Summary
In many neurons, subthreshold somatic depolarization can spread electrotonically into the axon and modulate subsequent spike-evoked transmission. Although release probability is regulated by intracellular Ca2+, the Ca2+-dependence of this modulatory mechanism has been debated. Using paired recordings from synaptically connected molecular-layer interneurons (MLIs) of the rat cerebellum, we observed Ca2+-mediated strengthening of release following brief subthreshold depolarization of the soma. Two-photon microscopy revealed that, at the axon, somatic depolarization evoked Ca2+ influx through voltage-sensitive Ca2+ channels (VSCCs) and facilitated spike-evoked Ca2+ entry. Exogenous Ca2+ buffering diminished these Ca2+ transients and eliminated the strengthening of release. Axonal Ca2+ entry elicited by subthreshold somatic depolarization also triggered asynchronous transmission that may deplete vesicle availability and thereby temper release strengthening. In this cerebellar circuit, activity-dependent presynaptic plasticity depends on Ca2+ elevations resulting from both sub- and suprathreshold electrical activity initiated at the soma.
Short-term alteration in the strength of neurotransmission can result from the electrotonic spread of subthreshold depolarization from the soma to presynaptic sites of release on the axon1–3. Mechanisms that trigger or modulate neurotransmission are often mediated through control of VSCCs because the likelihood of vesicle fusion is largely determined by the intracellular [Ca2+]. Although high concentration Ca2+ elevations are required to rapidly trigger exocytosis4,5, low concentration elevations can augment subsequent release via vesicle recruitment, priming, and sensitization6. Because of their large amplitude, action potentials are efficient triggers for VSCC activation7–10 and release. However, direct recordings from calyceal nerve terminals show that even slight depolarization can open VSCCs, albeit at low probability, and strengthen subsequent spike-evoked neurotransmission11,12. In contrast, in dentate granule cells of the hippocampus, short-term strengthening of release elicited by modest somatic depolarization may be Ca2+-independent1,13. Whether somatic depolarization enhances action potential-evoked release by activating axonal VSCCs in inhibitory neurons has not been addressed, although asynchronous release is augmented in a Ca2+-dependent manner in young animals14.
MLIs of the cerebellum, including stellate and basket cells, make inhibitory GABAergic synapses onto Purkinje cells as well as other MLIs15. Depolarizing potentials originating in the somatodendritic compartment of MLIs passively propagate into their axonal arbor16,17 and open VSCCs18. However, action potentials are required to rapidly coordinate release from presynaptic sites, thereby limiting the voltage range for VSCC activation to subthreshold depolarization. Whether axonal Ca2+ entry evoked by subthreshold somatic depolarization is sufficient to alter spike-evoked neurotransmission has not been directly determined in MLIs.
Here, we investigated the mechanisms that mediate somatic voltage control of axonal transmitter release between MLIs using paired electrical recording and two-photon laser scanning microscopy (2PLSM). Subthreshold somatic depolarization was sufficient to activate axonal VSCCs, elicit Ca2+ influx and strengthen both action potential-evoked and asynchronous transmitter release. Enhancement of release was diminished or eliminated by chelating intracellular Ca2+ with EGTA or by blocking VSCCs, indicating that there is a direct connection between somatic voltage-control of neurotransmission and Ca2+ entry at the site of release. This suggests that release plasticity elicited by subthreshold somatic depolarization depends on presynaptic VSCCs.
Results
Subthreshold depolarization enhances AP-evoked release
GABAA receptor (GABAAR)-mediated synaptic transmission was examined between pairs of MLIs in rat cerebellar slices using simultaneous whole-cell recording. Action potentials elicited in presynaptic cells by somatic current injection (100 – 600 pA, 3 – 5 ms) evoked time-locked inward currents in voltage-clamped postsynaptic cells (ECl- ≈ 0 mV) that were blocked by picrotoxin (100μM; 3.8 ± 1.8 % of control; n = 5). Irreversible rundown of evoked transmission, commonly observed in paired MLI recordings19, was eliminated by presynaptic perforated patch recording20 (see Methods). To determine whether somatic depolarization was sufficient to alter the strength of neurotransmission, presynaptic neurons were briefly depolarized from rest (−73.3 ± 0.3 mV; n = 40) to a potential just subthreshold for spiking (−56.1 ± 0.9 mV, 300 ms). This depolarization was followed by action potential stimulation (10 ms delay). IPSCs evoked by action potentials preceded by subthreshold depolarization were larger than interleaved control IPSCs evoked by action potentials alone (121.1 ± 4.8% of control amplitude; n = 40, P < 0.001; Fig. 1a). Furthermore, the paired-pulse ratio (PPR = IPSC2/IPSC1) of IPSCs evoked by two action potentials (250 ms interval; PPR = 1.000 ± 0.064, n = 37; Fig. 1a, 1b) following subthreshold depolarization was significantly reduced compared to non-depolarized control trials (P = 0.002; Fig. 1b, 1c). This indicates that subthreshold somatic depolarization causes a short-term alteration in presynaptic release resulting from the electrotonic spread of the somatic depolarization into the axon, as observed in other neurons1–3,13.
Figure 1. Subthreshold somatic depolarization enhances spike-evoked release in MLIs.
(a) In a MLI paired recording, action potentials in the presynaptic neuron evoked GABAAR-mediated IPSCs in the postsynaptic neuron. Subthreshold somatic depolarization (300 ms) elicited prior to spiking enhanced release. Control action potential responses are in red and test responses preceded by subthreshold depolarization are in black. An amplified view of the first IPSC is shown in the inset. (b) Paired-pulse IPSCs from the same neuron normalized to the peak of the first response. (c) Summary data show that the PPR decreases with somatic depolarization of the presynaptic neuron. A significant difference (paired t-test, P < 0.05) between groups is indicated by asterisk with mean values ± SEM. (d) Linear regression analysis indicates that release enhancement elicited by subthreshold depolarization was correlated with the basal PPR. (e and f) Differences in release enhancement for facilitating and depressing responses measured in 1.5 and 3.0 mM extracellular Ca2+. ANOVA analysis with post-hoc Tukey’s multiple comparison tests indicated significance (P < 0.05) with mean values plotted ± SEM. For clarity, spontaneous IPSCs were blanked prior to averaging in a (inset) and b.
