Abstract
Alzheimer’s disease (AD) was first described little more than 100 years ago. It is the most common cause of dementia with an estimated prevalence of 30 million people worldwide, a number that is expected to quadruple in 40 years. There currently is no effective treatment that delays the onset or slows the progression of AD. However, major scientific advances in the areas of genetics, biochemistry, cell biology, and neuroscience over the last 25 years have changed the way we think about AD and offer hope that, if the disease is detected prior to the onset of overt symptoms and signs, it is possible that treatments based on knowledge of underlying pathogenesis can and will be effective.
Overview
Alois Alzheimer, a German psychiatrist, reported at a meeting in Tübingen, Germany, in 1906 his clinicopathological study of a woman who presented with a “peculiar” dementia at age 51 years. Alzheimer correlated the woman’s cognitive and behavioral features with histopathological findings of “miliary foci” and neurofibrillary change in the cerebral cortex following her death at 55 years (1). In the 105 years since Alzheimer’s original case report and particularly in the past 3 decades, much has been learned about Alzheimer’s disease (AD). In this review, we will describe the clinical and pathological features of AD and highlight its importance as a global public health problem of immense proportions, both today and in decades to come. We will also discuss the challenges in translating scientific findings into new treatments to improve cognitive function, slow progression, delay the onset, and prevent AD. Studies of AD molecular pathology provided the findings that guided geneticists in the late 1980s to important initial discoveries. In turn, the basic understanding of AD started with the identification of genetic changes and their associated cellular and molecular consequences that are either causative or alter risk of developing AD Current and future genetic studies may lead to new insights into pathogenesis and therapeutic targets by gene identification using state of the art genomic techniques combined with biomarkers. AD is thought to be a disorder of protein aggregation in which the aggregation and accumulation in the brain of two proteins, amyloid-β (Aβ) and tau are key players in AD pathophysiology. However, there are many additional cellular pathways, processes, and molecules involved in AD pathogenesis that have emerged and will continue to be discovered, that play important roles in the disease. A key insight that is changing diagnostic and therapeutic approaches to AD is that pathological changes that underlie the brain degeneration and cognitive loss seen in patients with AD begin at least 10–20 years prior to dementia onset. Recent advances in the identification of biomarkers of AD have already made it possible to detect aspects of AD-pathology in cognitively normal individuals such as Aβ deposition and neurodegeneration. With scientific advances, promising potential therapies are being developed, but these therapies are not yet being tested in attempts to delay the onset or prevent the cognitive symptoms from appearing. A major challenge for the field will be to identify patient populations at high risk for converting from cognitively normal to impaired over a 3–4 year window and to target this population for clinical trials. It will be necessary to change the way we design clinical trials and incorporate the wealth of pathophysiological, genetic, and biomarker data to identify such high risk populations and to target them with mechanistically based therapies in order to have a reasonable chance to make a real impact on this devastating disorder.
Clinical features of AD
Dementia is an acquired syndrome characterized by a loss or decline in memory and other cognitive abilities. It represents a decline from a person’s previously established level of intellectual function that is sufficient to interfere with the everyday performance of that individual. AD is the most common cause of dementia, estimated to contribute to about 60–70% of cases (2). There are many other diseases, however, that can cause or contribute to dementia. The more common causes of dementia, in addition to AD, include cerebrovascular disease (strokes), Lewy body dementia, and frontotemporal dementia. Sometimes depression can be confused with the early phases of dementia and a large percent of patients with dementia are also depressed. Mixed dementia such as AD along with strokes or Lewy body dementia is common in the elderly. Currently, there are an estimated 5.3 million Americans with AD (www.alz.org) or ~ 1 in 8 people over the age of 65. Because of the amount of care required over extended periods of time for individuals with AD, the estimated cost of care in the US in 2010 for AD and other dementias was ~172 billion dollars. With an estimated worldwide prevalence of AD of ~30 million and a quadrupling of numbers expected by 2050, AD and other dementias are likely to become one of the most important global public health issues. These numbers are probably an underestimate as they do not include those with the earliest clinical stages of the illness.
When a person first presents for dementia evaluation, it is important to rule out structural brain lesions that can cause dementia, such as a brain tumor or subdural hematoma; other disorders such as thyroid disease, vitamin B12 deficiency, and chronic infections rarely may produce dementia. However, usually the clinical history of AD allows it to be distinguished from other dementias. The essential feature of AD, intra-individual decline in cognitive abilities, has an insidious onset over several months with subsequent gradual but relentless progression though successive stages of dementia severity. The time course of AD dementia averages 7–10 years and inevitably the illness culminates in death. Impaired recent memory usually is an initial symptom of AD but other cognitive deficits, such as executive dysfunction manifested by changes in attention and problem solving abilities, are typically also present. As dementia progresses, language dysfunction, visuospatial difficulty, loss of insight, and personality changes (withdrawal, decreased initiative, occasionally depression) frequently are apparent. Although close relatives and friends are aware that the affected individual has declined from previously attained levels of cognitive function, the person often maintains activities in the community (including driving a motor vehicle), although generally not as well as before, and is independent in self-care. Thus, the person may appear “normal” to casual acquaintances. This early stage of mild AD dementia usually lasts from 2–5 years. The moderate stage (lasting 2–4 years) is characterized by more obvious difficulty with memory (now including long term memory) and other cognitive functions and the loss of the ability to operate independently in the community. There also is impaired ability to function at routine tasks at home, such that even basic activities of daily living (e.g., dressing, bathing, grooming) require supervision or assistance. In the severe stage of Alzheimer dementia, individuals are totally dependent on caregivers for all activities of daily living and, in advanced disease, often become mute, non-ambulatory, and unable to swallow or control bladder and bowel function.
A useful staging system for dementia severity is the Clinical Dementia Rating, or CDR (3). The CDR designates individuals who are cognitively normal (CDR=0), very mildly impaired (CDR=0.5), mildly impaired (CDR=1), moderately impaired (CDR=2), or severely impaired (CDR=3). The CDR is derived from semi-structured interviews with the individual and independently from someone who knows them well (typically, a spouse or other close relative/friend) to capture intra-individual decline in cognitive and functional abilities (i.e., the patient is used as his or her own control). The obtained information is combined with the examination of the individual, then synthesized by the clinician to determine the presence or absence of dementia and, when present, its severity. This CDR has been useful for research studies as a global dementia rating scale and can track dementia progression in the clinic or in research. It should be noted that many but not all individuals rated as having very mild dementia (CDR 0.5) also meet criteria for what is termed mild cognitive impairment (MCI) (4). A large percentage of people diagnosed with MCI have AD as an underlying cause for their cognitive impairment although other disorders can also cause MCI.
The presence of AD-pathology such as amyloid plaques and neurofibrillary tangles (see section below on neuropathology and biomarkers) begin to accrue many years before the clinical symptoms and signs of very mild dementia or MCI that is due to underlying AD. Recent advances in fluid and imaging biomarkers can detect the presence or absence of AD pathology in humans. For example, with the use of radiolabeled molecular probes that can bind to amyloid plaques in the brain that can be imaged with positron emission tomography (PET) scans, the absence or presence and the amount of amyloid can be detected in the brain of living people (5, 6). In addition, the levels of the Aβ42 and tau proteins in the cerebrospinal fluid (CSF) can accurately determine the presence not only of amyloid deposition in the brain but also the likely presence of neurodegeneration (7). Though these tests are mostly being utilized for research purposes, they will likely be utilized more widely for clinical purposes in the near future for assisting in the diagnosis of patients with very mild dementia/MCI to determine if the cognitive impairment is most likely due to AD or another cause. These tests are already proving useful now for clinical trials. A challenge for the field is whether such tests should be utilized more widely now in patients or whether to restrict their use to clinical trials and clinical research until there is an effective disease modifying therapy.
Even at the very mild dementia (CDR 0.5)/MCI stage caused by AD, the neuropathology of AD already is extensive (8–10). In fact, by ~ age 75, ~25–30% of individuals who die when they are still cognitively normal (CDR 0) already have substantial Alzheimer’s type cerebral lesions (e.g., amyloid plaques and neurofibrillary tangles; neuronal loss) (Figure 1B–E) (10, 11). Based on these and other findings, it is estimated that AD pathology probably begins 10–20 years prior to the onset of clinical symptoms (7, 12). Thus, elucidating the initial events in the pathogenic cascade beginning and progressing all the way up to clinical disease onset is likely to be critical for understanding disease pathogenesis and for developing new treatments.
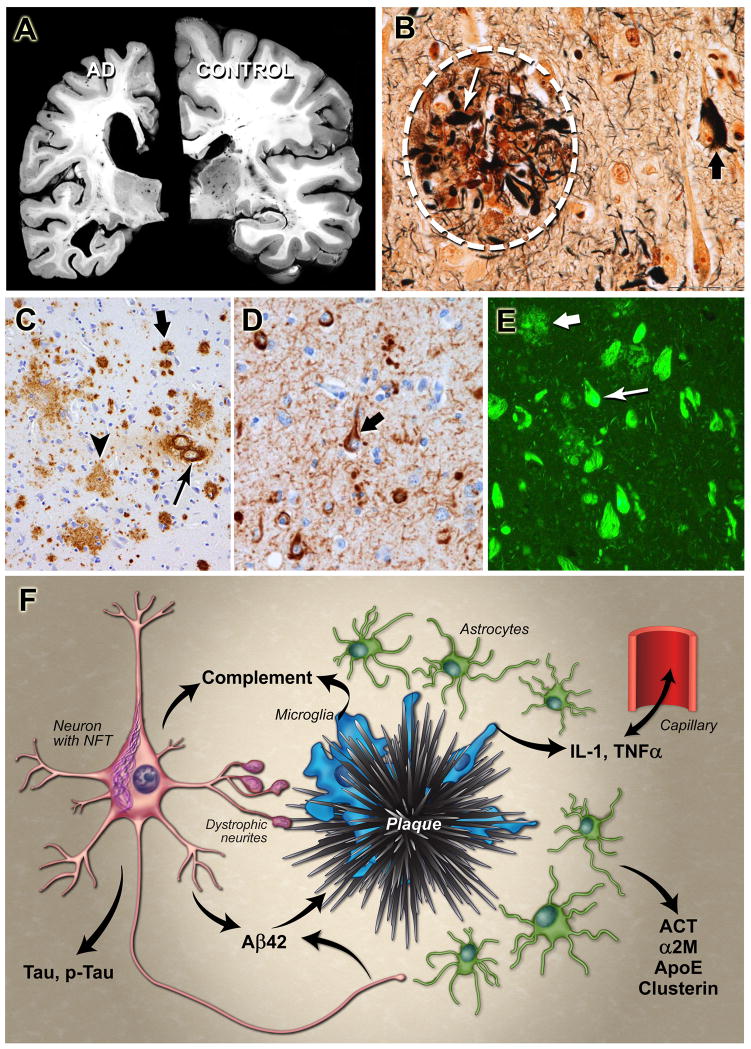
Figure 1. Pathology of Alzheimer’s disease (AD).
