Abstract
Background:
Impaired brachial flow-mediated dilation (FMD) is associated with risk for subsequent cardiovascular events in patients after myocardial infarction (MI). These patients often have obstructive sleep apnea (OSA). We tested the hypothesis that patients with OSA post MI will exhibit more severe impairment in FMD.
Methods:
We studied 64 patients with MI admitted to our hospital. OSA was determined using polysomnography. FMD was measured using high-resolution ultrasonography, with researchers blind to the OSA diagnosis.
Results:
The mean age was 60 ± 11 years, and the mean BMI was 29 (26, 32 kg/m2), 84% of patients were men, 39% had moderate to severe OSA (apnea-hypopnea index [AHI] > 15), and 31% of the patients had mild OSA (5 ≤ AHI < 15). FMD was severely impaired in patients with moderate to severe OSA (0.8% ± 0.7%) as compared with patients without OSA (4.7% ± 0.8%, P = .001) and with mild OSA (3.9% ± 0.8%, P = .015). Linear regression showed that FMD was associated with log nocturnal nadir oxygen saturation (minSao2) (β = 31.17, P = .0001), age (β = −0.11, P = .006). MinSao2 was an independent predictor of FMD after adjustment for possible confounders (β = 26.15, P = .001).
Conclusions:
FMD is severely impaired in patients with moderate to severe OSA post MI, which may be partially related to nocturnal hypoxemia. Patients with OSA may, therefore, be at higher risk for subsequent cardiovascular events after an MI. Identifying and treating OSA may have important implications in the long-term prognosis of patients post MI. Further studies are necessary to determine if the presence of OSA would affect the long-term occurrence of cardiovascular events after an MI.
The Bottom Line
How does this work advance the field?
This study reveals a correlation between the severity of obstructive sleep apnea and endothelial dysfunction that may be, in part, related to the severity of nocturnal oxygen desaturations. These findings are important in that they highlight a potential area for treatment.
What are the clinical implications?
The presence of severe obstructive sleep apnea in patients after a myocardial infarction is associated with impaired flow-mediated dilation, which may confer increased long-term risk. The identification and appropriate treatment of obstructive sleep apnea might have important implications for the prognosis of patients after a myocardial infarction.
Obstructive sleep apnea (OSA) is highly prevalent in patients with established coronary artery disease.1 We have reported that OSA is also a common comorbidity, affecting approximately two-thirds of patients who had a recent myocardial infarction (MI).2 However, the prognostic implications of OSA after an MI remain unknown.
Endothelial dysfunction is an early marker of vascular function impairment and is also predictive of future cardiovascular events.3 Measurement of flow-mediated dilation (FMD) is recognized as a measure of endothelial dysfunction and has been used to determine risk factors for cardiovascular disease in several clinical studies.4 Moreover, FMD is closely related to coronary endothelial function.5
Prior studies have shown diffuse endothelial dysfunction in patients with long-term6 and short-term coronary syndromes.7 Epidemiologic and experimental data suggest that patients with OSA have impaired endothelial function, a mechanism that may help explain the association between OSA and cardiovascular diseases.8,9 However, to our knowledge, there have been no previous studies examining endothelial function in patients with OSA post MI. This would be important information in establishing prognoses in this patient population. We, therefore, tested the hypothesis that patients with OSA post MI will exhibit severe impairment in FMD.
Materials and Methods
Study Population
We conducted a cross-sectional study of 74 patients who had a recent MI (1-3 months). The diagnosis of MI was made by the patient’s attending physician. While consecutive patients were eligible, recruitment was based on the availability of research personnel and patients consenting to participate. The exclusion criteria included a previous diagnosis of OSA under treatment with continuous positive airway pressure and ≥ 50% of disordered breathing events classified as central apnea and/or a Cheyne-Stokes pattern of respiration. The study was approved by the Mayo Clinic Institutional Review Board (IRB 2156-03), and all patients gave written informed consent.
Polysomnography
The subjects underwent comprehensive (overnight) polysomnography (PSG) to identify the presence and severity of OSA. PSG was performed using an attended system (Compumedics Siesta802 Wireless digital PSG recorder; Compumedics; Abbotsford, Victoria, Australia) that included EEG, electrooculography, electromyography, pulse oximetry, thermistor and transduced nasal pressure measurements of airflow, and respiratory-inductance plethysmography.
