Abstract
Background:
A major purported benefit of minimally-invasive spinal surgery (MIS) technique is less disruption of paraspinal soft tissues, but there is little quantifiable evidence of this in medical literature. Postoperative C-reactive protein (CRP) levels been shown to become more significantly elevated with larger surgical procedures, and this may allow for more measurable appreciation of any benefits of MIS verses open spinal surgery.
Methods:
CRP values were measured prior to and at multiple time points following surgery in patients undergoing posterior spinal fusion using both open and minimally invasive techniques.
Results:
Peak postoperative CRP was significantly lower in the 35 single-level minimally invasive procedures compared with the 11 single-level open procedures (13.5 vs. 21.3, P <0.01) and lower in the 12 two-level minimally invasive surgeries compared with 16 two-level open procedures (20.5 vs. 31.8, P <0.01).
Conclusions:
MIS lumbar fusion is associated with a lower peak in postoperative CRP compared with open surgery. This appears to support the notion that minimally invasive spine surgery technique leads to a measurable reduction in paraspinal soft tissue destruction mediated inflammation in the immediate postoperative period.
Keywords: C-reactive protein, infection, lumbar fusion, minimally invasive surgery, prospective, spine surgery
INTRODUCTION
Minimally invasive surgery (MIS) techniques are rapidly gaining popularity to treat a variety of spinal conditions. MIS procedures typically achieve exposure of the spine via tubular access ports placed after sequential, blunt, muscle dilation rather than wide dissection of paraspinal tissues and the purported advantages include reduced blood loss and postoperative pain. In addition, it has been suggested that MIS may lower the incidence of postoperative infections.[2,3]
C-reactive protein (CRP) is a non-specific marker of inflammation that has been shown to be valuable in the diagnosis of postoperative infections and for surveillance during treatment of established spinal infections.[6–8,10] CRP is manufactured in the liver and the normal plasma level is less than 1 mg/L. Within a 4-6 hr after initial tissue injury levels begin to rise. CRP half-life is less than 24 hours, and levels return to normal much faster than the erythrocyte sedimentation rate (ESR) making it more attractive as a marker of active inflammation or infection.[4–6,8,16] Normative values following lumbar spinal fusion surgery have not been definitively established, but evidence suggests that the peak values increase with the extent of surgical tissue destruction.[12,15] Al-Jabi noted that following neurosurgery, extent to which postoperative levels of CRP are elevated seem to depend upon the magnitude of the procedure.[1] Reduced soft tissue dissection in minimally invasive lumbar fusion may lower the expected values of postoperative CRP when compared with conventional open surgery, and awareness of any such difference may prove helpful in assessing infectious complications.
In this prospective study, we recorded the value of CRP prior to and at multiple time points following surgery in patients undergoing posterior spinal fusion using both open and minimally invasive techniques. Statistical analysis was employed to compare the results of theses two groups as well the results of subgroups undergoing single or two-level surgery.
MATERIALS AND METHODS
Over an eighteen month period at a university tertiary care center, patients undergoing one or two-level lumbar fusion with instrumentation had CRP tested prior to surgery and postoperatively at days 1, 3, 7, and at subsequent 3-week intervals until normalized. Indications for surgery were lumbar stenosis with spondylolisthesis; radiculopathy with foraminal stenosis, foreshortening of the disc space, and facet arthropathy; and recurrent disc herniation. Selection of operative technique was based upon surgeon preference. The majority of open surgery patients were treated in the early months of the study period before the senior authors practice had principally shifted to minimally invasive technique. Data was compiled on patient age, sex, level of surgery, medical comorbidities, and history of prior lumbar surgery.
MIS surgery was accomplished through a paramedian incision, typically 4cm off the midline, utilizing a blunt, muscle-splitting approach using a tubular dilators prior to placement of a working channel anchored to the OR table. All MIS patients underwent transforaminal lumbar interbody fusion (TLIF) with total or partial laminectomy, placement local autograft supplemented with cancellous allograft chips in the anterior third of the disc space, and interbody placement of a polyetheretherketone (PEEK) spacer filled with a portion of BMP-2 sponge (infuse, Medtronic Inc., Minneapolis) sized to loosely fit the cage cavity. The intertransverse gutter was not exposed for graft placement. Internal fixation with bilateral titanium pedicle screws was then inserted percutaneously under fluoroscopic guidance. The skin incision for a single-level MIS procedure was typically 28-30mm, and was extended several millimeters for the two-level procedures. Surgical drains were not used in any MIS patient.
