Abstract
(Pro)renin receptor (PRR), the newest member of the renin–angiotensin system (RAS), is turning out to be an important player in the regulation of the cardiovascular system. It plays a pivotal role in activation of the local RAS and stimulates signalling pathways involved in proliferative and hypertrophic mechanisms. However, the role of PRR in the brain remains unknown. Thus, our objective in this study was to determine whether a functional PRR is present in neurons within the brain. Neuronal co-cultures from the hypothalamus and brainstem areas of neonatal rat brain express PRR mRNA. Immunoreactivity for PRR was primarily localized on the neuronal cell soma and in discrete areas in the neurites. Treatment of neurons with renin, in the presence of 2 μM losartan, caused a time- and dose-dependent stimulation of phosphorylation of extracellular signal related kinase ERK1 (p44) and ERK2 (p42) isoforms of mitogen-activated protein kinase. Optimal stimulation of fourfold was observed within 2 min with 20 nM renin. Electrophysiological recordings showed that treatment of the neurons with renin, in the presence of 2 μM losartan, resulted in a steady and stable decrease in action potential frequency. A 46% decrease in action potential frequency was observed within 5 min of treatment and was attenuated by co-incubation with a PRR blocking peptide. These observations demonstrate that the PRR is present in neurons within the brain and that its activation by renin initiates the MAP kinase signalling pathway and inhibition of neuronal activity.
Recent discovery of (pro)renin receptor (PRR) has revolutionized the field of renin–angiotensin system (RAS) physiology and has underscored the critical role of the local and tissue RAS in the development and establishment of cardiac, vascular and renal pathophysiology linked to hypertension and diabetes (Ichihara et al. 2007; Nguyen, 2007). (Pro)renin receptor is a 350 amino acid protein with a single transmembrane domain that binds and actives prorenin. Prorenin contains a ‘handle’ region that conceals the active site of renin (Suzuki et al. 2003). Prorenin binding to PRR results in non-proteolytic activation of the enzyme by inducing a conformational change at the ‘handle’ region, exposing the active site (Nguyen et al. 2002; Danser et al. 2007). In addition, renin binding to the PRR increases its intrinsic activity. Thus, prorenin bound to PRR is many-fold more efficient in the conversion of angiotensinogen to angiotensin I, subsequently leading to increased angiotensin II (Ang II) at the tissue level (Nguyen et al. 2002; Nguyen, 2007). Studies have established that PRR plays a dual role in the regulation of RAS activity: it binds to prorenin/renin to facilitate the formation of Ang I and II, and the binding initiates an intracellular signal transduction pathway involving mitogen-activated protein kinases (MAPK). The latter action is presumably associated with increased synthesis of profibrotic molecules such as plasminogen activator inhibitor-1 (PAI-1), fibronectin, collagen and transforming growth factor-β (TGF-β; Huang et al. 2006; Danser et al. 2007; Nguyen, 2007). The importance of PRR in the cardiovascular system is further evident from pathophysiological and transgenic studies. For example: (i) inhibition of PRR by a ‘handle’ region peptide has been shown to protect the heart and kidney against tissue damage induced by hypertension (Ichihara et al. 2004, 2006); (ii) activation of PRR selectively promotes pathological retinal neovascularization (Satofuka et al. 2007); and (iii) overexpression of PRR in rats results in elevated blood pressure and increased plasma aldosterone (Burcklé et al. 2006). Collectively, these observations indicate that PRR may be a critical member of the RAS in the maintenance of normal cardiovascular physiology.
