Abstract
Background. A competitive Luminex Immunoassay (cLIA) has been developed to measure neutralizing antibodies against human papillomavirus (HPV) types 6, 11, 16 and 18.
Methods. In a cohort of 974 women from the Guanacaste Natural History Study, we studied the relationship of baseline cLIA and virus-like particle (VLP) enzyme-linked immunosorbent assay (ELISA) (HPV16 and HPV18 only) seropositivity to measures of HPV exposure, HPV DNA positivity, number of sexual partners, cytology findings, and age. We then studied immunity against subsequent infection with HPV6, 11, 16, 18 and related types over a 7-year period.
Results. cLIA seroprevalence varied with previous exposure; the prevalence of cLIA results positive for HPV16 and HPV18 was lower than the prevalence of positive VLP ELISA responses. cLIA and VLP ELISA positivity predicted protection from subsequent infections with concordant types. The combined odds ratio for HPV16 and HPV18 cLIA positivity was 0.41 (95% confidence interval [CI], 0.21–0.80), and the combined odds ratio for the HPV16 and HPV18 VLP ELISA positivity was 0.65 (95% CI, 0.46–0.93). Of individual types, statistical significance was only reached for HPV16 cLIA positivity (odds ratio, 0.44; 95% CI, 0.15–0.94).
Conclusions. Both assays showed an association between positive results and significant protection from subsequent infections for HPV16 and HPV18 combined. cLIA seroprevalence was lower than VLP ELISA, suggesting that the assay detects a subset of antibodies following natural infection that are specifically linked to immunity against subsequent HPV infection.
Almost all cervical cancers are caused by persistent infections due to carcinogenic human papillomavirus (HPV) types [1]. Cervical cancer develops through characteristic stages, from HPV infection to cervical precancer and cancer [2]. Most HPV infections are transient and resolve after a few months. The host immune system plays an important role in preventing, controlling, and eliminating HPV infection of the cervix [3]. Neutralizing antibodies prevent the initial internalization of the virus in basal cells [4, 5], whereas clearance of a transient infection is thought to be mainly mediated by cellular immune components [6, 7].
Because antibody responses are central to preventing HPV infections, serological assays measuring antibodies directed against HPV may be helpful to identify individuals exposed to HPV and/or protected from subsequent HPV infection [8]. Virus-like particle (VLP) enzyme-linked immunosorbent assays (ELISAs) measure a polyclonal response against HPV VLPs and cannot differentiate between neutralizing and nonneutralizing antibodies. Only approximately one-half of women with results positive for HPV DNA at the cervix experience seroconversion to that HPV type; higher seroconversion rates have been observed with longer duration of infection [9–11]. Most studies have reported similar seroprevalence for HPV16 and HPV18, despite the much higher prevalence of HPV16 infection [10]. Our previous studies in the Guanacaste Natural History Study have not convincingly demonstrated that natural antibody titers against HPV are protective against subsequent infections [9]. A recent VLP ELISA study conducted in the Costa Rica Vaccine Trial has suggested that there is some protection by natural antibody responses, especially at higher antibody titers [12].
HPV vaccination induces high antibody titers against HPV L1 epitopes that correlate with protection from new infections among virtually all women naive to that type of HPV [13]. The vaccine-induced antibodies are polyclonal, and only a subset of the polyclonal response represents neutralizing antibodies [14, 15].
Natural HPV antibody titers are lower than vaccine-induced titers, and it is unclear whether the composition of natural serologic responses is similar to that of vaccine-induced responses. We do not know whether protection observed in vaccinated women is mainly related to high titers or to a higher proportion of neutralizing antibodies. Neutralization assays can measure protective neutralizing antibodies with high specificity but suboptimal sensitivity; they are not applicable in large epidemiologic studies [16].
A new high-throughput competitive Luminex Immunoassay (cLIA) permits epidemiologic studies of HPV serology [17]. The assay measures the competition of antibodies in serum samples with neutralizing antibodies targeting HPV6, HPV11, HPV16, and HPV18 in parallel. We compared results obtained with the cLIA assay in a cohort of women from the Guanacaste Natural History Study with previous results obtained with a VLP ELISA assay.
MATERIAL AND METHODS
Study Population
We selected enrollment plasma samples from 974 women from the 10,000-woman Guanacaste Natural History Study (NHS). We focused on the 4 types covered by the cLIA assay: HPV6, HPV11, HPV16, and HPV18. To evaluate cross-protection, we enriched the sample with serum specimens from cases of infections with types closely related to HPV16 (HPV31, HPV33, HPV52, and HPV58) and HPV18 (HPV45).
The sampling strategy is shown in detail in the Consolidated Standards of Reporting Trials diagram. Among women from the full cohort who had follow-up HPV test results, we included as cases of new infection all individuals with results negative for HPV16, HPV18, HPV6, and HPV11 at baseline who developed an incident infection with 1 of the 4 types (including transient and persistent infections lasting at least 1 year, or a CIN2 or greater (CIN2+) with concomitant detection of the type) (n = 294). We sampled half of all women negative at baseline for HPV31, HPV33, HPV45, HPV52, and HPV58 who had an incident transient infection with at least 1 of these types (n = 119) and all women with an incident persistent infection or CIN2+ related to 1 of these 5 types (n = 116) (Figure 1A).
