Introduction
Many researchers use MAL (Maackia amurensis leukoagglutinin) and MAH (M. amurensis hemagglutinin), the seed lectins from M. amurensis, as glycoanalytical tools to probe biological targets for α2-3-linked sialic acids. The carbohydrate-binding specificities of these lectins have been carefully defined in several independent studies (Table I). However, investigators using M. amurensis lectins (MALs) for glycoanalysis often cite these specificities incorrectly (Table II). One reason for this discrepancy is that the M. amurensis seed lectins have been given a variety of different, but highly similar, names (Table I). This makes it particularly difficult to extract the correct binding specificities of these two distinct lectins (termed MAL and MAH herein) from the literature. Another reason for this discrepancy is that the technical specifications provided by some commercial vendors' cite binding specificities that differ from those defined in direct scientific studies (Tables I and II). Thus, researchers who rely on vendor's specifications can be misinformed. The confusion regarding the binding specificities of MAL and MAH and its impact on the accurate use of M. amurensis seed lectins for glycoanalysis was previously noted in two publications (Nicholls et al. 2007; Varki and Varki 2007). However, no communication has focused specifically on this topic. Having experienced serious confusion in this area, we thought it would be useful to review the binding properties of MAL and MAH and illustrate their utility as glycoanalytical tools in a controlled lectin-blotting assay.
Table I.
Maackia amurensis seed lectin-binding specificities defined by direct studies
|
MAL, MAM, MAL-I, MAA-1, MAA |
MAH, MAL-II, MAA-2, MAA |
|||
|---|---|---|---|---|
| Preferred binding substrates | Siaα2-3Galβ1-4GlcNAc†‡ (Refs 1–8) | Siaα2-3Galβ1-3(±Siaα2-6)GalNAc† (Refs 1, 10–14) | 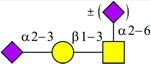 |
|
| SO4-3-Galβ1-4GlcNAc† (Refs 5,8) | SO4-3-Galβ1-3(±Siaα2-6)GalNAc† (Refs 12, 14, 15) | 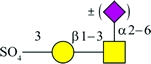 |
||
| SO4-3-Galβ (Refs 12, 14) | ||||
| Weak binding substrates | Galβ1-4GlcNAc† (Refs 4–6) | None clearly identified | ||
| SO4-3-Galβ (Refs 5, 8) | ||||
| Haptenic inhibitors | Neu5Acα2-3Galβ1-4Glc (Refs 3, 4, 6) | Galβ1-4Glc (lactose) (Ref. 9) | ||
| Galβ1-4Glc (lactose) (Refs 3, 4, 9) | ||||
| Galβ1-4GlcNAc (Ref. 4) | N-acetylneuraminic acid (Ref. 9) | |||
| N-acetylneuraminic acid (Ref. 9) | ||||
| Positive control | Murine laminin (used for purification) (bovine fetuin) | Human glycophorin A (also known as PSA-1) (bovine fetuin) | ||
References: 1, Imberty et al. (2000); 2, Johansson et al. (1999); 3, Kaku et al. (1993); 4, Knibbs et al. (1991); 5, Nicholls et al. (2007); 6, Wang and Cummings (1988); 7, Yamamoto et al. (2005); 8, CFG glycan array for MAL; 9, Kawaguchi et al. (1974b); 10, Brinkman-Van der Linden et al. (2002); 11, Konami et al. (1994); 12, Maenuma et al. (2008); 13, Maenuma et al. (2009); 14, CFG glycan array for MAH; 15, Bai et al. (2001).
†Some researchers investigated binding specificity using glycans with Glc instead of GlcNAc on the reducing end.
‡MAL also recognizes the structure (Siaα2-8)nSiaα2-3Galβ1-4GlcNAc, but not polysialic acid (Siaα2-8)n per se.
Table II.
