Abstract
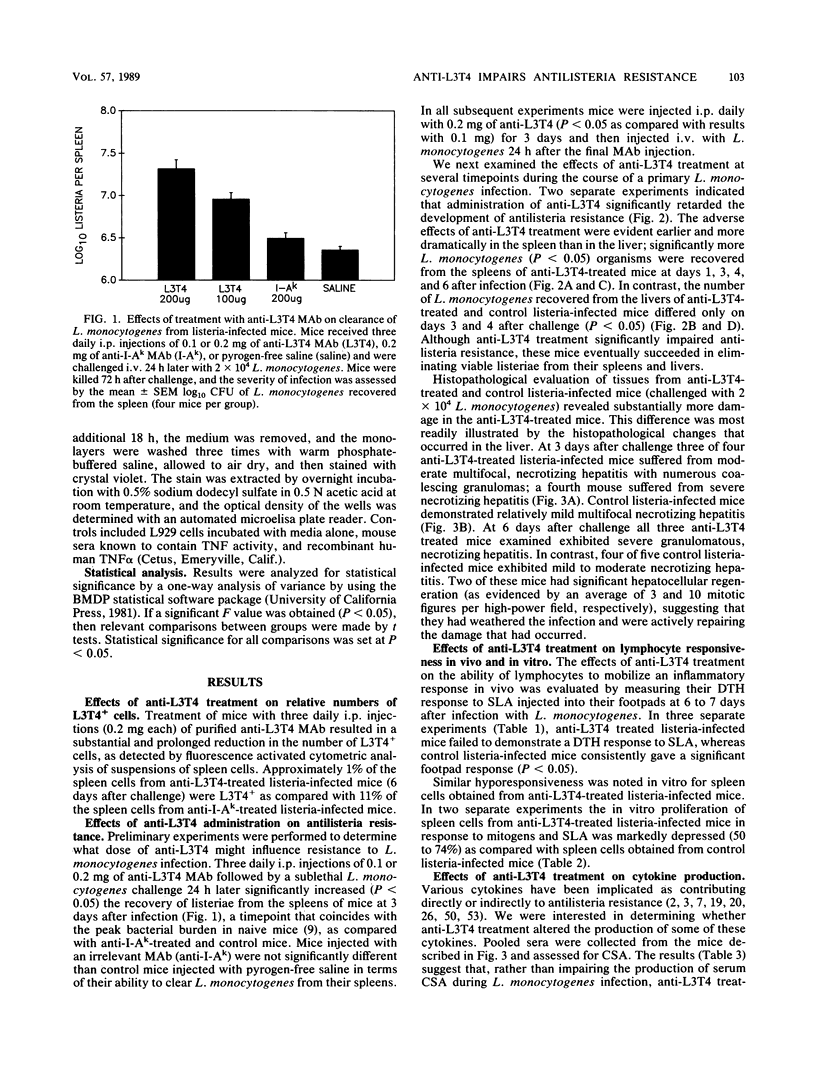
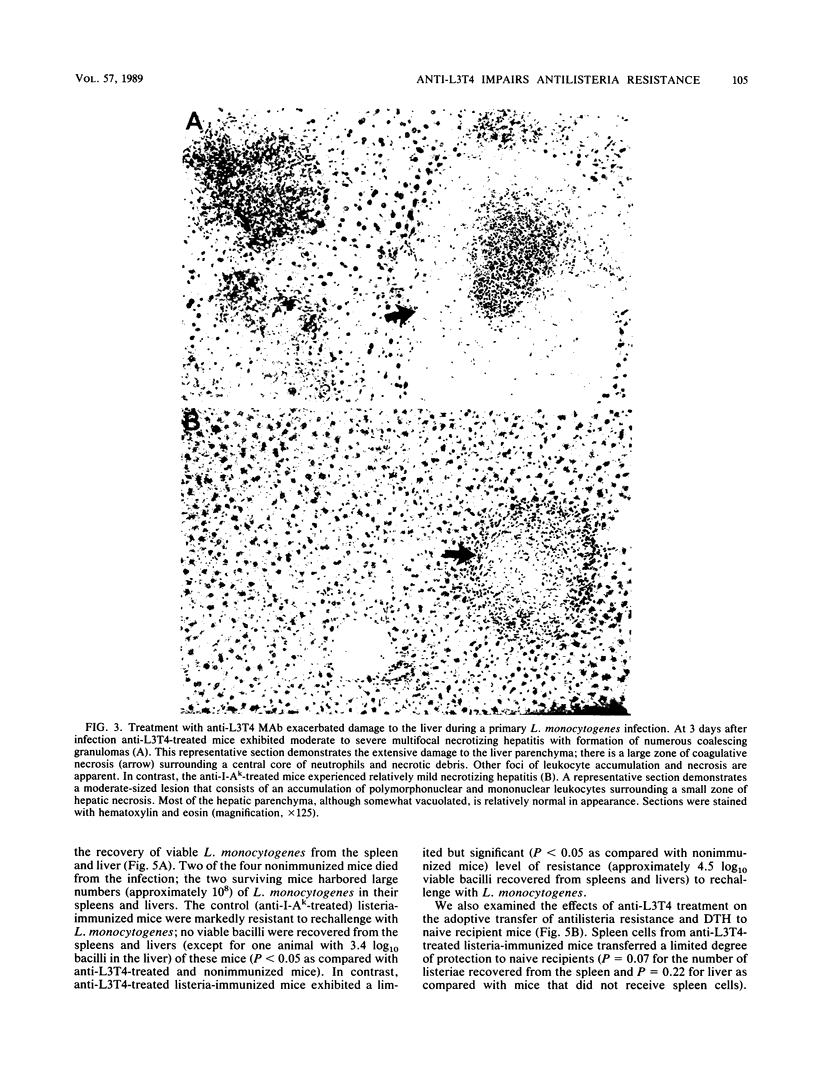
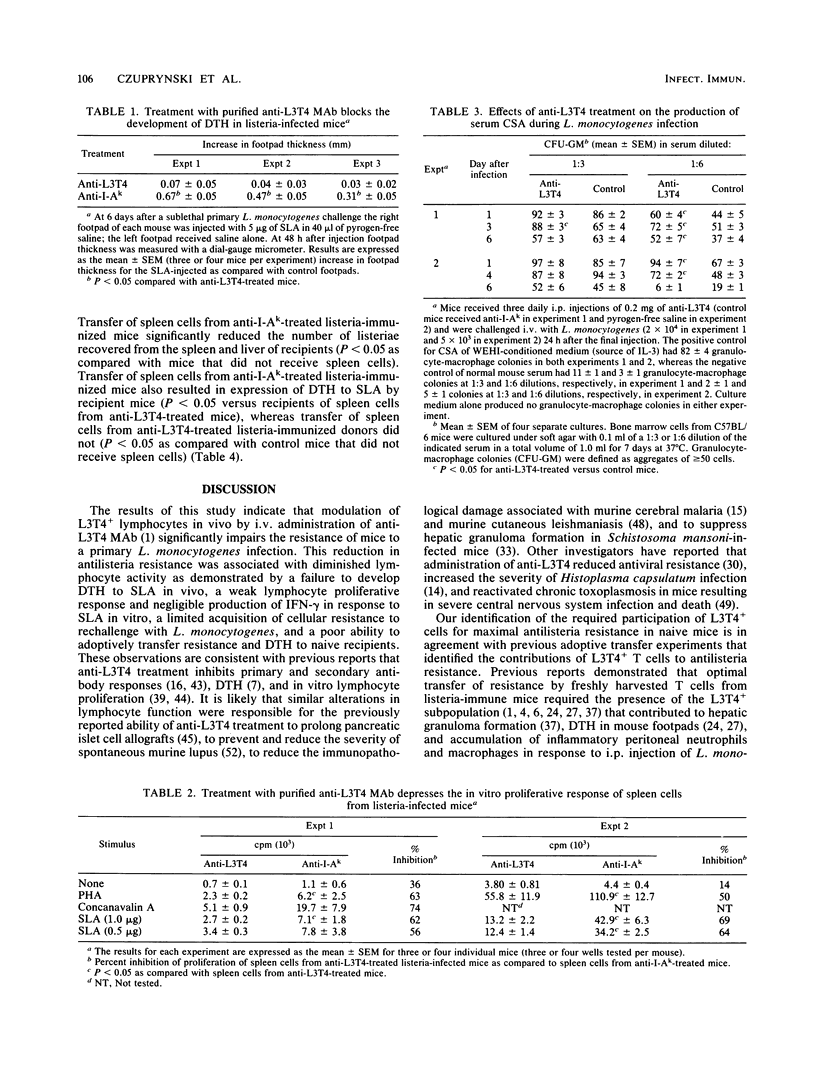
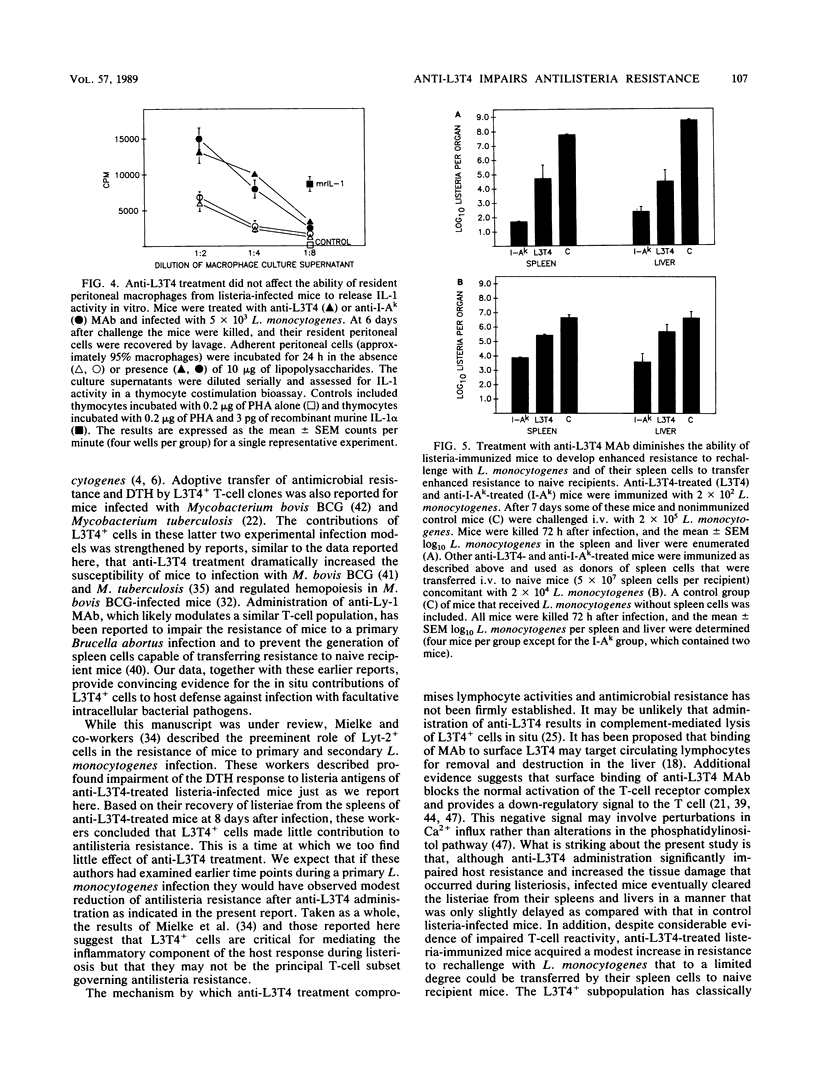
Anti-L3T4 monoclonal antibody (GK1.5) treatment significantly impaired the antilisteria resistance of mice as manifested by increased recovery of listeriae from the spleens and livers of anti-L3T4-treated mice and by greater severity of damage to the liver and other organs. Anti-L3T4-treated mice demonstrated a profound decrease in their T cell-mediated responses to Listeria monocytogenes and its products; they failed to develop delayed type hypersensitivity to soluble listeria antigens in vivo, and their spleen cells proliferated poorly in response to stimulation by either mitogens or listeria antigens in vitro. Spleen cells from control listeria-infected mice produced significant amounts of gamma interferon when stimulated with listeria antigens in vitro, whereas under the same conditions spleen cells from anti-L3T4-treated listeria-infected mice failed to produce detectable gamma interferon. Anti-L3T4 treatment resulted in a slight increase in serum colony-stimulating activity as compared with control listeria-infected mice, probably as a result of the increased bacterial burden in these animals. Despite the dramatic decrease in T-cell activities, anti-L3T4-treated mice succeeded in clearing L. monocytogenes from the spleen and liver in a manner that was only slightly delayed as compared with control listeria-infected mice. In addition, anti-L3T4-treated listeria-immunized mice exhibited a moderate degree of enhanced resistance to rechallenge with L. monocytogenes, and their spleen cells were able to transfer a limited degree of antilisteria resistance to naive recipient mice. Both of these responses, however, were diminished as compared with those of control listeria-immunized mice in the same experiments. Although these observations establish a critical role for L3T4+ cells in the development of maximal resistance to listeriosis, they also suggest that compensatory mechanisms may allow mice to develop considerable antilisteria resistance even when L3T4+ cell activities are substantially reduced.