A. Postmortem brain section from an AD case (left) compared with that of a cognitively normal individual (right) reveals severe brain atrophy in AD. B. High power photomicrograph of a neuritic amyloid plaque (encircled by dashed white lines), with dystrophic neurites (small arrow) and a neurofibrillary tangle (large arrow) (photomicrograph imaged at 400X magnification; silver stain). C. Anti-Aβ antibody immunohistochemical staining of an AD brain reveals diffuse plaques containing Aβ (large arrow), compact plaques (medium size arrow) and cerebral amyloid angiopathy (smallest arrow) (photomicrograph imaged at 100X magnification). D. Anti-phospho tau antibody immunohistochemical staining reveals hyperphosphorylated tau accumulation in neuronal cell bodies (large arrow) and throughout the neuropil in neuropil threads (neuronal processes, smaller arrow, photomicrograph imaged at 200X magnification). E. Thioflavin-S stain reveals Aβ in a β-pleated sheet structure in amyloid plaques (larger arrow) and tau in a β-pleated sheet structure in neurofibrillary tangles (smaller arrow, photomicrograph imaged at 200X magnification). F. Diagram illustrating aspects of the neuropathology of AD. Aβ aggregates in the extracellular space of the brain in the form of plaques. The plaques are surrounded by astrocytes and microglial cells that secrete a variety of cytokines and other molecules including complement components, interleukin-1 (IL-1), tumor necrosis factor-α (TNFα), α-1 antichymotrypsin (ACT), α -2 macroglobulin (α2M), apolipoprotein E (apoE), and clusterin. Many neurons develop intracellular aggregates of tau in neurofibrillary tangles (NFT), and there is an increase in the amount of total tau and phosphorylated forms of tau (p-tau) into the CSF. Transport of molecules via capillaries and other blood vessels across the blood-brain barrier regulates a variety of AD-related processes. Photomicrographs in A–E courtesy of Nigel Cairns, Washington University. Panel F reprinted with permission from R. J. Perrin et al., Nature 461, 916–922 (2009).
The pathological process in AD
The key neuropathological elements of AD were described by Alois Alzheimer in 1906 and at about the same time by Oskar Fischer (13). At the macroscopic level, there is gross atrophy of the brain (Figure 1A). At the microscopic level, the hallmarks of the disease are amyloid plaques, neurofibrillary tangles, and extensive neuronal loss (Figure 1B–E). Amyloid plaques are accumulations of molecules in the extracellular space of the brain. The principal proteinaceous component of plaques is the amyloid-β (Aβ) peptide, a 38–43 amino acid peptide derived from a much larger protein, the amyloid precursor protein (APP) (Figure 2) (14, 15). Within plaques, Aβ is present in aggregated (insoluble) forms including fibrils as well as oligomers (16, 17). When Aβ is deposited and aggregated in a non-β sheet (non-fibrillar) conformation, it is detected via immunohistochemical techniques as “diffuse” plaques (Figure 1C). When Aβ is aggregated in deposits in the extracellular space in fibrillar forms with a β-sheet conformation as identified by electron microscopy, it also can be recognized by light microscopy with stains such as Congo red and Thioflavin-S (Figure 1E). These “neuritic” plaques are surrounded by swollen, degenerating neurites (axons and dendrites) (Figure 1B). In areas surrounding neuritic plaques, there is also “gliosis” with hypertrophy and an alteration of the morphology as well as the proliferation of astrocytes and microglia (immune cells of the CNS). It is likely that this inflammatory response contributes to brain injury although there is evidence that glial cells also play a protective role (18, 19) (Figure 1F). When Aβ aggregates are found in blood vessel walls independently of deposition in the neuropil (which occurs in many cases of AD), these deposits are called cerebrovascular plaques or cerebral amyloid angiopathy (CAA) (20)(Figure 1C). Although CAA occurs in some individuals without large numbers of parenchymal amyloid plaques, most often it accompanies the pathology of AD and can be a cause of lobar hemorrhage (bleeding into the cortex as opposed to deeper brain structures) and rarely inflammatory vasculitis (21). CAA also may contribute to ischemic damage to the brain (22).
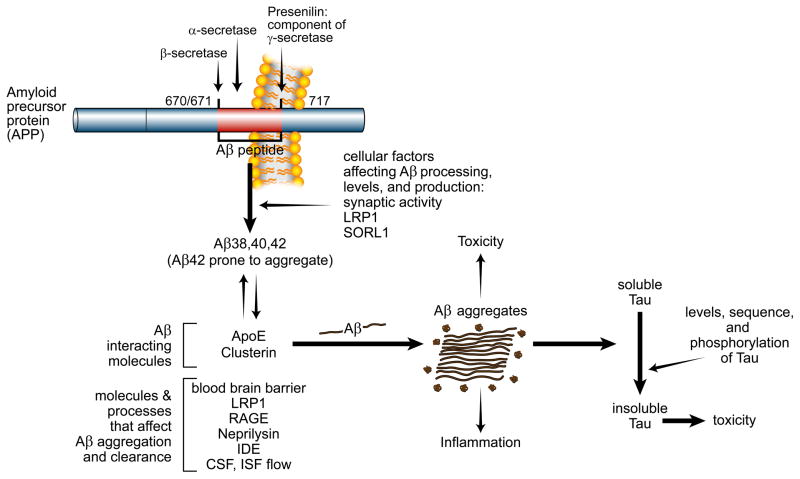
Figure 2.
Model of AD pathophysiology: How Aβ, tau, and other factors interact to contribute to the pathogenesis of AD. The Aβ peptide is produced from the transmembrane protein APP via cleavage by 2 enzymes, β-secretase and γ-secretase. Cleavage of APP by α-secretase prevents Aβ production. Presenilin is the active enzymatic component of the γ-secretase complex and cleaves APP at several sites within the membrane to produce Aβ peptides of different lengths such as Aβ38, Aβ40, and Aβ42. Several mutations in APP that are just outside of the Aβ region or within the coding sequence of Aβ cause forms of familial AD. Blue colored amino acids in APP/Aβ amino acid sequence represent the normal amino acids present in APP and the green colored amino acids below the normal sequence represent the amino acids that cause familial AD or CAA. Aβ is predominantly produced from APP within endosomes and a variety of molecules including the receptors LRP1 and SORL1 can influence Aβ levels. Synaptic activity also directly regulates Aβ levels. There is evidence that the Aβ aggregation is influenced by the Aβ binding molecules apoE and clusterin which likely interact in the extracellular space of the brain. A variety of molecules and processes affect Aβ clearance from the interstitial fluid (ISF) that is present in the extracellular space of the brain including neprilysin and insulin-degrading enzyme (IDE) as well as CSF and ISF bulk flow. LRP1 and RAGE appear to influence Aβ transport across the blood-brain-barrier. Aβ concentration and species (Aβ42 is more fibrillogenic) influences its aggregation. Once it aggregates into oligomers and fibrils, it can be directly toxic to cells, induce inflammation, and exacerbate the conversion of soluble tau to aggregated tau via unclear mechanisms. In addition to Aβ, a variety of factors influence tau aggregation and toxicity including tau levels, sequence, and its phosphorylation state.
In addition to the deposition of Aβ in plaques in AD, nerve cell bodies as well as their processes in specific brain regions develop neurofibrillary tangles (NFTs), neuropil threads, and neuritic dystrophy (Figure 1D–E) (23). NFTs (present in neuronal cell bodies) and neuropil threads (present in neuronal processes) are intracellular structures composed predominantly of a hyperphosphorylated, aggregated form of the microtubule binding protein, tau (24–27). Tau is synthesized and produced in all neurons and is also present in glia. The normal function of tau is to bind to tubulin and stabilize microtubules. However, in AD, tau becomes hyperphosphorylated and this form of tau disassociates from microtubules and has a tendency to self-aggregate forming NFTs in cell bodies and dystrophic neurites. These aggregates have a high β-sheet content that ultrastructurally appears as paired helical filaments (PHF) (28, 29). Data strongly suggest that neurofibrillary pathology contributes to neuronal dysfunction and correlates with the clinical progression of AD (Figure 2 and 3). Genetic, biochemical, and neurobiological studies suggest that it is the aggregation, change in conformation, and buildup of different forms of Aβ that play a central role in initiating AD pathogenesis and in directly damaging the brain, although a variety of additional changes, some of which may be downstream and others which may be independent of Aβ, appear to contribute to cognitive decline and progression of dementia. There is strong evidence that tau pathology contributes to clinical progression of AD; this is likely partly through pathways downstream of Aβ but there is also likely tau-related brain damage in AD that is independent of Aβ (30) (Figures 2 and 3).
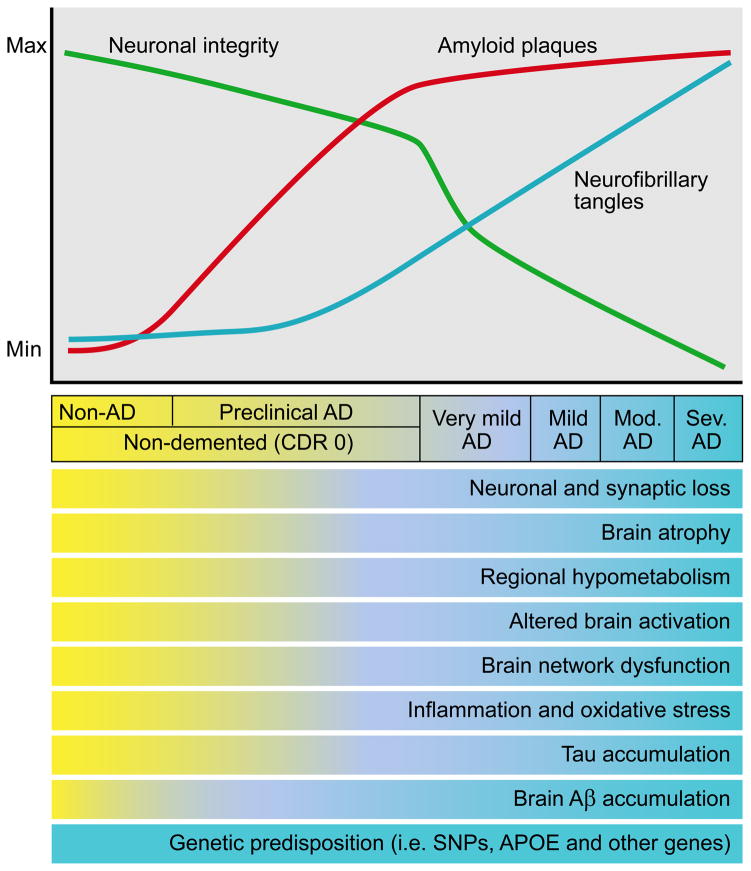
Figure 3.
Model of the time course of biomarker changes that occur in AD in relation to the cognitive and clinical changes. Over time, in cognitively normal people that is going to develop dementia due to AD, one of the first things that occurs is the initiation of Aβ aggregation in the brain in the form of amyloid plaques. Over years, while people are still cognitively normal, amyloid plaques continue to accumulate. At some point, perhaps ~ 5 years prior to any clearcut cognitive decline, tau accumulation begins to increase in the neocortex, inflammation and oxidative stress increase, and brain network connections and metabolism begin to decline. Neuronal and synaptic loss also begins to occur as well as brain atrophy. This period when AD-type pathology is building up yet a person is cognitively normal is termed “preclinical” AD. As these changes continue to accumulate, once there is enough neuronal and synaptic dysfunction as well as cell loss, very mild dementia becomes clinically detectable. At this time, amyloid deposition has almost reached its peak. As dementia worsens to mild, moderate (mod), and severe (sev) stages, there is increasing neurofibrillary tangle formation as well as increasing neuronal and synaptic dysfunction, inflammation, cell death, and brain atrophy. Modified figure reprinted with permission from R. J. Perrin et al., Nature 461, 916–922 (2009).
In addition to plaques and tangles, other neuropathological and neurochemical hallmarks of AD include loss of synapses and selective neuronal cell death as well as decreases in markers for certain neurotransmitters. Neurons that are particularly vulnerable in AD include those in layer II of the entorhinal cortex, the pyramidal layers (e.g. CA1) of the hippocampus, and certain areas of the temporal, parietal, and frontal neocortex. Although the majority of the vulnerable neurons use glutamate, the major neurotransmitter in the brain, there is also loss/dysfunction of certain subcortical projection neurons such as basal forebrain cholinergic neurons and noradrenergic neurons in the locus ceruleus. The dysfunction of cholinergic neurons (31, 32), which are involved in attention and memory, has been the basis for “cholinergic therapy” in AD. Centrally acting drugs that inhibit cholinesterase (the enzyme that breaks down the neurotransmitter acetylcholine) have been shown to provide modest symptomatic benefit in individuals with AD (33). There are also very modest symptomatic benefits, although limited to persons with moderate to severe AD, with memantine, a drug that modulates the NMDA subtype of glutamate receptors (34), among other properties. A challenge to the field is whether modification of other neurotransmitter systems and receptors can also offer symptomatic or other benefits in AD. A variety of systems/receptors are currently being tested in clinical trials.