Figure 1.
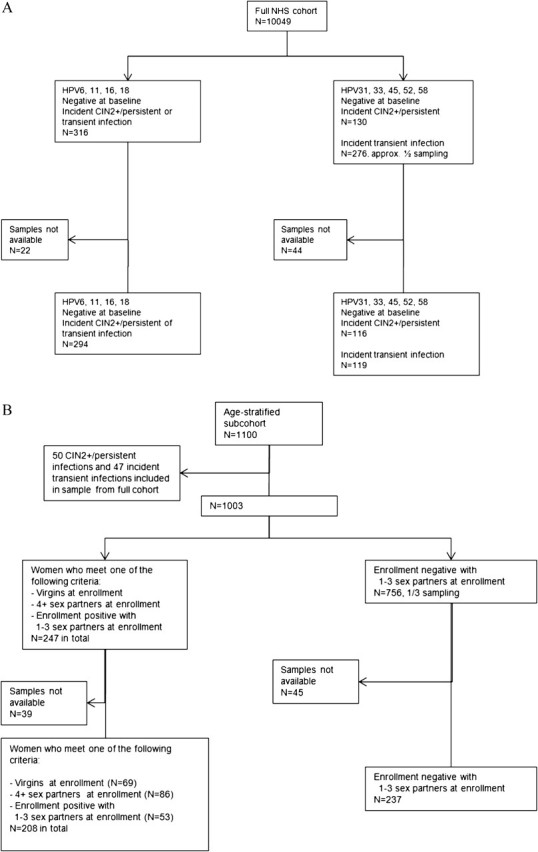
Consolidated Standards of Reporting Trials diagram describing the sample selection from (A) the complete Guanacaste Natural History Study (NHS) and (B) the 1100-women age-stratified subcohort.
We sampled additional women from an age-stratified random sample of 1100 women participating in the NHS enrollment visit, who had been previously tested for the prevalence of multiple sexually transmitted agents [18]. We specifically sampled women with varying characteristics related to past HPV infection: all women with 0 sexual partners, all women with ≥4 sexual partners, and all women with positive HPV DNA results for any of the 9 types at baseline (n = 208). In addition, we included one-third of all women who were HPV DNA negative at enrollment and had 1–3 sexual partners (n = 237) (Figure 1B). Determinants of cLIA seroprevalence were studied for all 974 selected women; 41 women without follow-up data and/or persistent infections at baseline were excluded from the analysis of risk of subsequent infections. We randomly selected a second aliquot from 56 samples for quality control testing, resulting in a total of 1030 cLIA assays.
HPV DNA testing was performed from cervical cells using the MY09-MY11 degenerate primer PCR and dot blot detection of type-specific DNA [19].
Plasma Conversion and cLIA
Heparinized plasma samples from NHS were converted to serum. A stock solution of 100 USP (NIH units)/mL bovine thrombin (Sigma Aldrich), 20 mg/mL protamine sulfate (Sigma Aldrich), and 50 ug/mL Atroxin (Sigma Aldrich) was freshly prepared. To each 1-mL plasma aliquot, 15 μL of the stock solution was added, and incubation was performed at 37° C for 30 min. Next, samples were centrifuged at 13,200 rpm for 30 min, and then the serum supernatant was transferred to a clean tube and heat inactivated at 56°C for 30 min.
Subsequently, the cLIA assay was performed as previously described [17]. In brief, VLPs for HPV6, HPV11, HPV16, and HPV18 were expressed in yeast, coupled to Luminex microspheres, and pooled. Type-specific antibodies binding to neutralizing epitopes were labeled with phycoerythrin and used at a final concentration of 0.5 μg/mL for H6.B10.5, 1.0 μg/mL for H11.B2, 1.0 μg/mL for H16.V5, and 1.25 μg/mL for H18.J4. Serum samples and VLP microspheres were incubated before PE-tagged antibodies were added to the well. After incubation over night at room temperature, serum samples were washed 3 times with phosphate-buffered saline. Mean fluorescence intensities (MFIs) were measured using a Luminex 100 instrument, and MFIs were converted to arbitrary mMU/mL values using standard curves. The cutoffs used for HPV6, HPV11, HPV16, and HPV18 were 20, 16, 20, and 24 [16].
VLP ELISA
All women included in the study had previous HPV16 and HPV18 serological measurements based on VLP ELISA assays [9, 10]. The cutoff for VLP ELISA serology results was calculated independently for each test batch, by comparison with the distribution of the values obtained for the concurrently tested women with 0 sexual partners in that batch. Seropositivity for each HPV type was defined as 5 standard deviations above the mean optical density (OD) obtained for the concurrently tested women with 0 sexual partners. In addition, we analyzed protection at lower cutoffs (3 standard deviations above the mean OD of concurrently tested women with 0 sexual partners in each batch) and higher cutoffs (titers above the median of positive values within each batch using the 5 standard deviation cutoff).
