Abstract
Background
In patients with urinary bladder cancer, brain metastases are quite rare and occur in only 1–7% of these patients. Of the urinary bladder cancers, large cell neuroendocrine carcinoma (LCNEC) is extremely rare; only 16 cases have been reported to date. In this report, a case of brain metastasis from LCNEC of the urinary bladder is described.
Case Description
A 74-year-old man was admitted with confusion and left-sided hemiparesis. Head magnetic resonance imaging demonstrated a ring-enhancing lesion in the right frontal lobe. Whole body computed tomography revealed a suspicious lesion in the urinary bladder. These findings were considered consistent with metastatic brain tumor. Craniotomy and tumor removal were performed. After craniotomy, the patient underwent cystoscopy and the bladder mass was biopsied. Histological and immunohistochemical examination of both the brain tumor and bladder mass revealed LCNEC. According to these findings, the patient was diagnosed with a brain metastasis from LCNEC of the urinary bladder.
Conclusion
To our knowledge, this is the first report of a patient with a brain metastasis from LCNEC of the urinary bladder.
Keywords: Brain metastasis, large cell neuroendocrine carcinoma, thyroid transcription factor-1, urinary bladder
INTRODUCTION
Brain metastases are quite rare in patients with urinary bladder cancer, and various case series in the literature have reported a 1–7% incidence of brain metastases in these patients.[4–6,10] Neuroendocrine carcinomas account for less than 1% of primary urinary bladder malignancies.[7,8,21] The most common neuroendocrine carcinoma of the urinary bladder is small cell carcinoma (SCC). Recently, a type of neuroendocrine carcinoma that is morphologically distinct from SCC was recognized as large cell neuroendocrine carcinoma (LCNEC). However, only 16 cases of LCNEC of the urinary bladder have been reported in the English language literature.[1–3,11,12,14,17–19,23,24] Moreover, to date, there have been no reports of brain metastasis from LCNEC of the urinary bladder. This report presents the first case of a brain metastasis from LCNEC of the urinary bladder.
CASE REPORT
The patient was a 74-year-old man who presented to our institution with a 2-week history of confusion and memory disturbance. On neurological examination, the patient was found to have disorientation and mild left-sided hemiparesis. Magnetic resonance imaging (MRI) of the head demonstrated a mass lesion, 4 cm in diameter, in the right frontal lobe with surrounding edema; the ring-enhancing lesion was strongly enhanced by gadolinium contrast on T1-weighted imaging [Figure 1]. Subsequent computed tomography (CT) of the chest, abdomen and pelvis revealed a suspicious lesion in the left lateral wall of the urinary bladder with small calcifications and an enlarged lymph node of the carina tracheae, but no mass lesions in the lung fields [Figure 2]. He denied any hematuria or other urologic symptoms. Preoperatively, these findings were considered consistent with a metastatic brain tumor. Craniotomy was performed and the tumor completely removed. Postoperatively, he regained consciousness, the left-sided hemiparesis improved, and the patient showed a remarkable improvement in quality of life.
Figure 1.
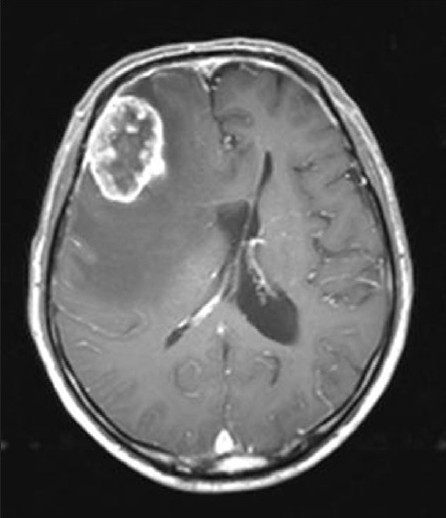
Magnetic resonance imaging showing the right frontal mass as a ring-enhancing lesion on T1-weighted imaging with gadolinium contrast enhancement
Figure 2.