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. K., Hinrichs D. J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987 Sep 15;139(6):2005–2009. [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Haigh A. M., Kelso A., Metcalf D., Stanley E. R., Young A. M. Production of colony-stimulating factors (CSFs) during infection: separate determinations of macrophage-, granulocyte-, granulocyte-macrophage-, and multi-CSFs. Infect Immun. 1988 Jan;56(1):247–251. doi: 10.1128/iai.56.1.247-251.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Woan M., Sajewski D. H., McGregor D. D. T-cell co-operation in the mediation of acquired resistance to Listeria monocytogenes. Immunology. 1985 Sep;56(1):33–42. [PMC free article] [PubMed] [Google Scholar]
- Chiplunkar S., De Libero G., Kaufmann S. H. Mycobacterium leprae-specific Lyt-2+ T lymphocytes with cytolytic activity. Infect Immun. 1986 Dec;54(3):793–797. doi: 10.1128/iai.54.3.793-797.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Dual regulation of anti-bacterial resistance and inflammatory neutrophil and macrophage accumulation by L3T4+ and Lyt 2+ Listeria-immune T cells. Immunology. 1987 Feb;60(2):287–293. [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F., Young K. M., Cooley A. J., Kurtz R. S. Effects of murine recombinant interleukin 1 alpha on the host response to bacterial infection. J Immunol. 1988 Feb 1;140(3):962–968. [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Enhanced accumulation of inflammatory neutrophils and macrophages mediated by transfer of T cells from mice immunized with Listeria monocytogenes. J Immunol. 1985 May;134(5):3449–3454. [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984 Feb;35(2):193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- De Libero G., Kaufmann S. H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986 Oct 15;137(8):2688–2694. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Gomez A. M., Bullock W. E., Taylor C. L., Deepe G. S., Jr Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988 Jul;56(7):1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P., Lambert P. H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [PubMed] [Google Scholar]