Wide variety of M. amurensis seed lectin-binding specificities cited in glycoanalytical studies or by commercial vendors
| Lectin | Binding specificities |
|
|---|---|---|
| MAL | Galβ1-4GlcNAc (Refs 1–6) | |
| Neu5Acα2-3Gal (Refs 7–11) | ||
| Galβ1-3GlcNAc (Refs 7, 9, 12–15) | ||
| Neu5Acα2-3 (Ref. 16) | ||
| Terminal Galactose (Refs 9, 17) | ||
| N-acetylglucosamine (Ref. 18) | ||
| MAH | Neu5Acα2-3Gal (Refs 4, 8, 9, 19) | |
| Neu5Acα2-3 (Refs 5, 14, 15, 20, 21) | ||
| Neu5Acα2-3Galβ1-4GlcNAc (Ref. 3) | ||
| Neu5Acα2-3GalNAc (Ref. 9) | ||
| MAA‡ | Neu5Acα2-3 (Refs 22–24) | |
| Neu5Acα2-3Gal (Ref. 25) | ||
| Sia containing glycoconjugates (Ref. 26) | ||
References: 1, Brownlee et al. (2007)†; 2, Dorscheid et al. (1999); 3, Meagher et al. (2005); 4, Yamada et al. (2006); 5, Vector labs catalog 2010†; 6, Bio-world catalog 2010; 7, Carpenter et al. (1996); 8, Chen et al. (2008); 9, Giannasca et al. (1997); 10, Legardinier et al. (2005); 11, Ohyama et al. (2004); 12, Dalmasso et al. (2000); 13, Grange et al. (2002); 14, Tatsuzuki et al. (2009); 15, Axxora catalog 2010†; 16, Pan et al. (2002); 17, Bio-world catalog 2010; 18, Nozaki et al. (2007); 19, Vercoutter-Edouart et al. (2008); 20, Hennet et al. (1998); 21, Yao et al. (2008); 22, Jarvis and Finn (1995); 23, Marchal et al. (2001); 24, Pilobello et al. (2005); 25, Roche DIG Glycan Differentiation Kit; 26, Sigma-Aldrich catalog 2010.
†“Tolerates” Neu5Ac linked to carbon 3 of galactose.
‡MAA is an undefined mixture of MAL and MAH.
Literature review
The presence of hemagglutinating activity in M. amurensis seed extracts was first described in the early 1960s (Boyd et al. 1961). Hemagglutinating proteins from M. amurensis seeds were subsequently isolated as a first fraction with relatively more potent hemagglutinating activity, which was called MAH, and a second fraction with relatively more potent lymphocyte mitogenic activity, which was called MAM (M. amurensis mitogen; Kawaguchi et al. 1974b). Initial studies suggested that MAH bound preferentially to sialylated O-linked glycans, whereas MAM bound preferentially to sialylated N-linked glycans (Kawaguchi et al. 1974a, 1974b; Kawaguchi and Osawa 1976).
Later, MAM was found to bind strongly to leukocytes and re-designated MAL (Wang and Cummings 1987), which is the term we will use henceforth. Subsequent studies showed that MAL bound glycans with sialic acids in α2-3-, but not α2-6-linkages to galactose (Wang and Cummings 1988). This result was confirmed and extended by several investigators who observed that MAL bound most preferably to terminal Siaα2-3Galβ1-4Glc(NAc) in N-linked glycans (Knibbs et al. 1991; Kaku et al. 1993; Johansson et al. 1999; Imberty et al. 2000; Yamamoto et al. 2005; Nicholls et al. 2007). Nicholls et al. (2007) also recently found that MAL can bind to the unsialylated glycan, SO4-3-Galβ1-4Glc(NAc).
MAL and MAH have very similar amino acid sequences (86% identity) and probably have similar secondary and tertiary structures, as well (Yamamoto et al. 1994, 1997; Imberty et al. 2000). Thus, it is not surprising that their binding preferences are somewhat similar. Since its initial characterization, MAH was found to bind preferentially to an O-linked, disialylated tetrasaccharide with the structure Siaα2-3Galβ1-3(Neu5Acα2-6)GalNAc, in which the α2-6-linked Neu5Ac is not required for binding (Konami et al. 1994; Imberty et al. 2000; Brinkman-Van der Linden et al. 2002; Maenuma et al. 2008, 2009). Like MAL, MAH also can bind unsialylated structures, such as glycans containing SO4-3-Galβ1-3GalNAc (Bai et al. 2001; Maenuma et al. 2008).
A defined glycan array also has been used to examine the binding specificities of MAL and MAH, and the results have been posted on the public website of the Consortium for Functional Glycomics (http://www.functionalglycomics.org/). Generally, the glycan array results confirmed the results of other direct studies and identified some new binding substrates. For example, they showed that MAL can bind the polysialylated structure Neu5Acα2-8Neu5Acα2-8Neu5Acα2- 3Galβ1- 4Glc, indicating that this lectin tolerates substitution at C8 of Siaα2-3Galβ1-4Glc. In addition, the array results showed that MAH can bind to all structures containing SO4-3-Galβ, underscoring the ability of this lectin to bind sulfated glycans. Table I summarizes the results of direct studies on the binding specificities of MAL and MAH, including the glycan array studies, lists haptenic inhibitors of lectin binding and includes suggested controls for lectin blotting experiments.