It is important to note that much of the cognitive dysfunction in AD is not due to loss of one neurotransmitter but rather to disruption of network connections between several key brain regions within the limbic system and specific areas of the neocortex. One such network preferentially affected early in AD has been termed the “default mode network” (35–38). The selective vulnerability to Aβ deposition of the specific brain regions in this network, which appears to be involved with internally directed behaviors and has reduced neuronal activity during the performance of goal-directed tasks (39), may provide key insights into the process of Aβ aggregation as well as early clinical deficits seen in AD. A clue to the relative specificity for Aβ deposition in this network may be the fact that this network has the highest baseline neuronal activity in the resting state and that Aβ levels are regulated by neuronal activity (40, 41). There is also a typical progression of tau pathology within specific brain networks in normal aging and in AD, starting first in areas such as the brainstem and transentorhinal region (23, 42) and then spreading throughout the limbic system and other regions of the neocortex. Although there is only partial overlap of the location of Aβ and tau pathology, there is clear evidence of interaction between the Aβ and tau pathways. In addition, there is emerging evidence from model systems that aggregates of both Aβ and tau once formed, may spread from brain region to brain region and from cell to cell in a prion-like fashion (43–46). For example, injections of human AD brain or mouse brain lysates that containing human Aβ aggregates into the brain of mice genetically modified to develop Aβ-containing amyloid plaques and CAA results in a much earlier onset of Aβ-related pathology than would otherwise occur (44). Further, the Aβ aggregates that deposit take on morphological characteristics of both the injected Aβ as well as the host Aβ. In the case of tau, injections of brain extract from genetically modified mice that have tau aggregates into the brain of mice that express wild-type human tau that would otherwise not develop tau aggregates results in assembly of wild-type tau into filaments and spreading of pathology from the site of injection to adjacent brain regions (46). Recent cell biological studies with tau demonstrate directly that tau aggregates that form in one cell can move to adjacent cells and cause the host cell’s tau to aggregate (43) A challenge to the field is to determine whether this mechanism of spreading of protein aggregation plays a major role in disease progression. If so, it will have important implications for developing new treatments.
AD genetics and link to pathophysiology
Despite the discovery by Alois Alzheimer in 1906 that “plaques” were a major feature of the neuropathology of AD, the identity of the main component of plaques remained unknown for 80 years. In 1984, the predominant proteinaceous component of CAA was isolated and termed the “β-protein” (47). In 1985, the same protein was isolated from amyloid plaque cores from patients with AD and Down’s syndrome (48). This led to the hypothesis that the gene encoding Aβ would be on human chromosome 21. It was already known at that time that 100% of individuals with Down’s syndrome caused by complete trisomy of chromosome 21 develop AD neuropathology with marked Aβ deposition in the brain by age 35. Subsequent studies showed that most if not all of these individuals develop dementia with a mean age of onset of 50 years (49, 50). The hypothesis that the gene encoding Aβ was located on chromosome 21 was shown to be correct when several groups cloned the cDNA encoding Aβ and found the coding sequence to be a small part of a much larger precursor protein termed the amyloid precursor protein (APP) (51–53). APP is an integral membrane protein that spans the membrane once (Figure 2). There are several isoforms of APP, the most common being 695, 751, or 770 amino acids in length. The 751 and 770 amino acid isoforms of APP contain a Kunitz protease inhibitor domain. These 2 isoforms are expressed preferentially outside the brain and these forms of APP function in the plasma in the coagulation pathway (54, 55). APP is highly expressed in the CNS, particularly by neurons. Aβ is derived from APP through the action of two proteases, β-secretase (BACE) and γ-secretase, and is normally secreted by many cell types (Figure 2). Aβ is relatively abundant in the cerebrospinal fluid (CSF) that bathes the CNS being present at concentrations of 10–20 ng/ml; it is present at much lower levels in the plasma (56–58). The finding that Aβ is normally present in physiological fluids suggested for the first time that physiological processing of APP occurred and set the stage for understanding the genetics of familial AD (FAD), see below. Amino acids 1–28 of Aβ are derived from a region of APP just outside the membrane; however, amino acids 29–43 of Aβ are derived from within the membrane-spanning region. There are 3 major sites within APP in which proteolysis occurs (Figure 2). If proteolysis occurs at the sites of β-secretase and γ-secretase action, Aβ is generated (Figure 2). If proteolysis occurs at the site of α-secretase action, Aβ cannot be generated but a smaller fragment (P3, Aβ 17–40 or Aβ17-42) is produced. Cleavage of APP by γ-secretase can occur at different sites within the membrane generating Aβ species that end in residues 38, 39, 40, 42, and 43 at the Aβ C-terminus as well as to a lesser extent, species that are shorter and longer. APP is also cleaved by γ-secretase at the ε site within the membrane at position 49 in relation to the Aβ sequence. This cleavage liberates an APP intracellular fragment (AICD) that appears to have a transcriptional function (59). The most abundant Aβ species produced in the brain and found in the CSF is Aβ1-40 (Aβ40). Aβ1-42 (Aβ42), is generally present in tissues and body fluids at levels 5–10% of those of Aβ40, yet it appears to be central to initiating Aβ aggregation as it is more hydrophobic and prone to aggregate.
Clinically, most cases of symptomatic AD begin after age 65 years with incidence increasing with age. These cases are often referred to as “sporadic” or “late-onset” AD (LOAD). This form of AD accounts for >99% of all cases. Genetic factors contribute strongly to the risk of developing LOAD. In addition to LOAD, a very small percentage (<1%) of AD occurs within families and is inherited in an autosomal dominant fashion and are termed FAD. In these families, dementia onset is usually between the ages of 30–60 years. APP was identified as the first gene in which mutations in the coding sequence cause either FAD, CAA, or a combination of the 2 disorders (60–62). The location of these mutations within the APP gene provided an important clue regarding the likely mechanism leading to increased Aβ accumulation in the brain in FAD.
Missense mutations in APP located at or near the sites of APP endoproteolysis by β and γ-secretases cause FAD (60, 63, 64), or a disease with CAA and cerebral hemorrhage (61, 65–67). Mutations near the C-terminus of the Aβ region, adjacent to the γ-secretase site lead to an increase in the ratio of Aβ42 to Aβ40 without having a significant effect on total Aβ levels (68, 69). Although Aβ40 is the most abundant Aβ species generated, Aβ42 is more fibrillogenic (70) and appears to be the species of Aβ that forms deposits first in AD (71). Thus, increased Aβ42 production leading to an increase in the Aβ42 to Aβ40 ratio caused by these mutations is likely over time to be central to AD pathogenesis. In contrast, another mutation, the APP Swedish FAD mutation (identified in a Swedish family with FAD), occurs near the β-secretase cleavage site (72). This mutation leads to increased production of all species of Aβ, and is associated with both AD and CAA (73, 74). Support for increased Aβ production leading to AD also comes from individuals with Down’s syndrome and from FAD families carrying a small duplication of chromosome 21 that encompasses the region containing the APP gene (75). Importantly, each of these families has a different duplication but the region of overlap contains the APP gene.
Some of the APP mutations that lead to FAD and CAA do not increase Aβ production and are located within the Aβ peptide itself. These mutations are C-terminal to the α-secretase cleavage site in APP and change the sequence of Aβ such that it increases the propensity of Aβ to oligomerize, fibrillize, or be cleared less effectively (66, 67, 76). Another recently described APP mutation exhibits autosomal recessive inheritance of AD and results in the deletion of one amino acid at position 22 in Aβ that results in a peptide that is markedly fibrillogeneic (77, 78). There is no current evidence that normal APP function is impaired by the APP mutations that cause FAD. This may be due to the fact that the function of APP is related to the regions of the intracellular and extracellular domains that do not include the Aβ region. In addition, there are homologues of APP such as APLP1 and APLP2 that likely provide some redundancy to APP function in vivo (79). Due to our current lack of full understanding of APP biology, it remains a formal possibility that APP mutations that cause familial AD do cause or contribute to AD not only via a mechanism(s) related to influencing Aβ metabolism. For example, although the normal function of APP is still not resolved, recent evidence suggests that APP plays a role in axonal pruning and neuronal migration during nervous system development (80, 81) as well as contributing to certain cognitive behaviors such as learning and memory (82). In particular, an N-terminal cleavage fragment of APP is important for its effects during development on axonal pruning (80). A challenge to the field will be to determine whether APP mutations that cause familial AD influence this N-terminal or other APP fragments to affect AD pathogenesis independently of Aβ. Whether Aβ itself, in a non-aggregated form, plays a normal function in the brain is as yet unresolved and remains another important area of inquiry. Though the amino acid sequence of APP is highly homologous throughout evolution, the sequence of the Aβ region changes greatly during evolution, even within mammals. This would seem to argue that APP is likely to have a normal function but argues against a normal function for monomeric Aβ. Intriguingly, there is evidence that Aβ generation is regulated in part by neuronal activity itself (40, 41), and in some experimental models, certain forms of Aβ appear to be able to suppress neuronal activity through interactions with glutamate receptors (83). If it turns out that monomeric Aβ that is produced by neurons throughout life does play a role in normal neuronal function, this may provide a challenge to target Aβ therapeutically.
The connection between APP, Aβ, and presenilins
In addition to APP, mutations in 2 other genes have been identified in which specific mutations result in the rare, autosomal dominant forms of FAD: presenilin-1 (PSEN1) (84) and presenilin-2 (PSEN2) (85). These genes encode highly homologous transmembrane proteins in which multiple mutations have been identified in FAD families (http://www.alzforum.org/res/com/mut/pre/default.asp; http://www.molgen.ua.ac.be/ADMutations/). Mutations in PSEN1 are the most common identified cause of FAD. Although mutations in APP, PSEN1 and PSEN2 were initially reported in families with early onset AD, more recent studies have demonstrated that some PSEN mutations are also associated with familial late-onset AD (86). In these families, the PSEN mutations may not segregate perfectly with disease either because of incomplete penetrance (mutation carriers who do not develop disease) or because of phenocopies (disease not caused by the mutation) (86, 87).
A molecular connection between presenilins and Aβ production was uncovered in the late 1990s. A normal function of presenilins is to form a gamma secretase complex with 3 other proteins, APH-1, PEN2, and nicastrin (88, 89). The gamma secretase complex directly cleaves the transmembrane protein Notch, APP, and other substrates, and its activity is required for Aβ formation (see below). Notch is a protein involved in cell fate decisions during development and in the adult animals. PSEN1 knockout mice die in utero and have a very similar phenotype to Notch knockout mice (90). The unique intramembranous enzymatic activity of γ-secretase is thought to depend on two aspartates within the transmembrane domains of presenilin, which are required for Aβ generation as well as Notch cleavage (91, 92). Gamma secretase has been a challenging drug target because of the side-effects resulting from cleavage of other, non-APP substrates such as notch. For example, inhibition of Notch cleavage results in toxicities related to altered cell proliferation in the gut, the immune system, and the skin (93). Despite this, many companies have developed gamma secretase inhibitors to decrease Aβ production as a potential treatment for AD. One gamma secretase inhibitor recently made it all the way into a phase III clinical trial in AD; however, the trial was halted due to treated patients worsening cognitively more than placebo-treated patients (94). Although the cause of this failure is unclear, it seems likely that the worsening was due to inhibition of notch or cleavage of other γ-secretase substrates. Whether it will be possible to relatively selectively inhibit cleavage of APP vs. other γ-secretase substrates with a γ-secretase inhibitor in vivo is not year clear. This is likely to be the most challenging issue going forward as to whether γ-secretase inhibitors can ever be developed to treat or prevent AD.
