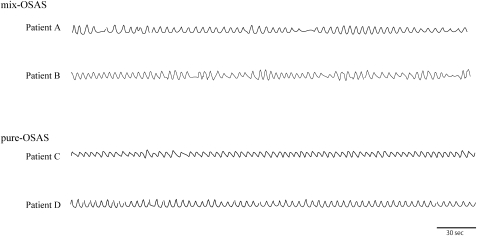Abstract
Background:
Mixed apneas share both central and obstructive components and are often treated as if they are obstructive events. The hypothesis is that patients with obstructive sleep apnea syndrome (OSAS) who exhibit a majority of mixed apneas will differ in ventilatory control from those with predominantly obstructive apneas during wakefulness; moreover, this difference could affect nasal continuous positive airway pressure (CPAP) adherence.
Methods:
In a retrospectively derived case-control study, 5 min of respiratory inductance plethysmography signals during wakefulness prior to sleep onset were extracted from a diagnostic polysomnogram in these groups: (1) mixed apnea-dominant OSAS (mix-OSAS) (n = 36), (2) obstructive apnea-dominant OSAS (pure-OSAS) (n = 20), (3) central apnea-dominant sleep apnea syndrome (pure-CSAS) (n = 6), and (4) control subjects (n = 10). Breathing patterning was compared between the groups using the coefficient of variation (CV) for breath-to-breath inspiration time (Ti), expiration time (Te), Ti + Te (Ttot), and tidal volume, and an information theory-based metric of signal pattern variability (sample entropy). Subsequent CPAP adherence over 12 months was determined in OSAS groups.
Results:
Breath-to-breath CV parameters and sample entropy in the mix-OSAS group were significantly greater as compared with the pure-OSAS and control groups. In a subanalysis, CV and sample entropy were similar in the mix-OSAS and the pure-CSAS groups. CPAP adherence was significantly poorer in mix-OSAS compared with pure-OSAS.
Conclusions:
During wakefulness, both breath patterning and sample entropy in mix-OSAS are similar to pure-CSAS and more variable than in pure-OSAS. In addition, CPAP adherence was decreased in patients with mix-OSAS, which may be related to basic differences in respiratory control.
The Bottom Line
How does this work advance the field?
Patients with mostly mixed apneas are diagnosed with and treated for obstructive sleep apnea syndrome. More irregular breathing during wakefulness and poor adherence to continuous positive airway pressure in patients with primarily mixed apneas identifies clinically important variability in the obstructive sleep apnea syndrome phenotype.
What are the clinical implications?
Patients with mixed apneas may have fundamental differences in respiratory control, which manifest as increased breathing pattern variability during wakefulness. An assessment of resting breathing pattern variability during wakefulness may be able to identify these patients who are less likely to adhere to subsequent continuous positive airway pressure therapy.
Obstructive sleep apnea syndrome (OSAS) is a major public health problem with a prevalence estimated at approximately 4% of adults in both Western and Asian countries.1,2 Nasal continuous positive airway pressure (CPAP) therapy for OSAS has been the most effective and widely used treatment.3-5 However, approximately 25% to 50% of patients with OSA will either refuse to try or will not tolerate CPAP therapy.6 Furthermore, some patients do not respond to CPAP treatment, either without symptom improvements or without reductions in overall respiratory events. Finally, central apneas can emerge with initiation of CPAP therapy, a condition that has been called “complex sleep apnea.”7 Taken together, these facts indicate significant variability of the OSAS phenotype.
Mixed apneas are characterized by a relative lack of respiratory effort during the initial event period followed by efforts against an occluded upper airway. According to the American Academy of Sleep Medicine Task Force in 1999, mixed apneas are pathophysiologically considered to be a part of obstructive apneas.8 Thus, patients whose apneas are mostly mixed receive the clinical diagnosis of OSAS, even though respiratory events in these patients may be primarily central as opposed to obstructive. This may contribute to the fact that some patients with OSAS do not show benefit from CPAP therapy.
