Abstract
OBJECTIVE
The purpose of this study was to reduce the cumulative radiation exposure from CT of patients with aneurysmal subarachnoid hemorrhage.
MATERIALS AND METHODS
Baseline data on 30 patients with aneurysmal subarachnoid hemorrhage were collected retrospectively for all CT examinations of the head performed throughout the hospital course. Radiation exposure estimates were obtained by recording dose–length products for each examination. As a departmental practice quality improvement project, an imaging protocol was implemented that included utilization guidelines to reduce radiation exposure in CTA and CT perfusion examinations performed to detect vasospasm in patients with aneurysmal subarachnoid hemorrhage. Ten months after implementation of this protocol, data on 30 additional patients were analyzed. Means, medians, and SD estimates were compared for cumulative radiation exposure and absolute numbers of each examination.
RESULTS
Sixty patients were included in the study: 30 patients at baseline and 30 patients after implementation of the quality improvement plan. These patients underwent 435 CT examinations: 248 examinations at baseline and 187 examinations with the new protocol. With the new algorithm, the mean number of CT examinations per patient was 5.8 compared with 7.8 at baseline, representing a decrease of 25.6%. The number of CT perfusion examinations per patient decreased 32.1%. Overall, there was a 12.1% decrease in cumulative radiation exposure (p > 0.05).
CONCLUSION
With the structured imaging algorithm, the cumulative radiation exposure and number of CT examinations of the head decreased among patients with aneurysmal subarachnoid hemorrhage because utilization guidelines defined the appropriate imaging time points for detection of vasospasm. Application of these methods to other patient populations with high use of CT may reduce cumulative radiation exposure while the clinical benefits of imaging are maintained.
Keywords: aneurysmal subarachnoid hemorrhage, CT, practice quality improvement, radiation safety, structured imaging algorithm
Since the introduction of CT in the 1970s, use of the technique has increased dramatically. From 1980 to 1990, the number of CT examinations in the United States increased from 3 million to 13 million, more than 300%. Data reported in 2002 revealed that CT accounted for approximately 15% of the imaging volume in hospitals in the United States [1]. With this rapid increase in the use of CT as an imaging technique, there has been a concurrent increase in patient exposure to ionizing radiation. As much as 75% of radiation exposure in medical imaging is attributed to CT [1]. In the United States, CT is considered responsible for 60% of the radiation exposure due to human activity [2].
Epidemiologic data suggest that increases in radiation exposure are associated with increased health risk to both the population exposed and its offspring [3–5]. There is growing public concern regarding carcinogenic effects and congenital defects occurring in the next generation. In response to these potential biologic effects, the radiologic community has embraced the as low as reasonably achievable (ALARA) principle of diagnostic imaging, whereby patients are exposed to the least possible ionizing radiation while image quality and patient care are maintained.
The development of imaging techniques such as CT angiography (CTA) and CT perfusion studies has improved the diagnostic utility of CT in the evaluation of cerebrovascular disease. These procedures, however, increase radiation exposure of patients because of the technical parameters, thin slice acquisition, and extended field of view, from the aortic arch to the skull vertex. Another factor contributing to increasing radiation exposure is the lack of guidelines for use of these imaging techniques in specific populations. Patients may be undergoing repeated CTA and CT perfusion examinations for uncertain indications and at suboptimal times for accurate diagnosis. In the care of patients with aneurysmal subarachnoid hemorrhage (SAH), guidelines for the use of CTA and CT perfusion imaging have not been fully developed. The implementation of CTA and CT perfusion techniques for the diagnosis of vasospasm has added exponentially to the radiation exposure levels in this patient population.
As a practice quality improvement (PQI) project to reduce radiation exposure in medical imaging, we developed an algorithm for the use of CTA and CT perfusion imaging of patients with aneurysmal SAH. This imaging algorithm is a guide to physicians regarding the most appropriate time points at which to detect vasospasm with CTA and CT perfusion imaging. Our hypothesis was that adherence to structured guidelines for the use of CT techniques reduces the overall amount of radiation exposure of individual patients and of the population. The purpose of this study was to evaluate the effect of our imaging algorithm on reducing radiation exposure of patients with aneurysmal SAH.