- Gutstein N. L., Seaman W. E., Scott J. H., Wofsy D. Induction of immune tolerance by administration of monoclonal antibody to L3T4. J Immunol. 1986 Aug 15;137(4):1127–1132. [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. T lymphocyte-macrophage interactions in cellular antibacterial immunity. Immunobiology. 1982 Apr;161(3-4):361–368. doi: 10.1016/S0171-2985(82)80093-4. [DOI] [PubMed] [Google Scholar]
- Haque S., Saizawa K., Rojo J., Janeway C. A., Jr The influence of valence on the functional activities of monoclonal anti-L3T4 antibodies. Discrimination of signaling from other effects. J Immunol. 1987 Nov 15;139(10):3207–3212. [PubMed] [Google Scholar]
- Havell E. A. Augmented induction of interferons during Listeria monocytogenes infection. J Infect Dis. 1986 May;153(5):960–969. doi: 10.1093/infdis/153.5.960. [DOI] [PubMed] [Google Scholar]
- Havell E. A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987 Dec 15;139(12):4225–4231. [PubMed] [Google Scholar]
- Jones B., Khavari P. A., Conrad P. J., Janeway C. A., Jr Differential effects of antibodies to Lyt-2 and L3T4 on cytolysis by cloned, Ia-restricted T cells expressing both proteins. J Immunol. 1987 Jul 15;139(2):380–384. [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. Function and antigen recognition pattern of L3T4+ T-cell clones from Mycobacterium tuberculosis-immune mice. Infect Immun. 1986 Nov;54(2):291–296. doi: 10.1128/iai.54.2.291-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., De Libero G. Specific lysis of Listeria monocytogenes-infected macrophages by class II-restricted L3T4+ T cells. Eur J Immunol. 1987 Feb;17(2):237–246. doi: 10.1002/eji.1830170214. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley V. E., Gaulton G. N., Strom T. B. Inhibitory effects of anti-interleukin 2 receptor and anti-L3T4 antibodies on delayed type hypersensitivity: the role of complement and epitope. J Immunol. 1987 May 1;138(9):2771–2775. [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Koga T., Mitsuyama M., Handa T., Yayama T., Muramori K., Nomoto K. Induction by killed Listeria monocytogenes of effector T cells mediating delayed-type hypersensitivity but not protection in mice. Immunology. 1987 Oct;62(2):241–248. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Antigen-specific production of colony-stimulating factors by Listeria monocytogenes-immune, L3T4-positive cells. J Infect Dis. 1988 May;157(5):941–949. doi: 10.1093/infdis/157.5.941. [DOI] [PubMed] [Google Scholar]