In this study, we hypothesized that even before sleep onset there would be a fundamental difference in the breathing pattern between patients with mixed apnea-dominant OSAS (mix-OSAS) and those with obstructive apnea-dominant OSAS (pure-OSAS); moreover, this difference may affect CPAP acceptance and compliance. To examine these hypotheses, the breathing pattern during wakefulness was analyzed using conventional (linear) analysis of tidal volume and frequency, as well as nonlinear analysis of the respiratory signal, an approach that does not depend on breath identification. In addition, subsequent CPAP compliance was compared between OSAS groups.
Materials and Methods
Subjects
Subjects were selected from 987 patients referred to a sleep laboratory with suspected sleep-disordered breathing who underwent diagnostic polysomnography between 2003 and 2008. The research database held values for the total number of apneas according to the each type (obstructive, central, and mixed) for each patient and measures of sleep latency, length, and state. Forty-four patients with mix-OSAS (4.4% of the sample) were identified and compared with patients with pure-OSAS (n = 20) and control subjects (n = 10) randomly extracted from the same database. A group with central apnea-dominant sleep apnea syndrome (pure-CSAS) (n = 7) was also identified. Data were collected on the Epworth sleepiness scale (ESS), medical history, and current medications.
Inclusion Criteria
Mix-OSAS was defined as an apnea-hypopnea index (AHI) > 20, in which the number of mixed apneas during the diagnostic study was > 30% of the total number of apneic events. Pure-OSAS was defined by an AHI > 20 in a patient in whom all of the apneas were obstructive apneas. The definition of pure-CSAS was a central apnea index > 5, where the total number of central apneas was greater than the number of obstructive apneas, or the presence of Cheyne-Stokes respiration. Patients with AHI < 5 were defined as control subjects.
Analysis of the Respiratory Signal
Approximately 5 min of stable respiratory signal data before sleep onset were extracted from the diagnostic polysomnography. Respiratory signals were generated by the sum of chest and abdominal signals using respiratory inductance plethysmography (RIP). The stable respiratory signal was identified using the respiratory signal itself as well as electromyogram (EMG) (chin and limb) to detect any body movements. When the amplitude of the EMG signal was high, that part of the signal was considered to be during movement and inappropriate for analysis. In the analytic phase of the study, investigators were blinded to the group assignment, and each 5-min record of respiratory signal during EEG staging of wakefulness was analyzed for breath-to-breath inspiration time (Ti), expiration time (Te), Ti + Te (Ttot), and tidal volume. To assess breathing irregularity, the coefficient of variation (CV) ([SD/mean] × 100) for each parameter was calculated.
Sample entropy is calculated from the same data sample (RIP-sum signals) but is not dependent on information of tidal volume or frequency per se. Rather, it is a statistical measure of the predictability or regularity of the data set and is defined as the logarithm of the difference between the probability that a vector X is within a chosen distance r in m-dimensional space and the probability that the vector X is within the same chosen distance r in m + 1-dimensional space.9 The probability densities are normally estimated using the method suggested by Grassberger and Procaccia.10 In the present study, sample entropy of the raw respiratory signal (sampled at 10 Hz) was calculated using standard parameters (m = 2 and r = 0.2 × SD).9 This information theory-based metric reflects linear and nonlinear determinants of temporal pattern variability with high values denoting less self-similarity and greater complexity. Comparisons of sample entropy of the original time series were made with the sample entropy of surrogate data sets (n = 19) to provide a statistical comparison between the surrogate data sets as well as to provide a means for comparing results of analyzing surrogate data and surrogate and original data through computational algorithms. Surrogate data were computed using the iterated amplitude-adjusted Fourier transform by moving the data into the frequency domain for adjustment and then back into the time domain while ensuring that both the frequency distribution (power spectrum/autocorrelation function) and the amplitude distribution are maintained.11,12 Sample entropy was computed over multiple time delays from unity up to one cycle length.13 Values were averaged across time lags excluding those with high linear correlations as defined by the first minimum of the mutual information function. Average sample entropy (excluding small lags) was reported for the surrogate and original data for each group.