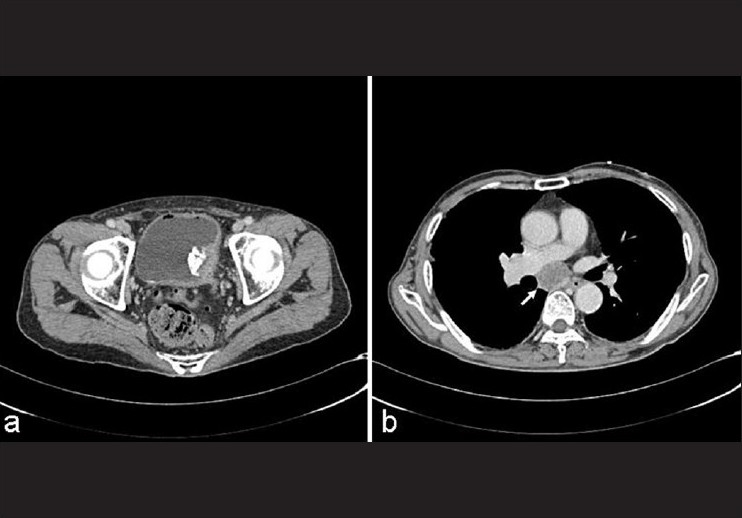
Contrast-enhanced computed tomography showing the tumor in the left lateral wall of the urinary bladder with small calcification (a) and enlarged lymph node of carina tracheae (arrow) (b)
Histological examination of the brain specimen revealed metastatic LCNEC characterized by diffuse proliferation of large atypical cells with coarse nuclear chromatin and frequent nucleoli [Figure 3a, b]. The carcinoma cells expressed CD56, chromogranin A, synaptophysin and thyroid transcription factor-1 (TTF-1) [Figure 3c–f].
Figure 3.
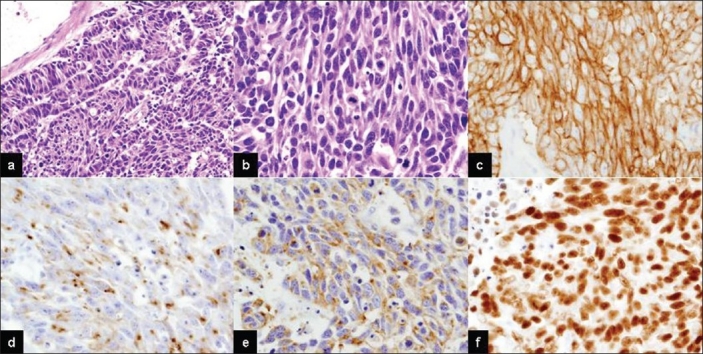
Microscopic findings of metastatic brain large cell neuroendocrine carcinoma. Neoplastic cells showing a sheet-like growth with trabecular and rosette-like patterns (a, b). Tumor cells demonstrating abundant cytoplasm, frequent salt-and-pepper like nuclear chromatin and numerous mitotic figures (H and E: a, ×100; b, ×200). Tumor cell expression of CD56 (c), chromogranin A (d), synaptophysin (e) and TTF-1 (f) (×200)
A urology consultation was requested. The patient underwent cystoscopy and the bladder mass was biopsied. Histological and immunohistochemical examination revealed LCNEC, the same findings as the brain specimen [Figure 4]. Finally, the patient was diagnosed with a brain metastasis from LCNEC of the urinary bladder.
Figure 4.