Statistical Analysis
We analyzed assay reproducibility using R2 for continuous measurements and percentage agreement for categorical measurements. For further analyses, cLIA results were treated as dichotomous variables, using the cutoff values described above. Percentage agreement, Cohen’s kappa, and McNemar’s test were calculated for agreement between dichotomous cLIA and VLP ELISA results for HPV16 and HPV18. Linear regression analysis was conducted to compare cLIA and VLP ELISA titers. We also analyzed mean and median cLIA values in 4 categories of VLP ELISA titers: <3 standard deviations above the titers in women with 0 sexual partners (category 1); between 3 and 5 standard deviations above the titers in women with 0 sexual partners (category 2); titers below the median of those called positive at a cutoff of 5 standard deviations above the titers in women with 0 sexual partners (category 3); and titers above the median of those classified as positive at a cutoff of 5 standard deviations above the titers in women with 0 sexual partners (category 4). To identify determinants of seropositivity, univariate associations for HPV16 and HPV18 seropositivity were assessed for the following variables: age (<25, 25–29, 30–44, 45–64, and ≥65 years); sexual activity (women with 0, 1, 2–3, or ≥4 sexual partners); baseline cytological test result (< Atypical squamous cells of undetermined significance [ASCUS] or ≥ASCUS); baseline HPV test result (negative for 13 carcinogenic types, positive for any of 13 carcinogenic types); baseline HPV16 test result (positive or negative). Odds ratio estimates with 95% confidence intervals (CIs) were calculated to identify determinants of cLIA seropositivity. To study the association between seropositivity and subsequent risk of HPV infection, we calculated crude and age-adjusted odds ratios (ORs) (continuous age at enrollment) for each type combining transient infections, persistent infections, and CIN2+ into a single category. Infections and disease outcomes were ascertained over a 7-year period after the baseline measurement. Including or excluding women with transient HPV infection at baseline did not change the point estimates. To estimate the combined protection related to seropositivity measured for type combinations, we computed the summary log OR based on log ORs and 95% CIs. We then computed a pooled summary estimate assuming a fixed-effects model using the Mantel-Haenszel method.
We report the sampling-adjusted ORs that account for the stratified sampling. We created disjoint strata that reflected the subcohorts from which we sampled, based on stratification variables (Figure 1). For each stratum, we calculated sampling fractions as the ratio of numbers of stratum members in the stratified sample and the cohort. With these sampling fractions, we constructed an estimate of any characteristic of the full NHS cohort from our stratified random sample, just as we could with a standard simple random sample. Specifically, to obtain the sampling-adjusted ORs, we used weighted logistic regression. The reciprocal of the sampling fraction for a stratum serves as the weight for every individual in the stratum. We used PROC surveylogistic in SAS to do our weighted logistic regression and to adjust the variances accordingly. All statistical analyses were run in SAS, version 9.1; SPSS, version 15.0; and Stata, version 11.0.
RESULTS
cLIA Reproducibility
We evaluated the reproducibility of the cLIA assay for all 4 types in 56 blinded quality control samples. In the quantitative evaluation, the R2 was 0.84 for HPV6 cLIA, 0.84 for HPV11 cLIA, 0.78 for HPV16 cLIA, and 0.96 for HPV18 cLIA. In the categorical evaluation, only 1 sample each for HPV11 cLIA, HPV16 cLIA, and HPV18 cLIA was classified differentially between the repeat measurements (with 4, 17, and 8 positive samples in total, respectively), translating to an overall agreement of 98% for these 3 types. Five samples were classified differentially in the HPV6 cLIA assay (with 15 samples with positive results), which had an overall agreement of 91%.
cLIA Seropositivity
Among women with positive cLIA results, the HPV6 cLIA titers ranged from 20 to 246, with a median value of 45; the HPV11 cLIA titers ranged from 16 to 440, with a median value of 25; the HPV16 cLIA titers ranged from 20 to 414, with a median value of 45; and the HPV18 cLIA titers ranged from 24 to 857, with a median value of 51.
Comparison of cLIA and VLP ELISA Results for HPV16 and HPV18
The prevalence of positive HPV16 VLP ELISA results (182 of 974 women; 18.7%) was almost twice as high as that of positive HPV16 cLIA results (94 of 974 women; 9.7%). The overall agreement between HPV16 VLP ELISA and HPV16 cLIA was 86.9%, with a kappa of 0.47 (95% CI, 0.38–0.55). For the HPV18 VLP ELISA, 175 of 974 women (18.0%) were positive, which is almost 4 times higher than the prevalence that was measured with the HPV18 cLIA (43 of 974 women; 4.4%). Of note, these percentages ignore the stratified sampling design and are presented to contrast the 2 assays. The overall agreement between HPV18 VLP ELISA and HPV18 cLIA was 79.2%, with a kappa of 0.29 (95% CI, 0.18–0.40). As expected, in both comparisons, most of the women with discordant results were positive according to the VLP ELISA (P<.001, by McNemar’s test). Linear regression analysis of the titers measured in cLIA and ELISA showed a moderate correlation between titers for the raw data (r = 0.53; R2 = 0.28). We also analyzed the cLIA titers by VLP ELISA categories, defined by the titers measured in women with 0 sexual partners in each batch, as outlined in the Methods section. The mean and median cLIA titers in the 4 VLP ELISA categories were as follows: 7.3 and 5.5 in category 1; 8.7 and 5.5 in category 2; 17.5 and 5.5 in category 3; and 53.7 and 29.5 in category 4.