Hypoxic exposure during sleep was quantified using two different variables. Nocturnal nadir oxygen saturation (minSao2), a measure of maximal severity of hypoxemia, was obtained by averaging the nadir oxyhemoglobin (HbO2) saturations associated with the severest desaturations during sleep. Second, the time with oxygen saturation (Sao2) < 90%, a measure of hypoxemia duration, was calculated as the percentage of total sleep time associated with HbO2 saturation ≤ 90% (T90Sao2).
Apneas were defined as a ≥ 90% drop in the peak thermal sensor excursion from baseline for ≥ 90% of the event duration, where the event duration is ≥ 10 s. Hypopneas were defined as a > 30% drop from baseline in nasal pressure signal excursions for ≥ 90% of the event duration, where the event duration is ≥ 10 s and the event is accompanied by a ≥ 4% pulse oximetry desaturation as measured from the pre-event baseline. Apneas unaccompanied by evidence of respiratory effort were scored as central, while those accompanied by respiratory effort were labeled obstructive. For the purposes of our study, mixed apneas were counted as obstructive. Arousals were considered respiratory related if associated with apneas, hypopneas, or other indicators of airflow limitation lasting ≥ 10 s but not meeting the criteria for apneas or hypopneas.10 After classification, disordered breathing events were quantified using the apnea-hypopnea index (AHI) and reported as the mean number of events per hour. Using the AHI, groups were defined a priori as patients without OSA (AHI < 5), with mild OSA (5 ≤ AHI < 15), and with moderate to severe OSA (AHI ≥ 15). All PSG data were analyzed by an experienced registered PSG technologist (C. v. d. W.). PSG montage and scoring were done in accordance with the American Academy of Sleep Medicine standards.10
Brachial Artery Vascular Reactivity
All measures were obtained in the morning, between 6:30 am and 7:30 am, just after the sleep studies. Patients were fasting and abstinent from caffeine and tobacco for 24 h prior to the procedure. We followed expert guidelines to assess FMD as measured by the percentage increase of diastolic diameter of the brachial artery before and after forearm cuff inflation to induce ischemia in the ipsilateral hand (reactive hyperemia).11 A continuous three-lead ECG was recorded and used to identify the time of diastole. A BP cuff was placed on the right distal forearm. The right brachial artery images were obtained above the antecubital fossa using B-mode imaging in the longitudinal plane of the artery using a 6-MHz linear transducer (Acuson Sequoia C512; Siemens Medical Solutions, Inc; Issaquah, Washington).
FMD was induced by inflating the forearm cuff to 50 mm Hg over the systolic BP or to 200 mm Hg, whichever was greater, for 5 min and then releasing it. The diameter of the brachial artery was assessed for 60 to 90 s after deflation of the cuff, was measured at the onset of the R wave, and was averaged from three measurements. All images were stored digitally for later analysis. All images were acquired by experienced investigators, and measurements were made manually off-line on a workstation with the researchers blinded to the OSA diagnosis and the patients’ clinical characteristics, including BMI. The magnitude of FMD was obtained by determining the percentage of change in the brachial artery diameter relative to the baseline diameter before occlusion. The intraobserver reproducibility and the interobserver reproducibility in our laboratory are 93% and 91%, respectively.
Statistical Analysis
Data are summarized as frequencies for categorical variables and means with SD (mean ± SD) for continuous variables or median with interquartile range (IQR) for skewed data. First, the three groups were compared using a Kruskal-Wallis test. When the overall F statistics were significant, pairwise Wilcoxon rank-sum tests were applied. Differences in proportions were tested using the χ2 test or Fisher exact test (when expected frequencies below 5 h occurred).
Covariates of interest as predictors of FMD were AHI, minSao2, T90Sao2, age, gender, BMI, mean arterial BP (MAP), total cholesterol, glucose, and presence of peripheral vascular disease. The covariates were first investigated as univariate predictors of change in brachial artery FMD using simple linear regression analysis. Multiple regression models (least squares regression) were used to assess whether the variables associated with OSA severity, namely AHI, minSao2, and T90Sao2, were independent predictors of FMD. Log-transformation of the data was used for the variables that had a skewed distribution, and residual distributions of the regression models were examined to assess the validity of model assumptions. Bonferroni correction was used to adjust for multiple comparisons, and two-tail P values < 0.016 were considered significant. Analyses were performed using JMP, version 7 (SAS Institute; Cary, North Carolina).
