Abstract
The aim of the present work was to compare the genomes of 21 strains of intestinal spirochetes, which were isolated from patients suffering intestinal disorders, with those of Treponema hyodysenteriae (strain P18), the known etiological agent of swine dysentery (bloody scours), and of a nonpathogenic strain (M1) of Treponema innocens. The percent guanine-plus-cytosine value of the 23 DNAs was found to be 25.5 to 30.1, as determined by a double-labeling procedure based on nick-translation by DNA polymerase I. The genome size of two spirochetal strains, of human and porcine origin, was found to be similar (4 x 10(6) base pairs) and close to that of the reference bacterium Escherichia coli (4.2 x 10(6) base pairs). Restriction analysis showed the presence of two modified bases in spirochetal DNA. Methyladenine was present in the GATC sequence of DNA from 15 spirochetes of human origin, and methylcytosine was present in several sequences occurring in all strains. The DNA of T. hyodysenteriae displayed a 30 to 100% homology with respect to that of 21 spirochetes from humans, thus suggesting the occurrence of a genetic heterogeneity in the latter group. These data indicate that the intestinal spirochetes analyzed in the present work are related; hence there is a possibility of domestic animals being reservoirs of microorganisms pathogenic for humans. A classification of intestinal treponemes into subgroups has been proposed on the basis of restriction analysis and hybridization experiments.
Full text
PDF


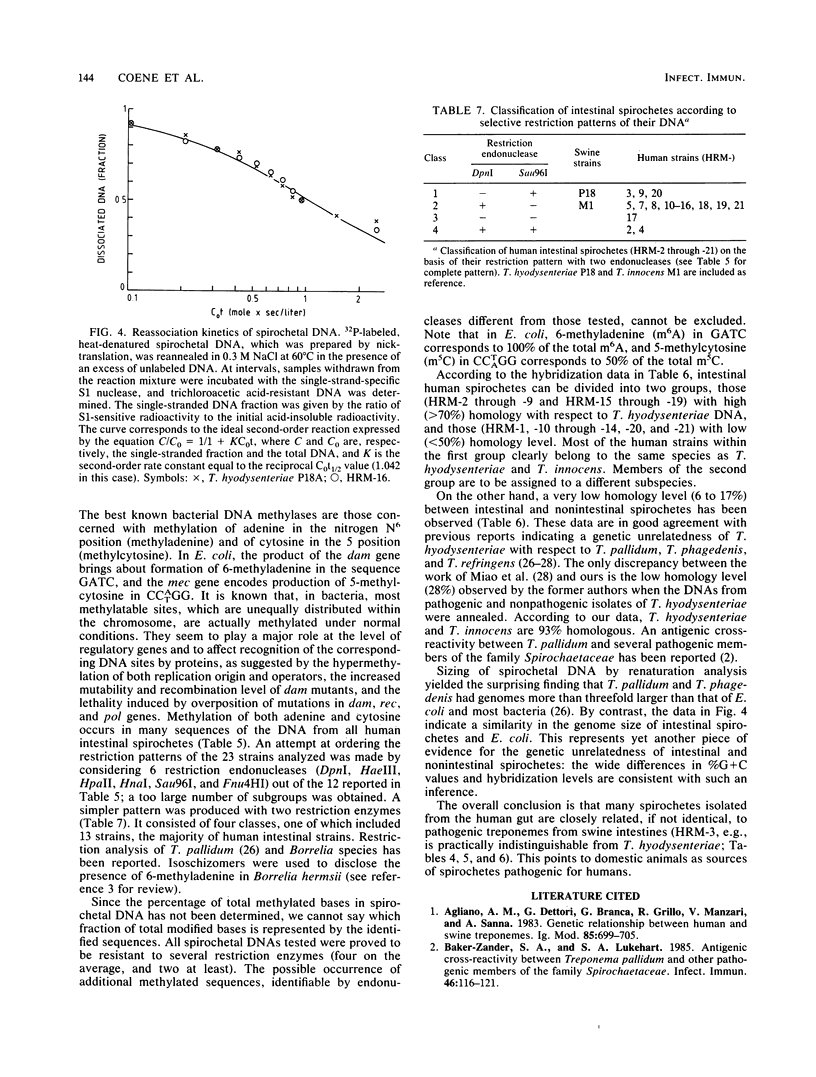

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker-Zander S. A., Lukehart S. A. Antigenic cross-reactivity between Treponema pallidum and other pathogenic members of the family Spirochaetaceae. Infect Immun. 1984 Oct;46(1):116–121. doi: 10.1128/iai.46.1.116-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F. Biology of Borrelia species. Microbiol Rev. 1986 Dec;50(4):381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coene M., Cocito C. A microanalytical procedure for determination of the base composition of DNA. Eur J Biochem. 1985 Aug 1;150(3):475–479. doi: 10.1111/j.1432-1033.1985.tb09046.x. [DOI] [PubMed] [Google Scholar]
- Davis C. P., McAllister J. S., Savage D. C. Microbial colonization of the intestinal epithelium in suckling mice. Infect Immun. 1973 Apr;7(4):666–672. doi: 10.1128/iai.7.4.666-672.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Mulcahy D., Takeuchi A., Savage D. C. Location and description of spiral-shaped microorganisms in the normal rat cecum. Infect Immun. 1972 Aug;6(2):184–192. doi: 10.1128/iai.6.2.184-192.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettori G., Amalfitano G., Polonelli L., Rossi A., Grillo R., Plaisant P. Electron microscopy studies of human intestinal spirochetes. Eur J Epidemiol. 1987 Jun;3(2):187–195. doi: 10.1007/BF00239758. [DOI] [PubMed] [Google Scholar]
- Douglas J. G., Crucioli V. Spirochaetosis: a remediable cause of diarrhoea and rectal bleeding? Br Med J (Clin Res Ed) 1981 Nov 21;283(6303):1362–1362. doi: 10.1136/bmj.283.6303.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland W. A., Lee F. D. Intestinal spirochaetosis. Br Med J. 1967 Sep 16;3(5567):718–719. doi: 10.1136/bmj.3.5567.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Hottat F., Coene M., Cocito C. DNA methylation in leprosy-associated bacteria: Mycobacterium leprae and Corynebacterium tuberculostearicum. Med Microbiol Immunol. 1988;177(1):33–45. doi: 10.1007/BF00190309. [DOI] [PubMed] [Google Scholar]