Materials and Methods
We performed a retrospective study that included patients with aneurysmal SAH consecutively admitted to our institution from October 2007 to June 2008 and composing the baseline group in our PQI project. Institutional review board approval was obtained. Patients were identified as having aneurysmal SAH according to the admitting diagnosis obtained from chart review. Data collection was focused on obtaining the number and type of CT examinations of the head performed on each patient during the hospital course, such as unenhanced CT, CTA, and CT perfusion imaging. The mean number of CT examinations performed on each patient also was calculated for the baseline group and for each examination type. The radiation exposure for each CT examination was recorded in the form of the dose–length product (DLP), which is an indication of the energy imparted to organs and can be used to assess the overall radiation burden associated with a CT examination [6]. DLP has been used as a measure of assessing radiation exposure in specific patient populations in several studies [7–9]. The mean DLP was calculated for the baseline group and for each examination type. The SD was calculated as a measure of variability.
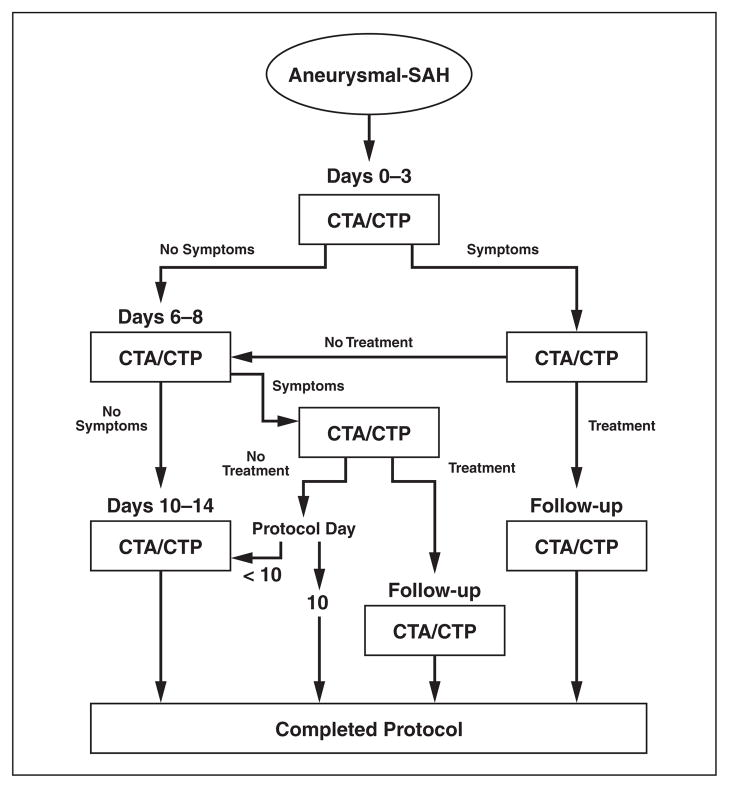
As a departmental PQI project, we developed an imaging algorithm (Fig. 1) to reduce radiation exposure during CTA and CT perfusion examinations performed for the detection of vasospasm in patients with aneurysmal SAH. This PQI project was implemented after the CT parameters for unenhanced head CT, CTA, and CT perfusion imaging were optimized according to the ALARA principle. In this imaging algorithm, patients without symptoms undergo a maximum of three CTA or CT perfusion examinations at defined imaging time points during the hospitalization: 0–3, 6–8, and 10–14 days after aneurysmal SAH. Patients with symptoms undergo an additional CTA or CT perfusion examination on the day of symptom onset for improved diagnostic accuracy for vasospasm. Patients treated for vasospasm may undergo a follow-up CTA or CT perfusion examination 24–48 hours after treatment for assessment of adequate treatment response. To limit the radiation exposure of each patient, this algorithm defines termination points for imaging in which no further CTA or CT perfusion examinations are suggested.
Fig. 1.
Practice quality improvement project imaging algorithm for performance of CT angiographic (CTA) or CT perfusion (CTP) examinations for detection of vasospasm in patients with aneurysmal subarachnoid hemorrhage (SAH).