Sleep Study
Data acquisition started from 9:00 pm and continued until 6:00 am on the following morning. Polysomnography was performed using a polygraph system (EEG7414; Nihon Kohden; Tokyo, Japan). EEG (C3-A2, C4-A1), bilateral electrooculogram, submental EMG, ECG, and bilateral anterior tibial EMG were recorded. Airflow was monitored using an oronasal thermal sensor and/or nasal air pressure transducer. Thoracic and abdominal respiratory movements were monitored using RIP (Respitrace; Ambulatory Monitoring Inc; Ardsley, New York). Oxyhemoglobin saturation and pulse rate were monitored using pulse oximetry with a finger probe (OLV-3100; Nihon Kohden). All the signals were digitized and stored on a personal computer. Apneas were defined as an episode of complete airflow cessation measured from the thermal sensor lasting > 10 s. Hypopneas were defined by ≥ 30% reduction in amplitude of the RIP-sum signal lasting > 10 s with ≥ 3% oxygen desaturation. AHI was calculated as the average number of apnea-hypopnea events per hour over the total sleep period.
Continuous Positive Airway Pressure
Patients who had an AHI > 20 and any symptoms related to OSAS were initiated on nasal CPAP (REMstar Auto; Respironics; Pittsburgh, Pennsylvania, or GoodKnight 420E; Tyco Mallinckrodt; Plaisir, France) with auto-titrating mode. All patients treated with CPAP visited our sleep laboratory every month, and CPAP compliance was monitored every month using data extracted from the memory of the CPAP equipment for at least 12 months. At the monthly visit to the laboratory, CPAP settings, including pressure range or CPAP mode (auto or fix mode), were modified by an expert physician if it was necessary. Eventually, most of the patients used CPAP with auto-titrating mode during the follow-up period. Good CPAP compliance was defined by use in > 75% of days with > 4 h usage each night; otherwise, it was considered to be poor compliance. CPAP acceptance was defined by whether a patient refuses CPAP within 1 month after CPAP administration.
Statistical Analysis
The differences in age, sleep-disordered parameters, and CV values between three groups (mix-OSAS, pure-OSAS, and control subjects) were detected by one-way analysis of variance. When the analysis of variance was significant, probing of differences within the model was done by t tests of estimated marginal means (simple main effects) with adjustments for multiple comparisons made via the Bonferroni correction. The difference in categorical variables between the three groups and the difference in CPAP acceptance and compliance were detected by χ2 test for independence. For the subanalysis, comparison between pure-CSAS and mix-OSAS was done by t test. Differences with P < .05 were considered significant. All results were expressed as means ± SD. Statistical analysis was done with SPSS, version 10.0 for Windows software (SPSS Inc; Chicago, Illinois).
Results
Subject Characteristics
Among 44 patients with mix-OSAS, eight were excluded from the analysis because sufficient respiratory signal data could not be extracted because of noise; thus, 36 patients with mix-OSAS were enrolled in the study. Table 1 shows subject characteristics for each group. Significant differences in ESS were not observed. There were significant differences in age, AHI, and BMI between the three groups; however, a post hoc test did not indicate significant differences between mix-OSAS and pure-OSAS groups. In addition, four subjects suffered from arrhythmias, including chronic atrial fibrillation (n = 3) and atrioventricular block (n = 1), in the mix-OSAS group. Another two patients in the mix-OSAS group had a past history of cerebral infarction. In contrast, no patients had a history of arrhythmias or cerebral infarction in the pure-OSAS and control groups. The use of medications for hypertension, hyperlipidemia, and diabetes mellitus were similar between groups. No patients were using opioid or hypnotic medications.
Table 1.