- Marchal G., Milon G. Control of hemopoiesis in mice by sensitized L3T4+ Lyt2-lymphocytes during infection with bacillus Calmette-Guérin. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3977–3981. doi: 10.1073/pnas.83.11.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R. C., Boros D. L. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin-2 production in Schistosoma mansoni infection. Infect Immun. 1986 Dec;54(3):820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Ehlers S., Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988 Aug;56(8):1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Godal T. BCG induced CD4+ cytotoxic T cells from BCG vaccinated healthy subjects: relation between cytotoxicity and suppression in vitro. Clin Exp Immunol. 1987 Aug;69(2):255–262. [PMC free article] [PubMed] [Google Scholar]
- Müller I., Cobbold S. P., Waldmann H., Kaufmann S. H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987 Sep;55(9):2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näher H., Sperling U., Takacs L., Hahn H. Dynamics of T cells of L3T4 and Ly 2 phenotype within granulomas in murine listeriosis. Clin Exp Immunol. 1985 Jun;60(3):559–564. [PMC free article] [PubMed] [Google Scholar]
- Owens T., Fazekas de St Groth B. Participation of L3T4 in T cell activation in the absence of class II major histocompatibility complex antigens. Inhibition by anti-L3T4 antibodies is a function both of epitope density and mode of presentation of anti-receptor antibody. J Immunol. 1987 Apr 15;138(8):2402–2409. [PubMed] [Google Scholar]
- Pavlov H., Hogarth M., McKenzie I. F., Cheers C. In vivo and in vitro effects of monoclonal antibody to Ly antigens on immunity to infection. Cell Immunol. 1982 Jul 15;71(1):127–138. doi: 10.1016/0008-8749(82)90502-0. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T., Hug K., Louis J. A. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guérin in mice. Assessment by elimination of T cell subsets in vivo. J Immunol. 1987 Sep 15;139(6):2032–2037. [PubMed] [Google Scholar]