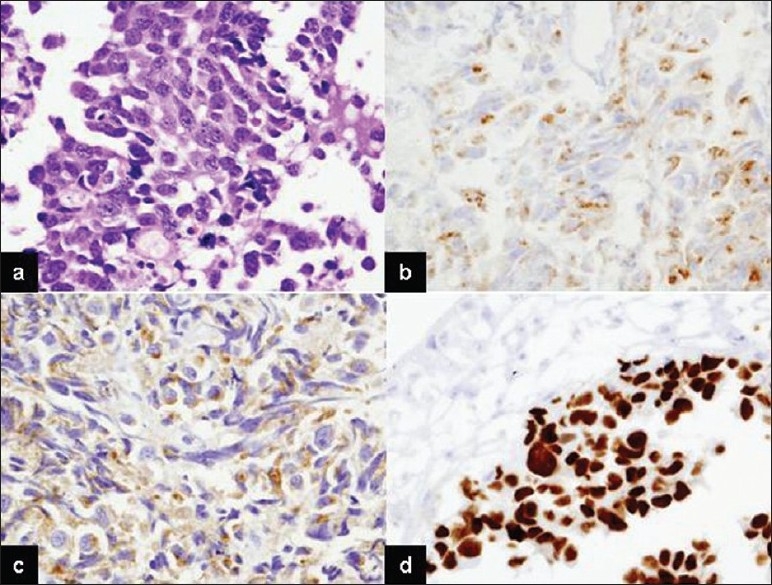
Microscopic findings of primary urinary large cell neuroendocrine carcinoma. Large tumor cells have abundant cytoplasm with large nuclei, vesicular nuclear chromatin, and prominent nucleoli (a) (H and E, ×200). Tumor cell expression of chromogranin A (b), synaptophysin (c) and TTF-1 (d) (×200)
Aggressive chemotherapy was initiated with carboplatin and etoposide. The patient also received whole brain radiotherapy. Head MRI done 4 months after surgical treatment showed complete removal of the brain metastasis and no evidence of recurrence or new lesions. However, the patient suddenly died of pulmonary embolism 5 months after surgical treatment.
DISCUSSION
Neuroendocrine tumors of the urinary bladder are relatively rare and include carcinoids, LCNEC, and SCC, the latter being by far the most common. Travis et al. first described LCNEC in the lungs[25] and they proposed the criteria for LCNEC. These criteria include cells large in size, polygonal in shape and with a low nuclear to cytoplasmic ratio; coarse nuclear chromatin and frequent nucleoli; mitotic activity in excess of 10 mitoses per 10 high-power fields with frequent necrosis; and immunohistochemical or ultrastructural evidence of neuroendocrine differentiation. To confirm the neuroendocrine features, tumor cells were stained with antibodies to CD56, synaptophysin, neuron-specific enolase, and chromogranin A.[20] Recently, a novel immunohistochemical marker, TTF-1, has been demonstrated in the neuroendocrine tumors. Among the pulmonary tumors, TTF-1 positivity is frequently found in SCCs (83–100%), atypical carcinoid tumors (100%), and LCNECs (25-75%).[13,16,22] Among SCCs of the urinary bladder, TTF-1 expression is reported to be 27–39%.[3,9,15] Interestingly, 4 (80%) of 5 cases of LCNEC of the urinary bladder (including the present case) that were examined immunohistochemically for TTF-1 demonstrated TTF-1 expression.[3,18,23] Therefore, it can be speculated that TTF-1 may be a useful marker for LCNEC of the urinary bladder.
Furthermore, LCNEC of the urinary bladder is extremely rare, with only 16 reported cases in the English language literature, to our knowledge.[1–3,11,12,14,17–19,23,24] The first description of an LCNEC of the bladder reported by Abenoza et al.[1] was of an LCNEC combined with adenocarcinoma. According to the literature, approximately half of the reported cases of LCNEC have been a mixture with other histological components, such as transitional cell carcinoma, and the rest of the cases have been reported as pure primary LCNEC of the bladder (including the present case). Because there have been only 16 previously reported cases of LCNEC of the urinary bladder, the biological behavior of this carcinoma remains to be determined. However, at least four cases have been reported to have widespread metastases despite treatment soon after diagnosis, suggesting aggressive behavior similar to SCC of the urinary bladder.[1,14,17,18] Distant metastases, either at diagnosis or later in the course of illness, have been observed in patients with LCNEC of the urinary bladder. Lung and liver were the most commonly reported sites of metastases. To our knowledge, this is the first reported case of a brain metastasis from LCNEC of the urinary bladder.
The low frequency of brain metastases in patients with bladder cancer may indicate a lack of metastatic potential for the brain. Moreover, the liver and lung act as filters to the right side of the heart, and this filtering before systemic circulation is probably partly responsible for the low incidence of metastasis to the brain. Review of the literature reveals that most patients with brain metastasis were identified after urinary bladder cancer was diagnosed. However, in the present case, there was no clinical evidence of urinary bladder cancer before the brain lesion was diagnosed. This observation is also quite rare and unusual. To date, routine neuroimaging screening is not performed on patients with a past history of urinary bladder cancer. Neuroimaging is undertaken generally only when symptoms such as headache, seizures, or focal deficits are present. This may have led to a lower frequency of brain metastases being detected. Patients′ complaints should be carefully noted, and detailed neuroimaging should be carried out promptly if such patients have symptoms including headache, seizures, altered consciousness or focal deficits.