—Subject Characteristics
| Characteristic | Mix-OSAS (n = 36) | Pure-OSAS (n = 20) | Control Subjects (n = 10) | P Value |
| Age, y | 56.8±13.9 | 49.9±11.9 | 42.5±9.6a | <.01 |
| AHI, per h | 65.8±17.8 | 59.1±15.8 | 3.2±1.3a | <.001 |
| ESS | 11.2±4.8 | 11.7±7.0 | 8.0±3.8 | NS |
| BMI, kg/m2 | 28.2±3.8 | 27.7±4.0 | 24.4±3.7b | <.05 |
| Arrhythmia | 4/36 (11.1) | 0/20 (0) | 0/10 (0) | NS |
| Hypertension | 15/36 (41.7) | 7/20 (35) | 2/10 (20) | NS |
| Hyperlipidemia | 8/36 (22.2) | 3/20 (15) | 1/10 (10) | NS |
| Diabetes mellitus | 6/36 (16.7) | 2/20 (10) | 1/10 (10) | NS |
| Past history of cerebral infarction | 2/36 (5.6) | 0/20 (0) | 0/10 (0) | NS |
Data are shown as mean ± SD or No. (%). AHI = apnea-hypopnea index; ESS = Epworth Sleepiness Scale; mix-OSAS = mixed apnea-dominant obstructive sleep apnea syndrome; NS = not significant; pure-OSAS = obstructive apnea-dominant obstructive sleep apnea syndrome.
Significant difference from mix-OSAS, P<.01.
Significant difference from mix-OSAS, P<.05.
Breathing Irregularity During Rest Before Sleep Onset
Figure 1 shows examples of RIP-sum signals during wakefulness for two subjects with mix-OSAS and two subjects with pure-OSAS. These tracings highlight the more irregular breathing pattern prior to sleep onset in mix-OSAS as compared with pure-OSAS. The average interval between the end of the extracted respiratory signal and sleep onset time in mix-OSAS, pure-OSAS, and control subjects was 10.0 ± 18.2, 7.5 ± 7.1, and 18.5 ± 32.8 min, respectively (data are not shown). This suggests that the irregularity of the extracted respiratory signal was not affected by drowsiness or an oscillation to sleep state. The CV values for Ti, Te, and Ttot in patients with mix-OSAS were significantly higher than in pure-OSAS and control subjects (Ti: 31.5 ± 18.8, 14.5 ± 10.2, 15.9 ± 7.5; Te: 41.9 ± 23.5, 18.5 ± 8.1, 19.5 ± 6.0; Ttot: 29.7 ± 16.6, 13.0 ± 6.5, 14.3 ± 6.0, respectively). Moreover, the CV of tidal volume in the mix-OSAS group was significantly higher than in pure-OSAS and control groups (37.0 ± 13.3, 18.8 ± 8.6, 23.1 ± 10.4, respectively) (Fig 2). Sample entropy for the respiratory signal in the mix-OSAS group was greater than that in both the pure-OSAS and control groups (1.42 ± 0.15, 1.20 ± 0.16, 1.19 ± 0.25, respectively). These findings suggest that there is greater complexity, less predictability, and thus greater variability in the mix-OSAS group as compared with both the control and pure-OSAS groups. However, this difference between groups remained when sample entropy of surrogate data were measured, suggesting that this difference may come from linear determinants of pattern variability in these two groups (Fig 3).
Figure 1.
Respiratory inductance plethysmography (RIP)-sum tracings prior to sleep onset in two patients with mix-OSAS and two patients with pure-OSAS. Respiratory patterns are more irregular in mix-OSAS as compared with pure-OSAS. mix-OSAS = mixed apnea-dominant obstructive sleep apnea syndrome; pure-OSAS = obstructive apnea-dominant obstructive sleep apnea syndrome.
Figure 2.
Coefficients of variation (CV) for breath-to-breath respiratory variables during resting breathing before sleep onset. Values are mean ± SD. A, Ti. B, Te. C, Ttot. D, Tidal volume. Resting breathing during wakefulness was more irregular in patients with mix-OSAS than in those with pure-OSAS and control subjects as expressed by higher CV values in all respiratory variables. Te = expiration time; Ti = inspiration time; Ttot = Ti1Te. See Figure 1 legend for expansion of other abbreviations.