In spite of its emerging involvement and importance in cardiac, renal and vascular pathophysiology, nothing is known about the role of PRR in the brain. This is particularly relevant in view of the fact that the brain RAS is integral in neural control of cardiovascular functions and its hyperactivity is linked to hypertension (Veerasingham & Raizada, 2003; Peterson et al. 2006; Osborn et al. 2007). Although the presence of all the components of the RAS is unquestionable in the brain, their cellular distribution and the interplay of different cell types (neurons, glia, etc.) in the generation of Ang II and the access of this hormone to the Ang II type 1 (AT1) receptor in cardiovascular-relevant neurons remains elusive. In addition, the presence of low levels of renin in the brain has been a consistent enigma in establishing an independent role of the brain RAS (Dzau et al. 1986; Baltatu et al. 1998). Discovery of the PRR is of great significance in this regard and may be critical in solving the puzzle of an intrinsic RAS in the brain and delineating the mechanism of neuronal Ang II actions. Thus, our objective in the present study was to determine whether neurons within the brain express a functional PRR, in at attempt to elucidate the role of the brain RAS in cardiovascular functions.
Methods
Preparation of neuronal and astroglial cultures from the Wistar–Kyoto (WKY) rat brain
Wistar–Kyoto rats were purchased from Charles River Laboratories (Wilmington, MA, USA) and used in our breeding colony to generate 1-day-old pups which were killed via overdose of pentobarbital. Hypothalamic and brainstem areas from 1-day-old rats were dissected, combined, brain cells dissociated and cells plated in poly-L-lysine precoated culture dishes for the establishment of neuronal and astroglial cultures, essentially as described previously (Raizada et al. 1995). These cultures have been very well characterized and data published (Raizada et al. 1995; Sun et al. 2005). Neuronal cultures contain >90% neurons (the remainder are primarily astroglia), while astroglial cultures contain >99% astroglial cells. These cultures were grown for 10–14 days prior to their use in experiments.
Measurement of PRR mRNA levels
Neuronal and astroglial cultures were processed for RNA isolation. Total RNA was isolated using RNAquous 4 polymerase chain reaction (PCR) kit (Ambion, Foster City, CA, USA) according to the manufacturer’s instructions, and 200 ng RNA was reverse transcribed with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). The PRR mRNA levels were analysed by quantitative real-time PCR using PRR specific primers and Taqman probe (Applied Biosystems, catalogue no. Rn01430718_m1) in the PRISM 7000 sequence detection system (Applied Biosystems). All cDNA samples were assayed in triplicate. Data were normalized to 18S RNA.
Immunocytochemistry of PRR
Neuronal cultures were fixed with 4% paraformaldhyde in phosphate-buffered saline (PBS) for 10 min at room temperature. After blocking non-specific binding with 1% bovine serum albumin in PBS for 30 min, cultures were rinsed with PBS containing 0.3% Triton X-100 (PBST). They were then incubated with a 1:100 dilution of goat anti-ATP6AP2, a PRR specific antibody (NOVUS Biologicals, Littleton, CO, USA) and a 1:100 dilution of mouse anti-neuronal nuclear antibody (NeuN, Chemicon, Temeaila, CA, USA). This was followed by rinsing them with PBST and incubation with secondary antibodies (Alexa Flour 488 donkey anti-goat IgG, 1:200 and Alexa Fluor 594 goat anti-mouse IgG, 1:200; Molecular Probes, Eugene, OR, USA). Cells were examined and photographed with the use of an Olympus BX41 microscope.
Measurement of phosphorylation of ERK1/2 MAP kinases
Western blot analysis was used to measure total and phosphorylated ERK1/2 MAP kinases as described previously (Yang et al. 1996). Neuronal cultures were incubated with or without recombinant human renin (Sigma-Aldrich, St Louis, MO, USA) in the presence of 2 μm losartan. Cultures were rinsed with ice-cold PBS containing phosphatase inhibitors (Active motif, Carlsbad, CA, USA), cells collected in radio-immunoprecipitation assay (RIPA) buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and proteins isolated as described previously (Sellers et al. 2005). Samples containing 15 μg of protein were separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose membrane and probed with mouse monoclonal antibody to phosphorylated ERK1/2 (Cell Signaling, Danvers, MA, USA) diluted at 1:2000 with 5% non-fat milk in Tris-buffered saline–Tween, essentially as described previously (Yamazato et al. 2007). Protein bands representing ERK1/2 MAP kinase were quantified using the NIH ImageJ program (Bethseda, Maryland, USA; http://rsb.info.nih.gov/ij/), and values normalized to total ERK1/2.