As mentioned in the Introduction, the use of several different, but similar names for the M. amurensis seed lectins has contributed to the confusion surrounding their carbohydrate-binding specificities. For example, MAL is sometimes called MAL-I (e.g. Bai et al. 2001), MAA (M. amurensis agglutinin, e.g. Ohyama et al. 2004) or MAA-1 (e.g. Nicholls et al. 2007), and MAH is sometimes called MAL-II (e.g. Hennet et al. 1998), MAA (e.g. Nefkens et al. 2007) or MAA-2 (e.g. Nicholls et al. 2007). Furthermore, the terms MAA (indicating M. amurensis agglutinin) and MAL (indicating M. amurensis lectin) have been used to designate either the individual lectins or mixtures of both, often with no distinction. In fact, some vendors (e.g. EY Laboratories Inc., San Mateo, CA; Roche Diagnostics, Indianapolis, IN; Sigma-Aldrich Corporation, St Louis, MO) provide undefined mixtures of M. amurensis seed lectins that can contain unequal amounts of MAL and MAH (Nicholls et al. 2007; Varki and Varki 2007). The binding specificities of these mixtures encompass those of both lectins, but can be more similar to that of either one of the individual lectins (Nicholls et al. 2007). Table II summarizes some incorrectly cited binding specificities for MAL and MAH from the scientific and commercial literature that have contributed to the confusion surrounding the use of these lectins for glycoanalysis.
Control experiment
To demonstrate that MAL and MAH can be used effectively as glycoanalytical probes, we performed a lectin blotting assay with commercial bovine fetuin as the target. We chose fetuin because it is an inexpensive, readily available glycoprotein that has both N- and O-linked glycans with well-characterized structures. Bovine fetuin has N-linked glycans with terminal Siaα2-3Galβ1-4GlcNAc, Siaα2-6Galβ1-4GlcNAc and unsubstituted Galβ1-4GlcNAc residues (Green et al. 1988). It also has O-linked glycans that include extended core 1 structures comprised of Siaα2-3Galβ1-3±(Neu5Acα2-6)GalNAc (Spiro and Bhoyroo 1974; Edge and Spiro 1987; Royle et al. 2002). Thus, bovine fetuin has N-glycans that should be recognized by MAL and O-glycans that should be recognized by MAH. In addition, fetuin has glycans that can be recognized by Sambucus nigra agglutinin (SNA, binds terminal Siaα2-6Gal; Shibuya et al. 1987) or Ricinus communis agglutinin I (RCA-I, binds terminal β-linked galactose; Baenziger and Fiete 1979; Itakura et al. 2007), which were used as controls in our lectin blotting assay.
Bovine fetuin (Sigma-Aldrich Corporation) was dissolved in ddH2O at a concentration of 5 μg/μL. For untreated samples, the fetuin solution was simply diluted 1:5 in water. For desialylation, samples of the fetuin solution were treated with Salmonella typhimurium α2-3 sialidase (New England Biolabs, Ipswich, MA) according to the manufacturer's instructions. Briefly, 20 μL of the fetuin solution was incubated overnight at 37°C with 50 U of sialidase in a final volume of 100 μL of 50 mM sodium citrate (pH 6.0), 100 mM NaCl and 100 μg/mL bovine serum albumin (BSA). For de-N-glycosylation, samples of the fetuin solution were treated with Flavobacterium meningosepticum PNGase F (New England Biolabs) according to the manufacturer's instructions. Briefly, 20 μL of the fetuin solution was mixed with 50 μL of ddH2O, 10 μL of 10× reaction buffer and 10 μL of 10× glycoprotein denaturing solution and incubated at 100°C for 10 min. Ten microliters of 10% NP-40 and 500 U of PNGase F were added and the final reaction mixture was then incubated overnight at 37°C.
For analysis, 5 μL of each treated or untreated protein sample were mixed with sodium dodecyl sulfate polyacrylamide gel electrophoresis loading buffer (yielding 50 mM Tris, pH 6.8, 10% glycerol, 1% SDS, 0.01% bromophenol blue, 1% β-mercaptoethanol), and the mixtures were incubated at 65°C for 10 min. The samples were then separated on 10% SDS-polyacrylamide gels, transferred to Immobilon P membranes (Millipore, Bedford, MA) for 3 h at 70 V, and the membranes were blocked with MAL buffer [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.5, 150 mM NaCl, 0.2% BSA, 0.2% Tween-20, 0.08% NaN3] for 1 h at room temperature. The membranes were probed overnight with 10 mL of 1 μg/mL biotinylated MAH or MAL in MAL buffer or 10 mL of 1 μg/mL biotinylated SNA or RCA-I lectin in SNA buffer (10 mM HEPES, pH 7.5, 150 mM NaCl, 1% BSA, 0.1% Tween-20, 0.08% NaN3, 1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2). All biotinylated lectins were purchased from Vector Laboratories, Burlingame, CA, which markets the lectin designated MAL herein as MAL-I with a binding preference for Galβ1-4GlcNAc and the lectin designated MAH herein as MAL-II with a binding preference for α2-3-linked sialic acids. The membranes were washed thrice for 5 min with 10 mL of the respective lectin-binding buffers and then probed for 1 h with 10 mL of 1 μg/mL streptavidin-alkaline phosphatase (Vector Laboratories) in the same buffers. The membranes were washed thrice again for 5 min with 10 mL of their respective lectin-binding buffers, and signals were developed using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate according to an established method (Sambrook et al. 1989).