- Hovind-Hougen K., Birch-Andersen A., Henrik-Nielsen R., Orholm M., Pedersen J. O., Teglbjaerg P. S., Thaysen E. H. Intestinal spirochetosis: morphological characterization and cultivation of the spirochete Brachyspira aalborgi gen. nov., sp. nov. J Clin Microbiol. 1982 Dec;16(6):1127–1136. doi: 10.1128/jcm.16.6.1127-1136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. R., Takeuchi A. Purulent rectal discharge associated with a nontreponemal spirochete. JAMA. 1979 Jan 5;241(1):52–53. [PubMed] [Google Scholar]
- Lemcke R. M., Burrows M. R. A comparative study of spirochaetes from the porcine alimentary tract. J Hyg (Lond) 1981 Apr;86(2):173–182. doi: 10.1017/s0022172400068881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier G. Vibrionic Dysentery of Swine in Ontario-Part I : 1. Clinical Aspects and Pathology. Can Vet J. 1962 Aug;3(8):228–237. [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Chiodini R. J., Hermon-Taylor J. Determination of genome size and DNA homology between an unclassified Mycobacterium species isolated from patients with Crohn's disease and other mycobacteria. J Gen Microbiol. 1987 Jan;133(1):211–214. doi: 10.1099/00221287-133-1-211. [DOI] [PubMed] [Google Scholar]
- Miao R. M., Fieldsteel A. H. Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J Bacteriol. 1980 Jan;141(1):427–429. doi: 10.1128/jb.141.1.427-429.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R. M., Fieldsteel A. H., Harris D. L. Genetics of Treponema: characterization of Treponema hyodysenteriae and its relationship to Treponema pallidum. Infect Immun. 1978 Dec;22(3):736–739. doi: 10.1128/iai.22.3.736-739.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K. Restriction analysis of DNA from Treponema pallidum, the causative agent of syphilis. Mol Gen Genet. 1983;191(1):126–131. doi: 10.1007/BF00330899. [DOI] [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Plasmid DNA in Treponema pallidum (Nichols): potential for antibiotic resistance by syphilis bacteria. Science. 1981 Jul 31;213(4507):553–555. doi: 10.1126/science.6264606. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Alexander T. J. The production of dysentery in swine by feeding cultures containing a spirochaete. Br Vet J. 1971 Nov;127(11):58–61. doi: 10.1016/s0007-1935(17)37282-2. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Blakemore W. F. Spirochaetal invasion of the colonic epithelium in swine dysentery. Res Vet Sci. 1971 Mar;12(2):177–179. [PubMed] [Google Scholar]
- Taylor D. J., Simmons J. R., Laird H. M. Production of diarrhoea and dysentery in pigs by feeding pure cultures of a spirochaete differing from Treponema hyodysenteriae. Vet Rec. 1980 Apr 12;106(15):326–332. doi: 10.1136/vr.106.15.326. [DOI] [PubMed] [Google Scholar]
- Tompkins D. S., Waugh M. A., Cooke E. M. Isolation of intestinal spirochaetes from homosexuals. J Clin Pathol. 1981 Dec;34(12):1385–1387. doi: 10.1136/jcp.34.12.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]