and static stability in hexapedal runners. J. Exp. Biol. 197, 251–269 [DOI] [PubMed] [Google Scholar]
- 43.Zill S., Schmitz J., Buschges A. 2004. Load sensing and control of posture and locomotion. Arthropod Struct. Dev. 33, 273–286 10.1016/j.asd.2004.05.005 (doi:10.1016/j.asd.2004.05.005) [DOI] [PubMed] [Google Scholar]
- 44.Carbonell C. S. 1947. The thoracic muscles of the cockroach Periplaneta americana (L.). Smithsonian Misc. Coll. 107, 1–23 [Google Scholar]
- 45.Jindrich D. L., Full R. J. 1999. Many-legged maneuverability: dynamics of turning in hexapods. J. Exp. Biol. 202, 1603–1623 [DOI] [PubMed] [Google Scholar]
- 46.Spence A., Revzen S., Seipel J., Mullens C., Full R. J. 2010. Insects running on elastic surfaces. J. Exp. Biol. 213, 1907–1920 10.1242/jeb.042515 (doi:10.1242/jeb.042515) [DOI] [PubMed] [Google Scholar]
- 47.Duerr V. 2001. Stereotypic leg searching movements in the stick insect: kinematic analysis, behavioural context and simulation. J. Exp. Biol. 204, 1589–1604 [DOI] [PubMed] [Google Scholar]
- 48.Full R. J., Tu M. S. 1990. Mechanics of 6-legged runners. J. Exp. Biol. 148, 129–146 [DOI] [PubMed] [Google Scholar]
- 49.Ekeberg O., Blumel M., Buschges A. 2004. Dynamic simulation of insect walking. Arthropod Struct. Dev. 33, 287–300 10.1016/j.asd.2004.05.002 (doi:10.1016/j.asd.2004.05.002) [DOI] [PubMed] [Google Scholar]
- 50.Chiel H. J., Beer R. D., Quinn R. D., Espenschied K. S. 1992. Robustness of a distributed neural network controller for locomotion in a hexapedal robot. IEEE Trans. Rob. Autom. 8, 293–303 10.1109/70.143348 (doi:10.1109/70.143348) [DOI] [Google Scholar]
- 51.Daley M. A., Usherwood J. R., Felix G., Biewener A. A. 2006. Running over rough terrain: guinea fowl maintain dynamic stability despite a large unexpected change in substrate height. J. Exp. Biol. 209, 171–187 10.1242/jeb.01986 (doi:10.1242/jeb.01986) [DOI] [PubMed] [Google Scholar]
- 52.Buschges A., Akay T., Gabriel J. P., Schmidt J. 2008. Organizing network action for locomotion: insights from studying insect walking. Brain Res. Rev. 57, 162–171 10.1016/j.brainresrev.2007.06.028 (doi:10.1016/j.brainresrev.2007.06.028) [DOI] [PubMed] [Google Scholar]
- 53.Schaefer P. L., Kondagunta G. V., Ritzmann R. E. 1994. Motion analysis of escape movements evoked by tactile stimulation in the cockroach Periplaneta americana. J. Exp. Biol. 190, 287–294 [DOI] [PubMed] [Google Scholar]
- 54.Camhi J. M., Johnson E. N. 1999. High-frequency steering maneuvers mediated by tactile cues: antennal wall-following by the cockroach. J. Exp. Biol. 202, 631–643 [DOI] [PubMed] [Google Scholar]
- 55.Holtje M., Hustert R. 2003. Rapid mechano-sensory pathways code leg impact and elicit very rapid reflexes in insects. J. Exp. Biol. 206, 2715–2724 10.1242/jeb.00492 (doi:10.1242/jeb.00492) [DOI] [PubMed] [Google Scholar]
- 56.Torres-Oviedo G., Macpherson J. M., Ting L. H. 2006. Muscle synergy organization is robust across a variety of postural perturbations. Jl Neurophysiol. 96, 1530–1546 10.1152/jn.00810.2005 (doi:10.1152/jn.00810.2005) [DOI] [PubMed] [Google Scholar]
- 57.Tresch M., Jarc A. 2009. The case for and against muscle synergies. Curr. Opin. Neurobiol. 19, 601–607 10.1016/j.conb.2009.09.002 (doi:10.1016/j.conb.2009.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graziano M. S. A., Aflalo T. N. S., Cooke D. F. 2005. Arm movements evoked by electrical stimulation in the motor cortex of monkeys. J. Neurophysiol. 94, 4209–4223 10.1152/jn.01303.2004 (doi:10.1152/jn.01303.2004) [DOI] [PubMed] [Google Scholar]
- 59.Kutch J., Kuo A., Bloch A. 2008. Endpoint force fluctuations reveal flexible rather than synergistic patterns of muscle cooperation. J. Neurophysiol. 100, 2455–2471 10.1152/jn.90274.2008 (doi:10.1152/jn.90274.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown I. E., Loeb G. E. 2000. A reductionist approach to creating and using neuromusculoskeletal movement. In Biomechanics and neural control of movement (eds Winters J. M., Crago P. E.). New York, NY: Springer [Google Scholar]
- 61.Arnold A. S., Anderson F. C., Pandy M. G., Delp S. L. 2005. Muscular contributions to hip and knee extension during the single limb stance phase of normal gait: a framework for investigating the causes of crouch gait. J. Biomech. 38, 2181–2189 10.1016/j.jbiomech.2004.09.036 (doi:10.1016/j.jbiomech.2004.09.036) [DOI] [PubMed] [Google Scholar]
- 62.Chen G. 2006. Induced acceleration contributions to locomotion dynamics are not physically well defined. Gait Posture 23, 37–44 10.1016/j.gaitpost.2004.11.016 (doi:10.1016/j.gaitpost.2004.11.016) [DOI] [PubMed] [Google Scholar]