Abstract
Objective
Plasminogen activator inhibitor-1 (PAI-1) production by adipose tissue is increased in obesity and its circulating levels are high in type 2 diabetes. PAI-1 increases cardiovascular risk by favoring clot stability and/or interfering with vascular remodeling. We investigated in obese diabetic persons whether an intensive lifestyle intervention for weight loss (ILI) would decrease PAI-1 levels independently of weight loss and whether PAI-1 reduction would be associated with changes in fibrinogen, an acute phase reactant, and/or fibrin fragment D-dimer (D-dimer), a marker of ambient coagulation balance.
Methods and Results
We examined 1-year changes in PAI-1, D-dimer and fibrinogen levels, adiposity, fitness, glucose and lipid control with ILI in 1,817 participants from Look AHEAD, a randomized trial investigating the effects of ILI, compared to usual care, on cardiovascular events in overweight/obese diabetic persons. Median PAI-1 levels decreased 29% with ILI, 2.5% with usual care (p<0.0001). Improvements in fitness, glucose control and HDL-cholesterol were associated with decreased PAI-1, independently of weight loss (p=0.03 for fitness, p<0.0001 for others). Fibrinogen and D-dimer remained unchanged.
Conclusions
Reductions in PAI-1 levels with ILI in obese diabetic individuals may reflect an improvement in adipose tissue health that could impact cardiovascular risk without changing fibrinogen or D-dimer levels.
Circulating plasminogen activator inhibitor-1 (PAI-1) levels are predictive of incident cardiovascular disease (CVD) in the general population.1 It is reasonable to expect elevation of PAI-1 to contribute to increased CVD risk in persons with type 2 diabetes (T2DM).2 Several mechanisms may explain the association of PAI-1 with CVD. PAI-1 favors intravascular fibrin deposition and promotes clot stability by inhibiting plasmin production from its inactive precursor, plasminogen.3 PAI-1 may also increase cardiovascular risk by inhibiting fibrinolysis in the vessel wall, interfering with vascular remodeling and promoting the development of an unstable plaque phenotype.4 In addition, PAI-1 is considered an acute phase protein5 and could, as an inflammatory mediator, increase CVD risk. Given the association of PAI-1 with CVD, we anticipate that an intervention that reduces PAI-1 could yield benefit through one or several of these pathways.
PAI-1 is synthesized in multiple tissues and its regulation is complex and incompletely understood. The secretion of PAI-1 by adipose tissue is increased in obese subjects due to an increase in adipose tissue mass6, 7 and to the activation of a pro-inflammatory phenotype within the adipose tissue microenvironment.8,9 In T2DM not only increased adipose tissue mass but other metabolic disturbances, including hyperinsulinemia, hyperglycemia and dyslipidemia, alter adipose tissue function and lead to an increased production and circulating levels of PAI-1.2, 6, 10, 11 Reductions in PAI-1 levels have been observed in obese non-diabetic individuals with weight loss,12,13 but the effects of weight loss in persons with T2DM and the independent contribution of changes in fitness and of improved glucose and lipid control on PAI-1 levels have not been evaluated in the setting of a clinical trial. The overall aim of this study was to investigate whether an intensive lifestyle intervention for weight loss (ILI) would, when compared to usual care, decrease PAI-1 levels in obese persons with T2DM and if an improvement in fitness and in metabolic factors known to impact adipose tissue function could contribute, independently of weight change, to the reduction in PAI-1 levels. Furthermore, to improve our understanding of the implications of PAI-1 reduction with ILI on cardiovascular risk in diabetic individuals and given the substantial epidemiological evidence supporting the association of fibrinogen and D-Dimer with cardiovascular disease (upper versus lower tertile risk of 1.8 for both),14, 15 we also investigated if the changes in PAI-1 with ILI were associated with changes in fibrinogen, an established acute phase reactant, and/or in fibrin fragment D-dimer (D-dimer), a marker of ambient coagulation balance. We hypothesized that despite the advanced degree of obesity and the metabolic disturbances commonly seen in T2DM, ILI would decrease PAI-1 levels to a greater extent than usual care. We also hypothesized that an improvement in metabolic factors and in fitness with ILI would, independently of adiposity changes, decrease PAI-1 levels. Our third and final hypothesis was that given that PAI-1 is a mild acute phase reactant and a major regulator of fibrinolysis, the reduction in PAI-1 levels with ILI would be associated with decreases in fibrinogen and D-dimer levels.
RESEARCH DESIGN AND METHODS
Study Design
We evaluated 1,817 individuals, generally corresponding to the first half of Look AHEAD (Action for Health in Diabetes) participants from 15 of 16 clinic sites, who had PAI-1 and fitness data at baseline and 1 year. Look AHEAD is a randomized clinical trial designed to examine whether a behavioral lifestyle intervention for weight loss will reduce cardiovascular events and overall mortality in overweight/obese subjects with T2DM.
The Look AHEAD study design, subject characteristics and components of the lifestyle intervention have been described.16 Briefly, subjects were randomized to ILI, aiming for a 7% weight loss from baseline, or to a diabetes, support and education (DSE) arm, which served as control. ILI participants attended 3 group sessions and 1 individual encounter per month during the first 6 months of the study, followed by 2 group sessions and 1 individual appointment per month thereafter, supporting behavioral change to increase physical activity to 175 weekly minutes of moderate-intensity exercise, reduce caloric and saturated fat intake and change macronutrient composition to improve glycemic control. The activity program relied on at home exercise, which for most participants consisted of brisk walking. Energy intake goal for persons <114 kg was 1200 –1500 kcal/day and 1500–1800 kcal/day for those ≥ 114 kg. Liquid meal replacement for 2 daily meals was encouraged during the first 6 months to help with portion control. Subjects were asked to keep food and physical activity diaries, counting only bouts of ≥ 10 minute-duration for the activity goal.16 DSE participants received 3 group health information sessions during the year. All participants continued care with their primary providers. The institutional review boards of the participating centers approved Look AHEAD and this ancillary study.