Similar to what is observed with many APP mutations, a selective and significant increase in the total level of Aβ42 or the ratio of Aβ42 to Aβ40 is found in the plasma (and in the media of cultured fibroblasts) from patients with PSEN mutations; this increase can be detected throughout life (95).. Importantly, transgenic mice expressing PSEN mutations have elevated Aβ42 in the brain and, when bred with transgenic mice expressing human APP harboring an FAD mutation, demonstrate accelerated Aβ deposition (96, 97). Neurons derived from PSEN1 knockout mice have a markedly reduced ability to generate Aβ due to a decrease in γ-secretase activity (the enzyme that cleaves Aβ from APP at its C-terminus giving rise to Aβ40 and Aβ42) (98). Data suggest that PSEN1 mutations cause forms of FAD from a partial but not complete loss of function that leads to a relative increase in Aβ42 production and lower production of shorter Aβ species such as Aβ40 (99). The γ-secretase enzyme cleaves APP sequentially generating different Aβ species of differing lengths, the most abundant being Aβ40. The partial loss of function induced by the PSEN1 mutations results in less efficient APP cleavage and relatively greater Aβ42 production than normal. This relative increase in Aβ42 results in a greater propensity for Aβ to aggregate earlier in the brain. The function of presenilins is required for survival and PSEN1 knockout mice die in the embryonic period due to a variety of developmental defects (98, 100, 101). These defects can be rescued by expressing either wild-type or PSEN1 carrying FAD mutations since these PSEN1 mutations do not block the ability of the γ-secretase to cleave notch(102, 103). While the PSEN1 mutations that cause FAD appear to result in a partial loss of function in γ-secretase activity, a few individuals have been reported to have dominant mutations in presenilin or other γ-secretase components that result in a complete loss of γ-secretase function in one allele (haploinsufficiency) and a skin disorder called acne inversa, but they do not have AD (104). Is it still possible that PSEN mutations that cause FAD do so via a non-Aβ-mediated mechanism? Such an explanation has not been formally ruled out. Though the clinical phenotypes seen with PSEN mutations have many features similar to LOAD, there are some differences in certain families such as a more rapid course and spinal cord involvement in rare situations (105).
Although mutations in APP, PSEN1, and PSEN2 suggest that overproduction of Aβ42 or an increase in the Aβ42 to Aβ40 ratio can cause FAD, recent experiments show that Aβ42, in the absence of APP, can cause Aβ accumulation in vivo and that Aβ40 can suppress this phenotype. Mice that only overproduce Aβ42, in the absence of APP, develop both neuritic plaques and CAA, whereas mice that overproduce Aβ40 alone do not develop AD-like pathology (106). When Aβ42 mice were crossed with the Aβ40 mice, there was reduced Aβ fibrillogenesis, which suggests that Aβ40 actually inhibits Aβ aggregation (107). Whether other abundant shorter species of Aβ such as Aβ38 also have this suppressive effect on Aβ aggregation is unknown. It will be important to investigate this further as potential new therapeutics such as γ-secretase modulators (GSMs) could be used to increase the ratio of shorter versus longer forms of Aβ. These drugs decrease Aβ42 and increase shorter Aβ species without decreasing total Aβ species (108). Importantly, GSMs have these effects without affecting overall γ-secretase activity, and thus do not appear to inhibit notch cleavage or cleavage of other gamma secretase substrates. Because of these attractive features, GSM’s have entered clinical trials and appear to be a promising class of compounds.
(109, 110)(90)(91, 92)(93)Although there are no known FAD families with mutations in β-secretase, the gene encoding this enzyme, the β-site APP cleaving enzyme 1 -BACE1, has been cloned (111). BACE-1, like γ-secretase, is also required for Aβ production. Mice lacking β-secretase produce very low levels of Aβ (111, 112). Although β-secretase knockout mice have defects in peripheral nerve myelination (113), BACE1 appears to be a potentially attractive therapeutic target for AD as inhibition of the enzyme in adults seems to have fewer side effects than inhibition of γ-secretase, which has many substrates. Numerous companies have developed BACE inhibitors and several have now entered clinical trials. With many treatments that target Aβ being developed including γ-secretase inhibitors, GSMs, BACE inhibitors, and anti-Aβ antibodies, one question that arises is what would make up the best human population to first demonstrate an effect targeting Aβ. Certainly, many trials have occurred and are ongoing in people with dementia due to late-onset AD. However, seems it seems most clear that Aβ42 over-production leads to most cases of FAD. Perhaps this would be the best population of individuals to do prevention trials in targeting Aβ. Large studies such as the dominantly inherited Alzheimer’s network have been formed which should enable such trials (105). This will be a challenge given the relatively small number of affected families worldwide though it should offer a unique opportunity to test compounds in people who are relatively young and generally not affected by other concurrent brain disorders seen commonly in the elderly.
ApoE, genes, the environment, and AD
In addition to the genetics of FAD in which mutations in specific genes “cause” AD, there are genes that confer risk for developing LOAD. For example, the gene encoding apolipoprotein E (APOE) has by far the greatest effect on risk for developing AD; the presence of specific apoE alleles has been correlated with the risk of developing LOAD and CAA. There are three common alleles of apoE in humans (ε2, ε3, and ε4) that only differ in sequence by one amino acid at either position 112 or 158 of the protein. The ε4 allele is a genetic risk factor for LOAD, whereas the ε2 allele is protective (114, 115). One copy of ε4 increases risk for AD by ~3-fold and two copies by ~12-fold (www.alzgene.org). APOEε4 alleles are also associated with a dose-dependent decrease in age at onset (~5yrs/ε4 allele) in both sporadic and familial forms of AD (114, 116). These findings have been substantiated in populations around the world. In CAA, the ε4 allele is associated with greater levels of cerebrovascular amyloid deposition and hemorrhage in the CNS (117, 118); the ε2 allele has been linked to a greater risk of CAA-associated vasculopathy and CNS hemorrhage (119, 120). Given that the common feature linking AD and CAA is the excessive deposition and accumulation of Aβ and that apoE is an Aβ binding molecule, it is likely that a major reason apoE is linked to both disorders is through direct effects on Aβ metabolism (121). ApoE is an important regulator of plasma lipoprotein metabolism (122, 123). In addition to expression in the liver, apoE is also produced at high levels within the CNS where it is present in high density lipoprotein (HDL)-like lipoproteins secreted predominantly by astrocytes (121). Although apoE can play a role in a variety of processes in the CNS including cholesterol transport, neuronal plasticity, and inflammation (121), its exact function in normal and disease conditions remains to be clarified.
There are several hypotheses about how apoE may influence AD pathogenesis. Accumulating in vitro and in vivo evidence suggests that apoE acts as an Aβ binding molecule influencing the clearance of soluble Aβ as well as the propensity for Aβ to aggregate by affecting Aβ seeding and polymerization (121). Both of these processes may directly influence Aβ aggregation in vivo. Both pathological and neuroimaging studies of cognitively normal humans as well as AD patients have found that Aβ deposition occurs earlier and to a greater extent in those who are ε4-positive versus those who are ε4-negative, with two copies of ε4 being significantly worse than one (124–126). This appears likely to initiate the Aβ pathophysiological cascade earlier in life. Studies using APP transgenic mice that develop Aβ deposition have provided direct evidence that apoE influences the level, amount, and structure of intraparenchymal deposits of Aβ in the brain as well as CAA in an isoform-specific fashion (ε4>ε3>ε2) (127–130). Recent studies in humans have confirmed that the APOE genotype is strongly associated with Aβ phenotypes such as Aβ deposition and CSF Aβ42 levels but not with CSF tau levels (125, 131). In addition to apoE, there are other Aβ-binding molecules such as apolipoprotein J/clusterin (132, 133), α2-macroglobulin (α2-M) (134), transthyretin (135), and α1-antichymotrypsin (136), that may play a role in Aβ aggregation in the brain by influencing cellular uptake and degradation of Aβ locally by cells, its local retention in the brain (137), and ultimately its removal from the brain into the systemic circulation.
Approximately 50% of individuals with AD carry an APOEε4 allele suggesting that other genetic factors must contribute to risk for disease. Despite hundreds of studies during the last fifteen years, few reported genetic associations have been replicated across studies (see www.alzgene.org) (138). The main reason for this is that other genetic risk factors have a much smaller impact on risk than that of the APOE genotype. As a result the sample sizes used in these earlier studies were too small to have the power to detect these genes. During the last few years several technological advances have transformed the landscape regarding the genetics of common complex traits such as AD (139, 140). The first of these was the development of genome-wide arrays that allowed the simultaneous evaluation of millions of single nucleotide polymorphisms (SNPs) in thousands of samples. The use of these arrays in thousands of samples from AD cases and non-demented elderly controls has resulted in compelling evidence for a number of new genetic risk factors including association with genes encoding clusterin (CLU), phosphatidylinositol-binding clathrin assembly protein (PICALM), complement receptor 1 (CR1), bridging integrator protein 1 (BIN1), and sialic acid binding Ig-like lectin (CD33) (141–144). Two subsequent studies each including over eight thousand cases and a similar number of controls have identified additional genes with genome-wide significant evidence for association (<5× 10−8) including membrane spanning 4A gene cluster (MS4A4A),CD2-associated protein (CD2AP), Ephrin receptor A1 (EPHA1), and ATP-binding cassette transporter (ABCA7) (145, 146). The functional alleles responsible for each of these associations have yet to be determined. However, the common SNPs identified in these genome-wide association studies (GWAS) have odds ratios of 1.1–1.2. The odds ratio is a measure of effect size, describing the strength of association or non-independence between two binary data values. This suggests that the effect of these risk alleles is much smaller than that of APOEε4 unless they are tagging rare alleles of larger effect. Estimates of the population attributable fractions for these new candidate genes are between 2.72–5.97%, with a cumulative population-attributable fraction for non-APOE loci estimated to be around 35%. However, the actual effect sizes are likely to be much smaller than these estimates because of the ‘winner’s curse’, a bias away from the null sometimes seen in GWAS studies. In comparison, the population-attributable fraction for APOEε4 is 20%. Five of these AD risk loci (CLU, CR1, ABCA7, CD33 and EPHA1) have putative functions in the immune system; four are involved in processes at the cell membrane, including endocytosis (PICALM, BIN1, CD33, CD2AP) and three are involved in lipid biology (APOE, CLU and ABCA7). These new gene discoveries provide new impetus for focused studies aimed at understanding the pathogenesis of AD (Table 1). Though some of the new genes appear to be involved in Aβ metabolism (e.g. CLU), the fact that several may influence inflammation, endocytosis, and lipid biology suggests the intriguing possibility that if one can effectively target the specific components of the pathways these gene products are telling us about, novel directions for drug discovery and avenues for treatment may be available distinct from directly targeting Aβ or tau. It is also possible that some of the new genetic discoveries are not targeting the nearby gene but actually targeting non-coding RNAs. If so, this could also provide new insights.
Table 1.