Electrophysiology
Spontaneous action potentials were recorded as previously reported (Sun et al. 2005). Briefly, all recordings were done at room temperature (~22°C) using whole cell patch-clamp configurations in bridge mode. Data were obtained using a Molecular Devices Axon 200A amplifier (Sunnyvale, CA, USA), filtered at 2 kHz and digitized using Molecular Devices Digidata 1322A at 10 kHz. Data were recorded using Axon pClamp 9.0 software and recorded onto a PC. Cells were bathed in Tyrode solution containing (mm): NaCl, 135; KCl, 5; CaCl2, 2; MgCl2, 2; NaH2PO4, 0.33; Hepes, 10; and dextrose, 10; pH was adjusted to 7.4 with NaOH. The recording pipette had resistance of 2–5 MΩ when filled with solution containing (mm): KCl, 150; MgATP, 4; Na2GTP (0.1 μm); and dextrose, 10; pH was adjusted to 7.2 with KOH. After obtaining access to the cytosol, cells were allowed to equilibrate with the pipette solution for a minimum of 10 min prior to all patch-clamp recordings. Subsequently, baseline was recorded for 5 min prior to treatment. The threshold for counting of action potentials was set to 0 mV. Owing of variability in basal action potential frequency (APF) and for simplification of analysis we chose to examine recordings where baseline levels average between one and three action potentials per second. This was the range of basal activity exhibited by the majority of our recordings. Neurons were considered to have a renin effect when changes in APF levels were greater than ±25%, which exceeds the variation in APF observed within naïve control neurons. Using this criterion, our recordings showed that 22 out of 32 neurons responded to renin. Input resistance was measure by passing a 10 s square-wave pulse of hyperpolarizing current of 0.2 nA during current-clamp recordings administered every 30 s, prior to and 5 min after renin treatment.
Synthesis of PRR blocking peptide (PRRB)
Use of the PRRB has been previously characterized (Nguyen et al. 2002; Ichihara et al. 2004). The PRRB is a decapeptide with the sequence NH3-RILLKKMPSV-COOH, conforming to the prorenin ‘handle’ region, with was synthesized and purified by the University of Florida Interdisciplinary Center for Biotechnology Research (ICBR; Gainesville, FL, USA). Peptide was dissolved in water at a stock concentration of 10 mm, aliquoted and frozen at −80°C until ready for use.
Statistical analysis
All data are expressed as means ± s.e.m. Statistical significance was evaluated with the use of one-way ANOVA and paired and unpaired Students t test. Differences were considered to be significant at P < 0.05.
Results
Expression of PRR in brain neurons
Neuronal cells in primary culture have been extensively used by us to elucidate the cellular and signalling mechanisms of angiotensin II-mediated central actions (Sumners et al. 1991; Veerasingham & Raizada, 2003; Sun et al. 2005). Thus, we used these cultures to study the cellular aspects of the PRR. Neuronal cultures abundantly expressed PRR mRNA, and its levels were 3.2-fold higher than those seen in astroglial cultures prepared from the same brain areas (Fig. 1A). Staining with PRR specific antibodies supports the neuronal localization of this receptor (Fig. 1B). Immunoreactivity for PRR was predominantly localized in neuronal cultures that co-stained with neuron specific antibody, NeuN. Observation of neuronal staining at higher magnification indicated that PRR was primarily localized on the somatic plasma membranes and clustered in certain regions of neurites (Fig. 1Bg ). Therefore, these observations indicated that PRR is localized in neurons.
Figure 1. Pro(renin) receptor expression in the WKY rat brain neuronal cultures.