Laboratory, Anthropometric and Fitness Determinations
PAI-1, D-dimer and fibrinogen were measured in the University of Vermont Laboratory for Clinical Biochemistry Research (LCBR) as described.17 Briefly, PAI-1 was measured, in duplicate, in platelet-free plasma by ELISA (Stago, Parsippany, NJ, Asserachrom # 00249). This assay is sensitive to all plasma forms of PAI-1 (average interassay coefficient of variation [CV] was 8.9% over 8 different controls). D-dimer was measured, by the STAR automated coagulation analyzer (Stago, Parsippany, NJ), using an immuno-turbidometric assay (Liatest D-DI) with two anti-human monoclonal antibodies specific to D-dimer and 4 controls (average interassay CVs for mean values of 2.18 µg/mL and 0.24 µg/mL were 6.3% and 12.3%, respectively, and estimated at 23% for the 25th percentile [0.18 ug/ml]). Fibrinogen was quantified, by the STAR automated coagulation analyzer, using a clot-rate method (Stago, Parsipanny, NJ; average interassay CV was 5.9% over 10 different controls).
Determination of fitness using sub-maximal effort on a graded exercise stress test in metabolic equivalents (METS) and procedures for obtaining anthropometric measures, Hemoglobin A1c (HbA1c), glucose and lipids in Look AHEAD have been described.18
Statistical Analysis
Descriptive statistics, including median and inter-quartile range (IQR), were determined for PAI-1, D-dimer and fibrinogen levels at baseline and for their 1-year changes from baseline. Differences between the ILI and DSE arms in variable 1-year changes were evaluated with the two-sample t-test or the Wilcoxon rank sum test. Bivariate associations of 1-year changes were evaluated with Spearman’s correlation coefficients, adjusting for age and gender with partial correlation analyses, and tested for trend across quartiles of change by treatment arm.
In the multivariable regression analysis, log-transformation was applied to PAI-1 to correct for its non-normal distribution and the difference between baseline and 1-year log-transformed PAI-1 values was calculated and treated as the outcome variable. Models were fitted to examine the effects of changes in metabolic variables of interest on PAI-1 change. Variables shown not to be significantly different between ILI and DSE in their 1-year changes were excluded. Changes in metabolic variables and in fitness were entered into separate regression models to evaluate their contribution to PAI-1 change, either alone or in combination, after adjusting for baseline PAI-1 level, demographics, clinic site, CVD history, diabetes duration, current smoking and treatment with statins and thiazolidinediones. A dichotomous indicator for treatment group (“ILI versus DSE”) was included in all models to examine the significance of the treatment effect. Multicolinearity between related metabolic variables was excluded using Spearman correlation coefficients prior to inclusion in the regression models (all ≤0.4). Type I error rate was fixed at 0.05 for all analyses. Analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
Participants were middle-aged, obese and sedentary, with mean fitness values below the 20th percentile for their age (Table 1). PAI-1 levels (Median [IQR]) were elevated at 45.42 ng/mL (25.26, 75.46 ng/mL; reference range: 4–43 ng/mL). Median (IQR) fibrinogen levels were in the high normal range at 376.5 mg/dL (330, 431 mg/dL; reference range: 203–404 mg/dL) and D-dimer median (IQR) levels were normal at 0.26 µg/mL (0.17, 0.39 µg/mL; reference range: 0.06–0.77 µg/mL). The geometric means for PAI-1 and D-dimer levels were 42.46 ng/mL and 0.27 µg/mL, respectively. Baseline characteristics of 230 participants, excluded from analyses because of missing data, were similar to those from the whole subset, except for CVD prevalence and diabetes duration differences, accounted for in the regression models. Because of change in age eligibility criteria during the second year of Look AHEAD recruitment, subjects in our sample had a slightly lower CVD prevalence than did those from the remaining cohort (12% and 15%, respectively).19
Table 1.