Late onset Alzheimer’s disease risk alleles that show genome-wide significant association in at least one study (adapted from Naj et al., Nature Genetics (in press).
| SNP | Gene | Odds Ratio (CI) |
|---|---|---|
| rs429358 | Apolipoprotein E (APOE4) | 3.77 (3.29–4.32)* |
| rs7412 | Apolipoprotein E (APOE2) | 0.55 (0.36–0.84)* |
| rs1532278 | Clusterin (CLU/APOJ) | 0.89 (0.85–0.92) |
| rs561655 | Phosphatidylinositol-binding clathrin assembly protein (PICALM) | 0.87 (0.84–0.91) |
| rs670713 | Complement receptor 1 (CRI) | 1.17 (1.12–1.23) |
| rs3752246 | ATP-binding cassette transporter (ABCA7) | 1.15 (1.09–1.21) |
| rs7561528 | Bridging integrator 1 (Bin1) | 1.17 (1.12–1.22) |
| rs4938933 | Membrane spanning 4A gene cluster (MS4A) | 0.89 (0.87–0.92) |
| rs9349407 | CD2-associated protein (CD2AP) | 1.11(1.07–1.15) |
| rs3865444 | Sialic acid binding Ig-like lectin (CD33) | 0.91 (0.88–0.93) |
| rs11767557 | Ephrin receptor A1 (EPHA1) | 0.90 (0.86–0.93) |
APOE Odds ratios from www.Alzgene.org
There has been much discussion in the literature about the “missing heritability” – the observation that new genes identified through GWAS do not explain all of the genetic heritability of most traits, which can be estimated in twin studies (147). One possibility is that the SNPs on the GWAS chips do not capture all of the genetic variation associated with AD; another possibility is that variants tagged by SNPs on the chips failed to reach genome-wide significance because the sample size used was insufficient. The second possibility can be overcome by increasing sample size through meta-analysis. The first possibility may be overcome either by genotyping GWAS chips with more SNPs or by DNA sequencing. Recent advances in sequencing technology have resulted in a sharp decrease in sequencing costs. Rare sequence variants that cause or increase risk for AD are likely to be found by whole exome and whole genome sequencing. This approach has already proved very effective in identifying causative genes for rare recessive disorders (148). Exome sequencing studies are likely to start soon in families with late-onset AD and will likely lead to the identification of new AD genes in the future. Though identification of new genes may lead to new insights into AD pathogenesis and new therapeutic targets, it may still be challenging to translate such targets into effective therapies. While APOE is the strongest genetic risk factor for AD, it is still not clear whether and how it can be directly targeted effectively.
Approaches for identifying new risk factors associated with other aspects of the LOAD phenotype are still in their infancy but include genetic studies for elucidating the rate of disease progression; age at onset; CSF or plasma biomarker measurements; and functional and structural imaging measures to mention just a few (149, 150). An advantage of these quantitative traits is the ability to select individuals from the extremes of the trait distribution. Sequencing of the known AD genes in individuals who are in the top and bottom 10% for Aβ42 concentration in CSF has led to the identification of a late onset AD family carrying a known PSEN1 mutation (86). In another study, SNPs within the PPP3R1 gene, encoding the regulatory subunit of calcineurin, were associated with higher CSF concentrations of tau and phosphorylated tau, and increased tangle pathology (151). In AD cases, the alleles associated with higher CSF tau and phosphorylated tau were also associated with a more rapid disease progression.
Genes obviously do not act in a vacuum. However, a major bottleneck has been that relatively little is yet understood about the way genetic and environmental factors combine to moderate or exacerbate the risk for AD. If such interactions can be better understood, it may suggest clearcut ways to alter AD risk in genetically predisposed populations. A challenge will be to study large number of cognitively normal middle aged individuals with differential genetic risk for AD to determine if common modifiable environmental and genetic factors clearly interact to increase risk. Some such studies are currently underway. Epidemiological studies have demonstrated that age, family history and head injury with loss of consciousness influence risk for AD. Overall, head injury is associated with an odds ratio of around 2 (152–154). However, when APOE genotype is incorporated into these analyses it is apparent that the risk associated with head injury is substantially higher (odds ratio 15–20) in individuals with an APOEε4 allele. The mechanisms underlying this interaction is still poorly understood. Higher education levels have been associated with a lower risk for AD (155), and the notion of brain or cognitive “reserve” (a greater number of neurons or connections) has been invoked to explain this difference (156). The concept of reserve suggests that some individuals have more capacity to tolerate AD neuropathology without becoming symptomatic; educational attainment is a surrogate for “reserve”. A recent imaging study has demonstrated that among individuals with Aβ deposition, those with higher education had better cognitive scores on a number of measures (157), consistent with the reserve hypothesis. APOE genotype was not examined in this study but may combine with education to moderate this effect on cognition. Animal studies have also demonstrated that environmental enrichment can delay Aβ deposition and improve cognition in mouse models (158, 159). Analyses in human subjects also demonstrate that active individuals who meet the exercise guidelines set by the American Heart Association have significantly less amyloid deposition in the brain(160). In this study the associations between exercise engagement and amyloid deposition were more prominent in APOEε4 non-carriers.
Connecting Aβ and tau
Mice that develop age-dependent Aβ accumulation and deposition in the brain have been useful in demonstrating the role of both Aβ as well as other molecules in disease pathogenesis. Multiple types of genetically modified mice have been generated that over-produce the human APP gene and Aβ peptide. Many of these models develop an age-dependent accumulation of Aβ in the brain as well as Aβ deposition in diffuse and neuritic plaques, often in region specific patterns similar to that seen in AD (161, 162). Subsequent to the development of increased Aβ production and deposition, many of the models develop neuritic plaques, microglial activation, astrocytosis, evidence of oxidative damage, and changes in neuronal cytoskeletal proteins including tau (163, 164). In addition, some but not all of the models show varying degrees of behavioral impairment (162). Mice that only over-produce and develop Aβ-related pathology generally do not develop marked neuronal loss nor typical NFTs; however, significant functional abnormalities in synapses do develop in the hippocampus and neocortex as Aβ deposition increases(165). Although these models have been very useful, they appear to model aspects of the human phase of “preclinical” or “presymptomatic” AD, a period when Aβ is accumulating in the absence of clinically detectable symptoms.(7). A major challenge to developing animal models for AD has been how to model not only brain changes associated with Aβ deposition, but also the additional changes seen in the AD brain including tau aggregation as well as neuronal, axonal, dendritic, and synaptic loss. Some progress is being made by creating genetically modified animals that express human Aβ and human tau (166). Since damage to axons, dendrites, and synapses that results in “disconnection” between brain regions is a fundamental problem not only in AD but in other neurodegenerative disorders, it may be that studying animal models with such features will be useful to develop and test new therapies that specifically target protecting neuronal connections (167) independent of Aβ or tau. Targeting aspects of neuroinflammation may also be a viable therapeutic approach; however, models with neuroinflammation and aspects of neurodegeneration need to be present in order to determine if altering specific aspects of the neuroinflammatory process truly decrease important aspects of neurodegeneration before moving new therapies into human trials.
Recent work on the genetics and biology of tau has led to insights into the connections between Aβ accumulation and exacerbation of tangle formation, “tauopathy”, and perhaps cognitive decline and neurodegeneration. Overall, this work suggests that tau aggregation is central to the clinical progression of AD. Tau is a microtubule-associated protein that plays an important role in microtubule stability. It is a major component and a primary constituent of NFTs that are found in AD as well as in a host of other neurodegenerative diseases such as certain frontotemporal dementias and progressive supranuclear palsy (168). Some families with autosomal dominant forms of a non-AD dementia, frontotemporal dementia with Parkinsonism (FTDP), have mutations in the tau gene on chromosome 17 (169–171). This disease does not feature Aβ accumulation. Expression of a mutant form of tau that causes FTDP in transgenic mice results in age-dependent tangle formation in some regions of the CNS as well as significant neuronal loss and neurological impairment (172, 173). Thus, abnormalities in tau, in and of itself, in the absence of Aβ deposition, can result in neurodegeneration and a non-AD dementing disorder. In AD, there is increasing evidence that at least a component of tau-related neurodegeneration in the brain acts downstream of Aβ. From the genetic standpoint, there are SNPs in the MAPT gene encoding tau (174) as well as in a major tau phosphatase, protein phosphatase B (calcineurin), (151) that do not affect risk for AD but affect its progression; tau concentrations in CSF are also markers of neurodegeneration. The effects of these SNPs, however, are only seen in people that already have brain Aβ accumulation. In animal models, groups have shown that Aβ appears to exacerbate the development of tau aggregation, NFTs, and evidence of neurodegeneration (166, 175–177). Although the initial biochemical events leading to plaques and tangles are different and each can occur in isolation, these studies suggest that the buildup of aggregated forms of Aβ exacerbates tangle formation. There are animal models that develop both Aβ and tau pathology (166), but a clear shortfall in this field is the availability of animal models that develop all aspects of AD pathology as previously mentioned.
The actual structure of Aβ and tau that leads to exacerbation of synaptic, neuritic, and behavioral abnormalities is not yet entirely clear. In addition to the insoluble Aβ and tau fibrils that are in plaques and tangles, oligomeric forms of Aβ and tau may play important roles in the toxicity of these molecules (for review, see (178, 179)). Oligomers of Aβ of different sizes have been shown to be toxic under certain conditions (e.g. dimers as well as larger oligomers) (16, 180–182). Monomeric, oligomeric, and fibrillar forms of Aβ and tau are probably present in equilibrium where local concentrations of oligomers surrounding larger aggregates may be quite high (17, 183). The extent to which oligomers, fibrils, or both are toxic in vivo remains unclear. This presents a challenge if it is critical to target a particular species of Aβ or tau to obtain a therapeutic effect. It is not yet clear if this is the case. At least in the case of Aβ, there is evidence that several cellular proteins may be mediating its damaging effects on neurons, glia, and the cells that make up the neurovascular unit, and these proteins include RAGE, Fyn, tau, NADPH oxidase, prion protein, and EphB2 among others (184–189). Some of these molecules are receptors, others are signaling molecules, and some are enzymes. Some may link Aβ toxicity to tau and in other cases they may directly modulate Aβ or tau toxicity independently of each other. Determining which molecules mediate neurodegeneration related to Aβ and tau in humans will be a challenge but may provide key insights into the development of new treatments.
Taken together, the available evidence favors a model in which diverse genetic variants in different ways lead to a similar biochemical abnormality, the conversion of the normally soluble Aβ peptide into both soluble and insoluble oligomeric, protofibrillar, and fibrillar toxic forms (Fig. 2). Subtle alterations in the production of soluble Aβ appear to result over many years in Aβ changing structure and forming deposits at an early age as occurs in FAD. However, control of the structure and concentration of Aβ after it is produced by cells through mechanisms such as degradation, clearance, and fibrillogenesis are likely also critical events that probably play a major role in Aβ aggregation in some forms of FAD and in LOAD. Finally, once Aβ oligomerizes and aggregates, this is likely to drive tau aggregation, which itself appears to play a key role in cell dysfunction and neurodegeneration in AD both downstream as well as independently of Aβ (30).
