Abstract
Siglecs (Sialic acid-binding Immunoglobulin Superfamily Lectins) are cell surface signaling receptors of the I-type lectin group that recognize sialic acid-bearing glycans. CD33-related-Siglecs are a subset with expression primarily in cells of hematopoietic origin and functional relevance to immune reactions. Earlier we reported a human-specific gene conversion event that markedly changed the coding region for the extracellular domain of Siglec-11, associated with human-specific expression in microglia (Hayakawa T, Angata T, Lewis AL, Mikkelsen TS, Varki NM, Varki A. 2005. A human-specific gene in microglia. Science. 309:1693). Analyzing human gene microarrays to define new patterns of expression, we observed high levels of SIGLEC11 transcript in the ovary and adrenal cortex. Thus, we examined human and chimpanzee tissues using a well-characterized anti-Siglec-11 mouse monoclonal antibody. Although adrenal expression was variable and confined to infiltrating macrophages in capillaries, ovarian expression of Siglec-11 in both humans and chimpanzees was on fibroblasts, the first example of Siglec expression on mesenchyme-derived stromal cells. Cytokines from such ovarian stromal fibroblasts play important roles in follicle development and ovulation. Stable transfection of SIGLEC11 into a primary human ovarian stromal fibroblast cell line altered the secretion of growth-regulated oncogene α, interleukin (IL)-10, IL-7, transforming growth factor β1 and tumor necrosis factor-α, cytokines involved in ovarian physiology. Probing for Siglec-11 ligands revealed distinct and strong mast cell expression in human ovaries, contrasting to diffuse stromal ligands in chimpanzee ovaries. Interestingly, there was a trend of increased Siglec-11 expression in post-menopausal ovaries compared with pre-menopausal ones. Siglec-11 expression was also found on human ovarian stromal tumors and in polycystic ovarian syndrome, a human-specific disease. These results indicate potential roles for Siglec-11 in ovarian physiology and human evolution.
Keywords: cytokine, mast cells, microarray, ovarian stroma, PCOS
Introduction
Siglecs (Sialic acid-binding Immunoglobulin Superfamily Lectins) are a family of sialic acid-binding members of immunoglobulin superfamily (Varki and Angata 2006; Crocker et al. 2007; Varki and Crocker 2009). Sialic acids are found mostly at the outermost position of glycan chains on cell surface and secreted glycoproteins in the Deuterostome lineage of animals (Varki 2007). Previous studies showed that interactions between Siglecs and sialic acids are involved in host-pathogen interactions as well as in host self-recognition (Varki and Angata 2006; Crocker et al. 2007; Varki and Crocker 2009). Accumulating evidence indicates that Siglecs play critical roles in immune signaling and functions. In this regard, a subset of Siglecs, called CD33-related Siglecs (CD33rSiglecs), has received attention due to their rapid evolution in mammalian species and multiple human-specific changes (Angata et al. 2004; Varki 2010a). CD33rSiglecs are generally expressed in hematopoietic and immune cells including monocytes, macrophages, dendritic cells, neutrophils, eosinophils, basophils, mast cells and natural killer cells, with a cell type-specific expression pattern in a given species (Crocker et al. 2007).
So far, only one instance of the non-hematopoietic expression of CD33rSiglecs is known, the human-specific expression of Siglec-6 on placental trophoblast cells (Brinkman-Van der Linden et al. 2007) potentially playing roles in slowing the tempo of the human birth process and/or in a human disease called preeclampsia (Winn et al. 2009). Here, we investigated gene expression profiles for human CD33rSiglec genes using whole-genome transcript arrays. Such arrays provide global signatures of expression patterns of de novo predicted transcripts. A recently built gene expression atlas targeted 44,775 human genes, representing a panel of mRNAs derived from 79 human tissues and cell lines, and has become a valuable resource and a widely used database for candidate gene study or genome-wide analyses (Su et al. 2004). Using this tool, as well as a custom microarray developed by a co-author (Lee et al. 2005), we found the predicted expression of SIGLEC11 transcript in human ovary and adrenal gland. This was unexpected, given our previous finding of Siglec-11 expression primarily on macrophages in many other human tissues (Angata et al. 2002) and given that the ovary and adrenal are not known to be especially enriched in macrophages. We here explore the biological and physiological significance of these findings, in the light of unusual features of the human condition.
Results
Microarray analysis suggests SIGLEC11 expression in the adrenal gland cortex and ovaries
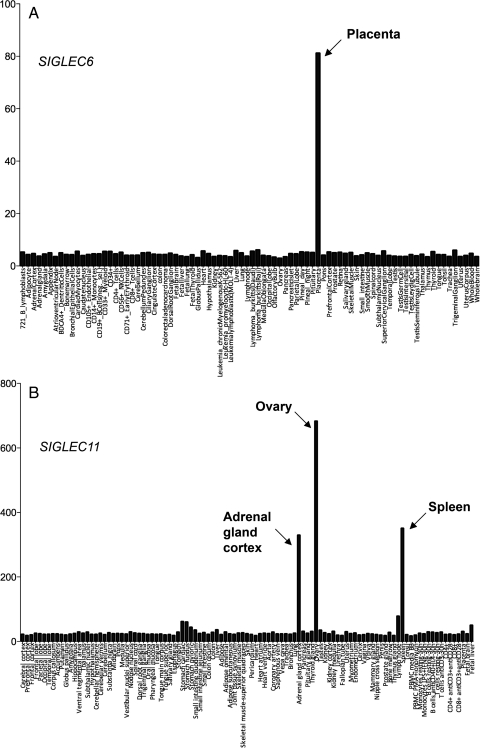
We used the web portal of Gene Atlas (http://biogps.gnf.org/) as well as an independent microarray Affymetrix U133 analysis (Lee et al. 2005) to check expression profiles for human CD33rSiglecs. Consistent with our previous report (Brinkman-Van der Linden et al. 2007), SIGLEC6 expression was prominently detected in the human placenta (Figure 1A). However, despite our previous report of human-specific microglial expression of Siglec-11 (Hayakawa et al. 2005), central nervous system (CNS)-derived tissues did not show elevated expression of this gene (Figure 1B). This is likely because transcript microarrays provide expression profiles for total homogenized tissues, and microglia represent only ∼1–2% of CNS cell types (Hanisch and Kettenmann 2007; Ransohoff and Perry 2009), with the signal thus being diluted by mRNAs from other CNS cell types. Surprisingly, we instead found high SIGLEC11 expression in human ovary and adrenal cortex based on the probe set 1552910_at of the custom array (Figure 1B). The expression was equivalent to, or greater than, that found in the spleen, where Siglec-11 is expressed on macrophages. Gene Atlas analysis also indicated clear expression of Siglec-11 in the adrenal gland and ovary (probe gnf1h01192; data not shown). Our earlier study using a Siglec-11-specific monoclonal antibody 4C4 had already reported macrophage-specific expression in multiple human tissues (Angata et al. 2002). Thus, we decided to characterize Siglec-11 expression in human ovary and adrenal gland using the same specific antibody.
