Abstract
Objectives
The purpose of this study was to assess whether (1) very small increases in troponin T, measured by a new highly sensitive assay for cardiac troponin T (hs-cTnT), may reflect ischemia without necrosis and (2) serial changes can discriminate ischemia from other causes of cTnT release.
Background
A new hs-cTnT assay offers greater sensitivity than current assays.
Methods
Nineteen patients referred for diagnostic catheterization underwent cannulation of the coronary sinus (CS). Serial CS and peripheral plasma samples were obtained at multiple timepoints during and after incremental rapid atrial pacing. cTnT was quantified using a standard and pre-commercial highly sensitive assay. Ischemia was determined by the presence of significant coronary atherosclerosis and myocardial lactate release with pacing.
Results
cTnT concentrations in CS blood increased from a median of 6.8 to 15.6pg/mL 60-minutes after termination of rapid atrial pacing (p<0.0001), changes that were mirrored at 180-minutes in peripheral blood (5.1 to 11.8pg/mL p<0.0001)]. Although peripheral cTnT concentrations tended to be higher at 180-minutes following pacing for patients with atherosclerosis and lactate elution (n=7) when compared to those without either marker (n=5) (25.0 vs. 10.2pg/mL, p=0.10), relative (1.7- vs 5.2-fold) and absolute (6.8 vs 8.8pg/mL, p=0.50) changes were similar between groups.
Conclusions
Brief periods of ischemia, without frank infarction, cause low-level cTnT release, and small increases are common after periods of increased myocardial work, even among patients without objective evidence of myocardial ischemia or obstructive atherosclerosis. Additional research is needed before hs-cTnT assays are widely adopted in the management of subjects with chest pain syndromes.
Keywords: ischemia, troponin T, cardiac biomarkers, coronary artery disease, rapid pacing
Introduction
Cardiac troponin I and T are currently the preferred biomarkers for the detection of myocardial infarction(1,2), having supplanted older biomarkers, such as creatine kinase (CK) and its MB fraction, due to superior sensitivity and specificity. Furthermore, troponin concentrations provide powerful prognostic information across a spectrum of disease states, even at the lower limit of detection of current assays(3–6).
Given the important information that is provided by the detection of low levels of troponin, interest has focused on using higher sensitivity assays to detect even lower concentrations of this biomarker. Recently, a highly sensitive assay for troponin T (hs-cTnT) has been shown to have favorable test characteristics compared with traditional less sensitive assays(7,8). Moreover, measurable troponin concentrations with these highly sensitive assays, below the detection range of standard assays, independently associate with an adverse prognosis in patients with acute coronary syndromes(9), chronic coronary artery disease(10), and chronic heart failure(11).
Although troponin elevation above thresholds of detection using current generation assays has become synonymous with “myonecrosis,” it is less clear whether low levels of troponin detected with more sensitive assays may result from ischemia without necrosis. Moreover, although it has been recommended that dynamic changes in troponin concentrations over short periods of time may be useful for distinguishing ischemia from other causes of troponin elevation(12), few data are available to support this recommendation. To address these questions, we sought (1) to quantify myocardial release of cTnT using a new highly sensitive assay during induced ischemia in a controlled human model and (2) to correlate troponin release with objective indicators of ischemia.
Methods
Patient population
Consecutive patients with stable angina referred for coronary angiography at Parkland Memorial Hospital from November, 2002, through April, 2004, were approached for enrollment in this study. Those with valvular disease, atrial fibrillation, previous CABG, a history of heart failure, acute coronary syndrome (ACS), or baseline left bundle branch block were excluded. The protocol was approved by the University of Texas Southwestern Institutional Review Board, and all subjects provided written informed consent
Study protocol
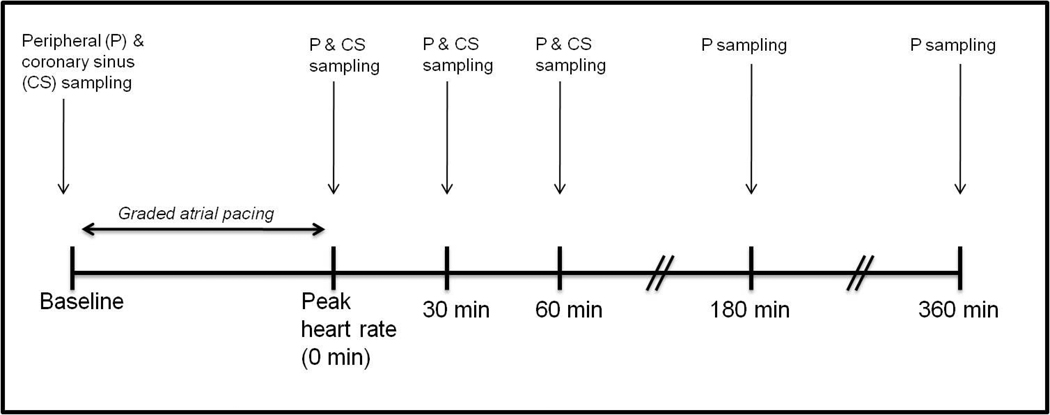
All patients had β-blockers and nitrates held for ≥ 24hrs before catheterization. A baseline ECG was recorded in all patients. A 6 Fr arterial cannula was placed in the brachial or femoral artery. A 7 Fr Zucker catheter was advance to the coronary sinus from a brachial vein, and its position was confirmed by fluoroscopy and oximetry. A baseline set of peripheral arterial and coronary sinus blood samples were obtained. Following the acquisition of baseline blood samples, the atrium was paced at 20 beats per minute (bpm) above the resting heart rate, and the pacing rate was increased by 20 bpm every 3 minutes until the patient developed (a) angina-like chest pain or (b) a target heart rate of 160 bpm. Atropine (0.5–1 mg) was administered as needed if atrioventricular block developed. A 12-lead ECG and a matched set of arterial and coronary sinus blood samples were obtained at this peak heart rate. Additional paired coronary sinus and arterial blood samples were obtained at 30 and 60 minutes following cessation of pacing, after which the CS catheter was removed. Coronary angiography was then performed to define coronary anatomy. Additional samples of peripheral blood were obtained at 180 and 360 minutes following cessation of pacing. The study schema is depicted in Figure 1.