Control recordings included both facilitating and depressing responses (Fig. 1c), indicating that synapses between MLIs with different release probabilities co-exist in cerebellum. Synaptic strengthening caused by subthreshold depolarization depended on basal release probability, as the amount of enhancement of IPSC1 was correlated with the PPR measured in control trials (Fig. 1d). Depressing synapses, on average, were not altered by subthreshold depolarization (P = 0.17). However, there was considerable variability within this group, with some pairs showing release enhancement while others were weakened (Fig. 1e). In 3 mM extracellular Ca2+ release enhancement caused by subthreshold depolarization was less than in 1.5 mM Ca2+ (Fig. 1e), but the increase was still significant (P = 0.03). High extracellular Ca2+ increased release probability, as reflected by larger control IPSCs (peak amplitude 306.0 ± 67.0 and 172.7 ± 22.5 pA, 3.0 and 1.5 mM Ca2+, respectively, n = 13 and 40, P = 0.02) and paired-pulse depression in all recordings (n = 11, Fig. 1f). Thus, strengthening of release by subthreshold depolarization is similar to other activity-dependent forms of release enhancement, such as spike-evoked facilitation and augmentation, in that these processes occur more readily in low release probability synapses.
Subthreshold depolarization and presynaptic Ca2+ entry
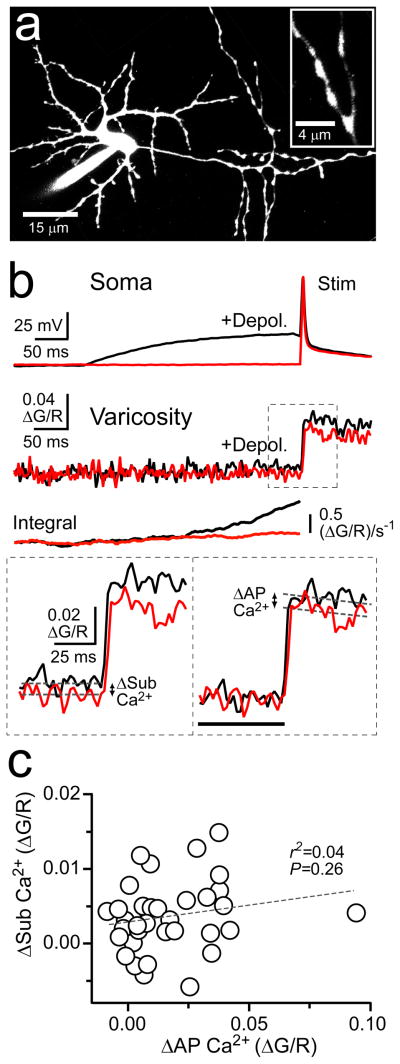
Intracellular Ca2+ is known to govern many aspects of transmitter release6. Therefore, we probed for presynaptic Ca2+ elevation evoked by subthreshold somatic depolarization using 2PLSM. MLIs were filled through the whole-cell patch pipette with Alexa Fluor 594 to visualize cell morphology and the Ca2+ indicator Fluo-5F to detect intracellular Ca2+ transients (Fig. 2a). Axon varicosities were targeted for optical recording. These varicosities are known to be hotspots of action potential-evoked Ca2+ entry and, therefore, are presumed to be en passant sites of release15,18,21,22, though it remains possible that these specializations may include non-synaptic sites.
Figure 2. Subthreshold somatic depolarization evokes and enhances axonal Ca2+ entry.
(a) Two-photon fluorescence image of a representative MLI filled via patch pipette with Alexa 594 (13 μM) and Fluo-5F (200 μM). On the right, a higher-magnification view shows axon varicosities. (b) Somatic current injection elicited control action potential-evoked responses (red) and test spikes paired with 300 ms subthreshold depolarizations (black). Shown directly below are the resulting Ca2+ transients recorded in an axon varicosity. Integration of the Ca2+ signals accentuates the difference between control and depolarized responses prior to spike firing. At the bottom left, amplified view of Ca2+ transients with dashed lines (100 ms epoch average) demarcating the amplitude of the Ca2+ response evoked by subthreshold depolarization. On the bottom right, action potential-evoked Ca2+ transients baselined prior to spike firing (black bar) isolated depolarization-dependent facilitation of the spike-evoked Ca2+ transient. Exponential fits to the spike-elicited transients were used to determine peak amplitudes. (c) Comparison of depolarization-evoked Ca2+ elevation and facilitation of action potential-elicited Ca2+ entry. Line fit of the linear regression is indicated by the dashed line.
In current-clamped MLIs, subthreshold somatic depolarization (300 ms) often evoked small Ca2+ transients in axon varicosities (Fig. 2b; ΔSub Ca2+). The amplitude of these Ca2+ transients varied across recording sites, including many varicosities without any apparent Ca2+ elevation (Fig. 2c). However, on average, Ca2+ responses were significantly larger than corresponding measurements obtained from interleaved control trials without subthreshold depolarization (ΔG/R −0.0010 ± 0.0004 and 0.0026 ± 0.0010, control and depolarized, respectively; P < 0.0001; n = 35). In addition, we observed that Ca2+ transients evoked by action potentials following subthreshold depolarization were larger than those without prior depolarization (Fig. 2b). Subtraction of the Ca2+ elevation evoked during the subthreshold depolarization revealed that the facilitation of spike-evoked Ca2+ (24.5 ± 4.5% increase in peak amplitude, P < 0.001) was not merely the result of the shift in baseline fluorescence, but rather a true enhancement of spike-induced Ca2+ influx (Fig. 2b;ΔAP Ca2+). The amount of spike-evoked Ca2+ facilitation was not correlated with the amplitude of Ca2+ influx during subthreshold depolarization (Fig. 2c). Whether this indicates that the two components contributing to Ca2+ elevation are not causally related is unclear because the extremely small amplitude of the subthreshold response interferes with our ability to accurately measure it. However, in 3 mM extracellular Ca2+, the Ca2+ transient resulting from subthreshold depolarization was larger than that in control conditions (Peak ΔG/R 0.0036 ± 0.0008 and 0.0061 ± 0.0030; 1.5 and 3.0 extracellular Ca2+, respectively; n = 35 and 74, P = 0.04) and was correlated with the facilitation of the spike-induced Ca2+ influx (r2 = 0.19; P = 0.0002), suggesting that the Ca2+ elevation during subthreshold depolarization caused the spike-induced facilitation.