Fig. 1.
Expression patterns of human CD33-related Siglec genes. (A) Expression profile of human Siglec-6 mRNA in 84 human tissues and cell lines, as acquired from BioGPS containing the HG_U133A/GNF1H gene atlas data set. Expression values are based on probe set 206520_x_at. (B) Expression profile of Siglec-11 mRNA in 105 human tissues and cell lines as acquired from microarray Affymetrix U133. Expression values are based on probe set 1552910_at.
Siglec-11 expression on ovarian stromal cells and adrenal macrophages
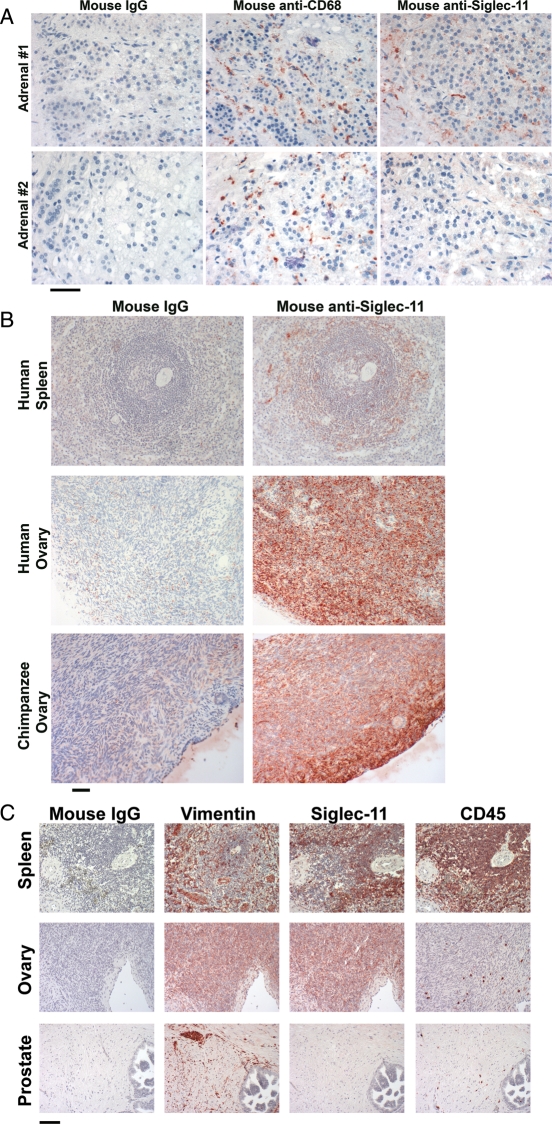
We next sought to demonstrate protein expression using the anti-Siglec-11 antibody 4C4. In keeping with our previous report (Angata et al. 2002), we confirmed that many tissues showed macrophage-specific staining (data not shown). In keeping with this, the strong adrenal cortex expression observed on microarray analysis was found to be present only in macrophage-like cells inside the capillaries of the glands (Figure 2A, upper panel). Furthermore, this expression varied widely between individual samples, with some samples being completely negative (see example in Figure 2A, lower panel). The reason for this variation is unknown, but these samples were all obtained at autopsy, and it is likely that major pathologies affect the adrenal cortex just prior to death, due to the systemic stress response, perhaps resulting in pre-mortem macrophage invasion related to autolysis. Interestingly, we found that expression of Siglec-11 in the ovary was not on the scattered hematopoietic cells (identified by CD45, a hematopoietic cell marker), but instead appeared to be on ovarian stromal cells, in both humans and chimpanzees (Figure 2B and C). This stromal cell expression was supported by parallel staining of vimentin (a stromal cell marker). In contrast, no expression of Siglec-11 was found on human prostate stromal cells (Figure 2C). We confirmed the presence of Siglec-11 on spleen and ovary using a commercially available biotinylated anti-human Siglec-11 polyclonal antibody and observed the same expression pattern (data not shown). To our knowledge, this is the first example of expression of a CD33rSiglec in a non-hematopoietic stromal cell of mesenchymal origin.
Fig. 2.
Detection of Siglec-11 protein expression in human and chimpanzee adrenals and ovaries. Mouse anti-Siglec-11 antibody 4C4 was used to detect the presence of Siglec-11 (shown in red) on paraffin-embedded tissue sections. The scale bar indicates 50 μm. (A) Expression of Siglec-11 is found in macrophage-like cells inside capillaries of human adrenal cortex (upper panel). Some adrenals had infiltrating macrophages but no expression of Siglec-11 (lower panel). Mouse IgG was used as the negative reagent control. CD68 is the macrophage marker. (B) Strong expression of Siglec-11 shows on ovarian stromal cells from human and chimpanzee ovaries. Human spleen sections are used as a positive control (macrophage staining only) and mouse IgG was used as the negative reagent control. (C) Comparison of Siglec-11 expression with Vimentin (stromal marker) and CD45 (hematopoietic cell marker) in human spleen, ovary and prostate. The spleen is the positive control for Siglec-11 expression on macrophages, and the prostate sample shows lack of non-specific staining on stromal cells in general.