Baseline Characteristics
| ILI (n=957) | DSE (n=860) | |
|---|---|---|
| Age, mean (SD), years | 57.5 (7.1) | 57.7 (7.2) |
| Gender, No. (%) | ||
| Males | 391 (40.9) | 356 (41.4) |
| Females | 566 (59.1) | 504 (58.6) |
| Ethnicity, No. (%) | ||
| White | 651 (68.1) | 574 (66.7) |
| African American | 124 (13.0) | 115 (13.4) |
| Hispanic | 91 (9.5) | 78 (9.1) |
| Native American | 58 (6.1) | 60 (7.0) |
| Asian/Pacific Islander | 8 (0.8) | 8 (0.9) |
| Other/Mixed | 24 (2.5) | 25 (2.9) |
| Duration of diabetes,a mean (SD), years | 6.5 (6.3) | 6.6 (6.2) |
| History of CVD,b No. (%) | 118 (12.3) | 99 (11.5) |
| Metabolic syndrome, No. (%) | 890 (93.0) | 797 (92.7) |
| Current tobacco use,a No. (%) | 34 (3.6) | 26 (3.0) |
| Statin therapy, No. (%) | 399 (41.7) | 343 (39.9) |
| Thiazolidinedione therapy, No. (%) | 243 (25.4) | 233 (27.1) |
| Insulin therapy, No. (%) | 146 (15.3) | 122 (14.2) |
| Estrogen replacement,a No. (%) | 168 (29.7) | 146 (29.0) |
| Weight, mean (SD), kg | 102.0 (20.1) | 101.4 (18.7) |
| BMI, mean (SD), kg/m2 | 36.3 (6.3) | 36.1 (5.9) |
| Waist circumference, mean (SD), cm | 114.6 (14.8) | 114.4 (14.2) |
| Fitness (submaximal), mean (SD), MET | 5.2 (1.5) | 5.1 (1.6) |
| Fasting glucose, mean (SD), mmol/L | 8.5 (2.4) | 8.7 (2.7) |
| HbA1c, mean (SD), % | 7.3 (1.2) | 7.4 (1.2) |
| Total cholesterol, mean (SD), mmol/L | 5.0 (1.0) | 4.9 (1.0) |
| LDL-C, mean (SD), mmol/L | 2.9 (0.8) | 2.9 (0.8) |
| HDL-C, mean (SD), mmol/L | 1.1 (0.3) | 1.1 (0.3) |
| Triglycerides, mean (SD), mmol/L | 2.1 (1.5) | 2.0 (1.4) |
| PAI-1 median (IQR), ng/mL | 46.53 (26.20, 75.63) | 44.61 (24.33, 75.14) |
| D-Dimer median (IQR), µg/mL | 0.26 (0.17, 0.39) | 0.26 (0.17, 0.39) |
| Fibrinogen, median (IQR), mg/dL | 376.0 (326.0, 431.0) | 379.0 (334.0, 431.0) |
SD: Standard deviation; cm: centimeters
Self-reported
Self-reported prior myocardial infarction, stroke, transient ischemic attack, angioplasty/stent, coronary artery bypass graft, carotid endarterectomy, abdominal aortic aneurysm or heart failure
Changes in Variables of Interest with ILI
ILI participants had significant improvements in adiposity, fitness, glucose and lipid control at 1 year when compared to those randomized to DSE, as observed for the overall Look AHEAD sample18 (Table 2). There were no differences in LDL-cholesterol between ILI and DSE at 1-year. 1-year PAI-1 levels (median [IQR]) dropped 13.4 (−38.6, 2.7) ng/mL from a baseline of 46.53 (26.2, 75.63) ng/mL in the ILI group (29% reduction) and 1.1 (−19.4, 20.8) ng/mL from a baseline median (IQR) of 44.61 (24.33, 75.14) ng/mL in the DSE group (2.5% reduction) (p<0.0001 for difference between groups). No change in D-dimer or decrease in fibrinogen levels was documented in either group.
Table 2.
Variable changes at 1-year by treatment arm
| ILI (n=957) | DSE (n=860) | p-value* | |
|---|---|---|---|
| Δ Weight, mean (SD), kg | −8.9 (7.6) | −0.8 (5.0) | <0.0001 |
| Δ BMI, mean (SD), kg/m2 | −3.2 (2.6) | −0.3 (1.8) | <0.0001 |
| Δ Waist circumference, mean (SD), cm | −7.7 (9.3) | −1.0 (7.7) | <0.0001 |
| Δ Fasting glucose, mean (SD), mmol/L | −1.23 (2.45) | −0.39 (2.59) | <0.0001 |
| Δ HbA1c, mean (SD), % | −0.7 (1.0) | −0.2 (0.9) | <0.0001 |
| Δ LDL-cholesterol, mean (SD), mmol/L | −0.11 (0.67) | −0.13 (0.74) | 0.66 |
| Δ HDL-C, mean (SD), mmol/L | 0.08 (0.18) | 0.04 (0.17) | <0.0001 |
| Δ Triglycerides, mean (SD), mmol/L | −0.36 (1.29) | −0.15 (1.1) | 0.0001 |
| Δ Fitness (submaximal), mean (SD), MET | 1.0 (1.4) | 0.2 (1.1) | <0.0001 |
| Δ PAI-1, median (IQR) ng/mL † | −13.4 (−38.6, 2.7) | −1.1 (−19.4, 20.8) | <0.0001 |
| Δ D- Dimer, median (IQR) µg/mL † | 0.0 (−0.1, 0.1) | 0.0 (−0.1, 0.1) | 0.57 |
| Δ Fibrinogen, median (IQR), mg/dL † | 5.0 (−33.0, 47.0) | 10.0 (−25.0, 42.0) | 0.21 |
Δ : change scores using the raw scale.
* p-values are unadjusted and evaluate treatment differences on variable changes using the unpaired-t test or the Wilcoxon Rank Sum Test (†).