Determinants of cLIA and VLP ELISA Positivity
We analyzed univariate predictors of seropositivity in both the cLIA and VLP ELISA assays (Table 1). There were no clear associations of seropositivity based on either assay with increasing age. Seropositivity using either assay was associated with markers of definite HPV exposure (ie, HPV DNA positivity or low grade squamous intraepithelial lesion [LSIL] cytology) or probable exposure (ie, number of sexual partners).
Table 1.
Univariate Predictors of Seropositivity as Measured in 974 Women With cLIA (HPV16, 18, 6, and 11) and VLP ELISA (HPV 16 and 18)
| HPV16 |
HPV18 |
HPV6 |
HPV11 |
||||||||||||||||
| Variable | Total | cLIA pos | % | OR (95% CI) | VLP ELISA pos | % | OR (95% CI) | cLIA pos | % | OR (95% CI) | VLP ELISA pos | % | OR (95% CI) | cLIA pos | % | OR (95% CI) | cLIA pos | % | OR (95% CI) |
| Age, years | |||||||||||||||||||
| <25 | 215 | 20 | 9.3 | Ref | 36 | 16.7 | Ref | 13 | 6.0 | Ref | 32 | 14.9 | Ref | 25 | 11.6 | Ref | 7 | 3.3 | Ref |
| 25–29 | 164 | 17 | 10.4 | 1.13 (.57–2.23) | 25 | 15.2 | 0.89 (.51–1.56) | 9 | 5.5 | 0.90 (.38–2.17) | 24 | 14.6 | 0.98 (.55–1.73) | 21 | 12.8 | 1.12 (.60–2.07) | 9 | 5.5 | 1.73 (.63–4.73) |
| 30–44 | 306 | 33 | 10.8 | 1.18 (.66–2.12) | 74 | 24.2 | 1.59 (1.02–2.47) | 12 | 3.9 | 0.63 (.28–1.42) | 63 | 20.6 | 1.48 (.93–2.36) | 54 | 17.6 | 1.63 (.98–2.71) | 25 | 8.2 | 2.64 (1.12–6.23) |
| 45–64 | 228 | 18 | 7.9 | 0.84 (.43–1.63) | 36 | 15.8 | 0.93 (.56–1.55) | 7 | 3.1 | 0.49 (.19–1.26) | 42 | 18.4 | 1.28 (.78–2.12) | 30 | 13.2 | 1.15 (.65–2.03) | 17 | 7.5 | 2.39 (.97–5.89) |
| ≥65 | 61 | 6 | 9.8 | 1.06 (.41–2.78) | 11 | 18.0 | 1.09 (.52–2.30) | 2 | 3.3 | 0.53 (.12–2.40) | 14 | 23.0 | 1.69 (.84–3.43) | 13 | 21.3 | 2.06 (.98–4.32) | 4 | 6.6 | 2.09 (.59–7.37) |
| No. of sexual partners | |||||||||||||||||||
| 0 | 69 | 0 | 0.0 | NA | 6 | 8.7 | NA | 0 | 0.0 | NA | 2 | 2.9 | NA | 4 | 5.8 | NA | 1 | 1.4 | NA |
| 1 | 401 | 32 | 8.0 | Ref | 53 | 13.2 | Ref | 12 | 3.0 | Ref | 53 | 13.2 | Ref | 43 | 10.7 | Ref | 18 | 4.5 | Ref |
| 2–3 | 306 | 39 | 12.8 | 1.68 (1.03–2.76) | 67 | 21.9 | 1.84 (1.24–2.74) | 13 | 4.2 | 1.44 (.65–3.20) | 64 | 20.9 | 1.74 (1.17–2.59) | 51 | 16.7 | 1.67 (1.08–2.58) | 22 | 7.2 | 1.65 (.87–3.13) |
| ≥4 | 185 | 21 | 11.4 | 1.48 (.83–2.64) | 54 | 29.2 | 2.71 (1.76–4.16) | 15 | 8.1 | 2.86 (1.31–6.24) | 55 | 29.7 | 2.77 (1.81–4.25) | 43 | 23.2 | 2.52 (1.58–4.02) | 20 | 10.8 | 2.58 (1.33–5.00) |
| Cytology baseline <ASCUS | 675 | 68 | 10.1 | Ref | 123 | 18.2 | Ref | 28 | 4.1 | Ref | 119 | 17.6 | Ref | 98 | 14.5 | Ref | 47 | 7.0 | Ref |
| Cytology baseline ≥ASCUS | 220 | 25 | 11.4 | 1.14 (.70–1.86) | 51 | 29.3 | 1.35 (.94–1.96) | 15 | 6.8 | 1.69 (.89–3.23) | 53 | 24.1 | 1.49 (1.03–2.15) | 38 | 17.3 | 1.23 (.82–1.85) | 14 | 6.4 | 0.91 (.49–1.68) |
| HR HPV baseline − | 699 | 63 | 9.0 | Ref | 122 | 17.5 | Ref | 30 | 4.3 | Ref | 125 | 17.9 | Ref | 107 | 15.3 | Ref | 46 | 6.6 | Ref |
| HR HPV baseline + | 195 | 30 | 15.4 | 1.84 (1.15–2.93) | 52 | 26.7 | 1.72 (1.19–2.50) | 13 | 6.7 | 1.59 (.81–3.12) | 47 | 24.1 | 1.48 (1.01–2.17) | 29 | 14.9 | 0.97 (.62–1.51) | 15 | 7.7 | 1.18 (.65–2.17) |
| Type at baseline − | † | 84 | 9.7 | Ref | 161 | 18.5 | Ref | 38 | 4.3 | Ref | 165 | 18.9 | Ref | 133 | 15.0 | Ref | 61 | 6.9 | Ref |
| Type at baseline + | ‡ | 9 | 36.0 | 5.26 (2.25–12.26) | 13 | 52.0 | 4.76 (2.13–10.64) | 5 | 25.0 | 7.33 (2.53–21.23) | 7 | 35.0 | 2.50 (.97–6.46) | 3 | 37.5 | 3.40 (.80–14.38) | 0 | 0.0 | NA (NA-NA) |
NOTE. Type at baseline indicates infection with the corresponding type at enrollment. Bold indicates statistical significance. CI, confidence interval; cLIA, competitive Luminex Immunoassay; HPV, human papillomavirus; HR, high risk; NA, not applicable; OR, crude odds ratio; Ref, reference; VLP ELISA, virus-like particle enzyme-linked immunosorbent assay; ASCUS, Atypical squamous cells of undetermined significance.