Expression of Siglec-11 in ovarian stromal fibroblasts alters cytokine expression
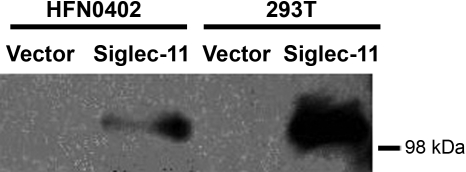
It is well known that ovarian cytokines play active roles in regulating ovarian cycle, ovulation, corpus luteum regression and follicular atresia (Vinatier et al. 1995). We obtained ovarian stromal fibroblast cell cultures from normal human ovaries (Quiros et al. 2008) and found that they did not express Siglec-11 at baseline growth by reverse transcriptase-PCR (data not shown). This is likely because Siglec-11 expression is a differentiated phenotype that is not maintained in tissue culture conditions. Regardless, this gave us the opportunity to ask if Siglec-11 expression alters cytokine production in these cells. We established stable expression of Siglec-11 in these cells by transfection of a pcDNA3.1-based Siglec-11 construct, followed by selection for G418 (Neo) resistance. Control cells were transfected with the pcDNA3.1 vector alone and selected identically with G418. Western blotting confirmed the expression of Siglec-11 in positively transfected cells (Figure 3). The delicate nature of these untransformed cells did not allow serum-free culture and also limited the stringency of G418 selection that could be used. However, immunohistology on formalin-fixed paraffin-embedded pellets of these cells showed that a minimum of 15–20% of cells are strongly transfected (data not shown). To ensure reproducibility of results, the primary human ovarian fibroblast cells were independently transfected twice, each time also with an empty vector control and each time subjected to the same G418 selection. The cytokine assays were also conducted twice in duplicates. The values from the duplicates were taken as average and used as one read for each assay. As shown in Table I, expression of Siglec-11 caused a statistically significant change in the pattern of cytokine secretion, specifically with up-regulation of growth-regulated oncogene α (GRO-α), interleukin (IL)-10, IL-7, transforming growth factor (TGF)-β1 and tumor necrosis factor (TNF)-α (P < 0.05), in comparison with the cells transfected with the empty vector control and selected with G418 in the same manner. The fact that stable transfection efficiency was not so high actually makes the difference even more likely to be significant.
Fig. 3.
Siglec-11 expression in transfected HFN0402 cells confirmed by western blot. Human ovarian fibroblast primary cell line HFN0402 was transfected with Siglec-11-pcDNA3.1 or vector only. G418 (100 µg/mL) was used to select positively transfected cells for 3 weeks. Transiently transfected 293T cells with Siglec-11-pcDNA3.1 or vector only were used as positive and negative controls, respectively. Cells were lysed with RIPA buffer (Cell Signaling Technology). Western blots of reduced cell lysates were probed with mouse anti-Siglec-11 antibody 4C4, and then goat anti-mouse IgG horseradish peroxidase.
Table I.
Effects of Siglec-11 expression on cytokine secretion by human ovarian stromal fibroblasts
| Cytokine | Siglec-11 (mean ± SD) | Control (mean ± SD) | Fold change | P-value |
|---|---|---|---|---|
| G-CSF | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.96 | 0.96 |
| GM-CSF | 0.05 ± 0.03 | 0.05 ± 0.03 | 1.04 | 0.69 |
| GRO | 0.92 ± 0.17 | 0.95 ± 0.04 | 0.98 | 0.81 |
| GRO-α | 0.96 ± 0.19 | 0.59 ± 0.27 | 1.61 | 0.01* |
| IFN-γ | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.85 | 0.69 |
| IL-10 | 0.24 ± 0.03 | 0.18 ± 0.01 | 1.32 | 0.03* |
| IL-13 | 0.02 ± 0.00 | 0.01 ± 0.01 | 1.66 | 0.41 |
| IL-15 | 0.01 ± 0.01 | 0.00 ± 0.01 | 1.29 | 0.69 |
| IL-1α | 0.07 ± 0.03 | 0.06 ± 0.02 | 1.19 | 0.60 |
| IL-2 | 0.01 ± 0.01 | 0.00 ± 0.01 | 2.61 | 0.47 |
| IL-3 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.75 | 0.17 |
| IL-5 | 0.00 ± 0.01 | 0.02 ± 0.01 | 0.04 | 0.05 |
| IL-6 | 1.03 ± 0.03 | 0.87 ± 0.13 | 1.18 | 0.08 |
| IL-7 | 0.12 ± 0.08 | 0.07 ± 0.07 | 1.68 | 0.01* |
| IL-8 | 0.86 ± 0.14 | 0.99 ± 0.07 | 0.87 | 0.27 |
| MCP-1 | 0.85 ± 0.08 | 0.88 ± 0.15 | 0.96 | 0.75 |
| MCP-2 | −0.02 ± 0.08 | 0.00 ± 0.01 | −4.89 | 0.65 |
| MCP-3 | 0.02 ± 0.03 | 0.00 ± 0.01 | 9.47 | 0.10 |
| MIG | 0.05 ± 0.03 | 0.03 ± 0.03 | 1.61 | 0.21 |
| RANTES | 0.31 ± 0.05 | 0.31 ± 0.03 | 0.99 | 0.77 |
| TGF-β1 | 0.04 ± 0.02 | 0.01 ± 0.01 | 3.71 | 0.01* |
| TNF-α | 0.16 ± 0.04 | 0.11 ± 0.04 | 1.44 | 0.02* |
| TNF-β | 0.04 ± 0.02 | 0.02 ± 0.01 | 1.71 | 0.12 |
G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; MCP, monocyte chemoattractant protein; MIG, monokine Induced by Interferon-gamma; RANTES, regulated upon activation, normal T cell expressed and secreted.
Human ovarian stromal fibroblast primary cell line HFN0402 was stably transfected in two independent experiments with Siglec-11-pcDNA3.1 or with vector only. In each case, selection was applied with G418 for 3 weeks. Collected supernatant from each transfected cell pool was assayed for various cytokine secretions in duplicate using Raybio human cytokine antibody array I (Raybiotech). Signal value for each cytokine was normalized by dividing with the average value of positive controls on each block. The mean and the standard deviation are derived from the reads (one read is the average of the duplicate) of two independent assays. Two-tailed P-values are estimated from a t-test.
*P < 0.05.