Figure 3.
Sample entropy values for original data set (black bar) and surrogate data set (gray bar). Values are mean±SD. See Figure 1 legend for expansion of abbreviations.
CPAP Acceptance and Compliance
In the mix-OSAS group (n = 36), all patients were treated with CPAP; however, one subject’s data for CPAP usage were not recorded. Excluding this subject, only 17 out of 35 patients with mix-OSAS (48.6%) had acceptable compliance and acceptance. On the other hand, in the pure-OSAS group (n = 20), three patients chose other treatments, including oral appliance, lateral positional sleep, and weight loss, rather than CPAP; thus 17 patients were treated with CPAP. One subject’s data for CPAP usage were not recorded. Thus, 16 patients with pure-OSAS were eligible for analysis of CPAP compliance. Among them, 13 patients (81%) had a good compliance and acceptance, whereas only three patients refused or could not tolerate CPAP within 1 month (Table 2). The main reasons for poor CPAP acceptance and compliance were an uncomfortable feeling with CPAP and having a sensation of it being hard to breathe and fall asleep. Some reported removing CPAP without awareness during sleep. Also, none of the three patients who refused or could not tolerate CPAP treatment felt any improvement in symptoms such as excessive daytime sleepiness, morning headache, and sleep quality.
Table 2.
—CPAP Compliance and Acceptance
| CPAP Usage | Mix-OSAS(n = 35)a | Pure-OSAS (n = 16)b | P Value |
| Good compliance | 17 | 13 | <.01 |
| Poor compliance | 16 | 1 | <.01 |
| Poor acceptance | 2 | 2 | NS |
CPAP = continuous positive airway pressure. See Table 1 legend for expansion of other abbreviations.
One patient was excluded from the analysis because of loss of CPAP compliance data.
Four patients were excluded from the analysis because three patients were given other treatments and one patient's CPAP data were lost
Comparison of Mix-OSAS and Pure-CSAS Groups
Among subjects with pure-CSAS, six patients were suitable for the analysis, as one patient did not have a sufficient length of stable respiratory signal for the analysis. The central apnea index for the pure-CSAS group was 15.3 ± 12.1. Their age and BMI were 64.7 ± 10.2 years and 25.3 ± 2.4, respectively, which were similar to the mix-OSAS group. Moreover, the CV of Ti, Te, Ttot, and tidal volume in pure-CSAS were 24.6 ± 6.5, 33.8 ± 9.2, 23.9 ± 7.6, and 34.6 ± 8.5, respectively, which were similar to the values for the mix-OSAS group. In addition, the sample entropy of the pure-CSAS group was 1.34 ± 0.17 bits, which was comparable to that observed in the mix-OSAS group (P = .24). However, the sample entropy of the surrogate data were 1.46 ± 0.20 bits in the pure-CSAS group, which was lower than that observed in the mix-OSAS group (P < .05). Taken together, these data suggest that the overall complexity and variability of the breathing pattern is similar between the pure-CSAS and the mix-OSAS groups, but that there are some differences in linear determinants of pattern variability.
Discussion
The present study suggests breathing irregularity during wakefulness, as quantified by both linear and nonlinear metrics, is greater in patients with mix-OSAS as compared with patients with pure-OSAS and control subjects. Additionally, a secondary comparison indicated that breathing irregularity in patients with mix-OSAS is similar to those with pure-CSAS. This finding suggests an intrinsic pathophysiology in the respiratory control system for breathing rhythm and depth in patients with mix-OSAS. Furthermore, this instability of breathing at rest might have some predictive importance in regard to CPAP acceptance and compliance, as there was significantly poorer CPAP adherence in the mix-OSAS group as compared with the pure-OSAS group.