A, PRR mRNA in neuronal and astroglial cultures from WKY rat brains. Neuronal and astroglial cells were established in primary culture as described in the Methods. Cells were collected, RNA isolated and subjected to quantitative real-time RT-PCR as described in the Methods. Data are means + S.E.M., n = 9 for neuronal cultures and 6 for astroglial cultures. *P < 0.001 versus astroglial cultures. B, localization of PRR immunoreactivity in neuronal cultures. Neuronal cultures from WKY rat brains were fixed and subjected to the immunocytochemical protocol described in the Methods with the use of anti-PRR and -NeuN antibodies. Cultures incubated without primary antibodies (Ba, b and c) were used as controls. Phase contrast (Ba), green (Bb) and red fluorescence (Bc) examination of control cultures. The PRR and NeuN staining is shown at lower (Bd and e) and higher magnifications (Bg and h), while Bf and i represent merged images. Arrows in Bg indicate localization of PRR immunoreactivity on cell body and neuronal processes (contrast was adjusted to enhance immunostained clusters). Scale bar represents 50 μm in Ba, b and c, 25 μm in Bd, e and f, and 10 μm Bg, h and i.
Effect of renin on ERK1 and ERK2 MAP kinases
Since phosphorylation of ERK1 and ERK2 MAP kinases has been linked to noradrenaline neuromodulation in these neuronal cultures (Yang et al. 1996), we proceeded to determine whether activation of PRR can result in phophorylation of these two isoforms. Figure 2 demonstrates that renin caused a dose- and time-dependent increase in the phosphorylation of ERK1 and ERK2 isoforms of MAP kinases. A maximal stimulation of fourfold was observed with 20 nm renin within 2 min. A twofold stimulation persisted for 10 min. This was followed by a gradual decrease in phosphorylation by 30 min.
Figure 2. Effects of renin on ERK1/2 phosphorylation in neuronal cultures.

A, dose–response relationship. Neuronal cultures, in triplicate, were incubated with the indicated concentrations of renin in the presence of 2 μM losartan for 2 min at 37°C. Proteins were isolated and subjected to 10% SDS-PAGE separation and Western blotting with the use of ERK1/2 and phosphorylated ERK1/2 antibodies as described in the Methods. Top left panel is a representative Western blot showing phosphorylated (p) ERK in each set of treatment conditions. Top right panel is a representative Western blot showing total ERK in each set of treatment conditions. Bottom panel shows quantification of phosphorylated bands that have been normalized with total ERK1/2 and compared with control, normalized to unity. Data are means + S.E.M. (n = 3), *P < 0.05 compared with control. B, time course. Neuronal cultures were incubated with 20 nM recombinant human renin in the presence of 2 μM losartan for the indicated time periods. Phosphorylated levels of ERK1/2 were quantified as described in A. Top left panel is a representative Western blot showing p-ERK in each set of treatment conditions. Top right panel is a representative Western blot showing total ERK in each set of treatment conditions. Bottom panel shows quantification of phosphorylated ERK1/2. Data are means + S.E.M. (n = 3). *P < 0.05 compared with control.
Effect of renin on action potential frequency
After establishing the effect of renin on MAP kinase phosphorylation in neurons, we proceeded to examine the effect of renin on neuronal excitability. Basal APF was measured after 10 min equilibration following access to the cell cytosol. Naïve, control neurons had a basal APF of 117 ± 21 events min−1 and after 20 min had an APF of 101 ± 22 events min−1 (Fig. 3A and C). Basal APF for the treatment group was 110 ± 12 events min−1. Treatment of neurons with 2.0 μm losartan, an AT1 receptor antagonist, had no significant effect on APF at 101 ± 12 events min−1 (Fig. 3B and C). However, treatment with 20 nm renin for 5 min resulted in marked inhibition of APF, down to 59 ± 7 events min−1 (n = 6; P < 0.05), without influencing resting membrane potential (Fig. 3D). Thus, renin treatment resulted in a 46% reduction in APF rate that was independent of AT1 receptor activation. Further examination showed that renin did have an effect on membrane input resistance when subjected to a 10 s hyperpolarizing pulse of 0.2 nA. In comparison with basal conditions, renin treatment resulted in a 37 ± 4% reduction in the change of resting membrane potential during administration of the hyperpolarizing pulse (Fig. 3E).
Figure 3. Effect of renin on APF in WKY rat brain neurons.