Different Siglec-11 binding sites in human and chimpanzee ovaries
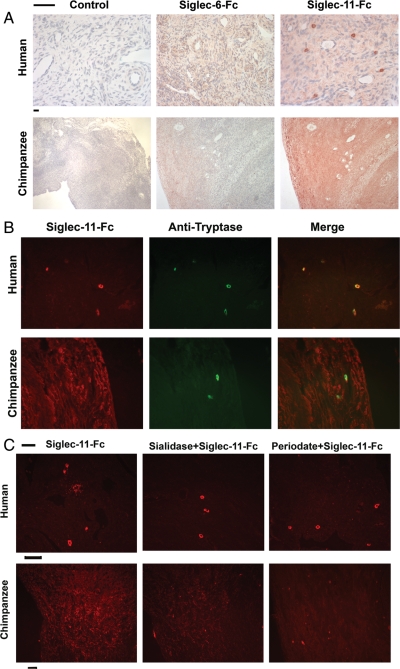
The functional roles of Siglec-11 in ovarian stromal cells are unknown, but are likely to be related to recognition of ligands present in the same tissue. The amino-terminal V-set domain of Siglec-11 is known to recognize sialic acids (Hayakawa et al. 2005). We used recombinant soluble human or chimpanzee Siglec-11-Fc chimera proteins to probe human or chimpanzee ovaries, respectively, for the presence of intrinsic Siglec-11 binding sites (potential ligands). While chimpanzee Siglec-11-Fc showed diffuse staining throughout the chimpanzee ovaries, human Siglec-11-Fc gave a markedly different pattern on human ovaries, showing only weak diffuse staining and strong staining of scattered cell types that had the appearance of mast cells (Figure 4A). The specificity of these interactions was further shown by lack of staining of either human or chimpanzee ovaries with a human Siglec-6-Fc chimera or IgG control (Figure 4A) or with a human Siglec-9-Fc chimera (not shown). This rules out Fc receptor binding or other non-specific interactions. Mast cell expression of human Siglec-11 ligands was confirmed by double staining with anti-tryptase antibody (Figure 4B). We also noted that human ovaries generally had larger numbers of mast cells than chimpanzee ovaries. Mast cells in other tissues such as tonsil and intestine were also labeled with human Siglec-11-Fc (data not shown).
Fig. 4.
Different Siglec-11 ligands in human and chimpanzee ovaries. Paraffin sections of human or chimpanzee ovaries were overlaid with human Siglec-11-FLAG-Fc or chimpanzee Siglec-11-FLAG-Fc, respectively. (A) Binding was detected using rabbit anti-FLAG and peroxidase-conjugated anti-rabbit IgG. Siglec-6-FLAG-Fc was used as a negative control for non-specific binding. Control slides had no added Siglec-Fc. The scale bars indicate 50 μm. Note that the human ovary slides are deliberately shown at a higher magnification, in order to highlight the bright staining of a few cells, which are shown below to be mast cells. (B) Siglec-11-FLAG-Fc binding was detected using rabbit anti-flag and Cy3-conjugated anti-rabbit IgG. Mast cells in human and chimpanzee ovaries were probed using a biotinylated mouse anti-human tryptase antibody, detected by Alexa-Fluor 488 labeled streptavidin. Rabbit IgG or mouse IgG was used as negative controls (not shown). The scale bar indicates 50 μm. (C) Sections were treated with sialidase or periodate before the overlay of Siglec-11-FLAG-Fc. Efficacy of both treatments was confirmed by loss of binding of SNA to the sections (not shown). The human Siglec-11 ligand on mast cells is not sensitive to either treatment. The chimpanzee Siglec-11 ligand on ovarian stroma is partially sensitive to both treatments.
As published previously a gene conversion event drastically changed the binding domain of human Siglec-11 after human/chimpanzee divergence (Hayakawa et al. 2005). Thus, cross-species studies of Siglec-11-Fc binding were not considered to be biologically meaningful. However, the fact that chimpanzee Siglec-11-Fc did not stain human mast cells (data not shown) indicates that the human-specific gene conversion event of Siglec-11 has generated new binding to potential ligands that already pre-existed in ancestral human mast cells. This finding may thus have relevance to the biology of potential mast cell interactions with Siglec-11 positive macrophages in other human tissues.
Sialic acid independence of Siglec-11 ligands in human ovaries
To ask if the human Siglec-11-Fc binding to ligands was sialic acid-dependent, we also used a human Siglec-11-Fc with R120A mutation that abrogates sialic acid binding. Interestingly, the mutant human protein could still recognize the endogenous human mast cell ligands with the same intensity as the wild-type protein (data not shown). Moreover, sialidase or mild periodate treatment before overlay of human Siglec-11-Fc had no impact on this mast cell staining (Figure 4C). The success of sialidase and periodate treatment was confirmed by the loss of binding of SNA (Sambucus nigra agglutinin, which recognizes terminal α2-6-linked sialic acids) on parallel tissue sections (data not shown). These results indicate that the interaction of human Siglec-11 and its ovarian ligands is not mediated by sialic acid recognition, but rather by protein–protein interactions and/or as yet unknown periodate-resistant glycans. In contrast, the diffuse staining on chimpanzee ovaries was partially sensitive to sialidase treatment or periodate treatment (Figure 4C).
Thus, there are two differences in Siglec-11 ligands between human and chimpanzee ovaries. First, while chimpanzees have diffuse Siglec-11 ligands throughout the ovary, human ligands are mostly confined to mast cells (which also appear increased in number). Second, while both sialic acid and non-sialic acid ligands are present in chimpanzee ovaries, the uniquely human mast cell ligands are sialic acid-independent.
Potential roles of Siglec-11 in human ovarian physiology and pathology
The marked difference between the Siglec-11 ligands in chimpanzee and human ovary suggest the possibility of unique human roles for ovarian Siglec-11. We noted a qualitative trend (data not shown) that while great ape (chimpanzee and gorilla) ovaries had uniform strong expression of Siglec-11, human ovary expression was much more variable. Thus, we also obtained initial data on the potential role of Siglec-11 in human ovarian pathologies. First, when we sorted 25 human ovarian samples based on the patient's age, there appeared to be a trend toward increased expression of Siglec-11 in older ages (data not shown). This is of interest because menopause was thought to be a human-specific process in comparison to the great apes (Varki and Altheide 2005). However, this has now become a controversial subject (Lacreuse et al. 2008; Atsalis and Videan 2009; Hawkes et al. 2009; Finch 2010; Finch and Holmes 2010; Hawkes 2010; Herndon and Walker 2010). Regardless, due to the somewhat subjective nature of histological quantitation and the technical challenges associated with obtaining quantitative western blot data from paraffin sections, we are not able to conduct a statistical significance analysis on the age-associated trend.