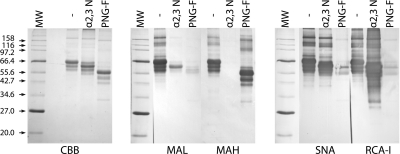
The lectin blotting assay results showed that MAL and MAH bound to untreated fetuin, as expected from its known glycan structures (Figure 1). Treatment with an α2-3-linkage-specific sialidase (Hoyer et al. 1991) slightly increased the electrophoretic mobility of fetuin, indicating that terminal sugars had been removed. The α2-3 sialidase treatment also strongly reduced MAL staining, reflecting the fact that Siaα2-3Galβ1-4GlcNAc is the preferred glycan-binding target for this lectin (Table I). Notably, a low level of residual MAL staining remained, illustrating that the underlying Galβ1-4GlcNAc moiety is a secondary binding target for MAL. The α2-3 sialidase treatment eliminated MAH staining, reflecting the fact that Siaα2-3Galβ1-3(±Neu5Acα2-6)GalNAc is the preferred glycan-binding target for this lectin (Table I). In contrast to the low level of residual binding observed with MAL, no residual binding was observed with MAH, illustrating that Galβ1-4GlcNAc is not a secondary binding target for this lectin. Importantly, the α2-3 sialidase treatment did not alter the level of SNA staining, confirming retention of the α2-6-linked sialic acids and, thereby, the α2-3-linkage specificity of the sialidase. The α2-3 sialidase treatment increased the level of RCA-I staining, as expected from the increased exposure of underlying β-linked galactose residues resulting from the removal of terminal sialic acids.
Fig. 1.
Lectin blots of untreated (−), S. typhimurium α2-3 sialidase treated (α2-3 N) or PNGase F (PNG-F) treated bovine fetuin. CBB, Coomassie brilliant blue stain; MAL, Maackia amurensis leukoagglutinin; MAH, Maackia amurensis hemagglutinin; SNA, Sambucus nigra agglutinin; RCA-I, Ricinus communis agglutinin I.
In addition to demonstrating their utility in detecting α2-3-linked sialic acids, the results shown in Figure 1 also demonstrated that one can use MAL and MAH to distinguish between N- and O-linked α2-3-sialylated glycans. Treatment with PNGase F, which removes only N-linked glycans (Tarentino et al. 1985), essentially eliminated MAL staining, illustrating the specificity of MAL for terminal Siaα2- 3Galα1-4GlcNAc moieties on N-linked glycans. The absence of residual MAL staining after PNGase F treatment highlighted the fact that this enzyme removes the entire N-glycan, including the secondary binding substrate Galβ1-4GlcNAc, not just the terminal α2-3-linked sialic acids. In contrast, treatment with PNGase F had no impact on the level of MAH staining, reflecting the specificity of this lectin for the O-linked glycan, Siaα2-3Galβ1-3(±Neu5Acα2-6)GalNAc, which cannot be removed by PNGase F. Like MAL staining, RCA-I and SNA staining were essentially eliminated by treatment with PNGase F, indicating that nearly all of their binding sites had been removed by this N-glycan specific reagent.
Conclusions
The binding preferences and specificities of the seed lectins from M. amurensis have been clearly defined in a wide variety of scientific studies and a clear understanding of this information is required in order to use these lectins effectively as probes for glycoanalytical assays. In addition to understanding their binding preferences and specificities, it is essential for investigators to know that other names have been used for the M. amurensis seed lectins designated MAL and MAH herein. Furthermore, it should be known that some scientific and commercial literature inaccurately or incorrectly cites their binding targets and that these lectins are sometimes sold as mixtures comprising different proportions of the two individual M. amurensis seed lectins.