Inhibition of VSCCs by a combination of antagonists (ω-conotoxin MVIIC 1μM, SNX 0.3 μM, nimodipine 20 μM, and mibefradil 10μM) confirmed that subthreshold depolarization-evoked Ca2+ entry occurs through VSCCs (Supplemental Fig. 1a, 1b) as previously reported18. The unblocked component presumably reflects toxin-resistant VSCCs22–24, as spike-evoked Ca2+ transients were blocked to a similar extent (Supplemental Fig. 1b). Subthreshold depolarization-induced facilitation of spike-evoked Ca2+ entry was also diminished by VSCC inhibition (8.7 ± 5.0% of control; n = 9, P = 0.002), again suggesting that this facilitation was dependent on an increase in intracellular Ca2+ elicited by the preceding subthreshold depolarization.
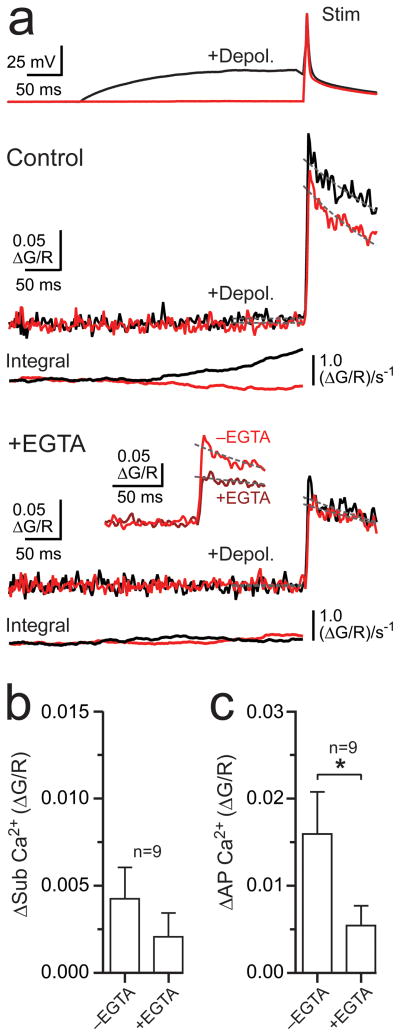
Release strengthening caused by subthreshold depolarization in MNTB calyceal nerve terminals is inhibited by Ca2+chelation, implying an alteration in an underlying Ca2+ elevation11,12. In MLI axonal varicosities, depolarization-evoked Ca2+ entry was not significantly altered by the cell-permeable Ca2+ buffer EGTA-AM (10–20 μM; >10 min; P = 0.08, Fig. 3a, 3b). However, in many cases subthreshold depolarization did not result in a measurable Ca2+ transient (see Fig. 2c) and, therefore, an effect of chelation overall could only be determined indirectly, using paired recording (see below). To test for a direct effect of chelation on Ca2+ elevation induced by subthreshold depolarization, we focused our analysis on large-amplitude responses (ΔG/R > 0.0050; n = 4; Fig. 3a). In this group, EGTA significantly reduced the Ca2+ transients (ΔG/R 0.0090 ± 0.0019 and 0.0043 ± 0.0027, control and EGTA, respectively, n = 4, P = 0.03). Facilitation of spike-evoked Ca2+ entry following subthreshold depolarization was greatly diminished by EGTA across all cells (Fig. 3c), indicating that, even in cases where depolarization did not produce a measurable increase in Ca2+, facilitation of the spike-evoked Ca2+ transient was Ca2+-dependent25,26. EGTA also reduced Ca2+ transients evoked by single action potentials alone (70.1 ± 3.3% of control; n = 9, P = 0.008; Fig. 3a), suggesting a competition between Fluo-5F and EGTA for Ca2+ binding27, that basal Ca2+ levels are sufficient to partially facilitate VSCC opening, or that there was a substantial rundown of VSCC opening.
Figure 3. Subthreshold depolarization-dependent Ca2+ signaling is EGTA-sensitive.
(a) Somatic recording of an action potential (red) and an action potential preceded by a 300 ms subthreshold depolarization (black). The resulting Ca2+ transients in an axon varicosity are shown below in control and following EGTA-AM application (10–20 μM; >10 min). Integrals of the Ca2+ responses are provided for each condition. The inset shows a comparison of action potential-evoked responses, elicited without preceding subthreshold depolarization, in control and with EGTA. Dashed lines indicate the response amplitude prior to spiking (100 ms epoch) and the exponential fits of action potential-evoked Ca2+ transients used to determine peak amplitudes. (b) The effect of EGTA on Ca2+ entry evoked by subthreshold depolarization. (c) EGTA inhibits facilitation of spike-evoked Ca2+ entry caused by subthreshold depolarization. Mean values are presented with error bars (+SEM); asterisks denote significance (paired t-test, P < 0.05).
Enhancement of release is Ca2+-dependent
In paired MLI recordings, the enhancement of IPSC1 by subthreshold depolarization was blocked by EGTA-AM (20 μM; >10 min; 122.2 ± 7.4 and 97.5 ± 6.9% of control amplitude, −EGTA and +EGTA, respectively, n = 9, P = 0.009; Fig. 4a, 4b). This indicates that the increase in spike-evoked release caused by subthreshold depolarization depends on the EGTA-sensitive Ca2+ elevation observed in axon varicosities. The block of release-strengthening by EGTA occurred mainly in recordings showing paired-pulse facilitation in control conditions. On average, IPSCs in depressing cell pairs were unaltered by subthreshold depolarization and remained unchanged with the addition of EGTA (Fig. 4b, top; see also Fig. 1e). It follows that the magnitude of the EGTA effect was correlated with the control PPR (Fig. 4b, bottom). This reinforces the conclusion that subthreshold depolarization differentially affects release at MLI synapses in a release probability-dependent manner.
Figure 4. EGTA inhibits enhancement of release elicited by subthreshold depolarization.
(a) Action potential-evoked IPSCs (red) recorded from pairs of connected MLIs in control and following EGTA-AM application. In alternating trials, action potentials were preceded by a 300 ms subthreshold depolarization (black). (b) Top summary graph shows that EGTA eliminates release strengthening caused by subthreshold depolarization in facilitating cell pairs. Release-strengthening was not apparent in cell pairs that depressed in basal conditions. In the bottom graph, linear regression analysis plots the difference between the percentage change in EGTA and in control relative to the basal PPR. (c) The effect of EGTA on paired-pulse responses from trials without preceding subthreshold depolarization. Amplified view of the first IPSC is shown on the inset. (d) EGTA altered paired-pulse responses in cell pairs that facilitated in basal conditions. Data are plotted as mean values with ± SEM and asterisks denote significance (paired t-test, P < 0.05). Spontaneous IPSCs were blanked prior to averaging in a and c.