Ten months after implementation of the imaging algorithm, we retrospectively reviewed the cases of patients with aneurysmal SAH admitted to our institution from September 2008 to April 2009, composing the postintervention group in the PQI project. Data collection was identical to that for the baseline group. The mean number of CT examinations performed on each patient and the mean DLP were calculated for the post-PQI group and for each examination type. The SD was calculated as a measure of variability. Percentage differences in the baseline and post-PQI groups were measured, and the two-tailed Student’s t test was used to determine statistical significance, which was considered p < 0.05.
Results
A total of 60 patients were included in this retrospective study: 30 in the baseline group (before implementation of the imaging algorithm) and 30 patients in the post-PQI group. The demographic data on the two groups are presented in Table 1. Length of stay was collected for each patient to represent the opportunity for CT in each group by comparing the number of days patients were hospitalized for aneurysmal SAH. In-hospital mortality rates were included for assessment of the severity of disease in the preintervention and postintervention groups.
TABLE 1.
Demographic Data
| Characteristic | All Patients (n = 60) | Baseline (n = 30) | Post-PQI (n = 30) |
|---|---|---|---|
| Age (y) | |||
| Median | 56 | 53 | 57.5 |
| Range | 24–92 | 39–92 | 24–88 |
| Sex | |||
| Male | 14 (23) | 8 (27) | 6 (20) |
| Female | 46 (77) | 22 (73) | 24 (80) |
| Length of stay (d) | |||
| Median | 21 | 24 | 20 |
| Range | 2–236 | 11–149 | 2–236 |
| In-hospital mortality (%) | 3 | 1 | 3 |
Note—Values in parentheses are percentages. PQI = practice quality improvement.
A total of 435 CT examinations were performed on all patients in the study. In the baseline group, 248 CT examinations were performed with a mean of 7.8 examinations for each patient (Tables 2 and 3). The post-PQI group (after implementation of the imaging algorithm) underwent 187 CT examinations with a mean of 5.8 examinations per patient, representing an overall decrease of 25.6% (p > 0.05) in the number of CT examinations performed for each patient in the post-PQI group (Table 3). Specifically, the mean number of CT perfusion examinations performed on the post-PQI group decreased 32.1% (Table 3). In further analysis of the decreased number of CT examinations, number of hospital days was used as the denominator in an attempt to correct for potential bias in the postintervention group that had a shorter length of stay compared with the baseline group. An 11.6% decrease in CT examinations performed per hospital day in the postintervention group was observed (Table 3).
TABLE 2.
Total Number of CT Examinations Performed
| Examination | Baseline | Post-PQI | Percentage Difference |
|---|---|---|---|
| Unenhanced CT | 146 | 93 | −36.3 |
| CT angiography | 41 | 58 | 41.5 |
| CT perfusion imaging | 61 | 36 | −41.0 |
|
| |||
| All CT examinations | 248 | 187 | −22.4 |
Note—PQI = practice quality improvement.
TABLE 3.
Mean Number of CT Examinations Performed per Patient
| Examination | Baseline | Post-PQI | Percentage Difference |
|---|---|---|---|
| CT examinations per hospital day | 0.30 (0.12) | 0.26 (0.13) | −11.6 |
| Unenhanced CT | 4.7 (3.33) | 3.1 (2.45) | −34.0 |
| CT angiography | 1.1 (0.80) | 1.9 (0.68) | 75.8 |
| CT perfusion imaging | 1.8 (0.77) | 1.2 (0.82) | −32.1 |
| All CT examinations | 7.8 (3.90) | 5.8 (2.95) | −25.6 |
Note—Values in parentheses are SD. PQI = practice quality improvement.