Results
Patient Characteristics
We recruited 74 patients, of whom 10 had ≥ 50% of disordered breathing events classified as of central origin and/or Cheyne-Stokes pattern of respiration, and were excluded from our data analysis. Our final sample included 64 patients, who were divided into three groups: those with no OSA (AHI < 5 events/h), mild OSA (5 ≤ AHI < 15 events/h), and moderate to severe OSA (AHI ≥ 15 events/h). These cutoffs were used to allow a representative sample size in each group, with 20, 19, and 25 patients, respectively. Eighty-four percent of the patients were men, with a mean age of 60 ± 11 years and a mean BMI of 29 (IQR 26, 32 kg/m2). Six patients (10%) had a history of peripheral vascular disease, seven (11%) had a history of diabetes, 33 (52%) had a history of hypertension, 42 (66%) had dyslipidemia, six (10%) had a previous MI, 47 (74%) had an ST-elevation MI, and percutaneous coronary intervention was the treatment used in the majority (95%) of patients. A comparison of the characteristics of patients is shown in Table 1. The groups had similar prevalences of comorbidities, except for the fact that patients with OSA had a higher BMI in comparison with patients without OSA. There was no difference between groups regarding medications being taken at the time of the study (Table 2).
Table 1.
—Patient Characteristics
| Characteristics | AHI < 5 events/h (n = 20) | 5 ≤ AHI < 15 events/h (n = 19) | AHI ≥ 15 events/h (n = 25) | P Value |
| Age, y | 59 ± 11 | 57 ± 9 | 64 ± 13 | .13 |
| Male | 16 (80) | 15 (78) | 23 (92) | .37 |
| MinSao2, mm Hg | 89.5 ± 3.3 | 86.4 ± 3.0a | 81.6 ± 4.6b | <.01 |
| T90Sao2 | 0.8 ± 0.4 | 1.3 ± 0.4 | 18.6 ± 5.5b | <.01 |
| BMI, kg/m2 | 27 (25, 29) | 31 (29, 33)a | 30 (27, 33)b | <.01 |
| ST-elevation MI | 15 (75) | 13 (68) | 19 (76) | .84 |
| PCI | 20 (100) | 17 (89) | 23 (92) | .36 |
| LVEF, % | 53 (45, 61) | 57 (45, 61) | 54 (44, 58) | .78 |
| Prior MI | 2 (10) | 1 (5) | 3 (12) | .77 |
| Hypertension | 9 (45) | 11 (58) | 13 (52) | .61 |
| Systolic BP, mm Hg | 118 (104, 130) | 115 (104, 130) | 116 (106, 135) | .85 |
| Diastolic BP, mm Hg | 66 (60, 73) | 64 (57, 69) | 66 (60, 76) | .54 |
| Hypercholesterolemia | 11 (55) | 14 (74) | 17 (68) | .32 |
| Cholesterol, mg/dL | 168 ± 37 | 171 ± 35 | 178 ± 53 | .59 |
| HDL cholesterol, mg/dL | 40 (35, 44) | 37 (34, 45) | 37 (33, 45) | .92 |
| LDL cholesterol, mg/dL | 103 (73, 123) | 92 (78, 133) | 107 (79, 159) | .77 |
| Triglycerides, mg/dL | 111 (68, 160) | 129 (93, 194) | 144 (103, 195) | .31 |
| Diabetes mellitus | 1 (5) | 2 (11) | 4 (16) | .51 |
| Fasting glucose, mg/dL | 109 (100, 115) | 108 (94, 118) | 108 (101, 126) | .79 |
| Peripheral vascular disease | 3 (15) | 1 (5) | 2 (8) | .58 |
Values are given as the mean ± SD, No. (%), or median (IQR). AHI = apnea-hypopnea index; HDL = high-density lipoprotein; IQR = interquartile range; LDL = low-density lipoprotein; LVEF = left-ventricular ejection fraction measured within 1 wk after myocardial infarction; MI = myocardial infarction; minSao2 = nocturnal nadir oxygen saturation; OSA = obstructive sleep apnea; PCI = percutaneous coronary intervention; T90Sao2 = percentage of total sleep time associated with oxyhemoglobin saturation ≤ 90%.