EGTA did not affect the amplitude of IPSCs evoked without preceding subthreshold depolarization (Fig. 4c; IPSC amplitudes 97.5 ± 4.8 and 82.8 ± 11.3% of control; n = 5 and 4, P = 0.47 and 0.16; facilitating, and depressing responses, respectively). This implies that the Ca2+ domain responsible for triggering exocytosis is tightly associated with release-competent vesicles28,29. However, EGTA altered the PPR, changing facilitation to depression, though it had no clear effect on depressing cell pairs (Fig. 4c, 4d; PPR for depressing cells 0.67 ± 0.09 and 0.97 ± 0.09, control and EGTA, respectively; n = 4, P = 0.13). This suggests that, in basal conditions, residual Ca2+ following an initial spike facilitates subsequent release27 and may mask depression30,31. EGTA did not induce a significant change in the PPR in these responses when subthreshold depolarization preceded spiking (PPR 0.79 ± 0.05 and 1.01 ± 0.11 EGTA and +EGTA, respectively; n = 5, P = 0.18), suggesting little additional or underlying modification of release in this condition.
Subthreshold depolarization and asynchronous release
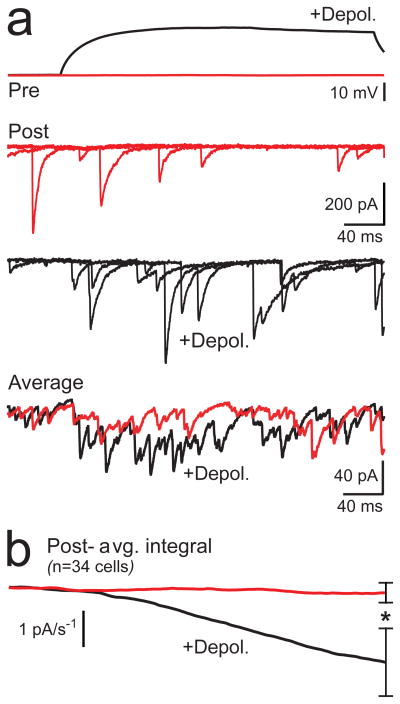
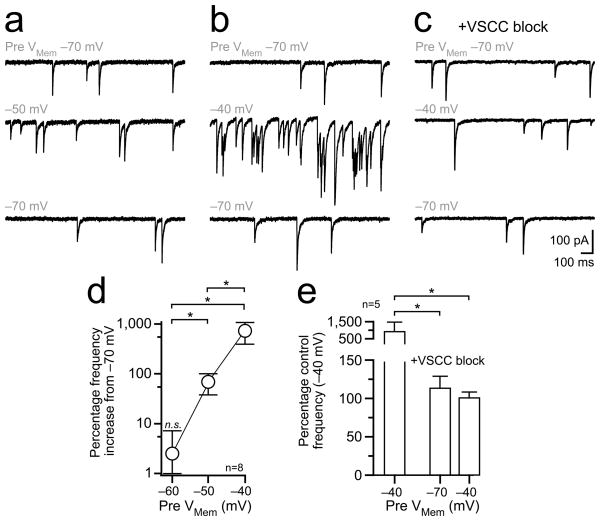
At some synapses, asynchronous release can result from the low concentration of Ca2+ remaining after spike firing32,33. In our paired recordings, there was often a small increase in the frequency of asynchronous IPSCs during subthreshold somatic depolarization (Fig. 5a) likely caused by the elevation of Ca2+ by the depolarizing stimulus. Although in many recordings, spike-evoked IPSCs from unclamped surrounding MLIs likely overwhelmed this small increase in asynchronous transmission, charge integration of averaged postsynaptic currents revealed a difference between control and depolarized trials across cells (Fig. 5b). The increase of asynchronous IPSCs by presynaptic somatic depolarization was much clearer when background action potential-driven release from spontaneously firing surrounding cells was blocked with TTX (1 μM) and the stimulus duration was prolonged (150 s; 3 mM extracellular Ca2+; Fig. 6a, 6b, and 6d). Block of VSCCs by Cd2+ and Ni2+ (100 μM) eliminated depolarization-evoked asynchronous release without affecting basal spontaneous transmission (Fig. 6c, 6e), demonstrating the connection between somatic depolarization, axonal VSCC activation, and asynchronous release.
Figure 5. Subthreshold depolarization evokes asynchronous release.
(a) Paired recording of MLIs show spontaneous IPSCs in unstimulated control responses (red) and recruitment of asynchronous events with a 300 ms presynaptic subthreshold depolarization (black). Three individual sweeps from the postsynaptic neuron are shown superimposed for each condition with the average response (27 and 28 sweeps, control and depolarized, respectively) at the bottom. (b) The average charge integration of the postsynaptic response across all cell recordings (± SEM). Asterisk denotes significance (paired t-test, P < 0.05).
Figure 6. Depolarization-dependent asynchronous release is triggered by VSCCs.
(a,b) Paired recording from MLIs in TTX with representative sweeps from the postsynaptic neuron showing recruitment of asynchronous IPSCs with presynaptic depolarization. The presynaptic holding potential is indicated in gray above each sweep. Responses were recorded in 3 mM extracellular Ca2+ to enhance release. (c) In the same cell, IPSC frequency is unaltered by presynaptic depolarization after VSCC block (100 μM Cd2+ and Ni2+). (d) Asynchronous IPSC frequency depends on the presynaptic holding potential. Mean values are plotted with error bars ± SEM. Asterisks denote significant differences (P < 0.05) between groups tests following ANOVA analysis (Tukey’s multiple comparison test). (e) Block of VSCCs eliminates the depolarization-dependent increase in asynchronous transmission. Mean values + SEM are shown with significance (asterisks P < 0.05) determined by ANOVA analysis.
Asynchronous release elicited by repetitive firing weakens subsequent action potential-evoked phasic transmission by depleting the pool of readily-releasable vesicles33,34. Vesicle availability may, in part, regulate release strengthening induced by subthreshold depolarization. To explore this idea, we reduced the releasable pool of vesicles with a train of presynaptic action potentials (10 spikes, 60 Hz) and then tested for subthreshold depolarization-induced enhancement of release (Fig. 7a). The amplitude of spike-evoked test IPSCs following burst firing was reduced compared to interleaved trials without the burst (77.3 ± 7.0% of control; n = 10, P = 0.05, Fig. 7b), a result consistent with depression induced by vesicle depletion. In addition, burst firing eliminated release-strengthening elicited by subthreshold depolarization (Fig. 7a, 7c). This indicates that in conditions that diminish the releasable pool of vesicles, subthreshold depolarization is less effective in altering release probability. By extension, asynchronous release elicited by subthreshold depolarization likely limits the strengthening of spike-evoked transmission by depleting the readily-releasable pool of vesicles. This may explain why in some cell pairs (4 of 10) the additive effect of subthreshold depolarization and bursting resulted in a weakening of transmission rather than enhancement (Fig. 7a, 7c).