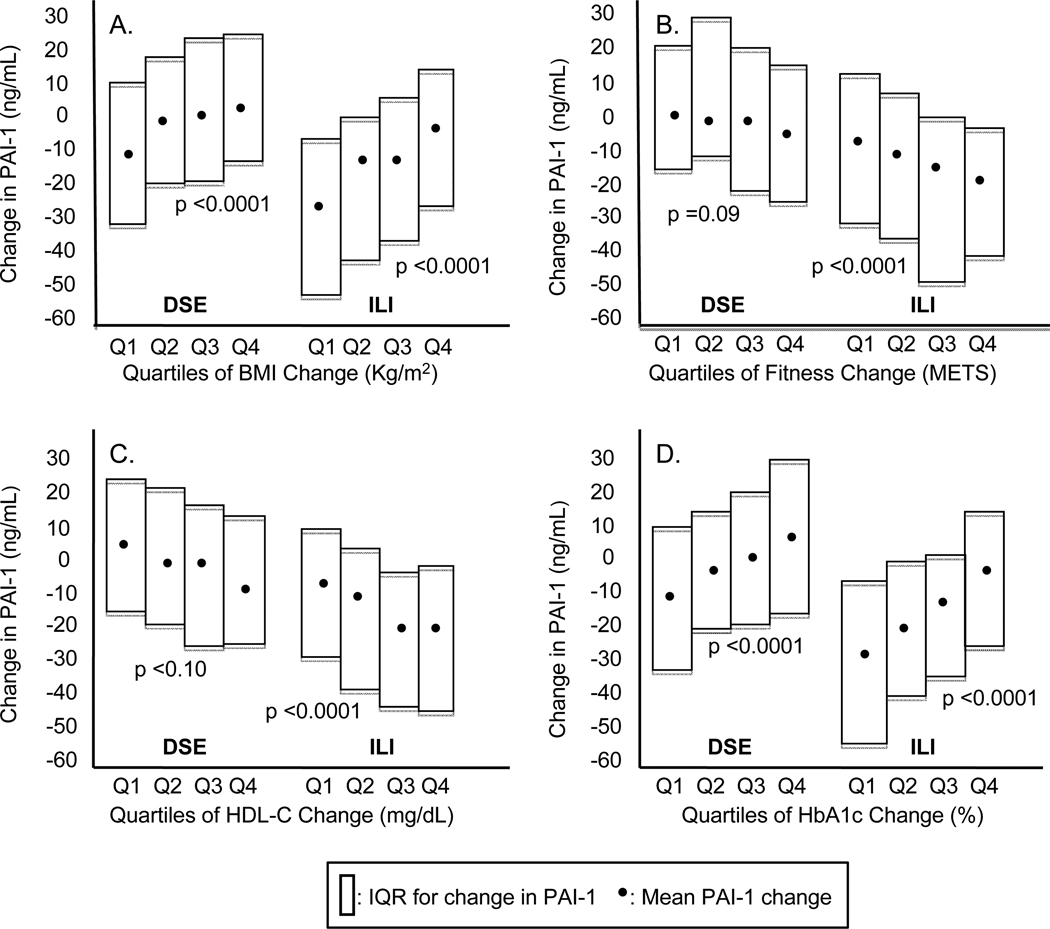
Changes in PAI-1 levels were not associated with changes in D-dimer or fibrinogen levels (Spearman’s correlation coefficients, adjusted for gender and age, of −0.03 [p = 0.22] and 0.03 [p = 0.24], respectively). Greater improvements in adiposity, but also in fitness, glucose control, HDL-cholesterol (HDL-C) levels (Figure 1), and triglycerides (not shown) with ILI, were found to be associated with greater decreases in PAI-1 levels (p for trend <0.0001 for all). Separate analysis in the DSE arm showed progressive change in PAI-1 across quartiles of change only for body mass index (BMI) and HbA1c (p for trend: <0.001 for both). These findings do not suggest important effect modification on the relation between treatment arm and PAI-1.
Figure 1.
1-year changes in PAI-1 in the DSE versus the ILI arm by quartiles (Q) of variable change
A: For DSE: Q1: < −1.11; Q2: −1.11 to < −0.15; Q3: −0.15 to < 0.64; Q4: ≥0.64; for ILI: Q1: < −4.32; Q2 −4.32 to < −2.75; Q3: −2.75 to < 1.39; Q4: ≥ 1.39
B: For DSE: Q1: < −0.4; Q2: −0.4 to 0.0; Q3: 0.0 to 0.8; Q4 > 0.8; for ILI: Q1: < 0.0; Q2: 0.0 to 0.8; Q3: 0.8 to 1.7; Q4: > 1.7
C: For DSE: Q1: < −2.0; Q2: −2.0 to < 1.0; Q3: 1.0 to < 5.0; Q4: ≥ 5.0; for ILI: Q1: < −1.0; Q2 −1.0 to < 3.0; Q3: 3.0 to < 7.0; Q4: ≥ 7.0
D: For DSE: Q1: < −0.6; Q2: −0.6 to < −0.2; Q3: −0.2 to < 0.3; Q4: ≥ 0.3; for ILI Q1: < −1.2; Q2 −1.2 to < −0.6; Q3: −0.6 to < −0.1; Q4: ≥ −0.1
Metabolic Predictors of 1-Year Change in PAI-1
Regression analyses showed that not only adiposity change (measured by BMI, weight and waist changes), but also each of the changes in glucose (HbA1c and fasting glucose) and lipid (triglycerides and HDL-C) control and in fitness with ILI predicted a decrease in PAI-1 levels (log-transformed for analysis) at 1 year (p<0.001 for all) (Table 3, Models A–I). Change in waist accounted for 5% of the variance in PAI-1 change (33% with ILI), after adjusting for baseline PAI-1 level, demographics, medical history and medication use, and was not a better predictor of PAI-1 change when compared to change in BMI or change in weight, which explained 6–7% of the variance in PAI-1 change (Models B and C; 34 and 35% of the variance in PAI-1 change with ILI). Given that change in BMI was a stronger predictor of change in PAI-1 than was change in waist, we chose to include change in BMI in the remainder of our analyses.
Table 3.