P < .016, no OSA vs mild OSA.
P < .016, no OSA vs moderate to severe OSA.
Table 2.
—Medications According to the Presence or Absence of OSA
| Characteristics | AHI < 5 events/h (n = 20) | 5 ≤ AHI < 15 events/h (n = 19) | AHI ≥ 15 events/h (n = 25) | P Value |
| Aspirin | 18 (90) | 18 (95) | 25 (100) | .11 |
| b-Blockers | 19 (95) | 18 (95) | 22 (96) | .33 |
| ACE inhibitors | 14 (70) | 11 (58) | 17 (68) | .83 |
| Statins | 20 (100) | 18 (95) | 24 (96) | .46 |
Data are given as No. (%). ACE = angiotensin converting enzyme. See Table 1 for expansion of other abbreviations.
Vascular Reactivity Study
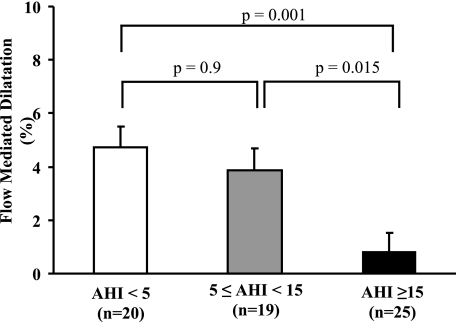
There were significant differences in FMD between the three groups. Patients with moderate to severe OSA had severely impaired endothelial function when compared with patients without OSA (0.8% ± 0.7% vs 4.7% ± 0.7%, P = .001) and with mild OSA (0.8% ± 0.7% vs 3.9% ± 0.8%, P = .015). However, there was no difference in FMD between the patients without OSA and those with mild OSA (4.7% ± 0.7% vs 3.9% ± 0.8%, P = .9) (Fig 1).
Figure 1.
Comparison of flow-mediated dilation of the brachial artery in patients without obstructive sleep apnea (OSA)(AHI<5 events/h), with mild OSA (5≤AHI<15), and with moderate to severe OSA (AHI≥15), showing a significant impairment in patients with moderate to severe OSA. AHI = apnea-hypopnea index.
Linear regression showed that FMD was positively associated with log minSao2 (R2 = 0.23, P = .0001) and negatively associated with log AHI (R2 = −0.17, P = .0008). Age was also significantly associated with FMD (R2 = 0.11, P = .006), and there was a trend for log T90Sao2 and the presence of peripheral vascular disease (Table 3). These variables, plus sex and BMI, because of their known clinical importance, were retained for inclusion in the multiple linear regression models. Results from this analysis are summarized in Table 4. Because of the strong correlations (r > 0.60) between AHI, minSao2, and T90Sao2, only the one that explained the greater amount of variability in FMD (minSao2, R2 = 0.23) was used in the multivariate models to avoid colinearity. We noted that in each of the four models, minSao2 and age remained significantly associated with FMD after adjustment for possible confounders (Table 4).
Table 3.
—Predictors of FMD by Univariate Linear Regression Analysis
| Predictors | R2 | b (95% CI) | P Value |
| Log minSao2 | 0.23 | 31.17 (16.42, 45.90) | .0001 |
| Log AHI | 0.17 | 21.21 (21.89, 20.52) | .0008 |
| Age, y | 0.11 | 20.11 (20.19, 20.03) | .006 |
| Log T90Sao2 | 0.10 | 20.66 (21.22, 20.11) | .02 |
| Peripheral vascular disease | 0.06 | 23.19 (26.46, 0.07) | .05 |
| Male sex | 0.04 | 22.14 (24.79, 0.50) | .11 |
| Log glucose | 0.03 | 24.43 (210.85, 1.98) | .17 |
| Total cholesterol, mg/dL | 0.03 | 0.005 (20.02, 0.03) | .62 |
| Log MAP | 0.01 | 23.71 (212.06, 4.63) | .38 |
| Log BMI | 0.002 | 21.17 (27.59, 5.24) | .71 |
| Ejection fraction | < 0.01 | <0.01 (20.10, 0.10) | .98 |
FMD = flow-mediated dilation; MAP = mean arterial BP. See Table 1 for expansion of other abbreviations.
Table 4.