Generally, MAL (also designated as MAM, MAL-I or MAA-1) should be used to detect N-linked or core 2 O-linked glycans containing the trisaccharide Siaα2-3Galβ1-4GlcNAc and MAH (also designated as MAL-II or MAA-2) should be used to detect O-linked glycans containing the trisaccharide Siaα2-3Galβ1-3GalNAc. Mixtures of MAL and MAH can be used to detect glycans containing α2-3-linked sialic acids, but they cannot be used to determine if the binding target is an N- or O-linked glycan. Furthermore, the binding assays must include sialidase-treated controls, which are required to determine if an observed MAL, MAH or MAL/MAH binding signal is specific for α2-3-sialylated glycans. Sialidase treatment will greatly reduce or eliminate the lectin-binding signal if it is specific for these carbohydrates. In contrast, sialidase treatment will have no impact on non-specific lectin–protein interactions or on MAL or MAH binding to glycans containing SO4-3-Galβ. Sialidase-insensitive MAL or MAH binding due to the presence of glycans containing SO4-3-Galβ can be assessed using additional approaches. For example, MAL or MAH signals resulting from SO4-3-Galβ binding in live cell surface staining assays should be reduced if the cells are grown in the presence of chlorate, an inhibitor of macromolecular sulfation (Bai et al. 2001). MAL or MAH signals resulting from SO4-3-Galβ binding in lectin blotting assays should be more completely reduced by desulfation and desialylation (e.g. by mild methanolysis; Slomiany et al. 1981) than by desialylation alone (Tsujii-Hayashi et al. 2002). In addition to using sialidase treatments as a negative control, a positive control should be included to validate negative signals if they are to be taken as an indicator of the absence of α2-3-sialylated glycans (see Table I for suggestions). However, negative results obtained using either MAL or MAH alone do not necessarily demonstrate the absence of α2-3 linked sialic acids, as a sample might contain α2-3-sialylated glycans that cannot be recognized by the individual lectins. For example, MAL would not stain samples that have α2-3-sialylated core 1 O-glycans, but lack α2-3-sialylated N-glycans and MAH would not stain samples that have α2-3-sialylated N-glycans, but lack α2-3-sialylated core 1 O-glycans. Finally, all experimental conditions (e.g. lectin and lectin target concentrations, blocking conditions, binding conditions and signal development times) need to be optimized to provide clear staining of the positive control in parallel with minimal or no staining of the negative control. In summary, MAL and MAH can serve as excellent glycoanalytical tools if one develops stringent experimental conditions, incorporates informative controls and clearly understands the glycan-binding specificities of these M. amurensis seed lectins.
Funding
This work was supported by Award Number R01GM49734 from the National Institute of General Medical Sciences.
Conflict of interest
None declared.
Abbreviations
BSA, bovine serum albumin; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; MAA, Maackia amurensis agglutinin; MAH, Maackia amurensis hemagglutinin; MAL, Maackia amurensis leukoagglutinin or Maackia amurensis lectin; MAM, Maackia amurensis mitogen; PNGase F, Peptide N-glycosidase F; SNA, Sambucus nigra agglutinin; RCA-I, Ricinus communis agglutinin I.
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
References
- Baenziger JU, Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979;254:9795–9799. [PubMed] [Google Scholar]
- Bai X, Brown JR, Varki A, Esko JD. Enhanced 3-O-sulfation of galactose in Asn-linked glycans and Maackia amurenesis lectin binding in a new Chinese hamster ovary cell line. Glycobiology. 2001;11:621–632. doi: 10.1093/glycob/11.8.621. doi:10.1093/glycob/11.8.621. [DOI] [PubMed] [Google Scholar]
- Boyd WC, Waszczenko-Zacharczenko E, Goldwasser SM. List of plants tested for hemagglutinating activity. Transfusion (Paris) 1961;1:374–382. doi: 10.1111/j.1537-2995.1961.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Brinkman-Van der Linden EC, Sonnenburg JL, Varki A. Effects of sialic acid substitutions on recognition by Sambucus nigra agglutinin and Maackia amurensis hemagglutinin. Anal Biochem. 2002;303:98–104. doi: 10.1006/abio.2001.5539. doi:10.1006/abio.2001.5539. [DOI] [PubMed] [Google Scholar]