- Pedrazzini T., Louis J. A. Functional analysis in vitro and in vivo of Mycobacterium bovis strain BCG-specific T cell clones. J Immunol. 1986 Mar 1;136(5):1828–1834. [PubMed] [Google Scholar]
- Ranges G. E., Cooper S. M., Sriram S. In vivo immunomodulation by monoclonal anti-L3T4. 1. Effects on humoral and cell-mediated immune response. Cell Immunol. 1987 Apr 15;106(1):163–173. doi: 10.1016/0008-8749(87)90159-6. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Burakoff S. J., Greenstein J. L. The role of the L3T4 molecule in mitogen and antigen-activated signal transduction. Cell. 1987 Jun 19;49(6):845–853. doi: 10.1016/0092-8674(87)90622-2. [DOI] [PubMed] [Google Scholar]
- Shizuru J. A., Gregory A. K., Chao C. T., Fathman C. G. Islet allograft survival after a single course of treatment of recipient with antibody to L3T4. Science. 1987 Jul 17;237(4812):278–280. doi: 10.1126/science.2955518. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tite J. P., Sloan A., Janeway C. A., Jr The role of L3T4 in T cell activation: L3T4 may be both an Ia-binding protein and a receptor that transduces a negative signal. J Mol Cell Immunol. 1986;2(4):179–190. [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Vollmer T. L., Waldor M. K., Steinman L., Conley F. K. Depletion of T-4+ lymphocytes with monoclonal antibody reactivates toxoplasmosis in the central nervous system: a model of superinfection in AIDS. J Immunol. 1987 Jun 1;138(11):3737–3741. [PubMed] [Google Scholar]
- Wing E. J., Barczynski L. C., Waheed A., Shadduck R. K. Effect of Listeria monocytogenes infection on serum levels of colony-stimulating factor and number of progenitor cells in immune and nonimmune mice. Infect Immun. 1985 Aug;49(2):325–328. doi: 10.1128/iai.49.2.325-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Analysis of the function of L3T4+ T cells by in vivo treatment with monoclonal antibody to L3T4. Immunol Res. 1986;5(2):97–105. doi: 10.1007/BF02917584. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J Immunol. 1987 May 15;138(10):3247–3253. [PubMed] [Google Scholar]
- Young A. M., Cheers C. Colony-forming cells and colony-stimulating activity during listeriosis in genetically resistant or susceptible mice. Cell Immunol. 1986 Feb;97(2):227–237. doi: 10.1016/0008-8749(86)90393-x. [DOI] [PubMed] [Google Scholar]