Metabolic Variables as Predictors of PAI-1 Change with 1-Year ILI
| Model * | B-Coefficient | SE | R2 | p |
|---|---|---|---|---|
| Model A | 0.28 | |||
| ILI v. DSE | − 0.47 | 0.034 | <0.0001 | |
| Model B | 0.34 | |||
| ILI v. DSE | − 0.20 | 0.038 | <0.0001 | |
| Change in BMI | 0.10 | 0.007 | <0.0001 | |
| Model C | 0.35 | |||
| ILI v. DSE | − 0.19 | 0.038 | <0.0001 | |
| Change in weight | 0.03 | 0.003 | <0.0001 | |
| Model D | 0.33 | |||
| ILI v. DSE | − 0.32 | 0.035 | <0.0001 | |
| Change in waist circumference | 0.02 | 0.002 | <0.0001 | |
| Model E | 0.34 | |||
| ILI v. DSE | − 0.41 | 0.033 | <0.0001 | |
| Change in fasting glucose | 0.01 | 0.0004 | <0.0001 | |
| Model F | 0.32 | |||
| ILI v. DSE | − 0.39 | 0.034 | <0.0001 | |
| Change in HbA1c | 0.17 | 0.018 | <0.0001 | |
| Model G | 0.29 | |||
| ILI v. DSE | − 0.46 | 0.034 | <0.0001 | |
| Change in triglycerides | 0.001 | 0.0002 | 0.0002 | |
| Model H | 0.30 | |||
| ILI v. DSE | − 0.45 | 0.034 | <0.0001 | |
| Change in HDL-C | − 0.02 | 0.003 | <0.0001 | |
| Model I | 0.30 | |||
| ILI v. DSE | − 0.40 | 0.035 | <0.0001 | |
| Change in Fitness | − 0.10 | 0.014 | <0.0001 | |
| Model J | 0.33 | |||
| ILI v. DSE | − 0.33 | 0.035 | <0.0001 | |
| Change in HbA1c | 0.15 | 0.018 | <0.0001 | |
| Change in Fitness | − 0.08 | 0.014 | <0.0001 | |
| Model K | ||||
| ILI v. DSE | − 0.16 | 0.038 | 0.37 | <0.0001 |
| Change in BMI | 0.08 | 0.008 | <0.0001 | |
| Change in HbA1c | 0.13 | 0.017 | <0.0001 | |
| Change in fitness | − 0.03 | 0.014 | 0.026 | |
| Model L | 0.37 | |||
| ILI v. DSE | − 0.16 | 0.038 | <0.0001 | |
| Change in BMI | 0.08 | 0.007 | <0.0001 | |
| Change in HbA1c | 0.13 | 0.018 | <0.0001 | |
| Change in triglycerides | 0.0001 | 0.0002 | 0.45 | |
| Model M | 0.38 | |||
| ILI v. DSE | − 0.15 | 0.038 | <0.0001 | |
| Change in BMI | 0.08 | 0.008 | <0.0001 | |
| Change in HbA1c | 0.12 | 0.018 | <0.0001 | |
| Change in HDL-C | −0.012 | 0.002 | <0.0001 | |
| Change in triglycerides | −0.0001 | 0.0002 | 0.67 | |
| Change in fitness | − 0.03 | 0.014 | 0.04 |
Each model (A–M) was analyzed independently and adjusted for baseline PAI-1 level, demographics, clinic site, history of CVD, diabetes duration, smoking and thiazolidinedione and statin use, with difference between baseline and 1-year log-transformed PAI-1 values as outcome variable.
Improvement in fitness with ILI, again in the presence of multiple covariates, explained 2% of the variance in PAI-1 change (Model I; 30% of the variance in PAI-1 change with ILI) and remained associated with PAI-1 change after adjusting for changes in glucose control (Model J, p<0.0001) and in adiposity (Model K, p=0.026). Change in triglycerides with ILI was not associated with PAI-1 change when changes in glucose control and BMI were taken into account (Model L, p= 0.45). Change in glucose control, HDL-C and fitness remained significantly associated with PAI-1 change in the full model after adjusting for adiposity change (Model M; p<0.0001 for HbA1c and HDL-C, p= 0.04 for fitness) and together accounted for 10% of the variance in PAI-1 change (38% of the variance in PAI-1 change with ILI) independently of baseline PAI-1 levels, individual demographic characteristics, history of CVD, diabetes duration, smoking and thiazolidinedione use.
DISCUSSION
Our study shows that, in obese individuals with T2DM, moderate weight loss with ILI sustained over a 1-year period was sufficient to achieve significant reductions in PAI-1 levels when compared to usual care and that improvements in fitness, glucose control and HDL-C with ILI contributed, independently of adiposity change, to the lowering of PAI-1 levels. Finally, and contrary to our initial hypothesis, ILI did not change fibrinogen or D-Dimer levels, pointing to complex physiological relationships between PAI-1 inflammation and coagulation balance.
PAI-1 levels are elevated in diabetes, in this study over twice those in healthy subjects in the Multi-Ethnic Study of Atherosclerosis (MESA; assays also performed at the Vermont LCBR)20 and higher than those seen in non-diabetic obese12 or pre-diabetic adults.13 In support of our main hypothesis, ILI effected a greater reduction in PAI-1 levels (29% from baseline) than did usual care (2.5% reduction) in our obese diabetic participants. The relatively large decline in PAI-1 levels seen in this study is similar to the reduction observed in less obese subjects without diabetes12, 13 and was associated with a reduction in measures of adiposity. ILI participants reduced baseline weight by 8.7%, whereas DSE participants experienced less than a tenth of that change. Reductions in measures of both central and overall obesity were associated with the decrease in PAI-1 levels. Of interest, ILI was able to decrease PAI-1 to levels well within the normal range despite the presence of persisting obesity after the intervention (mean BMI at 1-year with ILI was 33.1 kg/m2, down from 36.3). In obesity, activated macrophages and T cells accumulate in adipose tissue, shifting adipokine secretion towards a pro-inflammatory pattern.21, 22 Of these adipokines, tumor necrosis factor–α (TNF-α) and transforming growth factor (TGF)-B appear to play an important role.9,11 It has been postulated that the adipocyte microenvironment acquires a pro-inflammatory phenotype when local angiogenesis cannot keep up with the expanding adipose tissue mass.23 It is possible that moderate weight loss in individuals who remain obese is sufficient to allow adipose tissue to reach a new balance between tissue mass and perfusion, decreasing pro-inflammatory adipokine production and reducing PAI-1 levels.