- Brownlee IA, Knight J, Dettmar PW, Pearson JP. Action of reactive oxygen species on colonic mucus secretions. Free Radic Biol Med. 2007;43:800–808. doi: 10.1016/j.freeradbiomed.2007.05.023. doi:10.1016/j.freeradbiomed.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Carpenter GH, Proctor GB, Pankhurst CL, Linden RW, Shori DK, Zhang XS. Glycoproteins in human parotid saliva assessed by lectin probes after resolution by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Electrophoresis. 1996;17:91–97. doi: 10.1002/elps.1150170116. doi:10.1002/elps.1150170116. [DOI] [PubMed] [Google Scholar]
- Chen P, Liu Y, Kang X, Sun L, Yang P, Tang Z. Identification of N-glycan of alpha-fetoprotein by lectin affinity microarray. J Cancer Res Clin Oncol. 2008;134:851–860. doi: 10.1007/s00432-008-0357-7. doi:10.1007/s00432-008-0357-7. [DOI] [PubMed] [Google Scholar]
- Dalmasso AP, Benson BA, Johnson JS, Lancto C, Abrahamsen MS. Resistance against the membrane attack complex of complement induced in porcine endothelial cells with a Galα(1–3)Gal binding lectin: Up-regulation of CD59 expression. J Immunol. 2000;164:3764–3773. doi: 10.4049/jimmunol.164.7.3764. [DOI] [PubMed] [Google Scholar]
- Dorscheid D, Conforti A, Hamann K, Rabe K, White S. Characterization of cell surface lectin-binding patterns of human airway epithelium. Histochem J. 1999;31:145–151. doi: 10.1023/a:1003599916558. doi:10.1023/A:1003599916558. [DOI] [PubMed] [Google Scholar]
- Edge AS, Spiro RG. Presence of an O-glycosidically linked hexasaccharide in fetuin. J Biol Chem. 1987;262:16135–16141. [PubMed] [Google Scholar]
- Giannasca PJ, Boden JA, Monath TP. Targeted delivery of antigen to hamster nasal lymphoid tissue with M-cell-directed lectins. Infect Immun. 1997;65:4288–4298. doi: 10.1128/iai.65.10.4288-4298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange PA, Mouricout MA, Levery SB, Francis DH, Erickson AK. Evaluation of receptor binding specificity of Escherichia coli K88 (F4) fimbrial adhesin variants using porcine serum transferrin and glycosphingolipids as model receptors. Infect Immun. 2002;70:2336–2343. doi: 10.1128/IAI.70.5.2336-2343.2002. doi:10.1128/IAI.70.5.2336-2343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green ED, Adelt G, Baenziger JU, Wilson S, Van Halbeek H. The asparagine-linked oligosaccharides on bovine fetuin. Structural analysis of N-glycanase-released oligosaccharides by 500-megahertz 1H NMR spectroscopy. J Biol Chem. 1988;263:18253–18268. [PubMed] [Google Scholar]
- Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci USA. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. doi:10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer LL, Roggentin P, Schauer R, Vimr ER. Purification and properties of cloned Salmonella typhimurium LT2 sialidase with virus-typical kinetic preference for sialyl α2→3 linkages. J Biochem (Tokyo) 1991;110:462–467. doi: 10.1093/oxfordjournals.jbchem.a123603. [DOI] [PubMed] [Google Scholar]
- Imberty A, Gautier C, Lescar J, Pérez S, Wyns L, Loris R. An unusual carbohydrate binding site revealed by the structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides. J Biol Chem. 2000;275:17541–17548. doi: 10.1074/jbc.M000560200. doi:10.1074/jbc.M000560200. [DOI] [PubMed] [Google Scholar]
- Itakura Y, Nakamura-Tsuruta S, Kominami J, Sharon N, Kasai K-i, Hirabayashi J. Systematic comparison of oligosaccharide specificity of Ricinus communis agglutinin I and Erythrina lectins: A search by frontal affinity chromatography. J Biochem (Tokyo) 2007;142:459–469. doi: 10.1093/jb/mvm153. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Finn EE. Biochemical analysis of the N-glycosylation pathway in baculovirus-infected lepidopteran insect cells. Virology. 1995;212:500–511. doi: 10.1006/viro.1995.1508. doi:10.1006/viro.1995.1508. [DOI] [PubMed] [Google Scholar]
- Johansson L, Johansson P, Miller-Podraza H. Detection by the lectins from Maackia amurensis and Sambucus nigra of 3- and 6-linked sialic acid in gangliosides with neolacto chains separated on thin-layer chromatograms and blotted to PVDF membranes. Anal Biochem. 1999;267:239–241. doi: 10.1006/abio.1998.2982. doi:10.1006/abio.1998.2982. [DOI] [PubMed] [Google Scholar]