Figure 1. Study schema.
Biomarker assessment
Blood samples were collected into ethylenediaminetetraacetic acid (EDTA) and serum separator tubes and placed in an iced saline bath until processing occurred, which was within one hour of collection. Samples were centrifuged, after which the plasma and serum components were removed by pipette, aliquoted into plastic storage tubes, and stored at −70°C until assays were performed. Plasma samples underwent a single thaw cycle for all measurements. Concentrations of troponin T were determined using both a conventional fourth-generation assay (Elecsys cTnT, Roche Diagnostics, Indianapolis Indiana) and a precommercial highly sensitive assay (Elecsys-2010 Troponin T hs STAT, also from Roche Diagnostics). The lower limit of detection of the traditional assay is 0.01 ng/mL, whereas that of the hs-cTnT assay is 0.003 ng/mL (3pg/mL). Based on the manufacturer’s data from >1300 normal subjects, the 99th percentile for the upper limit of normal was reported to be 14 pg/mL for the hs-cTnT assay.
Assessment of myocardial ischemia
Lactate concentrations were quantified using mass spectrometric methods, as previously described [13]. Under normal aerobic conditions, the heart is a net consumer of lactate. During ischemia, areas of ischemic myocardium elute lactate from the anaerobic metabolism of pyruvate. As previously reported(14–16), net myocardial lactate elution was defined as
[arterial lactate concentration] - [coronary sinus lactate concentration] < 0
The presence of coronary artery disease (CAD) was defined as ≥ 75% luminal diameter narrowing of at least one major epicardial coronary artery.
Patients were categorized into one of three groups reflecting the strength of evidence to support pacing-induced ischemia: (1) no significant CAD and no net lactate elution after pacing [(CAD−/lactate−), n=5], (2) significant CAD but no net lactate elution after pacing [(CAD+/lactate−), n=7] and (2) significant CAD with pacing-induced lactate release [(CAD+/lactate+), n=7].
Statistical analysis
Clinical and procedural characteristics are presented as mean ± SD or percentages, as appropriate. Since cTnT values were non-normally distributed following pacing, we report values as median (25th, 75th percentile) and use non-parametric statistical methods for comparison. Between-group comparisons were performed using the Wilcoxon signed-rank test. Within-group changes in cTnT values over time were assessed by Friedman’s Chi-Square test. For cTnT values < 3.0 pg/mL, we assumed a value of 1.5 pg/mL for representation purposes. Because of missing data from 8 patients at the final (360-minute) timepoint, statistical analysis for peripheral biomarker release was instead performed on the 180-minute timepoint. No adjustments for multiple comparisons were made. Statistical significance was defined as a value of p < 0.05. All analyses were performed using SAS 9.2 with Enterprise Guide 4.1.
Results
Clinical characteristics
The baseline clinical characteristics of the 19 subjects are presented in Table 1. The mean peak heart rate with pacing was 146±16 bpm, with an average rate•pressure product of 21,458 ± 4,216 mmHg•beat/min. With pacing, 37% of subjects had net myocardial lactate elution. The median transcoronary lactate extraction was 0.36 (−0.05, 0.83) mg/dL at baseline and 0.25 (−0.25,0.99) mg/dL at peak heart rate. ST segment depression developed in 47% of patients at peak pacing.
Table 1.