Overall, there was a 12.1% decrease (p > 0.05) in the cumulative DLP for each patient in the post-PQI group (Table 4). During the postintervention phase of the PQI project, two changes in clinical practice at our institution affected our results. First, CTA was implemented as part of routine imaging in monitoring patients for development of vasospasm; the result was a 41.5% overall increase in the number of CTA examinations performed on the post-PQI group (Table 2). Second, the technical parameters of the CT perfusion examinations were revised at a departmental level to extend the acquisition time from 45 to 60 seconds for accurate data processing in the evaluation of patients with cardiac abnormalities. As a result of increasing the acquisition time, the estimated DLP for each CT perfusion examination also increased. Table 4 shows the corrected DLP in the post-PQI group, which was made by substituting the DLP from the revised CT perfusion examinations with the standard DLP from the baseline CT perfusion examinations. The DLP for a CT perfusion examination was a constant value in each group because our institution uses a standard CT perfusion scanning protocol for all aneurysmal SAH patients, including constant tube current and kilovoltage settings and a constant scanning distance of 20 mm. In an attempt to accurately compare the preintervention and postintervention groups, this correction would allow evaluation of the effect of the new imaging guidelines on reducing the number of CTA and CT perfusion examinations performed separately from the change in technical parameters that had been made for the postintervention group.
TABLE 4.
Mean Dose Length Product Estimated per Patient
| Examination | Baseline | Post-PQI | Corrected Post-PQI | Percentage Difference |
|---|---|---|---|---|
| Unenhanced CT | 1,183.18 (239.06) | 1,018.74 (226.63) | −13.9 | |
| CT angiography | 3,128.15 (1,356.8) | 3,167.04 (1,333.65) | 1.2 | |
| CT perfusion imaging | 1,991.80 (0.00) | 3,205.61 (823.59) | 1,991.80 (0.00) | 0 |
| All CT examinations | 13,385.76 (5,288.75) | 12,615.75 (5,450.76) | 11,767.00 (4,684.05) | −12.1 |
Note—Values in parentheses are SD. PQI = practice quality improvement.
Discussion
The clinical significance of increased radiation exposure has become a concern for both the population and its progeny. Epidemiologic data [10] show that 10–50 mSv from a single exposure or 50–100 mSv from protracted exposure are associated with a significant increase in cancer incidence. The risk of development of solid cancer is associated with a linear increase in radiation dose, known as the linear no-threshold theory, which states that there is no threshold below which radiation exposure is completely safe. According to the theory, increased health risks due to radiation begin with exposure to even the smallest dose of radiation and increase linearly from there [3–5]. This theory supports the belief that even low doses of radiation exposure, as in medical imaging at 2–10 mSv, contributes to these potential carcinogenic effects. In a 15-country epidemiologic study, Cardis et al. [11] found a significant association between radiation dose and all-cause mortality. Mortality from cancer represented the largest component in that study.
A review of the literature reveals that there is not a consensus belief in the linear no-threshold theory. Cohen [12] stated that the assumption of cancer risk associated with low-dose exposure is grossly overestimated and may in fact even be zero. In spite of the opposing opinions, most experts in the radiology community practice according to the ALARA principle. Some strategies have been focused on altering technical scanning parameters to decrease the radiation dose for each CT examination performed. In a 2008 study [13], DLP was reduced significantly by either z-axis modulation or x-y-z-axis modulation. Automatic tube current modulation combined with low tube voltage settings has been found [14] to significantly reduce radiation exposure under simulated conditions. Our strategy in this PQI project was to provide utilization guidelines to decrease radiation exposure of individual patients and the population by limiting the number of CT examinations performed.
The population in our study has poor clinical outcomes. The mortality is as high as 45%, and the morbidity among survivors is substantial [15–20]. Imaging is critical in the diagnosis of aneurysmal SAH, and CT is considered the cornerstone of immediate and accurate diagnosis. In the first 12 hours after hemorrhage, the sensitivity of CT is 98–100%, declining to 57–85% 6 days after hemorrhage [21–25]. CTA is an important diagnostic tool; the reported sensitivity for detection of aneurysms is 96–100% depending on the size of the aneurysm [26, 27].
After the initial diagnosis of aneurysmal SAH, delayed cerebral vasospasm is the most serious cause of morbidity and mortality. Patients with delayed cerebral vasospasm are at high risk of poor clinical outcome, including long-term disability and death [28–30]. Identification of patients with vasospasm is important for initiation of prompt treatment. The most widely used systemic therapy is hypertensive–hypervolemic–hemodilutional therapy. Intraarterial therapy with selective injection of vasodilatory medications or angioplasty is used conservatively in the care of patients with angiographic vasospasm. Even though current clinical management of cerebral vasospasm is largely focused on initiating early and aggressive treatment, not all patients with aneurysmal SAH are treated for vasospasm because of the serious systemic and neurologic risks associated with therapy, including cerebral edema, intraparenchymal hemorrhage, and pulmonary edema [31].