—Predictors of FMD by Multiple Variable Linear Regression
| Model 1 Adjusted (R2 = 0.31) |
Model 2 Adjusted (R2 = 0.34) |
Model 3 Adjusted (R2 = 0.36) |
Model 4 Adjusted (R2 = 0.36) |
|||||
| Predictors | β | P Value | β | P Value | β | P Value | β | P Value |
| Log minSao2 | 28.39 | .0006 | 27.68 | .0006 | 26.01 | .0006 | 26.15 | .001 |
| Age, y | 20.10 | .012 | 20.09 | .02 | 20.09 | .017 | 20.09 | .03 |
| Peripheral vascular disease | . . . | . . . | 22.08 | .15 | 22.10 | .14 | 22.11 | .15 |
| Male sex | . . . | . . . | . . . | . . . | 21.59 | .17 | 21.60 | .17 |
| Log BMI | . . . | . . . | . . . | . . . | . . . | . . . | 0.15 | .96 |
Discussion
Our main finding is that, after MI, patients with moderate to severe OSA have severely impaired endothelial function and that nocturnal hypoxemia may contribute to endothelial dysfunction in this patient population. Cardiovascular events post MI remain common despite significant advances in secondary prevention.12,13 OSA is a common comorbidity in patients with MI; however, there is no information about the implications of OSA for the long-term prognosis of patients post MI. Brachial artery FMD is strongly associated with coronary artery endothelial function5 and has been used in clinical practice as a measure of endothelial function. Among men who had an acute coronary syndrome without ST elevation, those who had FMD < 1.9% were more likely to have cardiovascular events.14 Our study patients with moderate to severe OSA had a mean FMD of 0.8%, suggesting higher risk of future cardiovascular events after an MI when compared with patients with mild OSA and with those without OSA. Our findings suggest that the presence of severe OSA may worsen the prognosis in patients after an MI.
Our findings are consistent with studies that have shown the presence of endothelial dysfunction in patients with OSA and without coronary artery disease.8,15-17 There is also evidence of an association between OSA and other markers of endothelial dysfunction, such as circulating levels of adhesion molecules18 and vascular endothelial growth factor.19 Previous studies in patients with OSA have also suggested that endothelial dysfunction is improved after treatment with nasal continuous positive airway pressure.9,20,21
The association between OSA and FMD could be confounded by the presence of hypertension, obesity, diabetes, smoking, and dyslipidemia.22 In our study, the patients’ characteristics, including comorbidities, cardiac function, and left-ventricular ejection fraction, were very similar in the three groups, with the exception of BMI, which was higher in patients with OSA in comparison with patients without OSA. However, univariate analyses showed that BMI, along with MAP, left-ventricular ejection fraction, and glucose and cholesterol levels, was not associated with FMD in our patient population. Moreover, minSao2 remained associated with FMD after adjustment for possible confounders and was the strongest variable predicting FMD in patients with OSA post MI. This finding is consistent with the finding of a prior study.23 The causal association between minSao2 and FMD is biologically plausible since endothelium-dependent vasodilation is mediated by nitric oxide, and its biosynthesis is an oxygen-dependent process. In fact, previous studies suggest that chronic sleep-related intermittent hypoxia might have effects on vascular nitric oxide synthesis or availability.16,19,24 It has also been shown that patients with OSA exhibit reductions in available nitric oxide and increases in reactive oxygen species.19,24 In addition to the severe endothelial dysfunction seen in subjects with moderate to severe OSA, recurrent hypoxemia might result in increased systemic inflammation and a prothrombotic state,25 which would further heighten the risk of future cardiovascular events.
Interestingly, our data also showed that patients with mild OSA exhibit similar FMD when compared with patients without OSA. A possible explanation is the higher T90Sao2 observed in patients with moderate to severe OSA post MI. Therefore, both minSao2 and hypoxemia duration might play a role in the development of endothelial dysfunction in this patient population.
The strengths of our study include the use of comprehensive, attended, overnight PSG to detect the presence and the severity of OSA. Moreover, the PSG data were analyzed by researchers who were blind to the FMD data. We used standard methods to assess and measure brachial artery FMD; these measurements were also made by researchers who were blind to the OSA diagnosis. A limitation of our study is the possibility of participation bias; however, we do not believe that this interfered with our primary results. Another possible limitation is the lack of BMI matching. However, minSao2 was still a significant predictor, even after adjustment for BMI in our multiple variable model. Chung and colleagues26 have shown that BMI was associated with FMD in patients who were middle-aged and had OSA, but not in patients who were elderly and had OSA.