Figure 7. Burst firing reduces the capacity for release enhancement caused by depolarization.
(a) In a recording from connected MLIs, high-frequency bursts of action potentials (10 spikes, 60 Hz) preceded a 300 ms subthreshold stimulation and test spikes in interleaved trials. Amplified views on the right show release enhancement caused by subthreshold depolarization and weakening of transmission when subthreshold excitation was paired with burst firing. (b) The effect of burst firing on the first IPSC in control trials without subthreshold stimulation is shown. (c) Burst firing eliminated release strengthening induced by subthreshold depolarization. Mean values are illustrated ± SEM with significance denoted by an asterisk (paired t-test, P < 0.05). For amplified views (a and b) spontaneous events were blanked prior to averaging.
Discussion
We report that subthreshold somatic depolarization passively spreads into the axon arbor of MLIs and strengthens subsequent action potential-evoked release of GABA onto neighboring cells. Therefore, subthreshold depolarization enhancement of release is not limited to excitatory neurons, but also includes inhibitory neurons as well. We show that for MLIs, this enhancement of release depends on the opening of VSCCs, which results in Ca2+ elevation as well as facilitation of spike-evoked Ca2+ influx, a novel finding. An increase in asynchronous release that accompanies the subthreshold depolarization is Ca2+-dependent and may decrease the capacity for release strengthening owing to vesicle depletion. Our results suggests the possibility that subthreshold potentials may modify neurotransmission at synapses throughout the nervous system in a Ca2+-dependent manner, as do other forms of short-term facilitation.
Somatic depolarization and Ca2+ influx at sites of release
Axons are not electrotonically isolated from the somatodendritic compartment, but rather support the electrotonic spread of subthreshold somatodendritic potentials1–3,16,18. As a result, the open probability of voltage-sensitive ion channels in axons can be modified by somatodendritic depolarization. Our Ca2+ measurements indicate that somatic depolarizations electrotonically spread into MLI axons and are sufficient to open VSCCs. Though not directly determined in this report, axonal length constants can exceed 400 μm in some neurons1–3,35. This parameter must be convolved with the number and composition of VSCCs at sites of release to determine subthreshold depolarization-mediated Ca2+ entry36. In calyceal terminals, P/Q-type VSCCs open at low probability following slight depolarization11 (<−60 mV), an activation range below that previously reported25,37. Although MLI varicosities express P/Q/N-type channels, among others22, the VSCC subtypes mediating depolarization-evoked Ca2+ influx remain unclear. It is possible that low-activation threshold VSCCs, such as R-type channels, preferentially mediate this Ca2+ influx, as predicted for hippocampal mossy fiber boutons36. It is unknown whether Ca2+-induced Ca2+ release from intracellular stores38 contributes to subthreshold depolarization-evoked Ca2+ transients.
Subthreshold depolarization not only induces direct influx of Ca2+ in MLI axons but also facilitates action potential-evoked Ca2+ entry. This effect is probably dependent on the preceding influx of Ca2+, because both inhibition of VSCCs and Ca2+ chelation diminished the facilitation of spike-evoked Ca2+ entry. At some synapses, VSCC-mediated currents are enhanced following Ca2+ binding by neuronal calcium sensor 1 (NCS-1), which induces shortening of the activation phase of VSCCs26,39. VSCC facilitation is elicited by both supra- and subthreshold depolarization-evoked Ca2+ elevation and is mitigated by exogenous Ca2+ buffering12,25,26. In MLIs, enhancement of spike-evoked Ca2+ entry following subthreshold depolarization could reflect a comparable Ca2+-dependent facilitation of VSCC activity, as MLIs express NCS-1 at presynaptic sites of release40,41. It remains possible that facilitation of action potential-evoked Ca2+ entry does not depend directly on the Ca2+ elevation evoked by the subthreshold depolarization. Alternatively, because spike-evoked Ca2+ influx in presynaptic terminals is, in part, controlled by the amplitude and duration of the action potential waveform9,42, facilitation of spike-evoked Ca2+ entry following subthreshold depolarization may reflect an alteration of the underlying action potential. Prolonged subthreshold somatic depolarization inactivates axonal Kv1 potassium channels in L5 pyramidal cells of visual cortex, leading to spike broadening3, which should in turn enhance Ca2+ entry42. However, potassium channel inactivation is not expected to be EGTA-sensitive or depend on VSCCs, arguing against this possibility.
Somatic depolarization and enhancement of release
Presynaptic receptor-mediated depolarization of calyceal nerve terminals elicits VSCC Ca2+ elevation and strengthening of release11,43. Our results extend this finding; subthreshold excitatory potentials need not only be elicited on or near terminals to enhance release. Depolarizing potentials evoked in the dendrites or soma spread through the axon, opening VSCCs, elevating Ca2+ at presynaptic specializations, and thereby increasing the likelihood of transmission. Small Ca2+ transients increase the probability of fusion by increasing microdomain Ca2+44,45, recruiting or sensitizing vesicles through Ca2+ binding by a high-affinity sensor distinct from the exocytosis trigger27,46, and saturating endogenous Ca2+ buffers44,45. If these mechanisms are mediated by residual Ca2+ following action potential firing, then they may also be triggered by Ca2+ influx during subthreshold depolarization12. Given that release probability is steeply dependent on the intracellular [Ca2+]4,5, enhancement of spike-evoked Ca2+ entry will also strengthen transmission12,25,26,42 unless the release process is saturated. If this occurs in MLIs, it may explain why high release probability synapses have a diminished capacity for release-strengthening induced by subthreshold depolarization.