Once metastasis to the brain occurs, prognosis is very poor, with most patients surviving less than 6 months. Surgical resection of a brain metastasis does not influence the underlying disease and may not increase long-term survival. On the other hand, surgical resection may enhance the quality of life, as occurred in the present patient with resolution of his paresis. Early detection and early treatment are desirable for improvement of outcomes in patients with brain metastases.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/84/82250
Contributor Information
Atsushi Tsugu, Email: tsugu@is.icc.u-tokai.ac.jp.
Michitsura Yoshiyama, Email: yossymichitsura0710@yahoo.co.jp.
Mitsunori Matsumae, Email: mike@is.icc.u-tokai.ac.jp.
REFERENCES
- 1.Abenoza P, Manivel C, Sibley RK. Adenocarcinoma with neuroendocrine differentiation of the urinary bladder. Arch Pathol Lab Med. 1986;110:1062–6. [PubMed] [Google Scholar]
- 2.Akamatsu S, Kanamaru S, Ishihara M, Sano T, Soeda A, Hashimoto K. Primary large cell neuroendocrine carcinoma of the urinary bladder. Int J Urol. 2008;15:1080–3. doi: 10.1111/j.1442-2042.2008.02168.x. [DOI] [PubMed] [Google Scholar]
- 3.Alijo Serrano F, Sanchez-Mora N, Angel Arranz J, Hernandez C, Alvarez-Fernandez E. Large cell and small cell neuroendocrine bladder carcinoma: Immunohistochemical and outcome study in a single institution. Am J Clin Pathol. 2007;128:733–9. doi: 10.1309/HTREM6QYQDYGNWYA. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RS, El Mahdi AM, Kuban DA, Higgins EM. Brain metastases from transitional cell carcinoma of urinary bladder. Urology. 1992;39:17–20. doi: 10.1016/0090-4295(92)90034-t. [DOI] [PubMed] [Google Scholar]
- 5.Anderson TS, Regine WF, Kryscio R, Patchell RA. Neurologic complications of bladder carcinoma: A review of 359 cases. Cancer. 2003;97:2267–72. doi: 10.1002/cncr.11354. [DOI] [PubMed] [Google Scholar]
- 6.Babaian RJ, Johnson DE, Liamas L, Ayala AG. Metastases from transitional cell carcinoma of urinary bladder. Urology. 1980;16:142–4. doi: 10.1016/0090-4295(80)90067-9. [DOI] [PubMed] [Google Scholar]
- 7.Bertaccini A, Marchiori D, Cricca A, Carofalo M, Giovannini C, Manferrari F, et al. Neuroendocrine carcinoma of the urinary bladder: Case report and review of the literature. Anticancer Res. 2008;28:1369–72. [PubMed] [Google Scholar]
- 8.Cheng L, Pan CX, Yang XJ, Lopez-Beltran A, MacLennan G, Lin H, et al. Small cell carcinoma of the urinary bladder: A clinicopathologic analysis of 64 patients. Cancer. 2004;101:957–62. doi: 10.1002/cncr.20456. [DOI] [PubMed] [Google Scholar]
- 9.Cheuk W, Kwan MY, Suster S, Chan JK. Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med. 2001;125:228–31. doi: 10.5858/2001-125-0228-IFTTFA. [DOI] [PubMed] [Google Scholar]
- 10.Committee of the Brain Tumor Registry of Japan: Metastatic brain tumors. In:Report of Brain Tumor Registry of Japan (1984-2000) Neurol Med Chir(Tokyo) Suppl. 2009;49:22–5. [Google Scholar]
- 11.Dundr P, Pesl M, Povysil C, Vitkova I, Dvoracek J. Large cell neuroendocrine carcinoma of the urinary bladder with lymphoepithelioma-like features. Pathol Res Pract. 2003;199:559–63. doi: 10.1078/0344-0338-00462. [DOI] [PubMed] [Google Scholar]
- 12.Evans AJ, Al-Maghrabi J, Tsihlias J, Lajoie G, Sweet JM, Chapman WB. Primary large cell neuroendocrine carcinoma of the urinary bladder. Arch Pathol Lab Med. 2002;126:1229–32. doi: 10.5858/2002-126-1229-PLCNCO. [DOI] [PubMed] [Google Scholar]