In summary, in patients post MI, the presence of severe OSA is associated with impaired FMD, related, at least in part, to the nocturnal hypoxemia. Identification and appropriate treatment of OSA might have important implications for the prognosis of patients after an MI.
Conclusions
Endothelial dysfunction in patients with MI and coexisting OSA suggests a poor long-term prognosis. Whether the presence and severity of OSA can significantly influence subsequent cardiovascular events and whether OSA treatment can improve long-term outcomes of patients post MI remain to be determined.
Acknowledgments
Author Contributions: The authors contributed significantly to the study and take full responsibility for the integrity and accuracy of the data. All authors agreed to the manuscript as written.
Dr Sert Kuniyoshi: contributed to study design and concept, data acquisition, data analysis and interpretation, and drafting of the manuscript.
Dr Singh: contributed to data analysis and interpretation and revised the manuscript critically.
Dr Gami: contributed to study design and concept, data acquisition, data analysis and interpretation, and revised the manuscript critically.
Dr Garcia-Touchard: contributed to study design, data acquisition, and revised the manuscript critically.
Ms van der Walt: contributed to data acquisition, polysomnography data interpretation, and revised the manuscript critically.
Dr Pusalavidyasagar: contributed to data acquisition and revised the manuscript critically.
Dr Wright: contributed to data interpretation and revised the manuscript critically.
Dr Vasquez: contributed to data interpretation and revised the manuscript critically.
Dr Lopez-Jimenez: contributed to data analysis and interpretation and revised the manuscript critically.
Dr Somers: contributed to study design and concept, data analysis and interpretation, and revised the manuscript critically.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Somers has served as a consultant for ResMed, Cardiac Concepts, Sova Pharmaceuticals, Apnex Medical, Merck, and Johnson and Johnson and has been a principal investigator or coinvestigator on research grants funded by the Respironics Foundation, the ResMed Foundation, Select Research, and the Sorin Corporation. Dr Somers is working with Mayo Health Solutions and their industry partners on intellectual property related to sleep and cardiovascular disease. Dr Sert Kuniyoshi became a full-time employee for Philips Respironics after the collection of the data provided in this article. Drs Singh, Gami, Garcia-Touchard, Pusalavidyasagar, Wright, Vasquez, and Lopez-Jimenez; and Ms van der Walt have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources or the National Institutes of Health. Information on the National Center for Research Resources is available at http://www.ncrr.nih.gov. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
Other contributions: The authors gratefully acknowledge Debra L. Pfeifer and Ann B. Peterson for their superb secretarial and administrative assistance; Diane E. Davison, RN, MA, for her expertise in coordinating the studies; Jo-Ellen Ehrsam, RDCS, for her assistance with the vascular reactivity study analysis; and Jan Bukartyk, MSc, for his technical assistance.
Abbreviations
- AHI
apnea-hypopnea index
- FMD
flow-mediated dilation
- HbO2
oxyhemoglobin
- IQR
interquartile range
- MAP
mean arterial blood pressure
- MI
myocardial infarction
- minSao2
nocturnal nadir oxygen saturation
- OSA
obstructive sleep apnea
- PSG
polysomnography
- Sao2
oxygen saturation
- T90Sao2
percentage of total sleep time associated with oxyhemoglobin saturation ≤ 90%
Footnotes
Funding/Support: This publication was made possible by the National Center for Research Resources [Grant 1 UL1 RR024150] a component of the National Institutes of Health, and the National Institutes of Health’s Roadmap for Medical Research. Dr Somers was supported by the National Institutes of Health [Grant HL65176]. Dr Sert Kuniyoshi was supported by the American Heart Association [Grant 09-20069G]. These studies were also supported by a gift to the Mayo Foundation by the Respironics Foundation for Sleep and Breathing.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest. 1996;109(3):659–663. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- 2.Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52(5):343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 4.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106(6):640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 5.Tarutani Y, Matsumoto T, Takashima H, Yamane T, Horie M. Brachial artery flow-mediated vasodilation is correlated with coronary vasomotor and fibrinolytic responses induced by bradykinin. Hypertens Res. 2005;28(1):59–66. doi: 10.1291/hypres.28.59. [DOI] [PubMed] [Google Scholar]