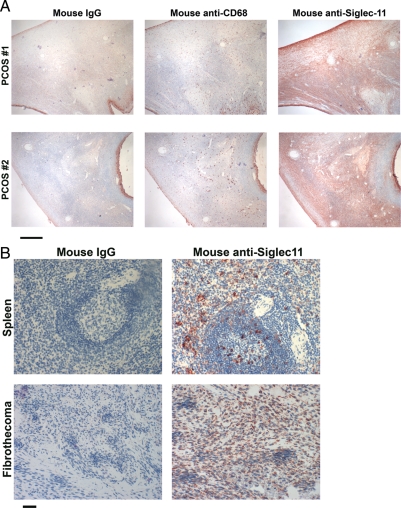
We also studied ovarian samples from patients with polycystic ovarian syndrome (PCOS), which is thought to be a human-specific disease (Mason et al. 2008; Yii et al. 2009; Glintborg and Andersen 2010), that can only be induced experimentally in other primates (DiGiacomo 1977; Zhou et al. 2005; Abbott et al. 2008; Abbott and Bird 2009). All 11 PCOS cases were from patients between the ages of 19 and 38, ages at which ovarian function is usually preserved in the unaffected population. We found strong Siglec-11 staining on stromal cells throughout the whole ovarian cortex surrounding the enlarged follicles in 9 of 11 of such samples (see Figure 5A for two examples). We also noted mast cell ligands for Siglec-11-Fc in PCOS ovaries, but with no obvious difference in pattern compared with normal ovaries (data not shown). Finally, we found clear expression of Siglec-11 in two samples of human ovarian stromal tumors (see Figure 5B for one example).
Fig. 5.
Expression of Siglec-11 in pathologic human ovaries. Paraffin sections of ovaries were overlaid with mouse anti-Siglec-11 antibody 4C4 to detect the presence of Siglec-11 (shown in red). Mouse IgG as the negative control. (A) Siglec-11 expression in human PCOS sample. CD68 is the macrophage marker. The scale bar indicates 500 μm. (B) Siglec-11 expression in one of two human ovarian stromal tumors. A spleen section is shown as a positive control. The scale bar indicates 50 μm.
Discussion
Siglec-11 belongs to a sialic acid-binding family of immunoglobulin superfamily lectins that are widely distributed throughout various cell types in the immune system of primates and rodents. We have previously shown the expression of Siglec-11 on tissue macrophages including microglial cells and infiltrating mononuclear leukocytes in inflammatory tissues (Angata et al. 2002; Hayakawa et al. 2005). Here, we report for the first time the high amount of SIGLEC11 mRNA in human ovarian tissues, based on the microarray expression data. Expression of Siglec-11 was then shown to be co-expressed with vimentin on ovarian stromal cells. Siglec-11 is known to interact with both SH2 domain-containing tyrosine phosphatase-1 (SHP-1) and SH2 domain-containing tyrosine phosphatase-2 (SHP-2). These phosphatases play critical roles in signaling pathways of innate immune responses against foreign antigens (Angata et al. 2002). The newly identified expression of Siglec-11 on ovarian stroma suggests the possibility of an unusual function in a cell type not in the immune system. This ovarian stromal expression of Siglec-11 is not human-specific since it was also observed in chimpanzee and gorilla ovaries. However, Siglec-11 ligands in the ovary show a very different distribution pattern between human and chimpanzee. While ligands for human Siglec-11 are located on mast cells only (which also seem increased in number), chimpanzee Siglec-11 ligands are diffusely present all over the chimpanzee ovary. Thus, there are potential implications for uniquely human aspects of ovarian physiology and pathology.
Ovaries have two interrelated functions: the production of gametes and the production of sex steroids. The majority of the cortex area in which ovarian follicles are distributed is occupied by ovarian stroma. Cortical stroma provides a microenvironment to support the development of follicles (Picton et al. 2008). A cyclic pattern of follicular maturation, ovulation and regression continues in parallel with the menstrual cycle before menopause. Although it has been noted that ovarian stroma is critical to support and nurture ovarian follicles, the functions and mechanisms provided by ovarian stromal cells in follicle maturation, menstrual cycle, menopause and ovarian disease have been addressed only to a limited extent. In the present study, we found that overexpression of Siglec-11 on ovarian stromal cells in vitro is able to cause significantly more secretion of TNF-α, IL-7, IL-10, TGF-β1 and GRO-α (Table I).
Cytokines appear to be important in folliculogenesis, ovulation and in the process of follicular atresia and corpus luteum regression through immune cell activation. TNF-α is one well-studied cytokine in mammalian ovaries, staying active through the whole menstrual cycle. During follicle maturation, TNF-α is able to guide the arrival of leukocytes into the developing follicles (Wang et al. 1992) by degrading collagens, as well as preventing premature luteinization of the follicle (Vinatier et al. 1995). During ovulation, TNF-α increases the local synthesis of prostaglandins, which is involved in ovulation and in the luteinization of granulosa cells (Vinatier et al. 1995). Moreover, TNF-α induces apoptosis of the endothelial cells of granulosa cells and of the luteal cells, finally leading to follicular atresia and luteolysis (Hughes and Gorospe 1991; Tilly et al. 1991). Like TNF-α, TGF-β1 is also a multifunctional cytokine in human ovary. First, TGF-β1 is active at the end of the follicular phase by facilitating differentiation of granulosa cells. Second, it stimulates progesterone production by promoting the differentiation of theca cells and maintains the corpus luteum. Third, like TNF-α, TGF-β1 is also able to induce apoptosis which leads to follicular atresia and luteolysis (Hughes and Gorospe 1991; Rotello et al. 1991; Tilly et al. 1991).
In the human ovarian cycle, the ovulation phase has features similar to an inflammatory reaction (Espey 1994). Histamine released by degranulation of the mast cells can facilitate the permeability of the endothelium and increase blood flow to the ovary accompanying the ovulation process (Vinatier et al. 1995). The concentration of histamine in the follicle was found to increase up to its maximum when ovulation occurs (Morikawa et al. 1981). Our study found that the ligand of Siglec-11 is located on human mast cells, which suggests the possibility that interaction between stromal Siglec-11 and its ligands on mast cells might stimulate the secretion of histamine right before ovulation. Another cytokine related to ovulation is GRO-α, which belongs to the C-X-C motif chemokine subfamily. An in vitro study showed that GRO-α is able to attract and activate neutrophils, as well as stimulate new blood vessel formation for the corpus luteum (Kawano et al. 2007). Proteolytic enzymes secreted by neutrophils, such as collagenase and elastase, can digest extracellular matrix proteins and conceivably contribute to the final stages of weakening the follicular wall that precedes ovulation (Kawano et al. 2007). Moreover, the function of neutrophils in ovulation has been supported by the observation that the number of ovulations is reduced in rats with neutrophils depleted by antineutrophil monoclonal antibodies (Brannstrom et al. 1995).