Baseline demographic and clinical characteristics of the study population.
| Clinical characteristic | Entire cohort (n=19) |
Ischemic subgroup | ||
|---|---|---|---|---|
| CAD− / Lactate elution− (n=5) |
CAD+/ Lactate elution− (n=7) |
CAD+/ Lactate elution+ (n=7) |
||
| Age (years) | 52±6 | 49±2 | 52±8 | 54±6 |
| Gender (no., % female) | 7 (37) | 4 (80) | 0 (0) | 3 (43) |
| Race/ethnicity | ||||
| White | 6 (32) | 2 (40) | 3 (43) | 1 (14) |
| Black | 8 (42) | 3 (60) | 2 (29) | 3 (43) |
| Hispanic | 5 (26) | 0 (0) | 2 (29) | 3 (43) |
| Hypertension (%) | 14 (74) | 5 (100) | 5 (71) | 4 (57) |
| Hyperlipidemia (%) | 13 (68) | 2 (40) | 6 (86) | 5 (71) |
| Diabetes mellitus (%) | 8 (42) | 0 (0) | 4 (57) | 4 (57) |
| Tobacco use (%) | 11 (58) | 3 (60) | 4 (57) | 4 (57) |
| Canadian Cardiovascular Society Angina Score | ||||
| I | 2 (11) | 1 (20) | 0 (0) | 1 (14) |
| II | 9 (47) | 1 (20) | 6 (86) | 2 (29) |
| III | 8 (42) | 3 (60) | 1 (14) | 4 (57) |
| LVEF [%, median (25th,75th)] | 53 (42,59) | 55 (45,55) | 50 (45,63) | 51 (38,63) |
| Creatinine [mg/dl, median (25th,75th)] | 1.0 (0.8,1.3) | 0.9 (0.8,1.2) | 0.9 (0.8,1.1) | 1.3 (0.8,1.8) |
| Chronic medications | ||||
| ACE-inhibitor/ARB | 12 (63) | 2 (40) | 4 (57) | 6 (86) |
| β-blocker* | 16 (84) | 4 (80) | 6 (86) | 6 (86) |
| Statin | 11 (58) | 1 (20) | 5 (71) | 5 (71) |
held for ≥24hrs prior to procedure
On subsequent angiography, 14 of 19 (84%) patients were found to have significant CAD. Nine of these 14 (64%) had single vessel disease, most often involving the left anterior descending coronary artery. Pacing and angiographic data are displayed in Table 2.
Table 2.
Angiographic and pacing-stress characteristics of the study population.
| Clinical characteristic | Entire cohort (n=19) |
Ischemic subgroup | ||
|---|---|---|---|---|
| CAD− / Lactate elution− (n=5) |
CAD+/ Lactate elution− (n=7) |
CAD+/ Lactate elution+ (n=7) |
||
| Angiography | ||||
| No. diseased vessels | ||||
| 0 | 5 (26) | 5 (100) | NA | NA |
| 1 | 10(53) | NA | 4 (57) | 6 (86) |
| 2 | 3 (16) | NA | 3 (43) | 0 |