In agreement with our second hypothesis, improvement in metabolic factors and fitness decreased PAI-1 levels independently of changes in adiposity. ILI achieved a mean 0.7% reduction in HbA1c, an increase of 4.4 mg/dL in HDL-C and a 19% increase in fitness. Improvement in each of these metabolic factors, after accounting for demographic, medical history and medication covariates, explained 2–6% the variance in PAI-1 change (30–34% of the variance in PAI-1 change with ILI) at one year. The effect of each of the metabolic factors persisted in the full model after accounting for adiposity change. Recent work suggests that in obesity, oxidative stress accumulates in adipose tissue leading to the increased production of pro-inflammatory adipokines and of PAI-1.24 Hyperglycemia is an kinducer of pro-inflammatory adipokine production and an oxidative mechanism has been shown to mediate these effects and the secretion of PAI-1.25, 26 The beneficial effects of fitness on PAI-1 levels in our participants may be associated with those of regular moderate physical activity on adipose tissue function, including the promotion of antioxidant mechanisms,27 an improvement in insulin sensitivity,28 the modification of autonomic tone29 and the redistribution of HDL subfractions with a shift from the smaller HDL-3 to the larger more cardioprotective HDL-2 subfraction.30 Although we did not measure lipoprotein sub-fractions, others have shown that HDL-3, but not HDL-2, increases adipocyte PAI-1 secretion in vitro.31 This differential effect of HDL subfractions on adipokine secretion is thought to be ceramide-mediated and adds to potential effects of HDL on adipocyte cholesterol and adipokine secretion.32, 33 HDL is also known to bind to macrophages, cells known to be present in the stromal fraction of adipose tissue, where the anti-inflammatory lipoprotein could contribute to lower PAI-1 levels by decreasing oxidative stress.34
Our data did not support our hypothesis that reductions in PAI-1 levels with ILI would be associated with changes in fibrinogen and D-dimer. Studies in non-diabetic individuals suggest that moderate weight loss does not alter fibrinogen levels12, 13 and that major weight loss, in the order of a 40% reduction from baseline,35 is necessary to see a decrease in levels. Fibrinogen is an acute phase protein, but unlike PAI-1, it is synthesized only in liver and not subject to adipose tissue control. We know, from previous work in Look AHEAD, that ILI decreases C-reactive protein (CRP), another marker of systemic inflammation.36 Recent data suggest that, like PAI-1, CRP is synthesized not only by hepatocytes but also by several non-hepatic cells including adipocytes.37, 38 The unaltered fibrinogen levels with ILI suggest that the effects of moderate improvements in adiposity, metabolic control and fitness on inflammation in obese people with T2DM may be derived from improvements in adipose tissue function rather than in relation to changes in the acute phase response. Although non-adipose tissue sources of PAI-1, including liver, endothelial cells and platelets, could have contributed to the change in PAI-1 levels with ILI in our study, studies investigating the origin of circulating PAI-1 suggest that the source of PAI-1 may differ by age and health status and that, in obesity, adipose tissue is a major source.8, 39, 40 Our results are also in agreement with a factor analysis in healthy individuals that showed that PAI-1 clustered with a body mass factor and not with an IL-6 dependent inflammatory factor that included fibrinogen.41
The absence of associated changes in D-dimer with ILI, despite the important reduction in PAI-1 levels, was unexpected. PAI-1 is a major regulator of fibrinolysis3 and D-dimer a measure of ambient coagulation balance that includes intraluminal fibrinolysis.42 Both coagulation (resulting in fibrin formation) and fibrinolysis (resulting in clot dissolution) have to occur for D-dimer to be formed.42 Similar findings have been observed with weight loss in younger less obese persons without diabetes.12 Our results may be explained by the relatively normal D-dimer levels found in our stable ambulatory participants with T2DM, levels that indicate that on-going fibrin formation and dissolution were not elevated. Based on these findings one could hypothesize that elevated PAI-1 may exert an effect on clotting only in the setting of a relatively large stimulation, such as that occurring in the presence of a ruptured atherosclerotic plaque. An alternative hypothesis would be that if there were to be a CVD benefit associated with a decline in PAI-1 with ILI it would not be through regulation of on-going, so-called “ambient” coagulant balance and blood-based clot formation, but rather through its effect in the vessel wall.4 PAI-1 expression in the vessel wall is increased in the presence of diabetes43 and Sobel has proposed a deleterious effect of elevated tissue-based PAI-1 on vessel remodeling, leading to an increased risk of plaque rupture.4
Our study has several limitations. First, our PAI-1 assay measured total PAI-1 and was not specific for the active form. However, preliminary experiments in our laboratory found that it correlates highly with two frequently used assays: one measuring uncomplexed PAI-1 (active and latent free PAI-1; in-house immunoassay; R= 0.82) and a commercial assay measuring total PAI-1 (BioPool Tintelize immunoassay;R = 0.80). Furthermore, PAI-1 antigen and activity are strongly correlated (R = 0.77).44 Given the relatively stringent blood collection requirements for an activity assay, coupled with the multi-center nature of Look AHEAD, and the fact that much of the epidemiological data linking PAI-1 to CVD was assembled with assays for either uncomplexed or total PAI-1 (e.g.,1), we chose the automated total PAI-1 assay. We also evaluated the use of citrate plasma compared to a specialty collection tube (Biopool Stabilyte, Trinity Biotech USA) and found excellent correlation (R=0.99). Second, Look AHEAD did not measure insulin levels and the effects of insulin on PAI-1 change could not be directly assessed. It is possible that the association of hyperglycemia and PAI-1 could be explained in part by the presence of hyperinsulinemia. However, there is ample evidence that hyperglycemia is able to increase PAI-1 secretion independently of insulin change.26, 45
In summary, our findings show that ILI decreases and normalizes PAI-1 levels in stable obese diabetic persons when compared to usual care and that the decrease is associated not only with moderate reductions in adiposity, but also with improvements in fitness, glucose and HDL-C levels, factors known to impact adipose tissue function and pro-inflammatory adipokine production. The absence of effects on fibrinogen, an acute phase reactant, supports the position that the decreases in PAI-1 levels with ILI result mainly from its effects on adipose tissue inflammation rather than as a consequence of systemic changes in inflammatory status. Finally, we show that despite the large reduction in PAI-1 with ILI, there were no changes in D-dimer, a marker of ambient coagulation balance. These results support expanding the role of PAI-1 to that of a marker of adipose tissue health. Future results from Look AHEAD will determine if decreases in PAI-1 levels with ILI will reduce cardiovascular events.