Some institutions are using CTA and CT perfusion imaging to improve the accuracy of detection of vasospasm in patients with aneurysmal SAH. The sensitivity and specificity of CTA for vasospasm depend on the degree of vessel narrowing, the improved diagnostic accuracy being 97.5% in severe vasospasm [32]. Similarly, the diagnostic accuracy of CT perfusion imaging in the detection of vasospasm varies according to the location and severity of disease [32–34]; 90% sensitivity, 100% specificity, and 92.3% accuracy have been achieved in the detection of severe vasospasm [32]. Several studies have shown that CT perfusion imaging findings of reduced cerebral blood flow and prolonged mean transit time in patients with symptoms are suggestive of a vasospasm diagnosis. The combination of using CTA and CT perfusion imaging data with a mean transit time threshold of 6.4 seconds has a negative predictive value of 93.6% and positive predictive value of 89.9% in the diagnosis of vasospasm [35]. Although CT plays an important role in both the diagnosis and the management of aneurysmal SAH, recommendations for guiding physicians in performing these examinations are lacking.
Our hypothesis was that structured guidelines for utilization of CT techniques will reduce the overall amount of radiation to which individual patients and the population are exposed. In our PQI project, there was an overall reduction in the estimated DLP and in the number of CT examinations performed after implementation of the imaging algorithm. The mean DLP and mean number of CT examinations performed for each patient decreased 12.1% and 25.6%. However, the number of CTA examinations performed increased in the post-PQI group because of a change in clinical practice at our institution consisting of implementation of CTA for routine imaging for monitoring of vasospasm. Nonetheless, these initial results of our study are promising and suggest that such imaging guidelines can assist in reducing radiation exposure.
Developing strategies for reducing radiation exposure must comprehensively consider both the health risks in a specific population and the benefits of CT. In our population, CT is critical in the initial diagnosis of aneurysmal SAH with unenhanced CT and CTA. During hospitalization, unenhanced CT examinations are used repeatedly for monitoring of recurrent hemorrhage, hydrocephalus, and infarction. CTA and CT perfusion examinations are being used increasingly to detect vasospasm earlier to prompt immediate treatment. The incidence of vasospasm varies from 30% to 70% and is strongly associated with increased mortality and severe disability [36]. Benefits to this population are focused on improved clinical outcome through prevention of delayed cerebral ischemia and death. However, the risks of CT in this population include cataract formation, alopecia, and skin damage in addition to the carcinogenic risk [9]. When the risks are assessed alongside the benefits, it is clear that the continued use of CT in the population of patients with aneurysmal SAH is a vital component of patient care. Therefore, providing guidelines to physicians on appropriate scanning techniques and the time points most valuable for imaging may limit radiation exposure of patients. Furthermore, our study showed promising results regarding reduction of radiation exposure, even in a population in which the benefits of CT outweigh the health risks of the technique.
There were several limitations to our study. First, it was retrospective and the sample was small. The small sample size likely contributed to the lack of statistical significance of the differences observed between the baseline and post-PQI groups. However, statistical significance of the data is not necessarily the primary goal in PQI projects as long as some improvement has been achieved. Second, the protocol revision of the CT perfusion technique implemented during the PQI project increased the estimated DLP for each CT perfusion imaging examination. A correction method was applied to the results (Table 4) in an attempt to effectively compare the baseline and post-PQI groups by use of the standard DLP from the baseline group. The rationale for performing this additional analysis is that the purpose of the imaging algorithm is to reduce the number of CTA and CT perfusion imaging examinations of each patient, not to address changes in the technical parameters of CT examinations. Last, it is not evident from this study that the reduction in radiation exposure would definitely decrease the health risks of medical imaging of this population. However, in accordance with the ALARA principle, any reduction in radiation exposure is considered an improvement in patient safety.