- 13.Folpe AL, Gown AM, Lamps LW, Garcia R, Dail DH, Zarbo RJ, et al. Thyroid transcription factor-1: Immunohistochemical evaluation in pulmonary neuroendocrine tumors. Mod Pathol. 1999;12:5–8. [PubMed] [Google Scholar]
- 14.Hailemariam S, Gaspert A, Komminoth P, Tamboli P, Amin M. Primary, pure, large-cell neuroendocrine carcinoma of the urinary bladder. Mod Pathol. 1998;11:1016–20. [PubMed] [Google Scholar]
- 15.Jones TD, Kernek KM, Yang XJ, Lopez-Beltran A, MacLennan GT, Eble JN, et al. Thyroid transcription factor 1 expression in small cell carcinoma of the urinary bladder: An immunohistochemical profile of 44 cases. Hum Pathol. 2005;36:718–23. doi: 10.1016/j.humpath.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann O, Dietel M. Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathology. 2000;36:8–16. doi: 10.1046/j.1365-2559.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee KH, Ryu SB, Lee MC, Park CS, Juhng SW, Choi C. Primary large cell neuroendocrine carcinoma of the urinary bladder. Pathol Int. 2006;56:688–93. doi: 10.1111/j.1440-1827.2006.02031.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee WJ, Kim CH, Chang SE, Lee MW, Choi JH, Moon KC, et al. Cutaneous metastasis from large-cell neuroendocrine carcinoma of the urinary bladder expressing CK20 and TTF-1. Am J Dermatopathol. 2009;31:166–9. doi: 10.1097/DAD.0b013e31818eba4c. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Outman JE, Mathur SC. Carcinosarcoma with large cell neuroendocrine epithelial component: First report of an unusual biphasic tumour of the urinary bladder. J Clin Pathol. 2004;57:318–20. doi: 10.1136/jcp.2003.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loy TS, Darkow GV, Quesenberry JT. Immunostaining in the diagnosis of pulmonary neuroendocrine carcinomas.An immunohistochemical study with ultrastructural correlations. Am J Surg Pathol. 1995;19:173–82. doi: 10.1097/00000478-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Mazzucchelli R, Morichetti D, Lopez-Beltran A, Cheng L, Scarpelli M, Kirkali Z, et al. Neuroendocrine tumours of the urinary system and male genital organs: Clinical significance. BJU Int. 2009;103:1464–70. doi: 10.1111/j.1464-410X.2009.08451.x. [DOI] [PubMed] [Google Scholar]
- 22.Ordonez NG. Thyroid transcription factor-1 is a marker of lung and thyroid carcinomas. Adv Anat Pathol. 2000;7:123–7. doi: 10.1097/00125480-200007020-00007. [DOI] [PubMed] [Google Scholar]
- 23.Oshiro H, Gomi K, Nagahama K, Nagashima Y, Kanazawa M, Kato J, et al. Urinary cytologic features of primary large cell neuroendocrine carcinoma of the urinary bladder: A case report. Acta Cytol. 2010;54:303–10. doi: 10.1159/000325039. [DOI] [PubMed] [Google Scholar]
- 24.Quek ML, Nichols PW, Yamzon J, Daneshmand S, Miranda G, Cai J, et al. Radical cystectomy for primary neuroendocrine tumors of the bladder: The University of Southern California experience. J Urol. 2005;174:93–6. doi: 10.1097/01.ju.0000162085.20043.1f. [DOI] [PubMed] [Google Scholar]
- 25.Travis WD, Linnoila I, Tsokos MG, Hitchcock CL, Cutler GB, Nieman L. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma: An ultrastructural, immunohistochemical and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15:529–53. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]