Supplementary Material
ACKNOWLEDGMENTS
Members of the Look AHEAD Research Study Group are listed in the Online Supplement (Appendix). The authors thank Elaine S. Cornell, also a member of the Look AHEAD Obesity, Inflammation and Thrombosis Research Group, for her support with the assays.
Funding sources
Look AHEAD is sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases and co-sponsored by the National, Heart, Lung and Blood Institute, National Institute of Nursing Research, Office of Research on Women’s Health, National Center on Minority Health and Health Disparities and Centers for Disease Control and Prevention. Additional sources of funding for Look AHEAD are listed in the Online Data Supplement. Work by the Look AHEAD Ancillary Study Group Obesity Inflammation and Thrombosis was supported by the National Heart, Lung and Blood Institute, Grants HL090514 (CMB) and HL090514-02S1 (LMB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no potential conflicts of interest to disclose.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association in New Orleans, June 2010.
REFERENCES
- 1.Thogersen AM, Jansson JH, Boman K, Nilsson TK, Weinehall L, Huhtasaari F, Hallmans G. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: Evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98:2241–2247. doi: 10.1161/01.cir.98.21.2241. [DOI] [PubMed] [Google Scholar]
- 2.Juhan-Vague I, Alessi MC, Vague P. Thrombogenic and fibrinolytic factors and cardiovascular risk in non-insulin-dependent diabetes mellitus. Ann Med. 1996;28:371–380. doi: 10.3109/07853899608999095. [DOI] [PubMed] [Google Scholar]
- 3.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000;342:1792–1801. doi: 10.1056/NEJM200006153422406. [DOI] [PubMed] [Google Scholar]
- 4.Sobel BE, Taatjes DJ, Schneider DJ. Intramural plasminogen activator inhibitor type-1 and coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1979–1989. doi: 10.1161/01.ATV.0000091250.53231.4D. [DOI] [PubMed] [Google Scholar]
- 5.Kruithof EK, Gudinchet A, Bachmann F. Plasminogen activator inhibitor 1 and plasminogen activator inhibitor 2 in various disease states. Thromb Haemost. 1988;59:7–12. [PubMed] [Google Scholar]
- 6.Loskutoff DJ, Samad F. The adipocyte and hemostatic balance in obesity: Studies of PAI-1. Arterioscler Thromb Vasc Biol. 1998;18:1–6. doi: 10.1161/01.atv.18.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson P, Reynisdottir S, Lonnqvist F, Stemme V, Hamsten A, Arner P. Adipose tissue secretion of plasminogen activator inhibitor-1 in non-obese and obese individuals. Diabetologia. 1998;41:65–71. doi: 10.1007/s001250050868. [DOI] [PubMed] [Google Scholar]
- 8.Alessi MC, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, Geel O, Juhan-Vague I. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes. 2000;49:1374–1380. doi: 10.2337/diabetes.49.8.1374. [DOI] [PubMed] [Google Scholar]
- 9.Sawdey MS, Loskutoff DJ. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J Clin Invest. 1991;88:1346–1353. doi: 10.1172/JCI115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calles-Escandon J, Ballor D, Harvey-Berino J, Ades P, Tracy R, Sobel B. Amelioration of the inhibition of fibrinolysis in elderly, obese subjects by moderate energy intake restriction. Am J Clin Nutr. 1996;64:7–11. doi: 10.1093/ajcn/64.1.7. [DOI] [PubMed] [Google Scholar]
- 11.Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: Links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26:2200–2207. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 12.Folsom AR, Qamhieh HT, Wing RR, Jeffery RW, Stinson VL, Kuller LH, Wu KK. Impact of weight loss on plasminogen activator inhibitor (PAI-1), factor VII, and other hemostatic factors in moderately overweight adults. Arterioscler Thromb. 1993;13:162–169. doi: 10.1161/01.atv.13.2.162. [DOI] [PubMed] [Google Scholar]
- 13.Hamalainen H, Ronnemaa T, Virtanen A, Lindstrom J, Eriksson JG, Valle TT, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Rastas M, Aunola S, Uusitupa M, Tuomilehto J Finnish Diabetes Prevention Study Group. Improved fibrinolysis by an intensive lifestyle intervention in subjects with impaired glucose tolerance. The Finnish Diabetes Prevention Study. Diabetologia. 2005;48:2248–2253. doi: 10.1007/s00125-005-1938-5. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 15.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Rumley A, Lowe GD. Fibrin D-dimer and coronary heart disease: Prospective study and meta-analysis. Circulation. 2001;103:2323–2327. doi: 10.1161/01.cir.103.19.2323. [DOI] [PubMed] [Google Scholar]
- 16.Look AHEAD Research Group; Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C, Hill JO, Kumanyika S The Look AHEAD study. A description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McBane RD, 2nd, Hardison RM, Sobel BE BARI 2D Study Group. Comparison of plasminogen activator inhibitor-1, tissue type plasminogen activator antigen, fibrinogen, and D-dimer levels in various age decades in patients with type 2 diabetes mellitus and stable coronary artery disease (from the BARI 2D trial) Am J Cardiol. 2010;105:17–24. doi: 10.1016/j.amjcard.2009.08.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Look Ahead Research Group. Bray G, Gregg E, Haffner S, Pi-Sunyer XF, Wagenknecht LE, Walkup M, Wing R. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) Study. Diab Vasc Dis Res. 2006;3:202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutsey PL, Cushman M, Steffen LM, Green D, Barr RG, Herrington D, Ouyang P, Folsom AR. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: The MESA study. J Thromb Haemost. 2006;4:2629–2635. doi: 10.1111/j.1538-7836.2006.02237.x. [DOI] [PubMed] [Google Scholar]