We show that axonal VSCC-mediated Ca2+ entry elicited during presynaptic depolarization is sufficient to evoke asynchronous release in MLIs. Ca2+-induced asynchronous fusion has a relatively high sensitivity to intracellular [Ca2+], with an elevation to as little as 1 μM triggering release4,5. If the Ca2+ sensor for asynchronous exocytosis at MLIs has a similar sensitivity, then the intracellular [Ca2+] reached at MLI varicosities may be much higher than depolarization-evoked Ca2+ elevation in the calyx11 (100 nM). The supra-linear Ca2+ cooperativity for exocytosis4,5,47 might suggest a greater amount of spike-evoked release enhancement following presynaptic depolarization and Ca2+ entry than we observed. However, asynchronous transmission can deplete the pool of release-ready vesicles, diminishing availability for phasic transmission33,34. The resulting short-term depression of release can develop concomitantly with facilitation, one effect masking the other30,31. In this way, the capacity for depolarization-evoked strengthening of transmission in MLIs is limited by the superimposition of Ca2+-dependent depression mediated by vesicle pool depletion. Though IPSC amplitudes were unchanged by subthreshold depolarization in EGTA, it may be that release enhancement was merely balanced by depression rather than fully blocked. EGTA only partially diminished depolarization-induced Ca2+ elevation suggesting that the Ca2+-dependent effects on release persisted in this condition. The expression of facilitation and depression will depend on the relative Ca2+ sensitivity of each mechanism.
As implied in our burst-firing experiment, the capacity for release-strengthening by subthreshold depolarization may be strongly limited by depletion. Non-homogenous pools of vesicles may populate presynaptic sites at MLIs, as they do at the calyx of Held48. Subthreshold depolarization may help recruit more reluctant vesicles, distinguished by their relative Ca2+ sensitivity and/or distance from VSCCs, to participate in release during subsequent action potential-evoked transmission. Bulk Ca2+ elevation evoked by repetitive firing may also recruit these vesicles, depleting their availability and thereby decreasing the capacity for subthreshold depolarization induced enhancement of release. That is, multiplicative non-linear processes are competing for overlapping vesicle pools to determine the expression of short-term plasticity. We expect that any reduction in the vesicle pool(s), mediated by preceding sub- or suprathreshold activity, would diminish enhancement of release induced by subthreshold depolarization.
Functional implications
Subthreshold depolarization-induced regulation of spike-evoked release is clearly important in a number of cell types, though the mechanisms underlying the regulation may vary across brain regions1–3,13,18,35. In MLIs, the phenomenon appears to be entirely dependent on axonal Ca2+, as observed for other forms of release-strengthening6. In contrast, at hippocampal mossy fiber synapses, it may be that the Ca2+-dependence is restricted to proximal portions of the axon, whereas at more distal sites other mechanisms of strengthening are involved13, such as potassium channel inactivation3,42. One clear difference between these two cell types is axonal length, mossy fibers being much longer than MLI axons. Even in MLIs, the increases in axonal Ca2+ are, on average, larger in proximal than in distal portions of the axon, as expected from cable properties18. However, there was no correlation between the depolarization-induced enhancement of release and distance between the somata of synaptically connected pairs (r2 = 0.06, P = 0.23, n = 27), though this comparison is likely compromised by the inaccuracy of this measurement in predicting axonal length. The present results suggest that Ca2+-dependent plasticity induced by somatic depolarization will affect the gain of most synapses in short axon cells and at least proximal synapses in other cell types. However, it is likely that depolarization-induced plasticity does not simply monotonically decay with distance from the soma given the amount of variance we observed in our Ca2+ imaging and paired recording experiments. The control of transmitter release by subthreshold synaptic input that precedes action potential initiation adds a new dimension to synaptic signaling and requires consideration when analyzing information transfer across synapses.
Methods
Slice preparation
Parasagittal slices from cerebellar vermi were prepared from Sprague Dawley rats (PND 14–20) in accordance with OHSU IACUC-approved protocols. Rats, following isoflurane anesthesia, were decapitated and the cerebellum was isolated. Slices (250–300 μm) were cut on a vibroslicer (Leica Instruments, Nussloch, Germany) in an ice-cold solution containing (in mM) 87 NaCl, 25 NaHO3, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 0.5 CaCl2, 10 glucose, and 75 sucrose. Slices were subsequently transferred to a holding chamber containing (in mM) 119 NaCl, 26.2 NaHO3, 2.5 KCl, 1 NaH2PO4, 2 MgCl2, 1 CaCl2, 11 glucose and maintained at 34°C for 30 min, thereafter, at 22–25°C until use. For whole-cell recording, slices were placed in a submersion chamber and superfused with the same solution (22–25°C) altered by elevating CaCl2 to 1.5 and reducing MgCl2 to 1.5 mM, except where noted. All solutions were oxygenated by pre- or continuous equilibration with carbogen gas (95% O2, 5% CO2).
Electrophysiology
Whole-cell recordings were obtained from MLIs identified with gradient-contrast infrared video microscopy49 based on their location within the molecular layer and distinct morphology15. Patch pipettes used for current-clamped MLIs contained a solution of (in mM) 128 K-gluconate, 2 KCl, 9 HEPES, 4 MgCl2, 4 NaATP, and 0.5 NaGTP and 9 K4-BAPTA. For paired recordings, perforated access50 to the presynaptic MLI was achieved by including amphotericin B (300 μg/ml, previously aliquoted in DMSO). Alexa Fluor 488 hydrazide (30 μM; Molecular Probes; Eugene, OR) was also included in the pipette to fluorescently monitor the integrity of the perforated recording with light microscopy. If the patch membrane ruptured, resulting in cell fluorescence, recording was terminated. For Ca2+ imaging experiments, K4-BAPTA was replaced with 200μM Fluo-5F and 13μM Alexa Fluor 594 hydrazide (Molecular Probes, Eugene, OR). Current-clamped cells were held near −70 mV with constant current injection. Action potentials were stimulated between 0.3 to 0.1 Hz (~25–60 trials per condition). MLIs were voltage-clamped with a pipette solution containing (in mM) 135 CsCl, 10 HEPES, 4 Mg2ATP, 0.3 NaGTP and 5 EGTA. The holding potential was kept at −60 mV giving rise to inward GABAAR-mediated currents (ECl- ≈ 0 mV). All pipettes had open-tip resistances of 3–12 MΩ.