Representative traces of bridge-mode recordings in neurons performed after 10 min equilibration between the cell cytosol and pipette solution. A, control cells depicting firing rate in basal conditions and 15 min after establishing basal. B, renin (20 nM)-treated cells showing basal firing, trace at 10 min in the presence of 2 μM losartan and trace with renin and losartan co-treatment (5 min). C, bar graphs comparing APF levels in neuronal recordings, from control neurons (left) compared with treated neurons (right) that were subjected to the indicated treatments. Means + S.E.M.; n = 6, *P < 0.01 versus control. D, expansion of the representative traces shown in A and B to compare changes in resting membrane potential from baseline in control neurons (left) and in losartan plus renin-treated neurons (right). E, representative trace recordings (left) and bar graph (right) showing change in resting membrane potential after hyperpolarizing pulse administration in neurons before and after renin treatment during current-clamp recordings. ΔRMP, change in resting membrane potential. Means + S.E.M.; n = 5, *P < 0.05 versus control.
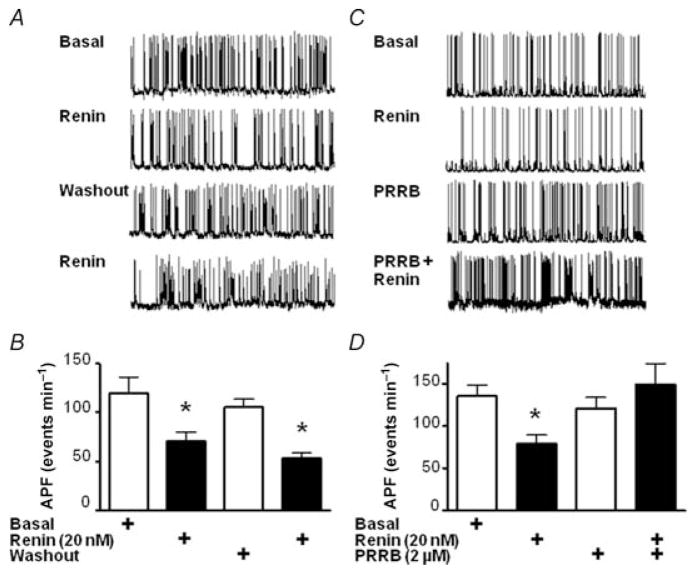
(Pro)renin receptor blocker (PRRB), a decapeptide previously used as a decoy, was tested to determine whether it could suppress the inhibitory effect of renin on APF. In these experiments, all recordings were made in the presence of 2.0 μm losartan. Neurons were initially screened for the inhibitory effect of renin on APF and then arbitrarily split between control and PRRB treatment groups (Fig. 4A, B and C). Control neurons were subjected to renin washout with recording buffer only for 30 min, while the treatment group had a 10 min washout, followed by 20 min treatment with 10 μm PRRB, prior to subsequent renin treatment. Control neuronal recordings recovered from renin treatment (APF 105 ± 8 events min−1). The treatment group also showed recovery, and no significant effect was observed before or after PRRB treatment (122 ± 10 and 121 ± 13 events min−1, pre- and 20 min post-PRRB, respectively). Pretreatment with PRRB, however, ablated the subsequent renin-induced decrease in APF levels, which was seen in control neurons after 5 min, with APF values of 149 ± 25 versus 54 ± 5 events min−1 PRRB-pretreated and control neurons, respectively (Fig. 4). Taken together, these results show that brain neurons are inhibited by renin acting on PRR.
Figure 4. Effect of PRR inhibitor peptide on renin-induced decrease in APF.
Representative traces of bridge-mode recordings performed after 10 min equilibration with pipette solution in control neurons (A) and PRRB-treated neurons (C). In both cases, neurons in the presence of 2 μM losartan were screened for the inhibitory effect of renin (20 nM). Control neurons were subjected to renin washout with recording buffer for 30 min, while the treatment group had a 10 min washout, followed by a 20 min treatment with 10 μM PRRB to determine whether the inhibitory peptide would block the effect of renin. Bar graphs are shown derived from bridge-mode recordings in neurons, representing APF levels for the indicated treatments, for the control (B) and PRRB-treated groups (D). Means + S.E.M. (n = 9). *P < 0.01 versus basal.