Patients treated with open surgery underwent a midline incision and laminectomy followed by traditional posterolateral, intertransverse fusion with local autograft supplemented with allograft cancellous chips and bilateral pedicle screw instrumentation. Three single-level open fusion patients also had placement of a PEEK interbody spacer filled with local autograft in addition to the graft in the posterolateral gutter. Subfascial surgical drains were placed in all open surgery patients and typically removed on the second postoperative day.
Any infectious complications were noted and excluded from analysis. Commercially available software (Graphpad Prism 4, Graphpad Software Inc., San Diego CA) was used to generate descriptive statistics analysis. The Mann-Whitney test was used to compare the peak values of CRP of patient subgroups.
RESULTS
Eighty-nine consecutive patients underwent surgery. Excluded from analysis were nine patients in whom a baseline CRP value failed to be drawn, three patients that failed to follow up with a minimum of three postoperative blood draws, and three patients that developed postoperative infections. The remaining 74 patients were the basis of the study, out of whom there were 41 female and 33 male patients a mean age of 59. Eight patients had undergone prior lumbar surgery, four of 27 (14%) patients undergoing open surgery and four of 47 (8%) of those undergoing MIS procedures. Fifty-two (70%) had lumbar spinal stenosis with a spondylolisthesis, sixteen (21%) had radiculopathy with foraminal stenosis associated with disc space foreshortening and facet arthropathy, and six (8%) had recurrent disc herniation. Demographic data and medical comorbidities of the four subgroups assessed in this study are detailed in Table 1.
Table 1.
Demographic and comorbidity data for patients undergoing lumbar decompression and fusion using either minimally invasive surgery or Open techniques
Data was collected according to protocol in only 40% of patients, with many patients missing specimen collection on the appropriate date and having additional collections on earlier and later days [Figure 1] Most of the deviation from protocol took place at the one and three week intervals, while over 80% of the day one and day three tests were successfully collected. Peak postoperative CRP was significantly lower in the 35 single-level minimally invasive procedures when compared with the 11 single-level open procedures (13.5 vs. 21.3, P <0.01) [Figure 2] and lower in the 12 two-level minimally invasive surgeries compared with 16 two-level open procedures (20.5 vs. 31.8, P <0.01). [Figure 3]. Detailed statistical data comparing subgroups is displayed in Tables 2 and 3. Peak value of CRP occurred at a mean 2.9 days after surgery and there was no significant difference in timing of peak values between various subgroups [Figure 4].
Figure 1.
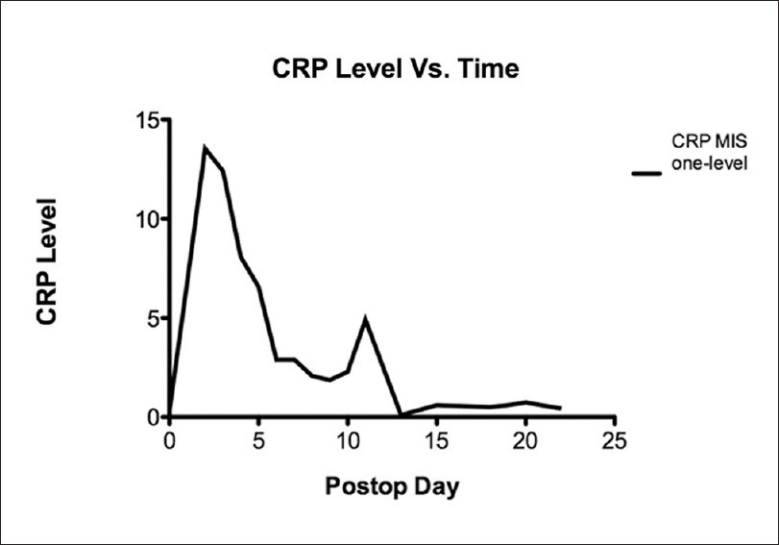
A graph demonstrating the value of C-reactive protein verses time following single level minimally invasive transforaminal lumbar interbody fusion procedures. The C-reactive protein rises rapidly to a peak generally seen at the third postoperative day after which it rapidly declines. Increasing C-reactive protein values subsequent to the fourth postoperative day should raise suspicion of a surgical infection
Figure 2.