- Kaku H, Mori Y, Goldstein IJ, Shibuya N. Monomeric, monovalent derivative of Maackia amurensis leukoagglutinin. Preparation and application to the study of cell surface glycoconjugates by flow cytometry. J Biol Chem. 1993;268:13237–13241. [PubMed] [Google Scholar]
- Kawaguchi T, Matsumoto I, Osawa T. Studies on competitive binding of lectins to human erythrocytes. Biochemistry. 1974a;13:3169–3173. doi: 10.1021/bi00712a026. doi:10.1021/bi00712a026. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Matsumoto I, Osawa T. Studies on hemagglutinins from Maackia amurensis seeds. J Biol Chem. 1974b;249:2786–2792. [PubMed] [Google Scholar]
- Kawaguchi T, Osawa T. Elucidation of lectin receptors by quantitative inhibition of lectin binding to human erythrocytes and lymphocytes. Biochemistry. 1976;15:4581–4586. doi: 10.1021/bi00666a006. doi:10.1021/bi00666a006. [DOI] [PubMed] [Google Scholar]
- Knibbs RN, Goldstein IJ, Ratcliffe RM, Shibuya N. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J Biol Chem. 1991;266:83–88. [PubMed] [Google Scholar]
- Konami Y, Yamamoto K, Osawa T, Irimura T. Strong affinity of Maackia amurensis hemagglutinin (MAH) for sialic acid-containing Ser/Thr-linked carbohydrate chains of N-terminal octapeptides from human glycophorin A. FEBS Lett. 1994;342:334–338. doi: 10.1016/0014-5793(94)80527-x. doi:10.1016/0014-5793(94)80527-X. [DOI] [PubMed] [Google Scholar]
- Legardinier Sb, Klett Dl, Poirier J-C, Combarnous Y, Cahoreau C. Mammalian-like nonsialyl complex-type N-glycosylation of equine gonadotropins in Mimic™ insect cells. Glycobiology. 2005;15:776–790. doi: 10.1093/glycob/cwi060. doi:10.1093/glycob/cwi060. [DOI] [PubMed] [Google Scholar]
- Maenuma K, Yim M, Komatsu K, Hoshino M, Tachiki-Fujioka A, Takahashi K, Hiki Y, Bovin N, Irimura T. A library of mutated Maackia amurensis hemagglutinin distinguishes putative glycoforms of immunoglobulin A1 from IgA nephropathy patients. J Proteome Res. 2009;8:3617–3624. doi: 10.1021/pr800816w. doi:10.1021/pr800816w. [DOI] [PubMed] [Google Scholar]
- Maenuma K, Yim M, Komatsu K, Hoshino M, Takahashi Y, Bovin N, Irimura T. Use of a library of mutated Maackia amurensis hemagglutinin for profiling the cell lineage and differentiation. Proteomics. 2008;8:3274–3283. doi: 10.1002/pmic.200800037. doi:10.1002/pmic.200800037. [DOI] [PubMed] [Google Scholar]
- Marchal I, Jarvis DL, Cacan R, Verbert A. Glycoproteins from insect cells: Sialylated or not? Biol Chem. 2001;382:151–159. doi: 10.1515/BC.2001.023. doi:10.1515/BC.2001.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher CK, Liu H, Moore CP, Phillips TE. Conjunctival M cells selectively bind and translocate Maackia amurensis leukoagglutinin. Exp Eye Res. 2005;80:545–553. doi: 10.1016/j.exer.2004.11.005. doi:10.1016/j.exer.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Nefkens I, Garcia J-M, Ling CS, Lagarde N, Nicholls J, Tang DJ, Peiris M, Buchy P, Altmeyer R. Hemagglutinin pseudotyped lentiviral particles: Characterization of a new method for avian H5N1 influenza sero-diagnosis. J Clin Virol. 2007;39:27–33. doi: 10.1016/j.jcv.2007.02.005. doi:10.1016/j.jcv.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Nicholls JM, Bourne AJ, Chen H, Guan Y, Peiris JS. Sialic acid receptor detection in the human respiratory tract: Evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir Res. 2007;8:73. doi: 10.1186/1465-9921-8-73. doi:10.1186/1465-9921-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Shimotani T, Uchida K. Gonadotropin-like and adrenocorticotropin-like cells in the pituitary gland of hagfish, Paramyxine atami; immunohistochemistry in combination with lectin histochemistry. Cell Tissue Res. 2007;328:563–572. doi: 10.1007/s00441-006-0349-3. doi:10.1007/s00441-006-0349-3. [DOI] [PubMed] [Google Scholar]
- Ohyama C, Hosono M, Nitta K, Oh-eda M, Yoshikawa K, Habuchi T, Arai Y, Fukuda M. Carbohydrate structure and differential binding of prostate specific antigen to Maackia amurensis lectin between prostate cancer and benign prostate hypertrophy. Glycobiology. 2004;14:671–679. doi: 10.1093/glycob/cwh071. doi:10.1093/glycob/cwh071. [DOI] [PubMed] [Google Scholar]