Prevention of Aβ and tau aggregation
The genetic, biochemical, and animal model data strongly support the idea that Aβ and tau accumulation is critical in ultimately driving AD pathogenesis. But proof of this in humans will require demonstrating that altering Aβ and tau aggregation (or any other mechanism) has a clinical effect on disease onset or progression. Currently, there is no convincing evidence that therapies directed against Aβ or tau influence symptomatic progression of disease in humans, although many clinical trials are underway to test these ideas. There is, however, strong evidence from preclinical studies that prevention or reversal of Aβ and tau aggregation has potential to serve as a way to delay the onset, slow the progression, or prevent AD. For example, there are ways to decrease soluble Aβ production, enhance soluble Aβ clearance, influence Aβ aggregation, neutralize Aβ toxicity, or remove existing Aβ aggregates in animal models. This includes using small molecules that inhibit Aβ production or block its toxicity in APP transgenic mice. There are also many reports showing that both active and passive immunization targeting Aβ in APP transgenic mice can decrease Aβ accumulation, in some cases remove existing Aβ deposits, and improve behavioral performance including learning and memory (190–196). It appears that anti-Aβ antibodies account for the beneficial effects in these immunization studies probably through several mechanisms involving clearing soluble and aggregated Aβ via cellular and non-cellular mechanisms as well as by blocking Aβ toxicity (190). Anti-Aβ antibodies and Aβ immunization techniques have demonstrated that by decreasing the toxic effects of Aβ in vivo, one can block some of the downstream effects of Aβ toxicity including neuritic dystrophy and early changes in tau phosphorylation that are precursors to tangle formation (177, 197). This suggests that targeting Aβ not only influences direct Aβ-related damage but also influences Aβ-induced changes to tau. Targeting Aβ with active immunization has been attempted in humans with AD but the first trials were halted due to a side effect of meningoencephalitis seen in a small percentage of patients. A subset of patients who entered the phase I trial were followed for several years after the trial was halted and their brains were assessed at autopsy. In several individuals, there was evidence of substantial amyloid clearance from the brain though no there was no obvious halting of clinical disease progression (198, 199). Since a full clinical trial was not done and even the current results are from an aborted phase I trial that was not powered to assess efficacy, it is difficult to know whether there would have been a clinical effect. Two large phase III passive immunotherapy trials are underway now to understand whether targeting Aβ with antibodies can show benefit. A current challenge in the field suggested by the animal data is that that if a treatment targeting Aβ is going to be maximally effective, it should begin before substantial tauopathy is present. That would mean beginning therapy during “preclinical” AD (Figure 3). Would treatments that specifically target tau or its phosphorylation state have the potential to be more effective than those that target Aβ? Genetic manipulations have shown that in mice that develop tauopathy, turning off the expression of tau even after tangles have developed prevents further brain atrophy and improves behavior (173, 200). In addition, decreasing phosphorylation of tau by certain enzymes such as GSK-3β has positive benefits in animal models (201). Thus, if tau can be safely and effectively reduced or dephosphorylated at certain sites or its aggregation can be inhibited, this may be an attractive therapeutic approach. A challenge for the field will be to determine whether tau levels, tau aggregation, and tau phosphorylation can be both targeted effectively and safely. A clinical trial of a tau aggregation inhibitor, methylene blue, is underway and will likely to be one of the first of many attempts to target tau.
The conundrum between biomarkers, pathology, and treatment
If therapies that target Aβ, tau, or other mechanisms are to be effective in humans, a key issue to consider is when in relation to the clinical and pathological course of AD would the treatments be effective. There is now compelling evidence that in terms of large scale quantitative changes, Aβ accumulation and deposition in the brain is a very early pathological process in AD and probably begins ~10–20 years prior to the onset of clinically detectable symptoms of impairment (8). Aβ buildup in the brain can now be detected by at least two methods in people, regardless of their clinical status. Imaging of amyloid plaques using small molecule radiotracers that bind to fibrillar forms of Aβ can detect the presence, amount, and location of these changes in the brain with PET scans (5, 6). In addition, measurement of CSF Aβ42 concentration is a very sensitive and specific way to determine the presence or absence of Aβ deposition in the brain particularly when combined with amyloid imaging (202–204). By the time the earliest symptoms and signs of cognitive decline caused by AD are detectable, Aβ accumulation in the brain is already substantial and approaching its maximal extent (Figure 3) (7, 12). However, the significant accumulation of NFTs, oxidative stress, inflammation, synaptic and network dysfunction, and ultimately neuronal cell death probably does not peak until the stages of moderate to severe dementia (Figure 3). Some tangle development clearly is present in the period prior to clinical onset of AD but the acceleration of tau aggregation and neurodegeneration may mark the transition period just prior to clinically detectable disease. This transition can be detected by increases in the CSF of total and phosphorylated forms of tau perhaps beginning ~ 3–4 years prior to the onset of very mild dementia (205–208). Support that these biomarker changes are clinically relevant comes from data showing that, in cognitively normal elderly individuals, a high ratio of CSF tau/Aβ42 or a high level of cerebral Aβ burden as shown by amyloid radiotracers both predict conversion to mild cognitive impairment (MCI) (208) or to very mild dementia over a 3–4 year period (205, 206, 209) and predict progression to more advanced stages of dementia in individuals characterized with MCI (210). By the time very mild dementia/MCI is present, in addition to substantial densities of plaques and tangles, there is also significant neuronal loss in certain brain regions (9, 211). This information together with some of the biomarker data just mentioned would suggest that to have the greatest potential to develop effective therapies that might influence the clinical onset and progression of AD, it would be best to begin therapies targeting Aβ or tau in the period preceding clinical symptoms of cognitive impairment and dementia. Although much more work is needed to characterize “preclinical AD”, this stage can now be accurately identified through the use of antecedent biomarkers (CSF and imaging). Clinical trials that take advantage of these advances have not yet taken place, although some are being designed, potentially for implementation in FAD individuals (for reviews of recent drug trials and consideration of future trial designs, see (212, 213)). Virtually all clinical trials of potential disease modifying therapies that target Aβ, tau, or other mechanisms have been in patients with symptomatic AD (i.e., patients with mild to moderate dementia) or with very mild dementia/MCI due to AD, when irreversible neuronal loss already is well established. Part of the failure to date of currently tested treatments may be due to the fact that drugs that have completed large phase III trials may not have been effectively hitting their target (e.g. decreasing Aβ in the CNS of humans to a significant degree). New methods to assess whether proposed disease-modifying agents are effectively hitting their target are now being incorporated in phase I and II trials in humans (214, 215). However, because of the time course of AD pathology and neurodegeneration prior to the onset of cognitive symptoms, it would seem critical and a major challenge to determine the best way to do clinical trials in cognitively normal people who are very likely to develop AD. Although it seems intuitive that the earlier one starts with therapy prior to cognitive decline, the more likely an effect will be seen, trials of large numbers of individuals, in the absence of screening techniques, would likely take 10–20 years to fully assess the benefits of treatment and would require very large financial resources. On the other hand, with current biomarker and genetic information, it is likely feasible to screen individuals greater than 60 years of age and select those at very high risk for cognitive decline over a 3–4 year period with biomarkers. Several large longitudinal studies such as the Alzheimer’s disease neuroimaging initiative (ADNI) as well as studies of adult children whose parents did or did not have AD are trying to better define exactly which biomarkers are the best at determining prognosis in such groups. In addition, the study of individuals with preclinical AD due to autosomal dominant mutations (such individuals are virtually certain to develop symptomatic AD) will define the predictive value of specific biomarkers in this population with the goal of ultimately enabling testing of promising therapies.
Summary
There have been tremendous advances in our understanding of the molecular pathogenesis of Alzheimer’s disease over the last 25 years. Overwhelming evidence suggests that AD is a disorder of protein aggregation in which the buildup of the normally soluble Aβ and tau drive AD pathogenesis. Chronic overproduction of Aβ, particularly Aβ42, as occurs in FAD cases, as well as altered propensity of Aβ to aggregate due to sequence changes, reproducibly leads to AD pathology and eventually results in dementia. In sporadic late-onset AD, current evidence does not support Aβ overproduction as a cause though there is emerging evidence suggesting deficits in Aβ clearance(216). It appears that molecules such as apoE, which play a role in Aβ aggregation and Aβ clearance as well as other genetic risk factors, are critical in determining whether Aβ will ultimately change conformation and form deposits in the brain. Once Aβ begins building up in the brain, a host of downstream events occur which probably play important roles in damaging axons, dendrites, synapses, intracellular signaling, synaptic transmission, and ultimately cell death. In particular, the accumulation and aggregation of tau appears to play a critical role in disease progression. Changes in tau phosphorylation and conformation as reflected in NFTs and neuropil threads are probably exacerbated by Aβ aggregation and are likely to contribute importantly to clinical progression in AD. How aggregated Aβ damages the brain and exacerbates tangle formation continues to be an important area for future studies. In LOAD, brain injury due to other disorders such as cerebrovascular disease, diabetes, and Lewy body pathology are common and a challenge will also be to mitigate the effects of these processes. There is a great need not only for continued refinement and development of antecedent biomarkers, which identify preclinical AD and increasingly appear to predict dementia due to AD, but also for markers that predict treatment effects for use in future clinical trials. Therapies based on decreasing production, increasing clearance, decreasing aggregation, and removing aggregates of both Aβ and tau are in development for AD. Such approaches as well as others that prevent or slow neurodegeneration through different mechanisms, including environmental manipulation (e.g. exercise, sleep (217)) offer promise to delay or perhaps even prevent the onset of symptomatic AD. Starting as early as possible prior to the appearance of cognitive symptoms is likely to be a critical strategy to make major headway against this devastating disease.