| 3 | 1 (5) | NA | 0 | 1 (14) |
| Diseased vessel | ||||
| LAD | 8 (57) | NA | 2 (29) | 6 (86) |
| LCx | 5 (36) | NA | 4 (57) | 1 (14) |
| RCA | 6 (43) | NA | 4 (57) | 2 (29) |
| Pacing-response | ||||
| Peak heart rate (bpm) | 146±16 | 150±18 | 144±11 | 145±22 |
| Rate•pressure product (bpm•mmHg) | 21458±4216 | 22566±3028 | 20810±4662 | 21313±4892 |
| Chest pain | 13 (68) | 2 (40) | 5 (71) | 6 (86) |
| ST-segment depression | 9 (47) | 3 (60) | 2 (29) | 4 (57) |
Transcoronary elution of cTnT in response to pacing-stress
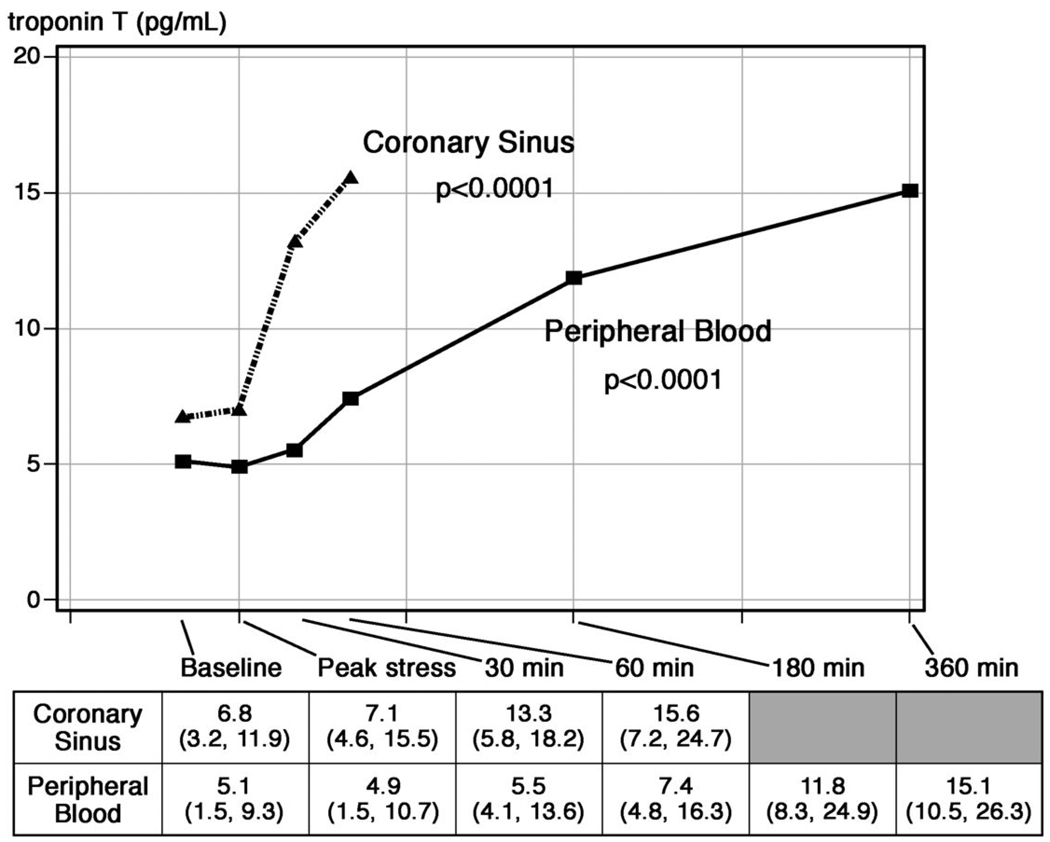
Median coronary sinus and peripheral blood concentrations of cTnT (as measured by the hs-cTnT assay) for the entire study population are shown in Figure 2. cTnT concentrations increased in coronary sinus blood by 5.8 pg/mL within the first 60 minutes following pacing. This was subsequently mirrored in the peripheral blood with an appropriate time delay due to the coronary sinus elution. The median increase in cTnT concentration from peripheral blood was 6.8 pg/mL 180 minutes after pacing.
Figure 2. Median levels of cTnT measured by the hs-cTnT assay in the coronary sinus and in peripheral blood in the entire study population.
An early rise in cTnT was observed in response to rapid pacing, which was eventually mirrored in the peripheral blood. The p-values refer to changes in biomarker levels across timepoints for both coronary sinus and peripheral samples.
Coronary sinus and peripheral cTnT levels stratified by ischemic subgroupings
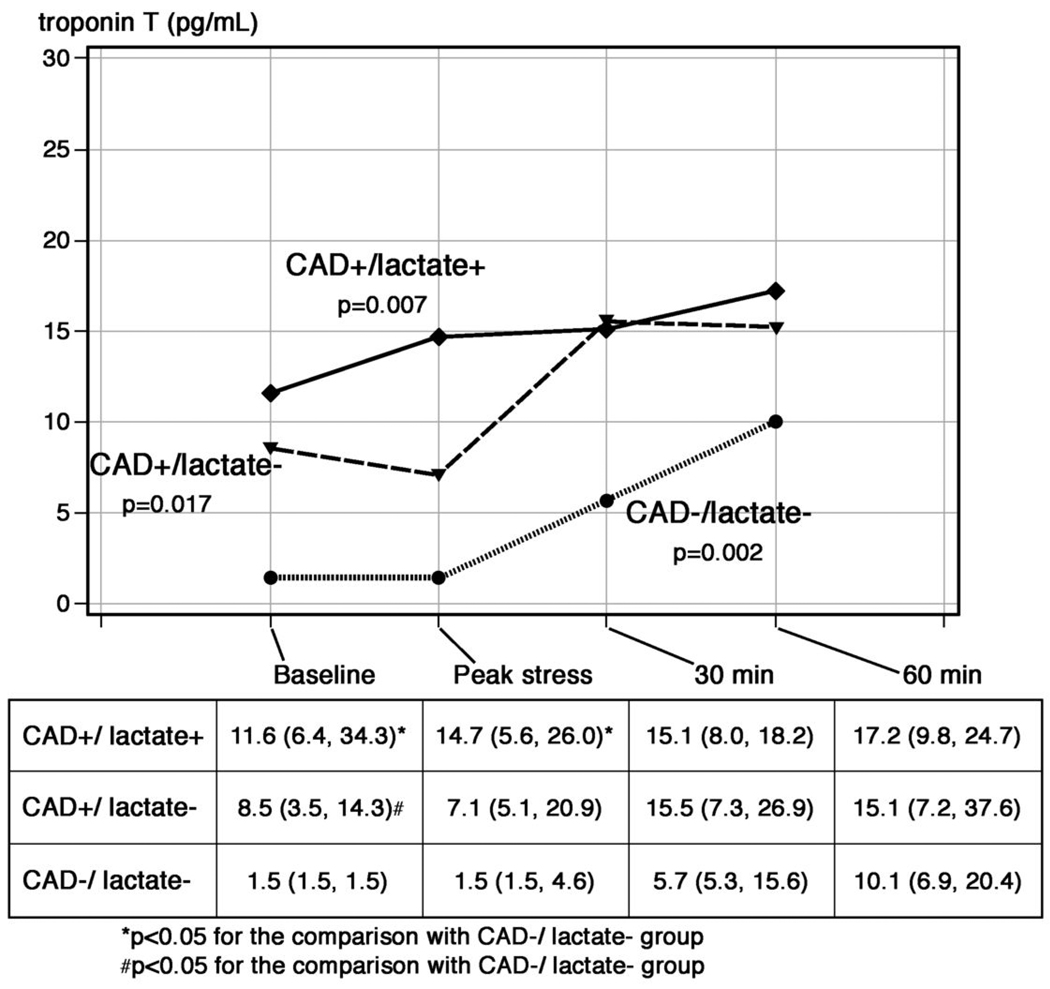
Figure 3 demonstrates the concentrations of cTnT from coronary sinus blood at each timepoint stratified by subgroup. Median baseline levels of cTnT were higher among patients in the lactate+/CAD+ subgroup compared with the lactate−/CAD− subgroup (11.6 vs. 1.5 pg/mL, p=0.02). At 60 minutes following cessation of pacing, however, the concentration of cTnT in the coronary sinus was not statistically different between patient groups (CAD+/lactate+: 17.2 pg/mL, CAD+/lactate−: 15.1 pg/mL, CAD−/lactate−: 10.1 pg/mL; p=0.87).