Breathing irregularity during wakefulness is associated with genetic diseases such as Rett syndrome,14,15 with certain environments such as high altitude,16,17 with treatment with opioid medications,18,19 and with medical conditions including heart failure20-22 and cerebral infarction.23,24 These phenomena reflect particular features of the respiratory control system involving respiratory rhythm generation and/or central and peripheral chemoreception. In the present study, we observed greater respiratory variability (as measured by CV of respiratory intervals) in the mix-OSAS group as compared with the pure-OSAS and control groups. This increase in breathing pattern variability was observed during a period of wakefulness, when it is rare for scoreable apnea and/or hypopnea events to occur. This finding suggests that the central respiratory control system in patients with mix-OSAS is different from those with pure-OSAS. To further investigate this difference, we quantified the morphology of the breathing pattern using sample entropy. This analysis identified a greater complexity and less predictability in the mixed group as compared with the control and obstructive groups. If this increased variability in the mixed group were due to nonlinear relationships in the data, we would expect that differences in sample entropy to be lost when looking at the surrogates. However, since these differences between the mixed and control and obstructive groups persisted on analysis of the surrogates, we concluded that the variability differences between the groups were primarily due to linear (stochastic) relationships in the data. The presence of these differences in the awake breathing patterns in these patients further supports the idea that there are fundamental differences in the respiratory control system in patients with mix-OSAS.
The pathogenesis for obstructive sleep apnea has been the focus of much study across the world. Anatomic features are key, but the neuromuscular control system also contributes to the pathogenesis of upper airway obstruction.25-27 In this regard, OSA is already a fairly complex disease. Moreover, it has been proposed that the interaction of respiratory output to the upper airway and diaphragm may determine the expression of apnea types, such as central and obstructive.28 Thus, individuals may manifest apneas with both obstructive and central components. The relative proportion of these components would depend on individual factors, which may be genetic or secondary to a medical condition. Taken together with our findings, we speculate that mixed apneas are closer to central apneas than to obstructive apneas. Although one can score each apnea as a mixed or obstructive apnea, the diagnosis must be OSAS because in the current American Academy of Sleep Medicine definition set a mixed apnea is considered as an obstructive apnea,8 and “mixed sleep apnea syndrome” has not been defined. The present findings also suggest that variability in the OSAS phenotype may be one reason for the variability in CPAP treatment effectiveness for this group.
Poor CPAP compliance in the mix-OSAS group compared with the pure-OSAS group suggests that just opening the upper airway with a pressure splint is not always effective in patients with mix-OSAS. Among 36 patients with mix-OSAS, none had nasal disease; however, four patients had arrhythmias including chronic atrial fibrillation and two patients had a past history of cerebral infarction. As arrhythmias as well as cerebral infarction could affect respiration, the analyses were also performed in a subgroup excluding the six patients with these conditions. However, the significant differences in CV values between the mix-OSAS and pure-OSAS groups remained, suggesting that the presence of the arrhythmias and past history of cerebral infarction might be a surrogate marker and not necessarily the main reason for the respiratory irregularity observed in the mix-OSAS group.
Complex sleep apnea syndrome (CompSAS) is a novel category of sleep-disordered breathing that describes patients with obstructive apneas who develop frequent central apneas or Cheyne-Stokes respiration after successful application of CPAP.29 It has been demonstrated that spectral analysis of ECG-based cardiopulmonary coupling distinguishes pure obstructive apnea from central or complex sleep apnea.30 Moreover, patients with CompSAS show poor CPAP adherence.31 Our study focused on mix-OSAS breathing detected during diagnostic PSG (before CPAP application). Although mixed-OSAS is distinct from CompSAS, similarities to CompSAS are relevant to our findings. Our study indicates that breath-to-breath analysis of breathing during wakefulness, which may be easier than spectral analysis of ECG-based cardiopulmonary coupling during sleep, might be able to not only distinguish mixed apnea dominant from pure obstructive sleep apnea but also predict CPAP adherence.