Even though our PQI project was focused on patients with aneurysmal SAH, the initial results are promising, showing that guidelines for utilization of CT can lead to reduced radiation exposure of individual patients and the population. Our overall goal is to apply to other patient populations this concept of imaging algorithms as utilization guidelines for CT. Further work is needed to build a framework for evaluating and implementing best clinical practice that supports both quality patient care and radiation safety.
Acknowledgments
This publication was made possible by grant 5K23NS058387-02 from the National Institute of Neurological Disorders and Stroke (NINDS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NINDS or NIH.
References
- 1.Wiest PW, Locken JA, Heintz PH, Mettler FA., Jr CT scanning: a major source of radiation exposure. Semin Ultrasound CT MR. 2002;23:402–410. doi: 10.1016/s0887-2171(02)90011-9. [DOI] [PubMed] [Google Scholar]
- 2.Linton OW, Mettler FA, Jr National Council on Radiation Protection and Measurements. National conference on dose reduction in CT, with an emphasis on pediatric patients. AJR. 2003;181:321–329. doi: 10.2214/ajr.181.2.1810321. [DOI] [PubMed] [Google Scholar]
- 3.UNSCEAR. Sources and effects of ionizing radiation. New York, NY: United Nations; 2000. [Google Scholar]
- 4.National Council on Radiation Protection and Measurements. Evaluation of the linear nonthreshold dose-response model for ionizing radiation. Bethesda, MD: National Council on Radiation Protection and Measurements; 2001. Report 136. [Google Scholar]
- 5.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–378. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 6.Smith AB, Dillon WP, Gould R, Wintermark M. Radiation dose-reduction strategies for neuroradiology CT protocols. AJNR. 2007;28:1628–1632. doi: 10.3174/ajnr.A0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 8.Huda W. Radiation doses and risks in chest computed tomography examinations. Proc Am Thorac Soc. 2007;4:316–320. doi: 10.1513/pats.200611-172HT. [DOI] [PubMed] [Google Scholar]
- 9.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. National survey of doses from CT in the UK: 2003. Br J Radiol. 2006;79:968–980. doi: 10.1259/bjr/93277434. [DOI] [PubMed] [Google Scholar]
- 10.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardis E, Vrijheid M, Blettner M, et al. The 15 country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation related cancer risks. Radiat Res. 2007;167:396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- 12.Cohen BL. Cancer risk from low-level radiation. AJR. 2002;179:1137–1143. doi: 10.2214/ajr.179.5.1791137. [DOI] [PubMed] [Google Scholar]
- 13.Smith AB, Dillon WP, Lau BC, et al. Radiation dose reduction strategy for CT protocols: successful implementation in neuroradiology section. Radiology. 2008;247:499–506. doi: 10.1148/radiol.2472071054. [DOI] [PubMed] [Google Scholar]
- 14.Herzog C, Mulvihill DM, Nguyen SA, et al. Pediatric cardiovascular CT angiography: radiation dose reduction using automatic anatomic tube current modulation. AJR. 2008;190:1232–1240. doi: 10.2214/AJR.07.3124. [DOI] [PubMed] [Google Scholar]
- 15.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124:249–278. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- 16.Hijdra A, van Gijn J, Nagelkerke NJ, Vermeulen M, van Crevel H. Prediction of delayed cerebral ischemia, rebleeding, and outcome after aneurysmal subarachnoid hemorrhage. Stroke. 1988;19:1250–1256. doi: 10.1161/01.str.19.10.1250. [DOI] [PubMed] [Google Scholar]
- 17.Hijdra A, Braakman R, van Gijn J, Vermeulen M, van Crevel H. Aneurysmal subarachnoid hemorrhage: complications and outcome in a hospital population. Stroke. 1987;18:1061–1067. doi: 10.1161/01.str.18.6.1061. [DOI] [PubMed] [Google Scholar]
- 18.Hop JW, Rinkel GJ, Algra A, van Gijn J. Changes in functional outcome and quality of life in patients and caregivers after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2001;95:957–963. doi: 10.3171/jns.2001.95.6.0957. [DOI] [PubMed] [Google Scholar]