- 6.Uren NG, Marraccini P, Gistri R, de Silva R, Camici PG. Altered coronary vasodilator reserve and metabolism in myocardium subtended by normal arteries in patients with coronary artery disease. J Am Coll Cardiol. 1993;22(3):650–658. doi: 10.1016/0735-1097(93)90172-w. [DOI] [PubMed] [Google Scholar]
- 7.Amir O, Jaffe R, Shiran A, Flugelman MY, Halon DA, Lewis BS. Brachial reactivity and extent of coronary artery disease in patients with first ST-elevation acute myocardial infarction. Am J Cardiol. 2006;98(6):754–757. doi: 10.1016/j.amjcard.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169(3):354–360. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 9.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169(3):348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 10.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 11.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: Implications for the assessment of endothelial function. J Physiol. 2005;568(Pt 2):357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: The emerging role of mitral regurgitation. Circulation. 2005;111(3):295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 13.Jokhadar M, Jacobsen SJ, Reeder GS, Weston SA, Roger VL. Sudden death and recurrent ischemic events after myocardial infarction in the community. Am J Epidemiol. 2004;159(11):1040–1046. doi: 10.1093/aje/kwh147. [DOI] [PubMed] [Google Scholar]
- 14.Karatzis EN, Ikonomidis I, Vamvakou GD, et al. Long-term prognostic role of flow-mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am J Cardiol. 2006;98(11):1424–1428. doi: 10.1016/j.amjcard.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102(21):2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 16.Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling : Association with the severity of apnea-induced hypoxemia during sleep. Chest. 2001;119(4):1085–1091. doi: 10.1378/chest.119.4.1085. [DOI] [PubMed] [Google Scholar]
- 17.Chami HA, Keyes MJ, Vita JA, et al. Brachial artery diameter, blood flow and flow-mediated dilation in sleep-disordered breathing. Vasc Med. 2009;14(4):351–360. doi: 10.1177/1358863X09105132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol. 2003;94(1):179–184. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 19.Lavie L, Hefetz A, Luboshitzky R, Lavie P. Plasma levels of nitric oxide and L-arginine in sleep apnea patients: effects of nCPAP treatment. J Mol Neurosci. 2003;21(1):57–63. doi: 10.1385/JMN:21:1:57. [DOI] [PubMed] [Google Scholar]
- 20.Chin K, Nakamura T, Shimizu K, et al. Effects of nasal continuous positive airway pressure on soluble cell adhesion molecules in patients with obstructive sleep apnea syndrome. Am J Med. 2000;109(7):562–567. doi: 10.1016/s0002-9343(00)00580-5. [DOI] [PubMed] [Google Scholar]
- 21.Imadojemu VA, Gleeson K, Gray KS, Sinoway LI, Leuenberger UA. Obstructive apnea during sleep is associated with peripheral vasoconstriction. Am J Respir Crit Care Med. 2002;165(1):61–66. doi: 10.1164/ajrccm.165.1.2009062. [DOI] [PubMed] [Google Scholar]
- 22.Corretti MC, Anderson TJ, Benjamin EJ, et al. International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. [published correction appears in J Am Coll Cardiol. 2002;39(6):1082] J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 23.Chung S, Yoon IY, Shin YK, et al. Endothelial dysfunction and C-reactive protein in relation with the severity of obstructive sleep apnea syndrome. Sleep. 2007;30(8):997–1001. doi: 10.1093/sleep/30.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz R, Schmidt D, Blum A, et al. Decreased plasma levels of nitric oxide derivatives in obstructive sleep apnoea: Response to CPAP therapy. Thorax. 2000;55(12):1046–1051. doi: 10.1136/thorax.55.12.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lévy P, Pépin JL, Arnaud C, et al. Intermittent hypoxia and sleep-disordered breathing: Current concepts and perspectives. Eur Respir J. 2008;32(4):1082–1095. doi: 10.1183/09031936.00013308. [DOI] [PubMed] [Google Scholar]
- 26.Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Ahn HJ. Endothelial dysfunction and inflammatory reactions of elderly and middle-aged men with obstructive sleep apnea syndrome. Sleep Breath. 2009;13(1):11–17. doi: 10.1007/s11325-008-0210-x. [DOI] [PubMed] [Google Scholar]