†HPV16: 869; HPV18: 874; HPV6: 886; HPV11: 889; ‡HPV16: 25; HPV18: 20; HPV6: 8; HPV11: 5.
When we examined the associations of seropositivity with ELISA only, when corresponding cLIA was negative, we observed residual and similar associations with markers of HPV exposure and sexual activity (data not shown). Thus, ELISA positivity was a more sensitive biomarker of past exposure; cLIA positivity represented a subset of exposures.
Protection Against Subsequent HPV Infection
We studied whether seropositivity according to cLIA and VLP ELISA was protective against subsequent infection with the 4 types measured by the cLIA assay (Table 2). A total of 151 incident HPV16 infections were observed. Both VLP ELISA and cLIA were associated with protection against subsequent HPV infections. Although the point estimate of the cLIA result showed stronger protection, the difference was not significant (HPV16 cLIA: OR, 0.44 [95% CI, 0.21–0.93]; HPV16 VLP ELISA: OR, 0.56 [95% CI, 0.33–0.93]; sampling-adjusted estimate for HPV16 cLIA: OR, 0.37 [95% CI, 0.15–0.94]; sampling-adjusted estimate for HPV16 VLP ELISA: OR, 0.54 [95% CI, 0.29–1.03]). At a lower cutoff value for HPV16 VLP ELISA seropositivity (3 standard deviations above the mean OD of concurrently tested women with 0 sexual partners in each batch), the OR was 0.60 (95% CI, 0.40–0.90) and at a higher cutoff value (titers above the median of positive values within each batch using the 5 standard deviation cutoff value), the OR was 0.48 (95% CI, 0.22–1.01), indicating a slight dose response effect, as previously described [12]. None of the women with a positive cLIA result developed an HPV16-associated CIN2, compared with 4 women with VLP ELISA positivity who developed HPV16-associated CIN2.
Table 2.
cLIA Serological Test Results, VLP ELISA Serological Test Results, and Risk of HPV Infection Detected as New During Follow-up in 933 Women
| HPV type, test result | Type− | Type+ | CIN2+ Type | Age-adjusted OR (95% CI) | Sampling-adjusted OR (95% CI) |
| HPV16 | |||||
| cLIA− | 696 | 122 | 21 | Ref | |
| cLIA+ | 86 | 8 | 0 | 0.44 (0.21–0.93) | 0.37 (0.15–0.94) |
| VLP ELISA − | 622 | 113 | 19 | Ref | |
| VLP ELISA + | 160 | 17 | 2 | 0.56 (0.33–0.93) | 0.54 (0.29–1.03) |
| HPV18 | |||||
| cLIA− | 787 | 99 | 4 | Ref | |
| cLIA+ | 41 | 1 | 1 | 0.33 (0.08–1.40) | 0.76 (0.17–3.33) |
| VLP ELISA − | 577 | 76 | 4 | Ref | |
| VLP ELISA + | 249 | 24 | 1 | 0.74 (0.46–1.19) | 0.81 (0.41–1.61) |
| HPV6 | |||||
| cLIA− | 734 | 59 | Ref | ||
| cLIA+ | 136 | 4 | 0.37 (0.13–1.04) | 0.41 (0.14–1.19) | |
| HPV11 | |||||
| cLIA− | 851 | 20 | Ref | ||
| cLIA+ | 60 | 2 | 1.60 (0.36–7.07) | 0.49 (0.10–2.38) | |
| HPV6/11/16/18 cLIA | 0.48 (0.28–0.80) | ||||
| HPV16/18 cLIA | 0.41 (0.21–0.80) | ||||
| HPV16/18 VLP | 0.65 (0.46–0.93) |
NOTE. The combined estimates show the pooled summary odds estimates for HPV16 and HPV18 or all 4 types combined based on the unweighted estimates. The sampling-adjusted OR estimates account for the stratified sampling, weighting back to the full NHS cohort excluding women censored at baseline and women without follow up HPV data, for a total population of 7811. CI, confidence interval; CIN2+Type, concurrent detection of CIN2 or greater and the corresponding type; cLIA, competitive Luminex Immunoassay; HPV, human papillomavirus; NHS, Guanacaste Natural History Study; OR, odds ratio adjusted for continuous age at enrollment; Ref, reference categories; Type−, negative for the corresponding HPV genotype in the cervix in follow up; Type+, positive for the corresponding HPV genotype in follow up, but no CIN2+ related to that type; VLP ELISA, virus-like particle enzyme-linked immunosorbent assay.