There are several potential limitations of the present work. First, arterial blood gas analysis was not performed. Thus, a possible effect of hypocapnia on the irregular breathing during wakefulness in mix-OSAS or in those with central apneas cannot be excluded. However, if such a difference were present it would be another reason to suggest that the root cause for breathing irregularity is different. Second, repeat polysomnography with CPAP was not performed at follow-up. Of note, three patients with mix-OSAS had relatively high AHI (roughly around 10.0) during routine CPAP use as documented in the adherence report generated by CPAP equipment and obtained from CPAP memory. Thus, it is possible that patients with mix-OSA may be more likely to develop CPAP-emergent central apneas. Third, this was a retrospective clinical sample. Given the 4% to 5% prevalence of mix-OSAS, it would be difficult to do a prospective study to examine this issue. However, we point out that the recordings were extracted before a sleep study, and the matching to other groups was randomly done and analyzed in the same manner. In this regard there may be a bias that the recordings were acquired before sleep. Although all records were scored for state (in this case wakefulness using standard criteria) by investigators blinded to the group assignment, there may be differences in cortical control of breathing in patients with mix-OSAS and central apneas as compared with those with purely obstructive events. Whether the structure of breathing is also different at other times of the day during quiet wakefulness would need to be studied separately.
In summary, we conclude that irregular breathing during wakefulness and poor adherence to CPAP in the mix-OSAS group suggest distinct features of mix-OSAS as compared with pure-OSAS and control subjects. Mixed apneas may be part of central apneas rather than obstructive apneas, and specific or additional treatment using CPAP may be needed to treat patients with mixed apnea-dominant sleep apnea. Furthermore, an assessment of resting breathing pattern variability during wakefulness might be not only a window to explore the central respiratory control system but also a new tool to distinguish clinically important OSAS phenotypes.
Acknowledgments
Author contributions: Dr Yamauchi: contributed to study concept and design; data acquisition, analysis, and interpretation; and drafting and revising the manuscript.
Dr Tamaki: contributed to study concept and design, data interpretation, and drafting and revising the manuscript.
Dr Yoshikawa: contributed to study concept and design, data interpretation, and drafting and revising the manuscript.
Dr Ohnishi: contributed to data acquisition and drafting and revising the manuscript.
Dr Nakano: contributed to data acquisition and drafting and revising the manuscript.
Dr Jacono: contributed to data analysis and drafting and revising the manuscript.
Dr Loparo: contributed to data analysis and drafting and revising the manuscript.
Dr Strohl: contributed to study concept and design and data interpretation and drafting and revising the manuscript.
Dr Kimura: contributed to study concept and design and data interpretation and drafting and revising the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The content is entirely the responsibility of the authors, and sponsors had no role in design or conduct of the study.
Other contributions: We thank Kaoru Senzaki, RPSGT, for her help with polysomnogram scoring.
Abbreviations
- AHI
apnea-hypopnea index
- CompSAS
complex sleep apnea syndrome
- CPAP
continuous positive airway pressure
- CV
coefficient of variation
- EMG
electromyogram
- ESS
Epworth sleepiness scale
- mix-OSAS
mixed apnea-dominant obstructive sleep apnea syndrome
- OSA
obstructive sleep apnea
- OSAS
obstructive sleep apnea syndrome
- pure-CSAS
central apnea-dominant sleep apnea syndrome
- pure-OSAS
obstructive apnea-dominant obstructive sleep apnea syndrome
- RIP
respiratory inductance plethysmography
- Te
expiration time
- Ti
inspiration time
- Ttot
inspiration time + expiration time
Footnotes