Figure 3. Median levels of cTnT measured by the hs-cTnT assay from the coronary sinus following pacing-stress stratified by the presence or absence of CAD and lactate elution.
At baseline there were significant differences in cTnT concentrations which diminished over time following pacing.
The p-values refer to changes in biomarker levels across timepoints for each group.
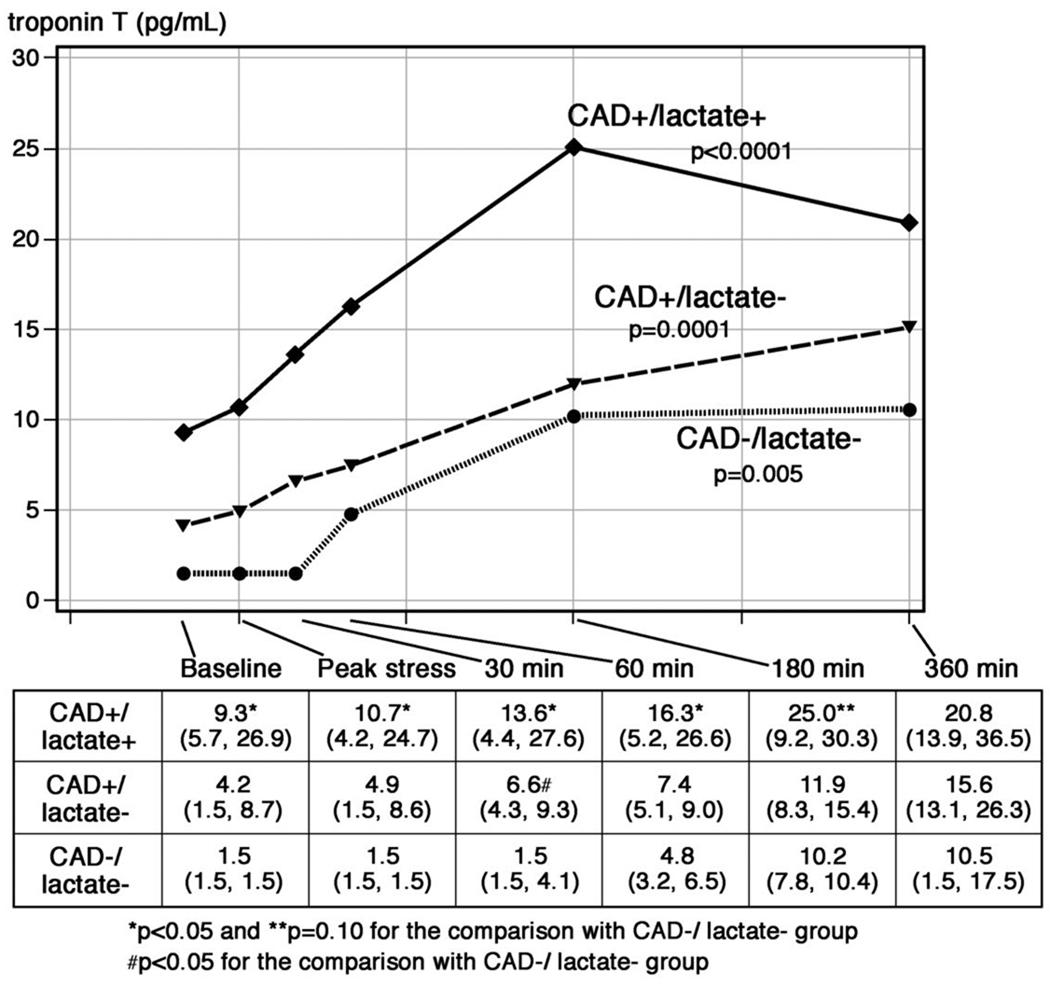
Median concentrations of cTnT measured from the peripheral blood using the hs-cTnT assay, stratified by ischemic subgroup, are shown in Figure 4. Significant increases in cTnT were seen over time for each subgroup in response to pacing. Similar to the results for the coronary sinus, baseline concentrations of cTnT were higher for patients in the lactate+/CAD+ subgroup compared with the lactate−/CAD− subgroup (9.3 vs. 1.5 (pg/mL, p=0.008). The differences in peripheral blood cTnT levels between these two subgroups remained statistically significant at later timepoints (30 and 60 minutes), and a trend towards higher cTnT levels remained after 180 minutes in the lactate+/CAD+ subgroup compared with the lactate−/CAD− subgroup (25.0 vs. 10.2 pg/mL, p=0.10).