- 21.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, Ballantyne CM. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 23.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 26.Rikitake Y, Liao JK. Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation. 2005;111:3261–3268. doi: 10.1161/CIRCULATIONAHA.105.534024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucker PS, Fisher-Wellman K, Bloomer RJ. Can exercise minimize postprandial oxidative stress in patients with type 2 diabetes? Curr Diabetes Rev. 2008;4:309–319. doi: 10.2174/157339908786241160. [DOI] [PubMed] [Google Scholar]
- 28.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E586–E594. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jae SY, Carnethon MR, Ahn ES, Heffernan KS, Choi YH, Lee MK, Fernhall B. Association between heart rate recovery after exercise testing and plasminogen activator inhibitor 1, tissue plasminogen activator, and fibrinogen in apparently healthy men. Atherosclerosis. 2008;197:415–419. doi: 10.1016/j.atherosclerosis.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Altena TS, Michaelson JL, Ball SD, Guilford BL, Thomas TR. Lipoprotein subfraction changes after continuous or intermittent exercise training. Med Sci Sports Exerc. 2006;38:367–372. doi: 10.1249/01.mss.0000185088.33669.fd. [DOI] [PubMed] [Google Scholar]
- 31.Lee MH, Hammad SM, Semler AJ, Luttrell LM, Lopes-Virella MF, Klein RL. HDL3, but not HDL2, stimulates plasminogen activator inhibitor-1 release from adipocytes: The role of sphingosine-1-phosphate. J Lipid Res. 2010;51:2619–2628. doi: 10.1194/jlr.M003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dagher G, Donne N, Klein C, Ferre P, Dugail I. HDL-mediated cholesterol uptake and targeting to lipid droplets in adipocytes. J Lipid Res. 2003;44:1811–1820. doi: 10.1194/jlr.M300267-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Le Lay S, Krief S, Farnier C, Lefrere I, Le Liepvre X, Bazin R, Ferre P, Dugail I. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J Biol Chem. 2001;276:16904–16910. doi: 10.1074/jbc.M010955200. [DOI] [PubMed] [Google Scholar]
- 34.Efrat M, Aviram M. Macrophage paraoxonase 1 (PON1) binding sites. Biochem Biophys Res Commun. 2008;376:105–110. doi: 10.1016/j.bbrc.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 35.Primrose JN, Davies JA, Prentice CR, Hughes R, Johnston D. Reduction in factor VII, fibrinogen and plasminogen activator inhibitor-1 activity after surgical treatment of morbid obesity. Thromb Haemost. 1992;68:396–399. [PubMed] [Google Scholar]
- 36.Belalcazar LM, Reboussin DM, Haffner SM, Hoogeveen RC, Kriska AM, Schwenke DC, Tracy RP, Pi-Sunyer FX, Ballantyne CM and the Look AHEAD Research Group. A one-year lifestyle intervention for weight loss in persons with type 2 diabetes reduces high C-reactive protein levels and identifies metabolic predictors of change, from the Look AHEAD (Action for Health in Diabetes) study. Diabetes Care. 2010 doi: 10.2337/dc10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, Nishizawa H, Maeda N, Kobayashi H, Hiraoka H, Matsuzawa Y. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 38.Calabro P, Chang DW, Willerson JT, Yeh ET. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: Linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46:1112–1113. doi: 10.1016/j.jacc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Brogren H, Sihlbom C, Wallmark K, Lonn M, Deinum J, Karlsson L, Jern S. Heterogeneous glycosylation patterns of human PAI-1 may reveal its cellular origin. Thromb Res. 2008;122:271–281. doi: 10.1016/j.thromres.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Serrano R, Barrenetxe J, Orbe J, Rodriguez JA, Gallardo N, Martinez C, Andres A, Paramo JA. Tissue-specific PAI-1 gene expression and glycosylation pattern in insulin-resistant old rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1563–R1569. doi: 10.1152/ajpregu.00093.2009. [DOI] [PubMed] [Google Scholar]
- 41.Sakkinen PA, Wahl P, Cushman M, Lewis MR, Tracy RP. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am J Epidemiol. 2000;152:897–907. doi: 10.1093/aje/152.10.897. [DOI] [PubMed] [Google Scholar]
- 42.Tracy RP. Atherogenesis and coronary heart disease. In: DeFronzo R, Ferannini E, Keen H, Zimmet P, editors. International Textbook of Diabetes. 3rd ed. Chichester, Wiley; 2003. pp. 1437–1448. [Google Scholar]
- 43.Sobel BE, Woodcock-Mitchell J, Schneider DJ, Holt RE, Marutsuka K, Gold H. Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients: A potential factor predisposing to thrombosis and its persistence. Circulation. 1998;97:2213–2221. doi: 10.1161/01.cir.97.22.2213. [DOI] [PubMed] [Google Scholar]
- 44.Juhan-Vague I, Pyke SD, Alessi MC, Jespersen J, Haverkate F, Thompson SG. Fibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. ECAT Study Group. European Concerted Action on Thrombosis and Disabilities. Circulation. 1996;94:2057–2063. doi: 10.1161/01.cir.94.9.2057. [DOI] [PubMed] [Google Scholar]
- 45.Pandolfi A, Giaccari A, Cilli C, Alberta MM, Morviducci L, De Filippis EA, Buongiorno A, Pellegrini G, Capani F, Consoli A. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta Diabetol. 2001;38:71–76. doi: 10.1007/s005920170016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.