Discussion
Our observations establish that neuronal cells from the hypothalamus and brainstem regions of normotensive rat brain in primary culture express PRR and that this PRR is functional. This is the first indication that a functional PRR may be involved in the neuronal control of cardiovascular functions.
Our previous studies and those of others have established that all the components of the RAS are not only present in neurons but also are generated in the brain (Raizada et al. 1995; Veerasingham & Raizada, 2003). In addition, increases in the levels of AT1 receptors and their coupling to the phosphatidylinositol-3 (PI3) kinase signalling system is linked to hypertension in a rat model of neurogenic hypertension (Sun et al. 2003; Veerasingham & Raizada, 2003; Veerasingham et al. 2005). Despite these overwhelming data and evidence from transgenic studies that establishes a key role of the brain RAS in cardiovascular functions and hypertension (Morimoto & Sigmund, 2002; Sakai & Sigmund, 2005), there has been a continuous debate about the presence of an intrinsic brain RAS. Skeptics of this proposal have argued that the brain levels of renin are too low to have any impact on Ang II formation and its actions (Bader et al. 2001; Lippoldt et al. 2001). In addition, a disparity in the cellular localization of angiotensinogen, ACE and AT1 receptors, coupled with invariant complexity of the brain, has made it difficult to reconcile the access of Ang II to cardiovascular-relevant neuronal circuits. Our study, demonstrating the presence of a functional PRR in neurons, is relevant in this respect because it, for the first time, opens a new direction in resolving these issues.
Emerging evidence suggests that the PRR functions by two distinct mechanisms. The PRR, by binding to prorenin, activates the enzyme activity of renin, resulting in the generation of Ang II at the cell/tissue level (Danser et al. 2007; Ichihara et al. 2007; Nguyen, 2007). Additionally, prorenin binding to the PRR initiates a cascade of signalling events, including activation of MAPK, that are associated with profibrotic and proliferative actions independent of Ang II (Danser et al. 2007; Nguyen, 2007). Our data provide evidence for a novel function of PRR in neurons. Stimulation of PRR in neurons invokes a signalling mechanism that results in the regulation of neuronal excitability. Furthermore, our findings indicate that renin, via PRR, activates MAPK pathways in neurons. Renin binding to PRR, which are localized in neurons, stimulates phosphorylation of ERK1 and ERK2 isoforms of MAPK. This effect does not appear to be mediated by the AT1 receptor, because all our experiments were carried out in the presence of losartan, which blocks the AT1 receptor (de Gasparo et al. 2000). Furthermore, AT1 receptor stimulation results in an increase rather than a decrease in neuronal excitability in our cultures. In addition, the inhibitory effect of renin is not likely to involve AT2 receptors, since their activation in our neuronal cultures is associated with an increase in neuronal excitability and inhibition of ERK1/2 (Huang et al. 1996; Zhu et al. 2001). Our data also show that the renin–PRR interaction is of physiological consequence, since it inhibits APF in neurons. Thus, it is reasonable to conclude that a neuronal PRR can mediate a signalling mechanism similar to that demonstrated for the neuronal AT1 receptor (Sun et al. 2003; Veerasingham & Raizada, 2003; Veerasingham et al. 2005).