Figure 4. Median levels of cTnT in peripheral blood measured by the hs-cTnT assay following pacing-stress stratified by the presence or absence of CAD and lactate elution.
At baseline there were significant differences in cTnT concentrations between the CAD+ and CAD− groups. Following pacing, significant and similar increases were observed in each group. The p-values refer to changes in biomarker levels across timepoints for each group.
As shown in Table 3, the median absolute increase in peripheral cTnT concentrations from baseline to 180-minutes after pacing for the entire cohort was 6.8 pg/mL. When stratified by indicators of ischemia, absolute changes in cTnT were similar in the lactate−/CAD− group (6.8 pg/mL) as compared with the lactate+/CAD+ (8.8 pg/mL, p=0.5)] or CAD+/lactate− (5.4 pg/mL, p=0.94) groups. Doubling or even tripling of cTnT values from baseline to 180 minutes following pacing-stress was frequently observed in all the groups. Notably, the majority of patients in the lactate−/CAD− subgroup demonstrated significant relative increases in cTnT values; however, despite the large relative increase in cTnT in this group, (median 5.2-fold increase) the peak value of cTnT did not exceed the upper limit of the normal range for the assay (>14 pg/mL) in any of the five patients.
Table 3.
Absolute and relative changes in plasma cTnT levels in peripheral blood (as measured by the hs-cTnT assay) from baseline to 180-minutes following pacing-induced stress among the entire cohort and when stratified by presence of CAD and lactate release.
| Entire cohort | CAD− / lactate − | CAD+ / lactate − | p- value |
CAD+ / lactate + | p- value |
|
|---|---|---|---|---|---|---|
| Absolute ΔcTnT [median (25th,75th)], pg/mL | 6.8 (3.4,9.1) | 6.8 (6.2,8.7) | 5.4 (3.3,9.1) | 0.50 | 8.8 (3.4,20.8) | 0.94 |
| Relative fold ΔcTnT [median (25th,75th)] | 2.3× (1.4×,4.2×) | 5.2× (2.3×,6.8×) | 2.0× (1.4×,3.3×) | 1.7× (1.1×,3.8×) | ||
| ≥2-fold increase [# of patients (%)] | 11/19 (58) | 4/5 (80) | 4/7 (57) | 3/7 (43) | ||
| ≥3-fold increase [# of patients (%)] | 7/19 (37) | 3/5 (60) | 2/7 (29) | 2/7 (29) |
Correlation between conventional and highly sensitive troponin T assays
No patient had elevation in cTnT at baseline detected using the conventional cTnT assay. Following the pacing-stress protocol, cTnT became detectable in peripheral plasma in 2 of the 19 patients by the conventional assay, both of whom had CAD on subsequent angiography. This contrasts with the highly sensitive assay: when using the previously established 99th percentile for the normal range, elevated cTnT levels (≥ 14 pg/mL) were detected in peripheral blood with the hs-cTnT assay in 3 of the 19 patients at baseline and 7 of the 19 at 180 minutes post pacing. All 7 of these patients had CAD.
Discussion
New highly sensitive troponin assays have demonstrated the ability to detect otherwise subclinical evidence of cardiac injury and to provide clinicians with additional prognostic information compared to standard assays(9–11). However, the improved sensitivity of these assays may come with the disadvantage of decreased specificity. Since cardiac troponins T and I are not found in skeletal muscle, their release into the bloodstream is thought to be indicative of myocyte necrosis. Theoretically, these properties should mitigate against problems with specificity. We undertook this analysis to address the question of whether small increases in cardiac troponin T could be detected by the highly sensitive assay, either in the peripheral circulation or directly eluted from the heart, in response to brief hemodynamic stress (i.e. without frank infarction) and whether this biomarker release was specific to those subjects who manifested angiographic or biochemical evidence of cardiac ischemia.
At baseline, cTnT levels varied considerably among the study population, and were significantly higher among patients with compared to those without CAD. This finding is consistent with previous reports that detection of low-level cTnT release is extremely common among patients with CAD using the highly sensitive cTnT assay(10). For the first hour following pacing, a clear release of cTnT into the coronary sinus, as detected by the highly sensitive assay, was noted. This was eventually mirrored in the peripheral plasma. Only rarely was troponin release detected with standard cTnT assays following pacing-stress.