When analyzing the interaction between HPV16 cLIA and HPV16 VLP ELISA, significant protection was only observed in the group of women positive for HPV16 by VLP ELISA and HPV16 by cLIA (OR, 0.33; 95% CI, 0.13–0.84) (Table 3).
Table 3.
Risk of Infection in Strata of HPV16 cLIA and HPV16 VLP ELISA in 933 Women
| No. (%) of participants |
||||||
| VLP ELISA | cLIA | HPV16− | HPV16+ | CIN2+ HPV16 | Total | OR (95% CI) for any HPV16 |
| Neg | Neg | 602 (82.0) | 113 (15.4) | 19 (2.6) | 734 | Ref |
| Pos | 17 (85.0) | 3 (15.0) | 0 | 20 | 0.81 (0.23–2.79) | |
| Pos | Neg | 91 (86.7) | 12 (11.4) | 2 (1.9) | 105 | 0.70 (0.39–1.27) |
| Pos | 69 (93.2) | 5 (6.8) | 0 | 74 | 0.33 (0.13–0.84) | |
NOTE. CI, confidence interval; CIN2+ HPV16, concurrent detection of CIN2 or greater and HPV16; cLIA, competitive Luminex Immunoassay; HPV, human papillomavirus; HPV16−, negative for HPV16 in follow-up; HPV16+, positive for HPV16 in follow-up but no CIN2+ related to HPV16; Neg, negative; OR, odds ratio adjusted for continuous age at enrollment; Pos, positive; VLP ELISA, virus-like particle enzyme-linked immunosorbent assay.
Although the OR point estimates for the HPV18 cLIA suggested a protective effect, the finding was not significant (Table 2). In contrast to HPV16, 1 CIN2+ with a concomitant HPV18 infection was detected in a woman positive for HPV18 by cLIA. Similar to HPV16, among strata of HPV18 VLP ELISA and HPV18 cLIA, the lowest OR point estimate was observed for women positive according to both assays (OR, 0.20; 95% CI, 0.03–1.47), but the protective effect was not significant (Table 4). For HPV18, lower or higher cutoff values for VLP ELISA seropositivity did not have an impact on the protection point estimates (data not shown).
Table 4.
Risk of Infection in Strata of HPV18 cLIA and HPV18 VLP ELISA in 933 Women
| No. (%) of participants |
||||||
| VLP ELISA | cLIA | Negative | HPV18 | CIN2+/18 | Total | OR for any HPV18 (95% CI) |
| Neg | Neg | 661 (87.8) | 89 (11.8) | 3 (0.4) | 753 | Ref |
| Pos | 5 (83.3) | 0 | 1 (16.7) | 6 | 1.44 (0.17–12.44) | |
| Pos | Neg | 123 (91.1) | 11 (8.1) | 1 (0.7) | 135 | 0.70 (0.37–1.32) |
| Pos | 36 (97.3) | 1(2.7) | 0 | 37 | 0.20 (0.03–1.47) | |
NOTE. CI, confidence interval; CIN2+ HPV18, concurrent detection of CIN2 or greater and HPV18; cLIA, competitive Luminex Immunoassay; HPV, human papillomavirus; HPV18−, negative for HPV18 in follow-up; HPV18+, positive for HPV18 in follow-up but no CIN2+ related to HPV18; Neg, negative; OR, odds ratio adjusted for continuous age at enrollment; Pos, positive; VLP ELISA, virus-like particle enzyme-linked immunosorbent assay.
A low but insignificant point estimate for risk of subsequent HPV6 infection was observed for the HPV6 cLIA (OR, 0.37 [95% CI, 0.13–1.04]; sampling-adjusted OR, 0.41 [95% CI, 0.14–1.19]).