References
- 1.Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiatr. 1907;64:146–148. [Google Scholar]
- 2.Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D, Duara R. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR., Jr Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 6.Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, Krautkramer MJ, Kung HF, Coleman RE, Doraiswamy PM, Fleisher AS, Sabbagh MN, Sadowsky CH, Reiman PE, Zehntner SP, Skovronsky DM. Use of florbetapir-PET for imaging beta-amyloid pathology. Jama. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Molec Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 9.Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer Disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 10.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goedert M. Oskar Fischer and the study of dementia. Brain. 2009;132:1102–1111. doi: 10.1093/brain/awn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golde TE, Eckman CB, Younkin SG. Biochemical detection of Ab isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochim Biophys Acta. 2000;1502:172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 15.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 16.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 17.Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 20.Smith EE, Greenberg SM. Beta-amyloid, blood vessels, and brain function. Stroke. 2009;40:2601–2606. doi: 10.1161/STROKEAHA.108.536839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg SM, Frosch MP. Life imitates art: Anti-amyloid antibodies and inflammatory cerebral amyloid angiopathy. Neurology. 2011;76:772–773. doi: 10.1212/WNL.0b013e31820e7bce. [DOI] [PubMed] [Google Scholar]
- 22.Soontornniyomkij V, Lynch MD, Mermash S, Pomakian J, Badkoobehi H, Clare R, Vinters HV. Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Pathol. 2010;20:459–467. doi: 10.1111/j.1750-3639.2009.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 24.Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988;85:4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wischik CM, Novak M, Edwards PC, Klug A, Tichelaar W, Crowther RA. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988;85:4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wischik CM, Novak M, Thogersen HC, Edwards PC, Runswick MJ, Jakes R, Walker JE, Milstein C, Roth M, Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988;85:4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kidd M. Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature. 1963;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- 29.Lee VM-Y, Balin BJ, Otvos L, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 30.Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 32.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 33.Farlow MR, Miller ML, Pejovic V. Treatment options in Alzheimer’s disease: maximizing benefit, managing expectations. Dement Geriatr Cogn Disord. 2008;25:408–422. doi: 10.1159/000122962. [DOI] [PubMed] [Google Scholar]
- 34.Areosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev. 2005:CD003154. doi: 10.1002/14651858.CD003154.pub3. [DOI] [PubMed] [Google Scholar]
- 35.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proc Natl Acad Sci U S A. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 41.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 42.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 43.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, Vigouret JM, Paganetti P, Walsh DM, Mathews PM, Ghiso J, Staufenbiel M, Walker LC, Jucker M. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 45.Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 48.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- 50.Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- 51.Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science. 1987;235:877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- 52.Kang J, Lemaire H-G, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K-H, Multhaup G, Beyreuther K, Müller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 53.Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, Van Keuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- 54.Van Nostrand WE, Wagner SL, Suzuki M, Choi BH, Farrow JS, Geddes JW, Cotman CW, Cunningham DD. Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid beta-protein precursor. Nature. 1989;341:546–549. doi: 10.1038/341546a0. [DOI] [PubMed] [Google Scholar]
- 55.Smith RP, Higuchi DA, Broze GJ., Jr Platelet coagulation factor XIa-inhibitor, a form of Alzheimer amyloid precursor protein. Science. 1990;248:1126–1128. doi: 10.1126/science.2111585. [DOI] [PubMed] [Google Scholar]
- 56.Golde TE, Estus S, Younkin LH, Selkoe DJ, Younkin SG. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992;255:728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- 57.Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 58.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 59.Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 60.Goate A, Chartier-Harlon MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton P, Roote K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 61.Levy E, Carman MD, Fernandez MIJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248:1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- 62.Van Broeckhoven C, Haan J, Bakker E, Hardy JA, Van Hul W, Wehnert A, Vegter-Van der Vlis M, Roos RA. Amyloid beta protein precursor gene and hereditary cerebral hemorrhage with amyloidosis (Dutch) Science. 1990;248:1120–1122. doi: 10.1126/science.1971458. [DOI] [PubMed] [Google Scholar]
- 63.Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353:844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- 64.Murrell J, Farlow M, Ghetti B, Benson M. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science. 1991;254:97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- 65.Davis J, Van Nostrand WE. Enhanced pathologic properties of Dutch-type mutant amyloid beta-protein. Proc Natl Acad Sci USA. 1996;93:2996–3000. doi: 10.1073/pnas.93.7.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grabowski TJ, Cho HS, Vonsattel JPG, Rebeck GW, Greenberg SM. Novel amyloid precursor protein mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann Neurol. 2001;49:697–705. doi: 10.1002/ana.1009. [DOI] [PubMed] [Google Scholar]
- 67.Van Nostrand WE, Melchor JP, Soon Cho H, Greenberg SM, Rebeck GW. Pathogenic effects of D23N Iowa mutant amyloid-protein. J Biol Chem. 2001;276:32860–32866. doi: 10.1074/jbc.M104135200. [DOI] [PubMed] [Google Scholar]
- 68.Haass C, Hung AY, Selkoe DJ, Teplow DB. Mutations associated with a locus for familial Alzheimer’s. J Biol Chem. 1994;269:17741–17748. [PubMed] [Google Scholar]
- 69.Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos LJ, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 70.Jarrett JT, Berger EP, Lansbury PT. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 71.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 72.Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of β-amyloid. Nature Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- 73.Cai X-D, Golde TE, Younkin SG. Release of excess amyloid protein from a mutant amyloid β protein precursor. Science. 1993;259:514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- 74.Citron M, Oltersdorf T, Haas C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 75.Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 76.Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Naslund J, Lannfelt L. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nature Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 77.Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, Takuma H, Kuwano R, Imagawa M, Ataka S, Wada Y, Yoshioka E, Nishizaki T, Watanabe Y, Mori H. A new amyloid beta variant favoring oligomerization in Alzheimer’s-type dementia. Ann Neurol. 2008;63:377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- 78.Cloe AL, Orgel JP, Sachleben JR, Tycko R, Meredith SC. The Japanese Mutant Abeta (DeltaE22-Abeta(1-39)) Forms Fibrils Instantaneously, with Low-Thioflavin T Fluorescence: Seeding of Wild-Type Abeta(1-40) into Atypical Fibrils by DeltaE22-Abeta(1-39) Biochemistry. 2011 doi: 10.1021/bi1016217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang P, Yang G, Mosier DR, Chang P, Zaidi T, Gong YD, Zhao NM, Dominguez B, Lee KF, Gan WB, Zheng H. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. J Neurosci. 2005;25:1219–1225. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Young-Pearse TL, Suth S, Luth ES, Sawa A, Selkoe DJ. Biochemical and functional interaction of disrupted-in-schizophrenia 1 and amyloid precursor protein regulates neuronal migration during mammalian cortical development. J Neurosci. 2010;30:10431–10440. doi: 10.1523/JNEUROSCI.1445-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, Wolfer DP, Muller UC. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei W, Nguyen LN, Kessels HW, Hagiwara H, Sisodia S, Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levy-Lahad E, Wasco W, Poorkag P, Romano DM, Oshima J, Pettingell WH, Yu C-E, Jondor PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 85.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin J-F, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning a a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 86.Kauwe JS, Jacquart S, Chakraverty S, Wang J, Mayo K, Fagan AM, Holtzman DM, Morris JC, Goate AM. Extreme cerebrospinal fluid amyloid beta levels identify family with late-onset Alzheimer’s disease presenilin 1 mutation. Ann Neurol. 2007;61:446–453. doi: 10.1002/ana.21099. [DOI] [PubMed] [Google Scholar]
- 87.Cruchaga C, Goate AM. unpublished data [Google Scholar]
- 88.Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- 89.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 90.Thinakaran G. The role of presenilins in Alzheimer’s disease. J Clin Invest. 1999;104:1321–1327. doi: 10.1172/JCI8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y-M, Xu M, Lai M-T, Huang Q, Castro JL, Dimuzio-Mower J, Harrrison T, Lellis C, Nadin A, NJG, Register RB, Sardana MK, SMS, Smith AL, Shi X-P, Yin K-C, Shafer JA, Gardell SJ. Photoactivated -secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 92.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 93.Liu Z, Turkoz A, Jackson EN, Corbo JC, Engelbach JA, Garbow JR, Piwnica-Worms DR, Kopan R. Notch1 loss of heterozygosity causes vascular tumors and lethal hemorrhage in mice. J Clin Invest. 2011;121:800–808. doi: 10.1172/JCI43114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schor NF. What the halted phase III gamma-secretase inhibitor trial may (or may not) be telling us. Ann Neurol. 2011;69:237–239. doi: 10.1002/ana.22365. [DOI] [PubMed] [Google Scholar]
- 95.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 APP mutations linked to familial Alzheimer’s disease. Nature Med. 1996;2:864–852. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 96.Borchelt DR, Ratovitski T, Van Lare J, Lee MK, Gonzales VB, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice co-expressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 97.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada C-M, Eckman C, Younkin S, Hsaio K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nature Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 98.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 99.De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8:141–146. doi: 10.1038/sj.embor.7400897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 101.Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJS, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LHT, Sisodia SS. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 102.Davis JA, Naruse S, Chen H, Eckman C, Younkin S, Price DL, Borchelt DR, Sisodia SS, Wong PC. An Alzheimer’s disease-linked PS1 variant rescues the developmental abnormalities of PS1-deficient embryos. Neuron. 1998;20:603–609. doi: 10.1016/s0896-6273(00)80998-8. [DOI] [PubMed] [Google Scholar]
- 103.Qian S, Jiang P, Guan X-M, Singh G, Trumbauer ME, Yu H, Chen HY, Van der Ploeg LHT, Zheng H. Mutant human presenilin 1 protectspresenilin 1 null mouse against embryonic lethality and elevates Aβ1-42/43 expression. Neuron. 1997;20:611–617. doi: 10.1016/s0896-6273(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 104.Wang B, Yang W, Wen W, Sun J, Su B, Liu B, Ma D, Lv D, Wen Y, Qu T, Chen M, Sun M, Shen Y, Zhang X. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi: 10.1126/science.1196284. [DOI] [PubMed] [Google Scholar]
- 105.Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, Salloway S, Sperling RA, Windisch M, Xiong C. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim J, Onstead L, Randle S, Price R, Smithson L, Zwizinski C, Dickson DW, Golde T, McGowan E. Abeta40 inhibits amyloid deposition in vivo. J Neurosci. 2007;27:627–633. doi: 10.1523/JNEUROSCI.4849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kounnas MZ, Danks AM, Cheng S, Tyree C, Ackerman E, Zhang X, Ahn K, Nguyen P, Comer D, Mao L, Yu C, Pleynet D, Digregorio PJ, Velicelebi G, Stauderman KA, Comer WT, Mobley WC, Li YM, Sisodia SS, Tanzi RE, Wagner SL. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer’s disease. Neuron. 2010;67:769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Serneels L, Van Biervliet J, Craessaerts K, Dejaegere T, Horre K, Van Houtvin T, Esselmann H, Paul S, Schafer MK, Berezovska O, Hyman BT, Sprangers B, Sciot R, Moons L, Jucker M, Yang Z, May PC, Karran E, Wiltfang J, D’Hooge R, De Strooper B. gamma-Secretase heterogeneity in the Aph1 subunit: relevance for Alzheimer’s disease. Science. 2009;324:639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C, Rogaev E, Smith M, Janus C, Zhang Y, Aebersold R, Farrer LS, Sorbi S, Bruni A, Fraser P, St George-Hyslop P. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 111.Vassar R, Brian D, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis J-C, Collins F, Treanor J, Rogers G, Citron M. b-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 112.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major b-secretase for generation of Ab peptides by neurons. Nature Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 113.Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 114.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 115.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pastor P, Roe CM, Villegas A, Bedoya G, Chakraverty S, García G, Tirado V, Norton J, Ríos S, Martínez M, Kosik KS, Lopera F, Goate AM. Apolipoprotein E4 modifies Alzheimer’s disease onset in an E280A PS1 kindred. Ann Neurol. 2003;54:63–69. doi: 10.1002/ana.10636. [DOI] [PubMed] [Google Scholar]
- 117.Schmechel DE, Saunders AM, Strittmattter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid β-peptide deposition in cerebral cortex as a consequence of apolipoprotein genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greenberg SM, Rebeck GW, Vonsattel JPG, Gomez-Isla T, Hyman BT. Apolipoprotein E e4 and cerebral hemorrhage associated with amyloid angiopathy. Ann Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 119.Greenberg SM, Vonsattel J-PG, Segal AZ, Chiu RI, Clatworthy AE, Liao A, Hyman BT, Rebeck GW. Association of apolipoprotein E e2 and vasculopathy in cerebral amyloid angiopathy. Neurology. 1998;50:961–965. doi: 10.1212/wnl.50.4.961. [DOI] [PubMed] [Google Scholar]