Following pacing, coronary sinus and peripheral concentrations of troponin T detected by the highly sensitive assay were noted to increase both in patients with and those without biochemical or angiographic correlates of coronary ischemia. Therefore, a significant proportion of patients without markers of ischemia (e.g. those with no angiographic CAD and no evidence of ischemia by lactate elution) still had large relative increases in their cTnT levels. This finding has important clinical implications for the proposed use of serial changes in cTnT concentrations (e.g. recent European Society of Cardiology recommendations)(17) to help determine whether troponin increases are caused by acute processes, such as myocardial ischemia(18), or rather reflect chronic elevations from underlying structural heart disease, renal insufficiency, or other processes(19). Our findings suggest that assessment of serial relative change may not solve the “specificity problem” of the hs-cTnT assays. For example, 3 of our 5 patients without CAD had a tripling of their baseline cTnT levels with pacing, which was similar to the proportion seen in patients with CAD. It is important to note, however, that for these patients with a large relative increase, the absolute cTnT value remained below the proposed MI diagnostic threshold for this assay (≥ 14 pg/mL). Thus, it remains possible that optimal strategies for interpreting serial troponin T values with the hs-cTnT assay may require integration of absolute levels and relative change. Our study is not large enough to address this possibility.
Recently, circulating levels of troponin I (using a new highly sensitive assay) were demonstrated to increase after exercise stress proportionate to the degree of ischemia found on nuclear imaging(20). Here, we demonstrate increasing levels of troponin T following a pacing-induced stress that caused a significant increase in the rate•pressure-product. Taken together, these studies challenge the traditional interpretation of cardiac troponin release in the context of a “threshold” effect, where the appearance of troponin has been considered synonymous with myonecrosis. These findings suggest that cardiac biomarker release may perhaps be viewed more accurately as occurring on a continuum from ischemia to infarction. This notion of gradations of myocardial work resulting in biomarker release is consistent with the previous finding of detectable troponin concentrations among healthy athletes following high-endurance exercise, e.g. marathons, without evidence of scar by cardiac MRI imaging(21–23). Although we cannot exclude the possibility that pacing resulted in microscopic infarction, the overall duration of pacing-stress was short and not greater than the level of stress that would be expected with moderate brief exercise. Our results demonstrate that the heart may commonly release troponin T when subjected to conditions of only moderate cardiac stress, even in the absence of underlying CAD or apparent ischemia. Further, this process occurs early following stress (i.e. detectable within 30 minutes from the coronary sinus effluent).
Limitations
The major limitation of this study is the relatively small sample size, which results from the complexity and length of the protocol. This increases the likelihood of a type II error, particularly given the heterogeneity of inter-individual responses to pacing. The second limitation relates to our clinical classification of cardiac ischemia. Although myocardial lactate elution is thought to be a highly specific marker of cardiac ischemia, it is relatively insensitive because (1) precise quantification requires concomitant assessment of coronary sinus flow, which is difficult to measure except during open heart surgery, and (2) some ischemic myocardial segments may be too small to induce a net-negative lactate gradient once integrated with the remainder of the coronary sinus effluent from areas of tissue that are not ischemic. For this reason, we also reported the intermediate group of patients who had CAD but no pacing-induced lactate elution. In this group, we could not exclude the presence of ischemia. We chose not to incorporate either ST-segment depression or chest pain into our classification, as they may be seen as an artifact of rapid pacing/tachycardia and hence lack sensitivity and specificity for diagnosing cardiac ischemia in this context(16,24–26). Finally, we did not include a control group that did not undergo pacing, and thus cannot completely exclude cardiac injury caused by placement of the pacing catheter as a source for troponin release.
Conclusions
Troponin T release in response to pacing-induced stress can be detected below the threshold of detection of conventional assay. Significant absolute and relative increases of troponin T as measured by the highly sensitive assay were noted even among patients without biochemical evidence of ischemia or significant CAD. The implications of these findings as more sensitive troponin assays becomes commercially available are (1) cTnT may be released in response to brief periods of ischemia, without frank infarction; (2) small increases in cTnT may be expected following periods of increased myocardial work, even among patients without clinical signs of myocardial ischemia or obstructive coronary artery disease; and (3) assessment of dynamic changes in cTnT, particularly below the threshold for MI definition, may not accurately discriminate increases in cTnT caused by myocardial ischemia from other causes of troponin release. These findings suggest that additional research is needed before highly sensitive troponin assays are widely adopted into clinical practice in the management of subjects with chest pain syndromes.
Acknowledgments
Funding Sources: This work was supported by National Institute of Health grant R01-HL098280-01 (Drs Gerszten and Sabatine). Troponin T assays were provided by Roche Diagnositics.
Abbreviations
- cTnT
cardiac troponin T
- hs-cTnT
high-sensitivity troponin T
- CAD
coronary artery disease
- CS
coronary sinus
- CK
creatine kinase
- LVEF
left ventricular ejection fraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. de Lemos has received grant support from Biosite and Roche Diagnostics and consulting fees from Johnson and Johnson and Tethys. Drs. Gerszten and Sabatine have received grant funding from the National Institutes of Health pertaining to biomarker research. All other co-authors report no relevant disclosures.