To analyze the protective effects of the cLIA and VLP ELISA assays across types, we computed summary OR estimates from the age adjusted ORs. The combined OR for HPV16 and HPV18 cLIA was 0.41 (95% CI, 0.21–0.80), and the combined OR for the HPV16 and HPV18 VLP ELISA was 0.65 (95% CI, 0.46–0.93). For all 4 cLIA types combined, we observed an OR of 0.48 (95% CI, 0.28–0.80) for subsequent infection with the respective types.
We explored whether HPV16 or HPV18 cLIA positivity was protective against infections with closely related types, suggesting cross-protection. We studied the risk of subsequent infection among women with HPV16 cLIA titers for the HPV16-related types HPV31, HPV33, HPV52, and HPV58 and observed no protective effect. In women with HPV18 cLIA titers, we observed a reduced OR point estimate for HPV45 infections of 0.52 (95% CI, 0.12–2.20) that was not statistically significant (Table 5).
Table 5.
Risk of Infection With Closely Related Types in Women With HPV16 or HPV18 cLIA Seropositivity Among a Cohort of 933 Women
| Type | No. of incident infections | OR (95% CI) |
| HPV31 | 114 | 1.17 (0.63–2.18) |
| HPV33 | 37 | 0.78 (0.24–2.59) |
| HPV52 | 121 | 0.98 (0.52–1.86) |
| HPV58 | 125 | 1.15 (0.63–2.10) |
| HPV45 | 78 | 0.52 (0.12–2.20) |
NOTE. Risk of incident infections with HPV31, 33, 52, and 58 in relation to HPV16 cLIA seropositivty and risk of infection with HPV45 in relation to HPV18 cLIA seropositivity is shown. Crude estimated odds ratios (ORs) are presented. CI, confidence interval; cLIA, competitive Luminex Immunoassay; HPV, human papillomavirus.
Characteristics of Women With Incident HPV16 and HPV18 Infections Despite cLIA Positivity
Eight women with positive HPV16 cLIA titers had a subsequent HPV16 infection, and 2 women with positive HPV18 cLIA titers had a subsequent infection with HPV18 (Table 1; online only). cLIA titers among the 10 women (median, 74; range, 33–289) were similar to the titers measured in all women (median, 45; range, 20–414). Six of the 10 women had positive VLP ELISA results, whereas 3 had only negative results. The time from the serological measurement to infection with HPV16 or HPV18 varied from 1 to 96 months. One woman who had a HPV18 infection at the time of CIN2+ detection and was positive according to cLIA had a strong HPV35 signal and a weak HPV18 signal, suggesting that HPV35 was the causal type.
DISCUSSION
We present the first population-based study analyzing serum responses against neutralizing HPV epitopes in unvaccinated women using an assay measuring antibodies against HPV6, HPV11, HPV16, and HPV18. The cLIA assay reproducibility was good. Median cLIA titers observed in our study were approximately 10-fold lower than were titers reported for vaccinated women measured with the same assay [20]. Although virtually all vaccinated women experience seroconversion when naive for the respective type, only 36% of women with HPV16 DNA detection at baseline and only 25% of women with HPV18 DNA detection at baseline had positive cLIA titers at that time.
We examined cLIA positivity as a measure of immunity and as a marker of previous exposure in comparison with a previously used VLP ELISA. A limitation of our VLP ELISA assay is the use of pre–vaccine era VLPs that were manufactured more crudely than were the VLPs included in current assays.
Notably, no CIN2+ related to HPV16 developed in 94 women with positive HPV16 cLIA titers over a 7-year period (Table 2). The combined OR estimate for all 4 types showed significant protection from subsequent infections, but individual estimates for HPV6 and HPV18 were not statistically significant, and the estimate for HPV11 was highly unstable and null (Table 2). Interestingly, women with positive HPV18 cLIA titers had a nonsignificantly reduced risk of incident HPV45 infections, suggesting that HPV18 antibodies may partly protect against HPV45 infections, as has been demonstrated in vaccination studies.
cLIA-based estimates of seroprevalence of HPV16 and HPV18, indicating past exposure, are lower than ELISA-based estimates. In the VLP ELISA, HPV16 and HPV18 seroprevalence was almost identical, whereas HPV18 cLIA seroprevalence was less than half of the HPV16 cLIA seroprevalence, better reflecting the prevalence of infections with these 2 types [9]. HPV6, HPV11, and HPV18 cLIA titers increased monotonically with more sexual partners, similar to the increase observed in positive VLP ELISA results, but this effect plateaued at 3 partners for HPV16 cLIA.
Although the cLIA seroprevalence was much lower than that of the VLP ELISA, the point estimate of protection was stronger, indicating that the cLIA is a more specific measure of immunity. Together, these findings corroborate that the cLIA assay measures a subset of the overall polyclonal response detected by the VLP ELISA. The proportion of cLIA-seropositive women with positive cLIA titers for multiple types was approximately 20%, in contrast to 60% reported for the VLP ELISA [10], suggesting that the cLIA assay has a higher type-specificity than does the VLP ELISA.