Funding/Support: This study was supported in part by Grant-in-Aid for Young Scientists (B) [21790781] from The Ministry of Education, Culture, Sports, Science and Technology, Japan; National Institutes of Health, National Heart, Lung, and Blood Institute [Grant R33HL087340-01]; and the Veterans Affairs Research Service.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119(1):62–69. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon S, Chung SA, Fargher T, Huterer N, Shapiro CM. Sleep apnea, hypertension, and the effects of continuous positive airway pressure. Am J Hypertens. 2005;18(5 pt 1):594–600. doi: 10.1016/j.amjhyper.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165(4):447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 6.Zozula R, Rosen R. Compliance with continuous positive airway pressure therapy: assessing and improving treatment outcomes. Curr Opin Pulm Med. 2001;7(6):391–398. doi: 10.1097/00063198-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29(9):1203–1209. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 8.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 9.Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278(6):H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- 10.Grassberger P, Procaccia I. Measuring the strangeness of strange attractors. Physica D. 1983;9(1-2):189–208. [Google Scholar]
- 11.Schreiber T, Schmitz A. Surrogate time series. Physica D. 2000;142(3-4):346–382. [Google Scholar]
- 12.Theiler J, Eubank S, Longtin A, et al. Testing for nonlinearity in time series: the method of surrogate data. Physica D. 1992;58(1-4):77–94. [Google Scholar]
- 13.Kaffashi F, Foglyano R, Wilson CG, et al. The effect of time delay on approximate & sample entropy calculations. Physica D. 2008;237(23):3069–3074. [Google Scholar]
- 14.Kerr AM. A review of the respiratory disorder in the Rett syndrome. Brain Dev. 1992;14(suppl):S43–S45. [PubMed] [Google Scholar]
- 15.Ogier M, Katz DM. Breathing dysfunction in Rett syndrome: understanding epigenetic regulation of the respiratory network. Respir Physiol Neurobiol. 2008;164(1-2):55–63. doi: 10.1016/j.resp.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netzer NC, Strohl KP. Sleep and breathing in recreational climbers at an altitude of 4200 and 6400 meters: observational study of sleep and patterning of respiration during sleep in a group of recreational climbers. Sleep Breath. 1999;3(3):75–82. doi: 10.1007/s11325-999-0075-7. [DOI] [PubMed] [Google Scholar]
- 17.Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131(2):595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teichtahl H, Wang D. Sleep-disordered breathing with chronic opioid use. Expert Opin Drug Saf. 2007;6(6):641–649. doi: 10.1517/14740338.6.6.641. [DOI] [PubMed] [Google Scholar]
- 19.Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747–758. doi: 10.1093/bja/aen094. [DOI] [PubMed] [Google Scholar]
- 20.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164(12):2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 21.Bradley TD, Floras JS. Sleep apnea and heart failure: part II: central sleep apnea. Circulation. 2003;107(13):1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 22.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30(3):291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 23.Hardavella G, Stefanache F, Ianovici N. Cheyne stokes respiration in stroke patients. Rev Med Chir Soc Med Nat Iasi. 2006;110(1):82–87. [PubMed] [Google Scholar]
- 24.Cherniack NS, Longobardo G, Evangelista CJ. Causes of Cheyne-Stokes respiration. Neurocrit Care. 2005;3(3):271–279. doi: 10.1385/NCC:3:3:271. [DOI] [PubMed] [Google Scholar]
- 25.Ayappa I, Rapoport DM. The upper airway in sleep: physiology of the pharynx. Sleep Med Rev. 2003;7(1):9–33. doi: 10.1053/smrv.2002.0238. [DOI] [PubMed] [Google Scholar]
- 26.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102(2):547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 27.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168(6):645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 28.Cherniack NS. Respiratory dysrhythmias during sleep. N Engl J Med. 1981;305(6):325–330. doi: 10.1056/NEJM198108063050606. [DOI] [PubMed] [Google Scholar]
- 29.Gilmartin GS, Daly RW, Thomas RJ. Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med. 2005;11(6):485–493. doi: 10.1097/01.mcp.0000183061.98665.b0. [DOI] [PubMed] [Google Scholar]
- 30.Thomas RJ, Mietus JE, Peng CK, et al. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep. 2007;30(12):1756–1769. doi: 10.1093/sleep/30.12.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pusalavidyasagar SS, Olson EJ, Gay PC, Morgenthaler TI. Treatment of complex sleep apnea syndrome: a retrospective comparative review. Sleep Med. 2006;7(6):474–479. doi: 10.1016/j.sleep.2006.04.005. [DOI] [PubMed] [Google Scholar]