References
- 1.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–e375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 3.Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett-Connor E. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52:450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang WHW, Wu Y, Nicholls SJ, et al. Subclinical Myocardial Necrosis and Cardiovascular Risk in Stable Patients Undergoing Elective Cardiac Evaluation. Arterioscler. Thromb. Vasc. Bio. 2010;30:634–640. doi: 10.1161/ATVBAHA.109.201210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Persistent cardiac troponin I elevation in stabilized patients after an episode of acute coronary syndrome predicts long-term mortality. Circulation. 2007;116:1907–1914. doi: 10.1161/CIRCULATIONAHA.107.708529. [DOI] [PubMed] [Google Scholar]
- 6.Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study. Circulation. 2006;113:1071–1078. doi: 10.1161/CIRCULATIONAHA.105.570762. [DOI] [PubMed] [Google Scholar]
- 7.Keller T, Zeller T, Peetz D, Tzikas S, et al. Sensitive Troponin I Assay in Early Diagnosis of Acute Myocardial Infarction. N Engl J Med. 361:868–877. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 8.Reichlin T, Hochholzer W, Bassetti S, et al. Early Diagnosis of Myocardial Infarction with Sensitive Cardiac Troponin Assays. N Engl J Med. 361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 9.Bonaca M, Scirica B, Sabatine M, et al. Prospective evaluation of the prognostic implications of improved assay performance with a sensitive assay for cardiac troponin I. J Am Coll Cardiol. 2010;55:2118–2124. doi: 10.1016/j.jacc.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 10.Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 12.Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: Analytical issues for biochemical markers of acute coronary syndromes. Circulation. 2007;115:e352–e355. doi: 10.1161/CIRCULATIONAHA.107.182881. [DOI] [PubMed] [Google Scholar]
- 13.Lewis GD, Wei R, Liu E, Yang E, et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J Clin Invest. 2008;118:3503–3512. doi: 10.1172/JCI35111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guth BD, Wisneski JA, Neese RA, et al. Myocardial lactate release during ischemia in swine. Relation to regional blood flow. Circulation. 1990;81:1948–1958. doi: 10.1161/01.cir.81.6.1948. [DOI] [PubMed] [Google Scholar]
- 15.Turer AT, Stevens RD, Bain JR, et al. Metabolomic profiling reveals distinct patterns of myocardial substrate use in humans with coronary artery disease or left ventricular dysfunction during surgical ischemia/reperfusion. Circulation. 2009;119:1736–1746. doi: 10.1161/CIRCULATIONAHA.108.816116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markham RV, Jr, Winniford MD, Firth BG, et al. Symptomatic, electrocardiographic, metabolic, and hemodynamic alterations during pacing-induced myocardial ischemia. Am J Cardiol. 1983;51:1589–1594. doi: 10.1016/0002-9149(83)90192-3. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Mair J, Katus H, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010 doi: 10.1093/eurheartj/ehq251. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Giannitsis E, Becker M, Kurz K, Hess G, Zdunek D, Katus HA. High-Sensitivity Cardiac Troponin T for Early Prediction of Evolving Non-ST-Segment Elevation Myocardial Infarction in Patients with Suspected Acute Coronary Syndrome and Negative Troponin Results on Admission. Clin. Chem. 2010;56:642–650. doi: 10.1373/clinchem.2009.134460. [DOI] [PubMed] [Google Scholar]
- 19.Wallace TW, Abdullah SM, Drazner MH, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006;113:1958–1965. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 20.Sabatine MS, Morrow DA, de Lemos JA, Jarolim P, Braunwald E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischaemia using an ultrasensitive assay: results from TIMI 35. Eur Heart J. 2009;30:162–169. doi: 10.1093/eurheartj/ehn504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mousavi N, Czarnecki A, Kumar K, et al. Relation of biomarkers and cardiac magnetic resonance imaging after marathon running. Am J Cardiol. 2009;103:1467–1472. doi: 10.1016/j.amjcard.2009.01.294. [DOI] [PubMed] [Google Scholar]
- 22.Mingels A, Jacobs L, Michielsen E, Swaanenburg J, Wodzig W, van Dieijen-Visser M. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem. 2009;55:101–108. doi: 10.1373/clinchem.2008.106427. [DOI] [PubMed] [Google Scholar]
- 23.Trivax JE, Franklin BA, Goldstein JA, et al. Acute cardiac effects of marathon running. J Appl Physiol. 2010;108:1148–1153. doi: 10.1152/japplphysiol.01151.2009. [DOI] [PubMed] [Google Scholar]
- 24.Hlatky MA, Pryor DB, Harrell FE, Jr, Califf RM, Mark DB, Rosati RA. Factors affecting sensitivity and specificity of exercise electrocardiography. Multivariable analysis. Am J Med. 1984;77:64–71. doi: 10.1016/0002-9343(84)90437-6. [DOI] [PubMed] [Google Scholar]
- 25.Güleç S, Ertaş F, Karaoŏuz R, Güldal M, Alpman A, Oral D. Value of ST-segment depression during paroxysmal supraventricular tachycardia in the diagnosis of coronary artery disease. Am J Cardiol. 1999;83:458–460. doi: 10.1016/s0002-9149(98)00888-1. [DOI] [PubMed] [Google Scholar]
- 26.Nelson SD, Kou WH, Annesley T, de Buitleir M, Morady F. Significance of ST segment depression during paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 1988;12:383–387. doi: 10.1016/0735-1097(88)90410-x. [DOI] [PubMed] [Google Scholar]