Electrophysiological potentials and currents were recorded with a Multiclamp 700B amplifier (Molecular Devices, Union City, CA). Electrode series resistance was compensated by bridge balance in current-clamped cells and was uncompensated in the voltage-clamp configuration. Analog signals were filtered at 3–10 kHz and digitized at 20–50 kHz with a 16-bit A/D converter (Instrutech Corp., Port Washington, NY). High access resistance (40–80 MΩ) in the presynaptic perforated recording also likely contributed to filtering and action potential attenuation, though, access often improved during an experiment due to continued partitioning of the antibiotic. Data were collected using custom software (J.S. Diamond) written in IgorPro (Wavemetrics, Lake Oswego, OR) and analyzed using Axograph (Axograph Scientific, Sydney, Australia). GABAAR-mediated synaptic responses were isolated with NBQX (10 μM) and D-AP5 (50 μM) (Tocris Cookson, Ellisville, MO) to block fast glutamate receptor-mediated responses. Picrotoxin (100 μM) was also included during imaging experiments to eliminate GABAAR-mediated transmission. EGTA-AM (10–20 μM, Molecular Probes, Eugene, OR), made from a stock 100 mM solution in DMSO, was applied for at least 10 minutes during which data acquisition ceased. Upon resumption, EGTA-AM was continuously applied until the end of the experiment. In amplified views shown in figures, spontaneous IPSCs that were not time-locked to the presynaptic action potential were blanked prior to averaging for illustrative purposes; events were only included within 1 ms after the action potential peak. In our analysis, however, spontaneous events were left unmodified.
Two-photon laser-scanning microscopy
Fluorescence was monitored with a lab-built 2PLSM using an Olympus (Melville, NY) upright microscope, objective (60X, 1.0 NA), and oil-immersion condenser (1.4 NA). A Ti:sapphire laser (Coherent, Santa Clara, CA) tuned to 810 nm was used for excitation. Emitted green and red fluorescence were simultaneously collected by photomultiplier modules (H8224, Hamamatsu, Hamamatsu City, Japan) in both epi- and transfluorescence pathways using a 565 nm dichroic and 525/50 and 620/60 bandpass filters (Chroma, Battleboro, VT). Images were acquired using ScanImage software(K. Svoboda). Line scans were obtained at 500 Hz. Cells were filled for a minimum of 20 minutes prior to acquisition to allow for dye equilibration (axon varicosities <150 μm from the soma). For varicosities >150 μm from the soma, longer equilibration times were used.
Imaging analysis was performed offline using ImageJ (National Institutes of Health) and Axograph. Fluorescence changes were quantified as increases in green fluorescence normalized to red fluorescence (ΔG/R). Peak amplitude measurements of subthreshold depolarization-evoked Ca2+ transients were determined by averaging a 100 ms epoch prior to action potential firing. Alternating trials in which no stimulation occurred were used to subtract background fluorescence levels; the difference reflecting the peak response. The peak of action potential-evoked Ca2+ transients were determined from exponential fits of the fluorescence decay following stimulation (measured at t = 0). Changes in the peak amplitude of action potential-evoked responses might be underestimated if the indicator were in the non-linear range. The Ca2+ transients evoked by subthreshold depolarization were usually very small. Therefore, for illustrative purposes only, we integrated these responses to accentuate the rise in Ca2+ from the baseline noise during the subthreshold stimulus (Fig. 2b, 3a). This method was not included in any quantification.
Statistical Analysis
Reported values are mean ± SEM. Excel (Microsoft) and InStat (GraphPad Software) were used for statistical analysis using paired and unpaired t-tests (two-tailed). ANOVA with post-hoc Tukey’s tests were used when making multiple comparisons. Differences were considered statistical significance with α values of P < 0.05. In figures, asterisks denote statistical significance.
Supplementary Material
Subthreshold depolarization evokes and enhances Ca2+ entry through VSCCs
(a) An action potential with (black) and without (red) a preceding subthreshold depolarization (300 ms) recorded in the soma. Shown below are the resulting Ca2+ transients recorded in an axon varicosity (3 mM extracellular Ca2+) in control and following inhibition with a combination of VSCC blockers (ω-conotoxin MVIIC 1μM, SNX 0.3 μM, nimodipine 20 μM, and mibefradil 10μM). (b) Inhibition of axonal Ca2+ transients by VSCC blockers. Mean values are presented with error bars (+SEM); asterisks denote that the reductions from control responses were significant (paired t-test, P < 0.05).
Acknowledgments
We thank Melissa Herman, Matt McGinley, Ben Nahir, and Jason Pugh for their helpful discussions and comments on the manuscript. This work was supported by National Institute of Health Grant NS066037 (CEJ).
Footnotes
Author Contribution
The authors contributed extensively to the design and implementation of the experiments, interpretation of the data, and writing of the manuscript.
References
- 1.Alle H, Geiger JRP. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. doi: 10.1126/science.1119055. [DOI] [PubMed] [Google Scholar]
- 2.Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–765. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- 3.Kole MHP, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control action potential waveform and synaptic efficacy. Neuron. 2007;55:633–647. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Bollmann JH, Sakmann B, Borst JGG. Calcium sensitivity of a glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- 5.Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 6.Zucker RS, Regehr WG. Short-term synaptic plasticity. Ann Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 7.Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule to Purkinje cell synapse. J Neurosci. 1997;17:3425–3435. doi: 10.1523/JNEUROSCI.17-10-03425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1998;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 9.Bischofberger J, Geiger JR, Jonas P. Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons. J Neurosci. 2002;22:10593–105602. doi: 10.1523/JNEUROSCI.22-24-10593.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenowitz SD, Regehr WG. Reliability and heterogeneity of calcium signaling at single presynaptic boutons of cerebellar granule cells. J Neurosci. 2007;27:7888–7898. doi: 10.1523/JNEUROSCI.1064-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Hori T, Takahashi T. Mechanisms underlying short-term modulation of transmitter release by presynaptic depolarization. J Physiol. 2009;587:2987–3000. doi: 10.1113/jphysiol.2009.168765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott R, Ruiz A, Henneberger C, Kullmann DM, Rusakov DA. Analog modulation of mossy fiber transmission is uncoupled from changes in presynaptic Ca2+ J Neurosci. 2008;28:7765–7773. doi: 10.1523/JNEUROSCI.1296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trigo FF, Bouhours B, Rostaing P, Papgeorgiou G, Corrie JET, Triller A, Ogden D, Marty A. Presynaptic miniature Gabaergic currents in developing interneurons. Neuron. 2010;66:235–247. doi: 10.1016/j.neuron.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Palay SL, Chan-Palay V. Cerebellar cortex: cytology and organization. Springer-Verlag; New York: 1974. [Google Scholar]