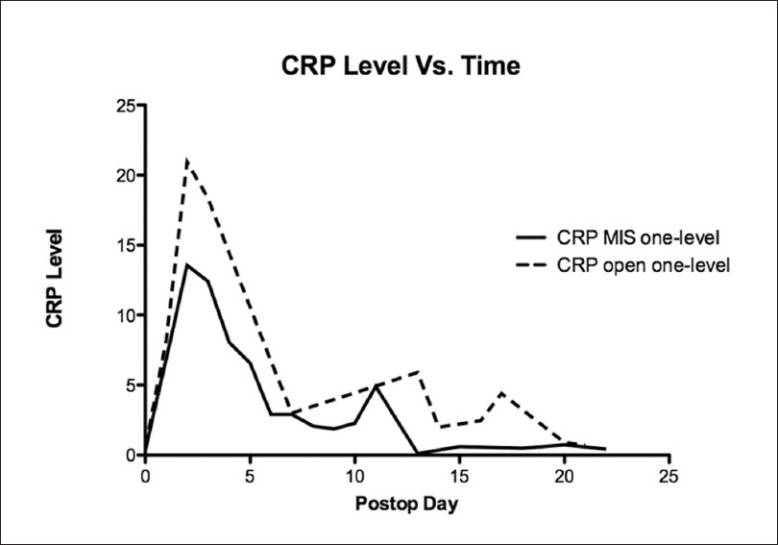
The peak value for C-reactive protein was significantly lower in patients undergoing single-level minimally invasive lumbar fusion (thick solid line) compared with open single-level lumbar fusion (thin solid line) (P<0.01)
Figure 3.
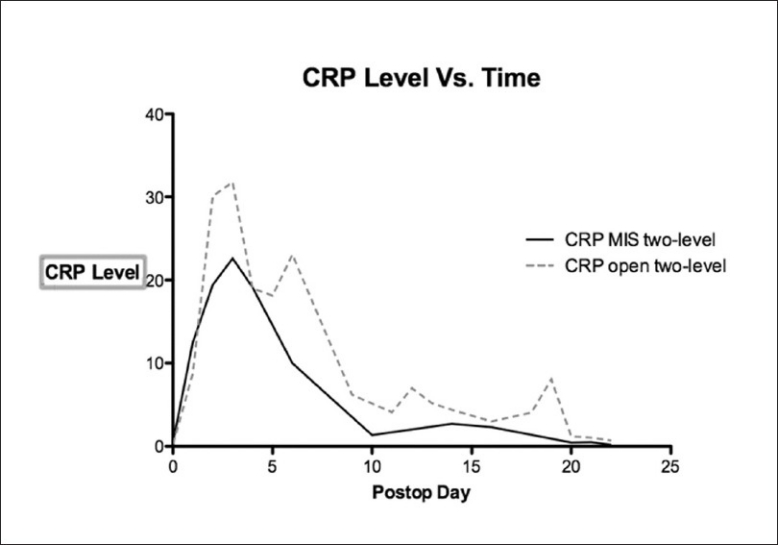
The peak value for C-reactive protein was significantly lower in patients undergoing Two-level minimally invasive lumbar fusion (thick broken line) compared with open two-level lumbar fusion (thin broken line) (P<0.01)
Table 2.
Statistical analysis of peak values of C-reactive protein in subgroups undergoing single-level open and minimally invasive surgery lumbar decompression and fusion surgery
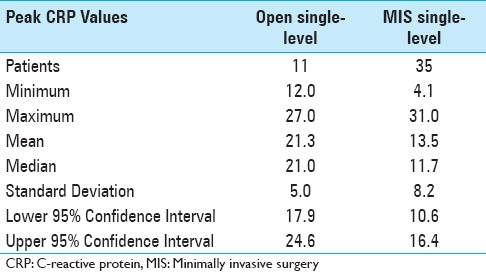
Table 3.
CRP data in two-level open verses minimally invasive transforaminal lumbar interbody fusion procedures
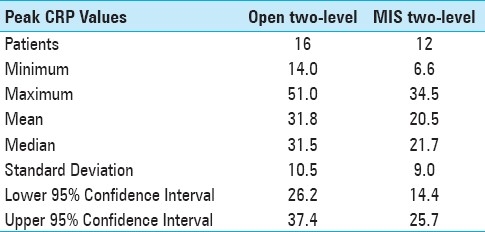
Figure 4.