- Pan T, Li R, Wong B-S, Liu T, Gambetti P, Man-Sun S. Heterogeneity of normal prion protein in two-dimensional immunoblot: Presence of various glycosylated and truncated forms. J Neurochem. 2002;81:1092–1101. doi: 10.1046/j.1471-4159.2002.00909.x. doi:10.1046/j.1471-4159.2002.00909.x. [DOI] [PubMed] [Google Scholar]
- Pilobello KT, Krishnamoorthy L, Slawek D, Mahal LK. Development of a lectin microarray for the rapid analysis of protein glycopatterns. ChemBioChem. 2005;6:985–989. doi: 10.1002/cbic.200400403. doi:10.1002/cbic.200400403. [DOI] [PubMed] [Google Scholar]
- Royle L, Mattu TS, Hart E, Langridge JI, Merry AH, Murphy N, Harvey DJ, Dwek RA, Rudd PM. An analytical and structural database provides a strategy for sequencing O-glycans from microgram quantities of glycoproteins. Anal Biochem. 2002;304:70–90. doi: 10.1006/abio.2002.5619. doi:10.1006/abio.2002.5619. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(α2–6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- Slomiany A, Kojima K, Banas-Gruszka Z, Slomiany BL. Structure of a novel sulfated sialoglycosphingolipid from bovine gastric mucosa. Biochem Biophys Res Commun. 1981;100:778–784. doi: 10.1016/s0006-291x(81)80242-2. doi:10.1016/S0006-291X(81)80242-2. [DOI] [PubMed] [Google Scholar]
- Spiro RG, Bhoyroo VD. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974;249:5704–5717. [PubMed] [Google Scholar]
- Tarentino AL, Gomez CM, Plummer TH. Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985;24:4665–4671. doi: 10.1021/bi00338a028. doi:10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Tatsuzuki A, Ezaki T, Makino Y, Matsuda Y, Ohta H. Characterization of the sugar chain expression of normal term human placental villi using lectin histochemistry combined with immunohistochemistry. Arch Histol Cytol. 2009;72:35–49. doi: 10.1679/aohc.72.35. doi:10.1679/aohc.72.35. [DOI] [PubMed] [Google Scholar]
- Tsujii-Hayashi Y, Kitahara M, Yamagaki T, Kojima-Aikawa K, Matsumoto I. A potential endogenous ligand of annexin IV in the exocrine pancreas. J Biol Chem. 2002;277:47493–47499. doi: 10.1074/jbc.M206572200. doi:10.1074/jbc.M206572200. [DOI] [PubMed] [Google Scholar]
- Varki NM, Varki A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab Invest. 2007;87:851–857. doi: 10.1038/labinvest.3700656. doi:10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercoutter-Edouart A-S, Slomianny M-C, Dekeyzer-Beseme O, Haeuw J-F, Michalski J-C. Glycoproteomics and glycomics investigation of membrane N-glycosylproteins from human colon carcinoma cells. Proteomics. 2008;8:3236–3256. doi: 10.1002/pmic.200800151. doi:10.1002/pmic.200800151. [DOI] [PubMed] [Google Scholar]
- Wang W-C, Cummings RD. An assay for leukoagglutinating lectins using suspension cultured mouse lymphoma cells (BW5147) stained with neutral red. Anal Biochem. 1987;161:80–84. doi: 10.1016/0003-2697(87)90654-3. doi:10.1016/0003-2697(87)90654-3. [DOI] [PubMed] [Google Scholar]
- Wang W-C, Cummings RD. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked α-2–3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–4585. [PubMed] [Google Scholar]
- Yamada S, Suzuki Y, Suzuki T, Le MQ, Nidom CA, Sakai-Tagawa Y, Muramoto Y, Ito M, Kiso M, Horimoto T, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature. 2006;444:378–382. doi: 10.1038/nature05264. doi:10.1038/nature05264. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ishida C, Saito M, Konami Y, Osawa T, Irimura T. Cloning and sequence analysis of the Maackia amurensis haemagglutinin cDNA. Glycoconj J. 1994;11:572–575. doi: 10.1007/BF00731308. doi:10.1007/BF00731308. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ito S, Yasukawa F, Konami Y, Matsumoto N. Measurement of the carbohydrate-binding specificity of lectins by a multiplexed bead-based flow cytometric assay. Anal Biochem. 2005;336:28–38. doi: 10.1016/j.ab.2004.09.030. doi:10.1016/j.ab.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Konami Y, Irimura T. Sialic acid-binding motif of Maackia amurensis lectins. J Biochem (Tokyo) 1997;121:756–761. doi: 10.1093/oxfordjournals.jbchem.a021650. [DOI] [PubMed] [Google Scholar]
- Yao L, Korteweg C, Hsueh W, Gu J. Avian influenza receptor expression in H5N1-infected and noninfected human tissues. FASEB J. 2008;22:733–740. doi: 10.1096/fj.06-7880com. doi:10.1096/fj.06-7880com. [DOI] [PubMed] [Google Scholar]