Not with standing the significance of these observations, many questions remain regarding the neuronal PRR. First, do cardioregulatory brain regions of adult rat express PRR? Detailed analysis of PRR distribution in the brain is needed to provide an answer to this question. However, our preliminary data indicate that PRR mRNA is abundant in the paraventricular nucleus of adult rat brain and its levels are 40% higher than the cortex. Second, does PRR participate in the generation of Ang II at neuronal level, as has been demonstrated in renal tissues (Kobori et al. 2007)? Some studies are consistent with this idea. Neurons express both ACE and AT1 receptors (Hermann et al. 1988; Phillips & Sumners, 1998; Veerasingham & Raizada, 2003). Thus, it is reasonable to assume that PRR can bind prorenin/renin and can hydrolyse angiotensinogen, produced from astroglia (McKinley et al. 2003), to Ang I and Ang II by neuronal ACE in a domain that is close to or on the same neuron as the AT1 receptors. Such an efficient mechanism has been demonstrated in peripheral tissues (Ichihara et al. 2007; Nguyen, 2007). Moreover, the high efficiency of this mechanism would attenuate the weight of the argument of low renin in the brain. Thus, a small amount of renin would be highly efficient in a membrane-bound, solid phase enzymatic formation of Ang II. Finally, our data show that activation of PRR by renin inhibits APF. This was a surprising observation, because we expected that it would increase APF, as Ang II does in these neurons (Sun et al. 2003). The physiological relevance of these observations of renin receptor function and its in vivo implications remain to be worked out in further studies. In this regard, it is relevant to point out one caveat in our experimental system. We have used mixed neuronal cultures from hypothalamus–brainstem which contain functionally heterogeneous neuronal types. Thus, it is quite likely that the PRR may exert distinct effects on different brain regions. In addition, the possibility that PRR is primarily localized in the regions (such as caudal ventrolateral medulla and nucleus tractus solitarii) that exert inhibitory influences in cardiovascular control cannot be ruled out at the present time. In vivo experiments will be needed to clarify this issue.
In summary, our observations demonstrate that the PRR is expressed in brain neurons and that it is capable of signalling via MAPK and regulation of neuronal activity. Thus, this study is a critical first step in defining the role of this newest member of the RAS in neuronal control of cardiovascular function.
References
- Bader M, Peters J, Baltatu O, Muller DN, Luft FC, Ganten D. Tissue renin-angiotensin systems: new insights from experimental animal models in hypertension research. J Mol Med. 2001;79:76–102. doi: 10.1007/s001090100210. [DOI] [PubMed] [Google Scholar]
- Baltatu O, Lippoldt A, Hansson A, Ganten D, Bader M. Local renin-angiotensin system in the pineal gland. Brain Res Mol Brain Res. 1998;54:237–242. doi: 10.1016/s0169-328x(97)00339-2. [DOI] [PubMed] [Google Scholar]
- Burcklé CA, Jan Danser AH, Müller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension. 2006;47:552–556. doi: 10.1161/01.HYP.0000199912.47657.04. [DOI] [PubMed] [Google Scholar]
- Danser A, Batenburg W, van Esch J. Prorenin and the (pro)renin receptor—an update. Nephrol Dial Transplant. 2007;22:1288–1292. doi: 10.1093/ndt/gfl846. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Dzau VJ, Ingelfinger J, Pratt RE, Ellison KE. Identification of renin and angiotensinogen messenger RNA sequences in mouse and rat brains. Hypertension. 1986;8:544–548. doi: 10.1161/01.hyp.8.6.544. [DOI] [PubMed] [Google Scholar]
- Hermann K, Phillips MI, Hilgenfeldt U, Raizada MK. Biosynthesis of angiotensinogen and angiotensins by brain cells in primary culture. J Neurochem. 1988;51:398–405. doi: 10.1111/j.1471-4159.1988.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Huang XC, Richards EM, Sumners C. Mitogen-activated protein kinases in rat brain neuronal cultures are activated by angiotensin II type 1 receptors and inhibited by angiotensin II type 2 receptors. J Biol Chem. 1996;271:15635–15641. doi: 10.1074/jbc.271.26.15635. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-β1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69:105–113. doi: 10.1038/sj.ki.5000011. [DOI] [PubMed] [Google Scholar]
- Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114:1128–1135. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Itoh H. The (pro)renin receptor and the kidney. Semin Nephrol. 2007;27:524–528. doi: 10.1016/j.semnephrol.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Nishiyama A, Inagami T, Hayashi M. Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension. 2006;47:894–900. doi: 10.1161/01.HYP.0000215838.48170.0b. [DOI] [PubMed] [Google Scholar]
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- Lippoldt A, Fuxe K, Luft FC. A view of renin in the brain. J Mol Med. 2001;79:71–73. doi: 10.1007/s001090100215. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–918. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Sigmund CD. Angiotensin mutant mice: a focus on the brain renin-angiotensin system. Neuropeptides. 2002;36:194–200. doi: 10.1054/npep.2002.0894. [DOI] [PubMed] [Google Scholar]
- Nguyen G. The (pro)renin receptor: pathophysiological roles in cardiovascular and renal pathology. Curr Opin Nephrol Hypertens. 2007;16:129–133. doi: 10.1097/MNH.0b013e328040bfab. [DOI] [PubMed] [Google Scholar]
- Nguyen G, Delarue F, Burcklae C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–241. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Sumners C. Angiotensin II in central nervous system physiology. Regul Pept. 1998;78:1–11. doi: 10.1016/s0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Raizada MK, Lu D, Sumners C. AT1 receptors and angiotensin actions in the brain and neuronal cultures of normotensive and hypertensive rats. Adv Exp Med Biol. 1995;377:331–348. doi: 10.1007/978-1-4899-0952-7_23. [DOI] [PubMed] [Google Scholar]
- Sakai K, Sigmund CD. Molecular evidence of tissue renin-angiotensin systems: a focus on the brain. Curr Hypertens Rep. 2005;7:135–140. doi: 10.1007/s11906-005-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satofuka S, Ichihara A, Nagai N, Koto T, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K, Itoh H, Oike Y, Ishida S. Role of nonproteolytically activated prorenin in pathologic, but not physiologic, retinal neovascularization. Invest Ophthalmol Vis Sci. 2007;48:422–429. doi: 10.1167/iovs.06-0534. [DOI] [PubMed] [Google Scholar]
- Sellers KW, Sun C, Diez-Freire C, Waki H, Morisseau C, Falck JR, Hammock BD, Paton JF, Raizada MK. Novel mechanism of brain soluble epoxide hydrolase-mediated blood pressure regulation in the spontaneously hypertensive rat. FASEB J. 2005;19:626–628. doi: 10.1096/fj.04-3128fje. [DOI] [PubMed] [Google Scholar]
- Sumners C, Tang W, Zelezna B, Raizada MK. Angiotensin II receptor subtypes are coupled with distinct signal-transduction mechanisms in neurons and astrocytes from rat brain. Proc Natl Acad Sci USA. 1991;88:7567–7571. doi: 10.1073/pnas.88.17.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Du J, Sumners C, Raizada MK. PI3-kinase inhibitors abolish the enhanced chronotropic effects of angiotensin II in spontaneously hypertensive rat brain neurons. J Neurophysiol. 2003;90:3155–3160. doi: 10.1152/jn.00222.2003. [DOI] [PubMed] [Google Scholar]
- Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res. 2005;96:659–666. doi: 10.1161/01.RES.0000161257.02571.4b. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Hayakawa M, Nakagawa T, Nasir UM, Ebihara A, Iwasawa A, Ishida Y, Nakamura Y, Murakami K. Human prorenin has “gate and handle” regions for its non-proteolytic activation. J Biol Chem. 2003;278:22217–22222. doi: 10.1074/jbc.M302579200. [DOI] [PubMed] [Google Scholar]
- Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerasingham SJ, Yamazato M, Berecek KH, Wyss JM, Raizada MK. Increased PI3-kinase in presympathetic brain areas of the spontaneously hypertensive rat. Circ Res. 2005;96:277–279. doi: 10.1161/01.RES.0000156275.06641.b2. [DOI] [PubMed] [Google Scholar]
- Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu D, Yu K, Raizada MK. Regulation of neuromodulatory actions of angiotensin II in the brain neurons by the Ras-dependent mitogen-activated protein kinase pathway. J Neurosci. 1996;16:4047–4058. doi: 10.1523/JNEUROSCI.16-13-04047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Sumners C, Gelband CH, Posner P. Chronotropic effect of angiotensin II via type 2 receptors in rat brain neurons. J Neurophysiol. 2001;85:2177–2183. doi: 10.1152/jn.2001.85.5.2177. [DOI] [PubMed] [Google Scholar]