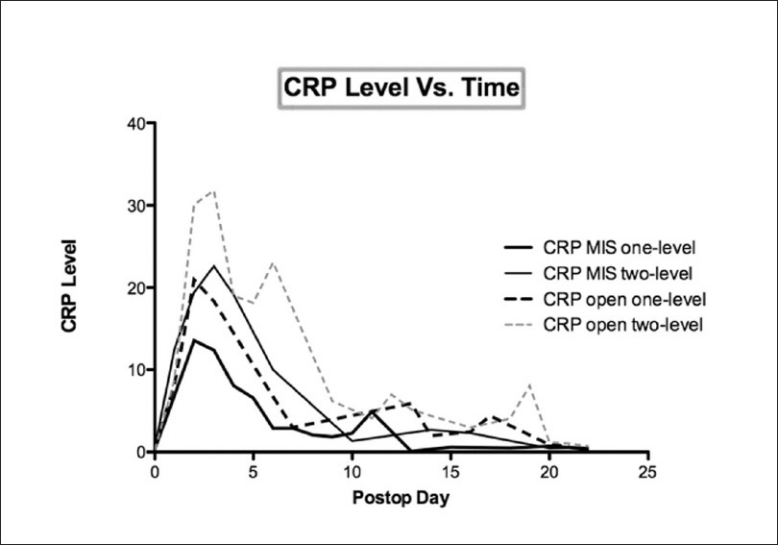
Postoperative C-reactive protein values for all four subgroups peaked at approximately the same duration of time following surgery. There was no statistical difference in the time necessary to return to normal values among the subgroups
Three patients suffered infectious complications: two subfascial infections in the two-level open group and one superficial infection in the single-level minimally invasive group. In the two deep infections, each of which necessitated operative debridement, CRP levels remained persistently elevated at three weeks post surgery, gradually returning to normal over prolonged courses of intravenous antibiotics. The superficial infection, which occurred in a patient after single-level minimally invasive fusion, presented with wound drainage and erythema at postop day seven; but the CRP was 1.3, only slightly above the upper limit of normal at 1.0, and within the range seen in the other patients in the single level MIS group at the same point in time.
DISCUSSION
A major theoretical benefit of minimally invasive spinal surgery technique is reduction in disruption of paraspinal soft tissues. There is little objective and quantifiable evidence in medical literature, however, to support this supposition. Stevens et al, examined postoperative MRI in patients undergoing conventional open and minimally invasive lumbar fusion surgery and found less T2 signal indicative of paraspinal edema in patients that had undergone MIS procedures.[14] Sasoka et al, compared the values of a panel of laboratory makers of inflammation including CRP in patients undergoing open verses minimally invasive single-level discectomy or laminotomy and found that the MIS patients had significantly lower values in the early post-operative phase.[12]
Our findings of significantly reduced peak values for CRP in MIS lumbar fusion procedures compared with open surgery supports the notion that there is measurable reduction in tissue destruction-mediated postoperative inflammation in MIS surgery. In addition, the pattern of decline in postoperative CRP values seen in this study may help in understanding normative values for MIS fusion surgery; and this may provide useful information when assessing a possible postoperative infection.[8]
A weakness of the study was the lack of rigid adherence to the study protocol. Although the study number permitted statistical analysis of the results, it is possible that better compliance with the protocol might have affected our results. Another potential confounding variable in interpreting our results would be the presence of any factors leading to elevated CRP beyond typical postoperative values. These would include infection, which was carefully excluded in this study, and activation of non-infectious etiology such as vasculitis or gout, though we did not identify patients with these conditions in the study.
One factor not controlled in this investigation was the use of bone morphogenic protein 2 (BMP-2). BMP-2 was used as the osteoinductive graft material in all patients in the MIS group and not used at all in the open surgery group. It is known that there is a substantial induction of tissue inflammation mediated by BMP-2 in the initial postoperative period, and the effect its use upon the systemic levels of CRP, if any, are not known.[9,11,13] Robin showed that local seroma fluid after posterior cervical fusion surgery contained impressive elevations of cytokines, especially IL-6 and IL-8. Thus, it can be speculated that the effect of BMP-2 use may have served to reduce rather than magnify any difference in CRP between the open and MIS groups and would not diminish the significance of our findings.
In conclusion, minimally invasive single and two-level posterior lumbar fusion surgery is associated with a lower peak in postoperative CRP compared with open surgery. This appears to support the notion that minimally invasive spine surgery technique leads to a measurable reduction in paraspinal soft tissue destruction mediated inflammation in the immediate postoperative period.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/94/82575
Contributor Information
John K. Houten, Email: jkhmd@yahoo.com.