The marked difference of Siglec-11 ligands between human and chimpanzee raises the possibility of roles unique to human physiology and pathology. In the pathological human ovary, such as in PCOS and in ovarian stromal tumors, normal ovarian functions may be disturbed through immune cells and cytokine perturbation. PCOS is one of the most common female endocrine disorders affecting ∼5–10% of women of reproductive age and is thought to be one of the leading causes of female infertility (Mason et al. 2008; Yii et al. 2009; Glintborg and Andersen 2010). The PCOS ovary contains arrested follicles with disturbed oocyte development and failed ovulation. In the present study, we found strong expression of Siglec-11 in human PCOS ovaries. Interestingly, somewhat increased expression of Siglec-11 was also observed in post-menopausal ovaries compared with pre-menopausal ones. PCOS and menopause are two different conditions, but they share some common features such as perturbed follicle growth and fertility deficiency. It is possible that Siglec-11 on ovarian stroma might be involved in follicular atresia and corpus luteum regression by modulating secretion of certain cytokines. It has been shown that TGF-β1 can promote fibroblast collagen biosynthesis and is critical to the development of fibrosis in a mouse model (Ong et al. 2009). TNF-α might also play a pathophysiologic role in human PCOS and insulin resistance with its overexpression being found in some cases (Hotamisligil et al. 1993; Naz et al. 1995; Spaczynski et al. 1999). The source of this increased TNF-α in PCOS in uncertain, and our finding of increased TNF-α production by a stromal cell line expressing Siglec-11 is potentially relevant for further study. Finally, it is of note that Gro-1 links RAS (RAt Sarcoma) oncogene signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis (Yang et al. 2006).
Given the data, we speculate that expression of Siglec-11 on stromal cells of human ovaries might help create a unique microenvironment of cytokines or hormones for ovarian follicles and leukocytes, which is critical to the process of normal ovarian function and cycle and potentially for unusual aspects of human ovarian physiology and pathology, such as menopause and PCOS. Additional studies are obviously needed to analyze these possibilities further. Such studies are truly challenging, given that great ape tissue samples are very difficult to obtain (Varki 2010b), and the fact that variations during the menstrual cycle in humans are very hard to control for, as we are confined to studying the random ovarian samples that do become available from various surgical procedures. On the other hand, the SIGLEC11 gene is not present in rodents, making mouse models irrelevant. Future studies must also consider the fact that a small minority of humans can also express Siglec-16, an activatory Siglec that has a nearly identical N-terminal sequence to Siglec-11 (Cao et al. 2008).
Finally, this paper emphasizes that CD33-related Siglecs should no longer be thought of as just involved in the immune system. These molecules need to be searched for also in non-immune cell types, not only by gene expression and western blot analyses, but also by carefully controlled immunohistology, which can pick up minor cell-type expression patterns missed by techniques that evaluate whole-cell extracts.
Materials and methods
Ovarian samples and cells
In total, 25 normal human and 13 chimpanzee ovarian samples, 11 PCOS ovarian samples, 10 human adrenal samples and 2 human ovarian fibrothecoma samples were investigated in this study. Human spleen or tonsil tissues were used as positive controls in all immunohistochemistry analyses. All samples were sectioned from paraffin-embedded blocks. Human ovarian fibroblast primary cell line HFN0402 was generated as previously described by a co-author (Quiros et al. 2008).
Immunostaining for Siglec-11 expression
Paraffin sections were deparaffinized, rehydrated, blocked for endogenous peroxidases, endogenous biotin and non-specific binding to extracellular matrix and overlaid with appropriate concentrations of mouse anti-Siglec-11 antibody 4C4, mouse universal IgG (DAKO, North America), mouse anti-vimentin (DAKO) or mouse anti-CD45, anti-CD68 (DAKO) following established protocols (Angata et al. 2002). The CSA kit was purchased from DAKO. A biotinylated polyclonal goat anti-human-Siglec-11 antibody was purchased from R&D systems. For fluorescent labeling, the last step used Alexa-Fluor 488 labeled Streptavidin (Molecular Probes) or Cy3 labeled Streptavidin (Jackson Laboratories). These slides were viewed by epifluorescence using a Nikon Axiophot microscope, with appropriate filters, and photographed using a Spot Camera and imaging software. Images were analyzed using Adobe Photoshop. Brightfield images were captured using an Olympus Magnfire digital camera mounted on an Olympus BH2 microscope.
Immunostaining for Siglec-11 ligands
Paraffin sections were deparaffinized, blocked for endogenous non-specific sites and overlaid with recombinant human or chimpanzee Siglec-11-FLAG-Fc chimera (Angata et al. 2002) at 1 µg/mL. Control sections were overlaid with human Siglec-6-FLAG-Fc or Siglec-9-FLAG-Fc chimera at 1 µg/mL or with blocking buffer alone. Slides were incubated overnight at 4°C, followed by washes before incubation with Rabbit anti-FLAG (Sigma, St Louis, MO) at 1:500, further washes and then incubated with peroxidase-labeled anti-rabbit or Cy3-labeled anti-rabbit IgG at 1:500 (Jackson Laboratories). Sialidase treatment was done by incubating sections with AUS (250 mU/mL) in sodium acetate (pH 5.5) for 2.5 h at 37°C in humid chambers before applying the Siglec-11-FLAG-Fc. Periodate treatment was done by incubating sections with cold 2 mM sodium periodate in Phosphate buffered saline in the dark for 30 min at 4°C. After additional washes, if enzyme labels were used, appropriate substrates were added, nuclei were counterstained and the sections were aqueous mounted for brightfield viewing. Fluorescent-tagged slides were analyzed using epifluorescence as above. Mast cells were detected using either rabbit anti-human tryptase (AbD Serotec) or a biotinylated monoclonal anti-human tryptase antibody (Promega) at 1 μg/mL. Sections of tonsil or spleen were included as tissue controls. Double staining was done with fluorescent tags, as above.