The cLIA assay is based on competition of serum antibodies with 1 specific neutralizing antibody for each type. Presumably, the cLIA assay does not measure neutralizing antibodies as accurately as a secreted alkaline phosphatase neutralization assay [21]. Additional neutralizing antibodies binding to different epitopes may not be detected by the assay. Conversely, competition by serum antibodies binding close to the neutralizing epitope could lead to false-positive results. We evaluated women who developed HPV infections despite cLIA titers and did not observe a specific characteristic, like lower titers, or incident infection after a long time, suggesting that cLIA titers may have waned. However, we cannot exclude that some of the infections are actually reactivated latent infections.
We benefitted from a large population-based cohort followed-up over a 7-year period from baseline with high-quality genotyping, good disease ascertainment, and previous VLP ELISA results. However, despite the large population base and the efficient sampling, our power to demonstrate protective effects for types other than HPV16 was limited. Although we observed lower ORs that were suggestive of better protection from subsequent HPV16 and HPV18 infections in the cLIA assay, compared with the VLP ELISA results, we could not assess the differences in protection between the 2 methods. Because only plasma samples were collected in NHS, we had to convert plasma samples to serum to run the cLIA assay.
A better quantification of the differences in protection from subsequent HPV infection between antibody titers measured with the cLIA assay and other tests would require a much larger study. A general problem in studying protective effects of HPV antibodies is the lack of a good exposure measurement; we cannot determine whether women who were not infected were actually exposed. We can only measure the failures and derive indirect measures of protection. Although the cLIA assay, at least for HPV16, indicates protection from subsequent infection and related disease, the assay is only a poor indicator of previous exposure to the HPV type. VLP ELISA assays that measure a broad spectrum of antibodies directed against L1 seem to be much better suited for this purpose. Our data suggest that naturally induced antibodies measured with the cLIA assay can protect against subsequent infection. Further studies are required to analyze which factors predict natural immunity and how long natural protection lasts.
Supplementary Data
Supplementary data are available at http://www.jid.oxfordjournals.org/ online.
Funding
The Intramural Research Program of the NIH and the National Cancer Institute.
Supplementary Material
References
- 1.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 3.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl 1):S16–S22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Roden RB, Weissinger EM, Henderson DW, et al. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J Virol. 1994;68:7570–4. doi: 10.1128/jvi.68.11.7570-7574.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roden RB, Armstrong A, Haderer P, et al. Characterization of a human papillomavirus type 16 variant-dependent neutralizing epitope. J Virol. 1997;71:6247–52. doi: 10.1128/jvi.71.8.6247-6252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welters MJ, de JA, van den Eeden SJ, et al. Frequent display of human papillomavirus type 16 E6-specific memory t-helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63:636–41. [PubMed] [Google Scholar]
- 7.Welters MJ, van der LP, van den Eeden SJ, et al. Detection of human papillomavirus type 18 E6 and E7-specific CD4+ T-helper 1 immunity in relation to health versus disease. Int J Cancer. 2006;118:950–6. doi: 10.1002/ijc.21459. [DOI] [PubMed] [Google Scholar]
- 8.Schiffman M, Safaeian M, Wentzensen N. The use of human papillomavirus seroepidemiology to inform vaccine policy. Sex Transm Dis. 2009;36:675–9. doi: 10.1097/OLQ.0b013e3181bce102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viscidi RP, Schiffman M, Hildesheim A, et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:324–7. doi: 10.1158/1055-9965.epi-03-0166. [DOI] [PubMed] [Google Scholar]
- 10.Wang SS, Schiffman M, Shields TS, et al. Seroprevalence of human papillomavirus-16, -18, -31, and -45 in a population-based cohort of 10000 women in Costa Rica. Br J Cancer. 2003;89:1248–54. doi: 10.1038/sj.bjc.6601272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SS, Schiffman M, Herrero R, et al. Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10 000 women in Costa Rica. Br J Cancer. 2004;91:1269–74. doi: 10.1038/sj.bjc.6602088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safaeian M, Porras C, Schiffman M, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst. 2010;102:1653–52. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24(Suppl 3):106–3. doi: 10.1016/j.vaccine.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 14.Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin. 2007;3:109–15. doi: 10.4161/hv.3.4.4058. [DOI] [PubMed] [Google Scholar]
- 15.Smith JF, Kowalski R, Esser MT, Brown MJ, Bryan JT. Evolution of type-specific immunoassays to evaluate the functional immune response to Gardasil: a vaccine for human papillomavirus types 16, 18, 6 and 11. Hum Vaccin. 2008;4:134–42. doi: 10.4161/hv.4.2.5261. [DOI] [PubMed] [Google Scholar]
- 16.Pastrana DV, Buck CB, Pang YY, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 17.Opalka D, Lachman CE, MacMullen SA, et al. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003;10:108–15. doi: 10.1128/CDLI.10.1.108-115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez AC, Castle PE, Smith JS, et al. A population based study of herpes simplex virus 2 seroprevalence in rural Costa Rica. Sex Transm Infect. 2003;79:460–5. doi: 10.1136/sti.79.6.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castle PE, Schiffman M, Gravitt PE, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68:417–23. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 20.Munoz N, Manalastas R, Jr, Pitisuttithum P, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 21.Kemp TJ, Garcia-Pineres A, Falk RT, et al. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008;26:3608–16. doi: 10.1016/j.vaccine.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