Adesh Tandon, Email: atandon@montefiore.org.
REFERENCES
- 1.Al-Jabi Y, El-Shawarby A. Value of C-reactive protein after neurosurgery: A prospective study. Br J Neurosurg. 2010;24:653–9. doi: 10.3109/02688697.2010.500408. [DOI] [PubMed] [Google Scholar]
- 2.Benglis DM, Elhammady MS, Levi AD, Vanni S. Minimally invasive anterolateral approaches for the treatment of back pain and adult degenerative deformity. Neurosurgery. 2008;63(3 Suppl):191–6. doi: 10.1227/01.NEU.0000325487.49020.91. [DOI] [PubMed] [Google Scholar]
- 3.Dhall SS, Wang MY, Mummaneni PV. Clinical and radiographic comparison of mini-open transforaminal lumbar interbody fusion with open transforaminal lumbar interbody fusion in 42 patients with long-term follow-up. J Neurosurg Spine. 2008;9:560–5. doi: 10.3171/SPI.2008.9.08142. [DOI] [PubMed] [Google Scholar]
- 4.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 5.Kang BU, Lee SH, Ahn Y, Choi WC, Choi YG. Surgical site infection in spinal surgery: Detection and management based on serial C-reactive protein measurements. J Neurosurg Spine. 2010;13:158–64. doi: 10.3171/2010.3.SPINE09403. [DOI] [PubMed] [Google Scholar]
- 6.Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6:311–5. doi: 10.1016/j.spinee.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Meyer B, Schaller K, Rohde V, Hassler W. The C-reactive protein for detection of early infections after lumbar microdiscectomy. Acta Neurochir (Wien) 1995;136:145–50. doi: 10.1007/BF01410617. [DOI] [PubMed] [Google Scholar]
- 8.Mok JM, Pekmezci M, Piper SL, Boyd E, Berven SH, Burch S, et al. Use of C-reactive protein after spinal surgery: Comparison with erythrocyte sedimentation rate as predictor of early postoperative infectious complications. Spine (Phila Pa 1976) 2008;33:415–21. doi: 10.1097/BRS.0b013e318163f9ee. [DOI] [PubMed] [Google Scholar]
- 9.Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10:e1–6. doi: 10.1016/j.spinee.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Mustard RA, Jr, Bohnen JM, Haseeb S, Kasina R. C-reactive protein levels predict postoperative septic complications. Arch Surg. 1987;122:69–73. doi: 10.1001/archsurg.1987.01400130075011. [DOI] [PubMed] [Google Scholar]
- 11.Robin BN, Chaput CD, Zeitouni S, Rahm MD, Zerris VA, Sampson HW. Cytokine-mediated inflammatory reaction following posterior cervical decompression and fusion associated with recombinant human bone morphogenetic protein-2: A case study. Spine (Phila Pa 1976) 2010;35:E1350–4. doi: 10.1097/BRS.0b013e3181e85756. [DOI] [PubMed] [Google Scholar]
- 12.Sasaoka R, Nakamura H, Konishi S, Nagayama R, Suzuki E, Terai H, et al. Objective assessment of reduced invasiveness in MED. Compared with conventional one-level laminotomy. Eur Spine J. 2006;15:577–82. doi: 10.1007/s00586-005-0912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 2006;31:542–7. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 14.Stevens KJ, Spenciner DB, Griffiths KL, Kim KD, Zwienenberg-Lee M, Alamin T, et al. Comparison of minimally invasive and conventional open posterolateral lumbar fusion using magnetic resonance imaging and retraction pressure studies. J Spinal Disord Tech. 2006;19:77–86. doi: 10.1097/01.bsd.0000193820.42522.d9. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi J, Ebara S, Kamimura M, Kinoshita T, Itoh H, Yuzawa Y, et al. Early-phase enhanced inflammatory reaction after spinal instrumentation surgery. Spine (Phila Pa 1976) 2001;26:1698–704. doi: 10.1097/00007632-200108010-00014. [DOI] [PubMed] [Google Scholar]
- 16.Thelander U, Larsson S. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. Spine (Phila Pa 1976) 1992;17:400–4. doi: 10.1097/00007632-199204000-00004. [DOI] [PubMed] [Google Scholar]