Western blotting
Western blotting was done using mouse anti-Siglec-11 antibody 4C4 (1:100) as primary and peroxidase-conjugated goat anti-mouse IgG (1:7500; Jackson Laboratories) as the secondary following established protocols (Angata et al. 2002).
Human cytokine antibody array
A human cytokine antibody array containing 23 cytokine-specific antibodies was purchased from Raybiotech (Norcross, GA). According to the manufacturer, this approach has several advantages over enzyme-linked immunosorbent assay (ELISA). In addition to detecting many cytokines simultaneously, sensitivity is greatly increased and the detection range is much greater than ELISA. Finally, variation between two spots ranges from 0 to 10% in duplicated experiments. Human ovarian fibroblast HFN0402 was cultured in Dulbecco's modified eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cells were transfected in 10 cm plate with Siglec-11-pcDNA3.1 or pcDNA3.1 vector by lipofectamine 2000 following the recommended procedure from Invitrogen. Twenty-four hours after transfection, G418 (100 μg/mL) was used to select positively transfected cells. After 3 weeks selection, DMEM with 0.2% FBS was used to culture cells for 4 h. Then, cells were cultured in fresh DMEM with 0.2% FBS for 48 h. The conditioned supernatant was collected and overlaid on human cytokine antibody arrays without dilution. The protocol recommended by Raybiotech was followed. The signals were developed on Hyblot CL autoradiography film (Denville Scientific Inc.). Image analysis was carried out with Genepix Pro 6.0 software (Molecular Devices Corporation).
Funding
NIH (U01CA128442) and the Mathers Foundation of New York for fiscal support.
Conflict of interest
None declared.
Abbreviations
CNS, central nervous system; DMEM, Dulbecco's modified eagle's medium; ELISA, enzyme-linked immunosorbent assay; FBS, fetal bovine serum; GRO, growth-regulated oncogene; IL, interleukin; PCOS, polycystic ovary syndrome; Siglecs, Sialic acid-binding Immunoglobulin Superfamily Lectins; SNA, Sambucus nigra agglutinin; TGF, transforming growth factor; TNF, tumor necrosis factor.
Acknowledgements
We acknowledge the expert assistance of Peter Hevezi in the expression analyses, and the Yerkes Primate Center for providing samples.
References
- Abbott DH, Bird IM. Nonhuman primates as models for human adrenal androgen production: Function and dysfunction. Rev Endocr Metab Disord. 2009;10:33–42. doi: 10.1007/s11154-008-9099-8. doi:10.1007/s11154-008-9099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Zhou R, Bird IM, Dumesic DA, Conley AJ. Fetal programming of adrenal androgen excess: Lessons from a nonhuman primate model of polycystic ovary syndrome. Endocr Dev. 2008;13:145–158. doi: 10.1159/000134831. doi:10.1159/000134831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angata T, Kerr SC, Greaves DR, Varki NM, Crocker PR, Varki A. Cloning and characterization of human Siglec-11. A recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J Biol Chem. 2002;277:24466–24474. doi: 10.1074/jbc.M202833200. doi:10.1074/jbc.M202833200. [DOI] [PubMed] [Google Scholar]
- Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci USA. 2004;101:13251–13256. doi: 10.1073/pnas.0404833101. doi:10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsalis S, Videan E. Reproductive aging in captive and wild common chimpanzees: Factors influencing the rate of follicular depletion. Am J Primatol. 2009;71:271–282. doi: 10.1002/ajp.20650. doi:10.1002/ajp.20650. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Bonello N, Norman RJ, Robertson SA. Reduction of ovulation rate in the rat by administration of a neutrophil-depleting monoclonal antibody. J Reprod Immunol. 1995;29:265–270. doi: 10.1016/0165-0378(95)00941-d. doi:10.1016/0165-0378(95)00941-D. [DOI] [PubMed] [Google Scholar]
- Brinkman-Van der Linden EC, Hurtado-Ziola N, Hayakawa T, Wiggleton L, Benirschke K, Varki A, Varki N. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 2007;17:922–931. doi: 10.1093/glycob/cwm065. doi:10.1093/glycob/cwm065. [DOI] [PubMed] [Google Scholar]
- Cao H, Lakner U, de Bono B, Traherne JA, Trowsdale J, Barrow AD. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur J Immunol. 2008;38:2303–2315. doi: 10.1002/eji.200738078. doi:10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. doi:10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- DiGiacomo RF. Gynecologic pathology in the rhesus monkey (Macaca mulatta). II. Findings in laboratory and free-ranging monkeys. Vet Pathol. 1977;14:539–546. doi: 10.1177/030098587701400601. [DOI] [PubMed] [Google Scholar]
- Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. doi:10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- Finch CE. Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: Roles of infection, inflammation, and nutrition. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1718–1724. doi: 10.1073/pnas.0909606106. doi:10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Holmes DJ. Ovarian aging in developmental and evolutionary contexts. Ann N Y Acad Sci. 2010;1204:82–94. doi: 10.1111/j.1749-6632.2010.05610.x. doi:10.1111/j.1749-6632.2010.05610.x. [DOI] [PubMed] [Google Scholar]
- Glintborg D, Andersen M. An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecol Endocrinol. 2010;26:281–296. doi: 10.3109/09513590903247873. doi:10.3109/09513590903247873. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. doi:10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Colloquium paper: How grandmother effects plus individual variation in frailty shape fertility and mortality: Guidance from human-chimpanzee comparisons. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8977–8984. doi: 10.1073/pnas.0914627107. doi:10.1073/pnas.0914627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K, Smith KR, Robson SL. Mortality and fertility rates in humans and chimpanzees: How within-species variation complicates cross-species comparisons. Am J Hum Biol. 2009;5:5–10. doi: 10.1002/ajhb.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Angata T, Lewis AL, Mikkelsen TS, Varki NM, Varki A. A human-specific gene in microglia. Science. 2005;309:1693. doi: 10.1126/science.1114321. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Walker LC. The grandmother effect and the uniqueness of the human aging phenotype. Gerontology. 