- 120.Biffi A, Sonni A, Anderson CD, Kissela B, Jagiella JM, Schmidt H, Jimenez-Conde J, Hansen BM, Fernandez-Cadenas I, Cortellini L, Ayres A, Schwab K, Juchniewicz K, Urbanik A, Rost NS, Viswanathan A, Seifert-Held T, Stoegerer EM, Tomas M, Rabionet R, Estivill X, Brown DL, Silliman SL, Selim M, Worrall BB, Meschia JF, Montaner J, Lindgren A, Roquer J, Schmidt R, Greenberg SM, Slowik A, Broderick JP, Woo D, Rosand J. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68:934–943. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 123.Plump AS, Breslow JL. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu Rev Nutr. 1995;15:495–518. doi: 10.1146/annurev.nu.15.070195.002431. [DOI] [PubMed] [Google Scholar]
- 124.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 125.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nature Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 128.Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. ApoE4 alters the amyloid- 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an APP transgenic model. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine apoE markedly influence Ab metabolism both prior and subsequent to plaque formation in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- 131.Kauwe JS, Cruchaga C, Bertelsen S, Mayo K, Latu W, Nowotny P, Hinrichs AL, Fagan AM, Holtzman DM, Goate AM. Validating predicted biological effects of Alzheimer’s disease associated SNPs using CSF biomarker levels. J Alzheimers Dis. 2010;21:833–842. doi: 10.3233/JAD-2010-091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi-Miura NH, Ihara Y, Fukuchi K, Takeda M, Nakano Y, Tobe T, Tomita M. SP-40,40 is a constituent of Alzheimer’s amyloid. Acta Neuropath. 1992;83:260–264. doi: 10.1007/BF00296787. [DOI] [PubMed] [Google Scholar]
- 133.DeMattos RB, O’dell MA, Parsadanian M, Taylor JW, Harmony JAK, Bales KR, Paul SM, Aronow BJ, Holtzman DM. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2002;10:10843–10848. doi: 10.1073/pnas.162228299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qiu WQ, Borth W, Ye Z, Haass C, Teplow DB, Selkoe DJ. Degradation of amyloid beta-protein by a serine protease-alpha2 -macroglobulin complex. J Biol Chem. 1996;271:8443–8451. doi: 10.1074/jbc.271.14.8443. [DOI] [PubMed] [Google Scholar]
- 135.Goldgaber D, Schwarzman AI, Bhasin R, Gregori L, Schmechel D, Saunders AM, Roses AD, Strittmatter WJ. Sequestration of amyloid beta-peptide. Ann NY Acad Sci. 1993;695:139–143. doi: 10.1111/j.1749-6632.1993.tb23042.x. [DOI] [PubMed] [Google Scholar]
- 136.Mucke L, Yu GQ, McConlogue L, Rockenstein EM, Abraham CR, Masliah E. Astroglial expression of human alpha(1)-antichymotrypsin enhances Alzheimer-like pathology in amyloid protein precursor transgenic mice. Am J Pathol. 2000;157:2003–2010. doi: 10.1016/s0002-9440(10)64839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 139.Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk of complex disease. Curr Opin Genet Dev. 2008;18:257–263. doi: 10.1016/j.gde.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 140.Cirulli ET, Goldstein DB. Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet. 2010;11:415–425. doi: 10.1038/nrg2779. [DOI] [PubMed] [Google Scholar]
- 141.Bertram L, Lange C, Mullin K, Parkinson M, Hsiao M, Hogan MF, Schjeide BM, Hooli B, Divito J, Ionita I, Jiang H, Laird N, Moscarillo T, Ohlsen KL, Elliott K, Wang X, Hu-Lince D, Ryder M, Murphy A, Wagner SL, Blacker D, Becker KD, Tanzi RE. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83:623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanche H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 144.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, Bis JC, Smith AV, Carassquillo MM, Lambert JC, Harold D, Schrijvers EM, Ramirez-Lorca R, Debette S, Longstreth WT, Jr, Janssens AC, Pankratz VS, Dartigues JF, Hollingworth P, Aspelund T, Hernandez I, Beiser A, Kuller LH, Koudstaal PJ, Dickson DW, Tzourio C, Abraham R, Antunez C, Du Y, Rotter JI, Aulchenko YS, Harris TB, Petersen RC, Berr C, Owen MJ, Lopez-Arrieta J, Varadarajan BN, Becker JT, Rivadeneira F, Nalls MA, Graff-Radford NR, Campion D, Auerbach S, Rice K, Hofman A, Jonsson PV, Schmidt H, Lathrop M, Mosley TH, Au R, Psaty BM, Uitterlinden AG, Farrer LA, Lumley T, Ruiz A, Williams J, Amouyel P, Younkin SG, Wolf PA, Launer LJ, Lopez OL, van Duijn CM, Breteler MM. Genome-wide analysis of genetic loci associated with Alzheimer disease. Jama. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JSK, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, St George-Hyslop P, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, DeCarli C, DeKosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin LW, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Parisi JE, Perl DP, Peskind E, Petersen RC, Poon WW, Quinn JF, Rajbhandary RA, Raskind M, Reisberg B, Ringman JM, Roberson ED, Rosenberg RN, Sano M, Schneider LS, Seeley W, Shelanski ML, Slifer MA, Smith CD, Sonnen JA, Spina S, Stern RA, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Williamson J, Woltjer RL, Cantwell LB, Dombroski BA, Beekly D, Lunetta KL, Martin ER, Kamboh MI, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Montine TJ, Goate AM, Blacker D, Tsuang DW, Hakonarson H, Kukull WA, Foroud TM, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD. Common variants in MS4A4/MS4A6E, CD2uAP, CD33, and EPHA1 are associated with late-onset Alzheimer’s disease. Nature Genet. 2011 doi: 10.1038/ng.801. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hollingworth, et al. Nature Genet. 2011 in press. [Google Scholar]
- 147.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, Nayir A, Bakkaloglu A, Ozen S, Sanjad S, Nelson-Williams C, Farhi A, Mane S, Lifton RP. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci U S A. 2009;106:19096–19101. doi: 10.1073/pnas.0910672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hinrichs AL, Mintun MA, Head D, Fagan AM, Holtzman DM, Morris JC, Goate AM. Cortical binding of pittsburgh compound B, an endophenotype for genetic studies of Alzheimer’s disease. Biol Psychiatry. 2010;67:581–583. doi: 10.1016/j.biopsych.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lambert JC, Sleegers K, Gonzalez-Perez A, Ingelsson M, Beecham GW, Hiltunen M, Combarros O, Bullido MJ, Brouwers N, Bettens K, Berr C, Pasquier F, Richard F, Dekosky ST, Hannequin D, Haines JL, Tognoni G, Fievet N, Dartigues JF, Tzourio C, Engelborghs S, Arosio B, Coto E, De Deyn P, Del Zompo M, Mateo I, Boada M, Antunez C, Lopez-Arrieta J, Epelbaum J, Schjeide BM, Frank-Garcia A, Giedraitis V, Helisalmi S, Porcellini E, Pilotto A, Forti P, Ferri R, Delepine M, Zelenika D, Lathrop M, Scarpini E, Siciliano G, Solfrizzi V, Sorbi S, Spalletta G, Ravaglia G, Valdivieso F, Vepsalainen S, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Hanon O, Piccardi P, Annoni G, Mann D, Marambaud P, Seripa D, Galimberti D, Tanzi RE, Bertram L, Lendon C, Lannfelt L, Licastro F, Campion D, Pericak-Vance MA, Soininen H, Van Broeckhoven C, Alperovitch A, Ruiz A, Kamboh MI, Amouyel P. The CALHM1 P86L polymorphism is a genetic modifier of age at onset in Alzheimer’s disease: a meta-analysis study. J Alzheimers Dis. 2010;22:247–255. doi: 10.3233/JAD-2010-100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cruchaga C, Kauwe JS, Mayo K, Spiegel N, Bertelsen S, Nowotny P, Shah AR, Abraham R, Hollingworth P, Harold D, Owen MM, Williams J, Lovestone S, Peskind ER, Li G, Leverenz JB, Galasko D, Morris JC, Fagan AM, Holtzman DM, Goate AM. SNPs associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer’s disease. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.O’Meara ES, Kukull WA, Sheppard L, Bowen JD, McCormick WC, Teri L, Pfanschmidt M, Thompson JD, Schellenberg GD, Larson EB. Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am J Epidemiol. 1997;146:373–384. doi: 10.1093/oxfordjournals.aje.a009290. [DOI] [PubMed] [Google Scholar]
- 153.Tang MX, Maestre G, Tsai WY, Liu XH, Feng L, Chung WY, Chun M, Schofield P, Stern Y, Tycko B, Mayeux R. Effect of age, ethnicity, and head injury on the association between APOE genotypes and Alzheimer’s disease. Ann N Y Acad Sci. 1996;802:6–15. doi: 10.1111/j.1749-6632.1996.tb32593.x. [DOI] [PubMed] [Google Scholar]
- 154.Jordan BD, Relkin NR, Ravdin LD, Jacobs AR, Bennett A, Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. Jama. 1997;278:136–140. [PubMed] [Google Scholar]
- 155.Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, Barnes LL, Bienias JL. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 156.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. Jama. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 157.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65:1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 159.Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer’s disease. Biol Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 160.Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 162.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Youkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 163.Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, Games D, Hyman BT. Aβ deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. 1997;17:7053–7059. doi: 10.1523/JNEUROSCI.17-18-07053.1997. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F β-amyloid precursor protein and Alzheimer’s disease. 1996;16:5795–5811. doi: 10.1523/JNEUROSCI.16-18-05795.1996. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 166.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 167.Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 168.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 169.Hutton M. Molecular genetics of chromosome 17 tauopathies. Ann NY Acad Sci. 2000;920:63–73. doi: 10.1111/j.1749-6632.2000.tb06906.x. [DOI] [PubMed] [Google Scholar]
- 170.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 171.Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- 172.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, PaulMurphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nature Genet; 2001;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 173.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kauwe JS, Cruchaga C, Mayo K, Fenoglio C, Bertelsen S, Nowotny P, Galimberti D, Scarpini E, Morris JC, Fagan AM, Holtzman DM, Goate AM. Variation in MAPT is associated with cerebrospinal fluid tau levels in the presence of amyloid-beta deposition. Proc Natl Acad Sci U S A. 2008;105:8050–8054. doi: 10.1073/pnas.0801227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 176.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 177.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 178.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 179.Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27:570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 180.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatsos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 183.Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 185.Chin J, Palop JJ, Yu GQ, Kojima N, Masliah E, Mucke L. Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J Neurosci. 2004;24:4692–4697. doi: 10.1523/JNEUROSCI.0277-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 187.Cisse M, Halabisky B, Harris J, Devidze N, Dubal DB, Sun B, Orr A, Lotz G, Kim DH, Hamto P, Ho K, Yu GQ, Mucke L. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature. 2011;469:47–52. doi: 10.1038/nature09635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175–193. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid- attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 192.Bard F, Cannon C, Barbour R, Burke R-L, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk DYT. Peripherally administered antibodies against amyloid b-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer’s disease. Nature Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 193.DeMattos RB, Bales KR, Cummins DJ, Dodart J-C, Paul SM, Holtzman DM. Peripheral anti-Ab antibody alters CNS and plasma Ab clearance and decreases brain Ab burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. 8810.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HTJ, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. Ab peptide immunization reduced behavioural impairment and plaques in a model of Alzheimer’ s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 195.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, Dicarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. Ab peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 196.Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Issazadeh S, Hancock WW, Selkoe DJ. Nasal administration of amyloid-b peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- 197.Brendza RP, Bacskai BJ, Cirrito JR, Simmons KA, Skoch JM, Klunk WE, Mathis CA, Bales KR, Paul SM, Hyman BT, Holtzman DM. Anti-Abeta antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J Clin Invest. 2005 doi: 10.1172/JCI23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 199.Holtzman DM. Alzheimer’s disease: Moving towards a vaccine. Nature. 2008;454:418–420. doi: 10.1038/454418a. [DOI] [PubMed] [Google Scholar]
- 200.Sydow A, Van der Jeugd A, Zheng F, Ahmed T, Balschun D, Petrova O, Drexler D, Zhou L, Rune G, Mandelkow E, D’Hooge R, Alzheimer C, Mandelkow EM. Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic tau mutant. J Neurosci. 2011;31:2511–2525. doi: 10.1523/JNEUROSCI.5245-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Terwel D, Muyllaert D, Dewachter I, Borghgraef P, Croes S, Devijver H, Van Leuven F. Amyloid activates GSK-3beta to aggravate neuronal tauopathy in bigenic mice. Am J Pathol. 2008;172:786–798. doi: 10.2353/ajpath.2008.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 203.Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, Marcus D, Morris JC, Holtzman DM. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, Foster NL, Petersen RC, Weiner MW, Price JC, Mathis CA. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D’Angelo G, Malone JP, Townsend RR, Morris JC, Fagan AM, Holtzman DM. YKL-40: A Novel Prognostic Fluid Biomarker for Preclinical Alzheimer’s Disease. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.08.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 207.Jack CR, Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, Bernstein MA, Gunter JL, Pankratz VS, Aisen PS, Weiner MW, Petersen RC, Shaw LM, Trojanowski JQ, Knopman DS. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 209.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 211.Gomez-Isla T, Price JL, McKeel DW, Morris J, Growdon CJH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s diseas. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aisen PS, Andrieu S, Sampaio C, Carrillo M, Khachaturian ZS, Dubois B, Feldman HH, Petersen RC, Siemers E, Doody RS, Hendrix SB, Grundman M, Schneider LS, Schindler RJ, Salmon E, Potter WZ, Thomas RG, Salmon D, Donohue M, Bednar MM, Touchon J, Vellas B. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011;76:280–286. doi: 10.1212/WNL.0b013e318207b1b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Aisen PS. Alzheimer’s disease therapeutic research: the path forward. Alzheimers Res Ther. 2009;1:2. doi: 10.1186/alzrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bateman RJ, Siemers ER, Mawuenyega KG, Wen G, Browning KR, Sigurdson WC, Yarasheski KE, Friedrich SW, Demattos RB, May PC, Paul SM, Holtzman DM. A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]