- 16.Mejia-Gervacio S, Collin T, Pouzat C, Tan YP, Llano I, Marty A. Axonal speeding: shaping synaptic potentials in small neurons by the axonal membrane compartment. Neuron. 2007;53:843–855. doi: 10.1016/j.neuron.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Glitsch M, Marty A. Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons. J Neurosci. 1999;19:511–519. doi: 10.1523/JNEUROSCI.19-02-00511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christie JM, Jahr CE. Dendritic NMDA receptors activate axonal calcium channels. Neuron. 2008;60:298–307. doi: 10.1016/j.neuron.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent P, Marty A. Fluctuations of inhibitory postsynaptic currents in Purkinje cells from rat cerebellar slices. J Physiol. 1996;494:183–199. doi: 10.1113/jphysiol.1996.sp021484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diana MA, Marty A. Characterization of depolarization-induced suppression of inhibition using paired interneuron-Purkinje cell recordings. J Neurosci. 2003;23:5906–5918. doi: 10.1523/JNEUROSCI.23-13-05906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llano I, Tan YP, Caputo C. Spatial heterogeneity of intracellular Ca2+ signals in axons of basket cells from the rat cerebellar slices. J Physiol. 1997;502:509–519. doi: 10.1111/j.1469-7793.1997.509bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forti L, Pouzat C, Llano I. Action potential-evoked Ca2+ signals and calcium channels in the axons of developing cerebellar interneurons. J Physiol. 2000;527:33–48. doi: 10.1111/j.1469-7793.2000.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tottene A, Moretti A, Pietrobon D. Functional diversity of P-type and R-type calcium channels in rat cerebellar neurons. J Neurosci. 1996;16:6353–6363. doi: 10.1523/JNEUROSCI.16-20-06353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metz AE, Jarsky T, Martina M, Spruston N. R-type calcium channels contribute to afterdepolarization and bursting in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:5763–5773. doi: 10.1523/JNEUROSCI.0624-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borst JGG, Sakmann B. Facilitation of presynaptic calcium currents in the rat brainstem. J Physiol. 1998;513:149–155. doi: 10.1111/j.1469-7793.1998.149by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuttle MF, Tsujimoto T, Forsythe ID, Takahashi T. Facilitation of the presynaptic calcium current at an auditory synapse in the rat brainstem. J Physiol. 1998;512:723–729. doi: 10.1111/j.1469-7793.1998.723bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Bucurenciu I, Bischofberger J, Jonas P. A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nature Neurosci. 2010;13:19–21. doi: 10.1038/nn.2461. [DOI] [PubMed] [Google Scholar]
- 30.Dittman JS, Kreitzer AC, Regehr WG. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J Neurosci. 2000;20:1374–1385. doi: 10.1523/JNEUROSCI.20-04-01374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller M, Goutman JD, Kochubey O, Schneggenburger R. Interaction between facilitation and depression at a large CNS synapse reveals mechanisms of short-term plasticity. J Neurosci. 2010;30:2007–2016. doi: 10.1523/JNEUROSCI.4378-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atluri PP, Regehr WG. Delayed release of neurotransmitter from cerebellar granule cells. J Neurosci. 1998;18:8214–8227. doi: 10.1523/JNEUROSCI.18-20-08214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu T, Trussell LO. Inhibitory transmission mediated by asynchronous transmitter release. Neuron. 2000;26:683–694. doi: 10.1016/s0896-6273(00)81204-0. [DOI] [PubMed] [Google Scholar]
- 34.Otsu Y, Shahrezaei V, Li B, Raymond LA, Delaney KR, Murphy TH. Competition between phasic and synchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses. J Neurosci. 2004;24:420–433. doi: 10.1523/JNEUROSCI.4452-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christie JM, Jahr CE. Selective expression of ligand-gated ion channels in L5 pyramidal cell axons. J Neurosci. 2009;29:11450–11441. doi: 10.1523/JNEUROSCI.2387-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Bischofberger J, Jonas P. Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J Neurocsi. 2007;27:13420–13429. doi: 10.1523/JNEUROSCI.1709-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu LG, Borst JGG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc Natl Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llano I, González J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nature Neurosci. 2000;12:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 39.Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T. Neural calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 2002;295:2276–2279. doi: 10.1126/science.1068278. [DOI] [PubMed] [Google Scholar]
- 40.Jinno S, Jeromin A, Roder J, Kosaka T. Immunocytochemical localization of neural calcium sensor-1 in the hippocampus and cerebellum of the mouse, with special reference to presynaptic terminals. Neurosci. 2002;113:449–461. doi: 10.1016/s0306-4522(02)00172-0. [DOI] [PubMed] [Google Scholar]
- 41.Jinno S, Jeromin A, Kasaka T. Expression and possible role of neuronal calcium sensor-1 in the cerebellum. Cerebellum. 2004;3:83–88. doi: 10.1080/14734220310025187. [DOI] [PubMed] [Google Scholar]
- 42.Geiger JRP, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 43.Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- 44.Felmy F, Neher E, Schneggenburger R. Probing the intracellular calcium sensitivity of transmitter release during synaptic facilitation. Neuron. 2003;37:801–811. doi: 10.1016/s0896-6273(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 45.Müller M, Felmy F, Schneggenburger R. A limited contribution of Ca2+ current facilitation to paired-pulse facilitation of transmitter release at the rat calyx of Held. J Physiol. 2008;586:5503–5520. doi: 10.1113/jphysiol.2008.155838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang Y, Schlumpberger T, Kim T, Lueker M, Zucker RS. Effects of mobile buffers on facilitation: experimental and computational studies. Biophys J. 2000;78:2735–2751. doi: 10.1016/s0006-3495(00)76819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heidelberger R, Heinemann C, Neher E, Matthews G. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 48.Wu L-G, Borst JGG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–832. doi: 10.1016/s0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- 49.Dodt HU, Eder M, Schierloh A, Zieglgansberger W. Infrared-guided laser stimulation of neurons in brain slices. Sci STKE. 2002:PL2. doi: 10.1126/stke.2002.120.pl2. [DOI] [PubMed] [Google Scholar]
- 50.Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subthreshold depolarization evokes and enhances Ca2+ entry through VSCCs
(a) An action potential with (black) and without (red) a preceding subthreshold depolarization (300 ms) recorded in the soma. Shown below are the resulting Ca2+ transients recorded in an axon varicosity (3 mM extracellular Ca2+) in control and following inhibition with a combination of VSCC blockers (ω-conotoxin MVIIC 1μM, SNX 0.3 μM, nimodipine 20 μM, and mibefradil 10μM). (b) Inhibition of axonal Ca2+ transients by VSCC blockers. Mean values are presented with error bars (+SEM); asterisks denote that the reductions from control responses were significant (paired t-test, P < 0.05).