- 19.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–1347. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 20.Sundt TM, Jr, Kobayashi S, Fode NC, Whisnant JP. Results and complications of surgical management of 809 intracranial aneurysms in 722 cases: related and unrelated to grade of patient, type of aneurysm, and timing of surgery. J Neurosurg. 1982;56:753–765. doi: 10.3171/jns.1982.56.6.0753. [DOI] [PubMed] [Google Scholar]
- 21.Morgenstern LB, Luna-Gonzales H, Huber JC, Jr, et al. Worst headache and subarachnoid hemorrhage: prospective, modern computed tomography and spinal fluid analysis. Ann Emerg Med. 1998;32:297–304. [PubMed] [Google Scholar]
- 22.van der Wee N, Rinkel GJ, Hasan D, van Gijn J. Detection of subarachnoid haemorrhage on early CT: is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatry. 1995;58:357–359. doi: 10.1136/jnnp.58.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidman R, Connolly E, Lemke T. Subarachnoid hemorrhage diagnosis: lumbar puncture is still needed when the computed tomography scan is normal. Acad Emerg Med. 1996;3:827–831. doi: 10.1111/j.1553-2712.1996.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 24.Sames TA, Storrow AB, Finkelstein JA, Magoon MR. Sensitivity of new-generation computed tomography in subarachnoid hemorrhage. Acad Emerg Med. 1996;3:16–20. doi: 10.1111/j.1553-2712.1996.tb03296.x. [DOI] [PubMed] [Google Scholar]
- 25.Tomasello F, d’Avella D, de Divitiis O. Does lamina terminalis fenestration reduce the incidence of chronic hydrocephalus after subarachnoid hemorrhage? Neurosurgery. 1999;45:827–831. doi: 10.1097/00006123-199910000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Wang J, Xing W, et al. Accuracy of 16-row multislice computerized tomography angiography for assessment of intracranial aneurysms. Surg Neurol. 2009;71:32–42. doi: 10.1016/j.surneu.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Tipper G, U-King-Im JM, Price SJ, et al. Detection and evaluation of intracranial aneurysms with 16-row multislice CT angiography. Clin Radiol. 2005;60:565–572. doi: 10.1016/j.crad.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Dorsch NW. Therapeutic approaches to vasospasm in subarachnoid hemorrhage. Curr Opin Crit Care. 2002;8:128–133. doi: 10.1097/00075198-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Kassell NF, Sasaki T, Colohan AR, et al. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- 30.Biller J, Godersky JC, Adams HP. Management of aneurysmal subarachnoid hemorrhage. Stroke. 1988;19:1300–1305. doi: 10.1161/01.str.19.10.1300. [DOI] [PubMed] [Google Scholar]
- 31.Sen J, Belli A, Albon H, et al. Triple-H therapy in the management of aneurysmal subarachnoid hemorrhage. Lancet Neurol. 2003;2:614–621. doi: 10.1016/s1474-4422(03)00531-3. [DOI] [PubMed] [Google Scholar]
- 32.Binaghi S, Colleoni ML, Maeder P, et al. CT angiography and perfusion CT in cerebral vasospasm after subarachnoid hemorrhage. AJNR. 2007;28:750–758. [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhary SR, Ko N, Dillon WP, et al. Prospective evaluation of multidetector-row CT angiography for the diagnosis of vasospasm following subarachnoid hemorrhage: a comparison with digital subtraction angiography. Cerebrovasc Dis. 2008;25:144–150. doi: 10.1159/000112325. [DOI] [PubMed] [Google Scholar]
- 34.Wintermark M, Ko NU, Smith WS, Liu S, Higashida RT, Dillon WP. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR. 2006;27:26–34. [PMC free article] [PubMed] [Google Scholar]
- 35.Nabavi DG, LeBlanc LM, Baxter B, et al. Dynamic CT perfusion imaging in subarachnoid hemorrhage–related vasospasm. Neuroradiology. 2001;43:7–16. [Google Scholar]
- 36.Schmidt JM, Wartenberg KE, Fernandez A, et al. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. J Neurosurg. 2008;109:1052–1059. doi: 10.3171/JNS.2008.109.12.1052. [DOI] [PubMed] [Google Scholar]