2010;56:217–219. doi: 10.1159/000253884. doi:10.1159/000253884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. doi:10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hughes FMJ, Gorospe WC. Biochemical identification of apoptosis (programmed cell death) in granulosa cells: Evidence for a potential mechanism underlying follicular atresia. Endocrinology. 1991;129:2415–2422. doi: 10.1210/endo-129-5-2415. doi:10.1210/endo-129-5-2415. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Furukawa Y, Fukuda J, Matsumoto H, Yuge A, Narahara H. The effects of platelet-activating factor on the secretion of interleukin-8 and growth-regulated oncogene alpha in human immortalized granulosa cell line (GC1a) Am J Reprod Immunol. 2007;58:434–439. doi: 10.1111/j.1600-0897.2007.00527.x. doi:10.1111/j.1600-0897.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Chennareddi L, Gould KG, Hawkes K, Wijayawardana SR, Chen J, Easley KA, Herndon JG. Menstrual cycles continue into advanced old age in the common chimpanzee (Pan troglodytes) Biol Reprod. 2008;79:407–412. doi: 10.1095/biolreprod.108.068494. doi:10.1095/biolreprod.108.068494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Hever A, Willhite D, Zlotnik A, Hevezi P. Effects of RNA degradation on gene expression analysis of human postmortem tissues. FASEB J. 2005;19:1356–1358. doi: 10.1096/fj.04-3552fje. [DOI] [PubMed] [Google Scholar]
- Mason H, Colao A, Blume-Peytavi U, Rice S, Qureshi A, Pellatt L, Orio F, Atkin SL. Polycystic ovary syndrome (PCOS) trilogy: A translational and clinical review. Clin Endocrinol (Oxf) 2008;69:831–844. doi: 10.1111/j.1365-2265.2008.03329.x. doi:10.1111/j.1365-2265.2008.03329.x. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Okamura H, Takenaka A, Morimoto K, Nishimura T. Histamine concentration and its effect on ovarian contractility in humans. Int J Fertil. 1981;26:283–286. [PubMed] [Google Scholar]
- Naz RK, Thurston D, Santoro N. Circulating tumor necrosis factor (TNF)-alpha in normally cycling women and patients with premature ovarian failure and polycystic ovaries. Am J Reprod Immunol. 1995;34:170–175. doi: 10.1111/j.1600-0897.1995.tb00934.x. [DOI] [PubMed] [Google Scholar]
- Ong VH, Carulli MT, Xu S, Khan K, Lindahl G, Abraham DJ, Denton CP. Cross-talk between MCP-3 and TGFbeta promotes fibroblast collagen biosynthesis. Exp Cell Res. 2009;315:151–161. doi: 10.1016/j.yexcr.2008.11.001. doi:10.1016/j.yexcr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction. 2008;136:703–715. doi: 10.1530/REP-08-0290. doi:10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- Quiros RM, Valianou M, Kwon Y, Brown KM, Godwin AK, Cukierman E. Ovarian normal and tumor-associated fibroblasts retain in vivo stromal characteristics in a 3-D matrix-dependent manner. Gynecol Oncol. 2008;110:99–109. doi: 10.1016/j.ygyno.2008.03.006. doi:10.1016/j.ygyno.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: Unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. doi:10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rotello RJ, Lieberman RC, Purchio AF, Gerschenson LE. Coordinated regulation of apoptosis and cell proliferation by transforming growth factor beta 1 in cultured uterine epithelial cells. Proc Natl Acad Sci USA. 1991;88:3412–3415. doi: 10.1073/pnas.88.8.3412. doi:10.1073/pnas.88.8.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaczynski RZ, Arici A, Duleba AJ. Tumor necrosis factor-alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61:993–998. doi: 10.1095/biolreprod61.4.993. doi:10.1095/biolreprod61.4.993. [DOI] [PubMed] [Google Scholar]
- Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. doi:10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly JL, Kowalski KI, Johnson AL, Hsueh AJ. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology. 1991;129:2799–2801. doi: 10.1210/endo-129-5-2799. doi:10.1210/endo-129-5-2799. [DOI] [PubMed] [Google Scholar]
- Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. doi:10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- Varki A. Colloquium paper: Uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci USA. 2010a;107(Suppl 2):8939–8946. doi: 10.1073/pnas.0914634107. doi:10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Fate of 'retired’ research chimps. Nature. 2010b;467:1047. doi: 10.1038/4671047e. doi:10.1038/4671047e. [DOI] [PubMed] [Google Scholar]
- Varki A, Altheide TK. Comparing the human and chimpanzee genomes: Searching for needles in a haystack. Genome Res. 2005;15:1746–1758. doi: 10.1101/gr.3737405. doi:10.1101/gr.3737405. [DOI] [PubMed] [Google Scholar]
- Varki A, Angata T. Siglecs–the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. doi:10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- Varki A, Crocker PR. In: Essentials of Glycobiology. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. pp. 459–474. I-type Lectins. [PubMed] [Google Scholar]
- Vinatier D, Dufour P, Tordjeman-Rizzi N, Prolongeau JF, Depret-Moser S, Monnier JC. Immunological aspects of ovarian function: Role of the cytokines. Eur J Obstet Gynecol Reprod Biol. 1995;63:155–168. doi: 10.1016/0301-2115(95)02227-9. doi:10.1016/0301-2115(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Brannstrom M, Robertson SA, Norman RJ. Tumor necrosis factor alpha in the human ovary: Presence in follicular fluid and effects on cell proliferation and prostaglandin production. Fertil Steril. 1992;58:934–940. doi: 10.1016/s0015-0282(16)55438-7. [DOI] [PubMed] [Google Scholar]
- Winn VD, Gormley M, Paquet AC, Kjaer-Sorensen K, Kramer A, Rumer KK, Haimov-Kochman R, Yeh RF, Overgaard MT, Varki A, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150:452–462. doi: 10.1210/en.2008-0990. doi:10.1210/en.2008-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Rosen DG, Zhang Z, Bast RCJ, Mills GB, Colacino JA, Mercado-Uribe I, Liu J. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci USA. 2006;103:16472–16477. doi: 10.1073/pnas.0605752103. doi:10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yii MF, Lim CE, Luo X, Wong WS, Cheng NC, Zhan X. Polycystic ovarian syndrome in adolescence. Gynecol Endocrinol. 2009;25:634–639. doi: 10.1080/09513590903015551. doi:10.1080/09513590903015551. [DOI] [PubMed] [Google Scholar]
- Zhou R, Bird IM, Dumesic DA, Abbott DH. Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:6630–6637. doi: 10.1210/jc.2005-0691. doi:10.1210/jc.2005-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]