Abstract
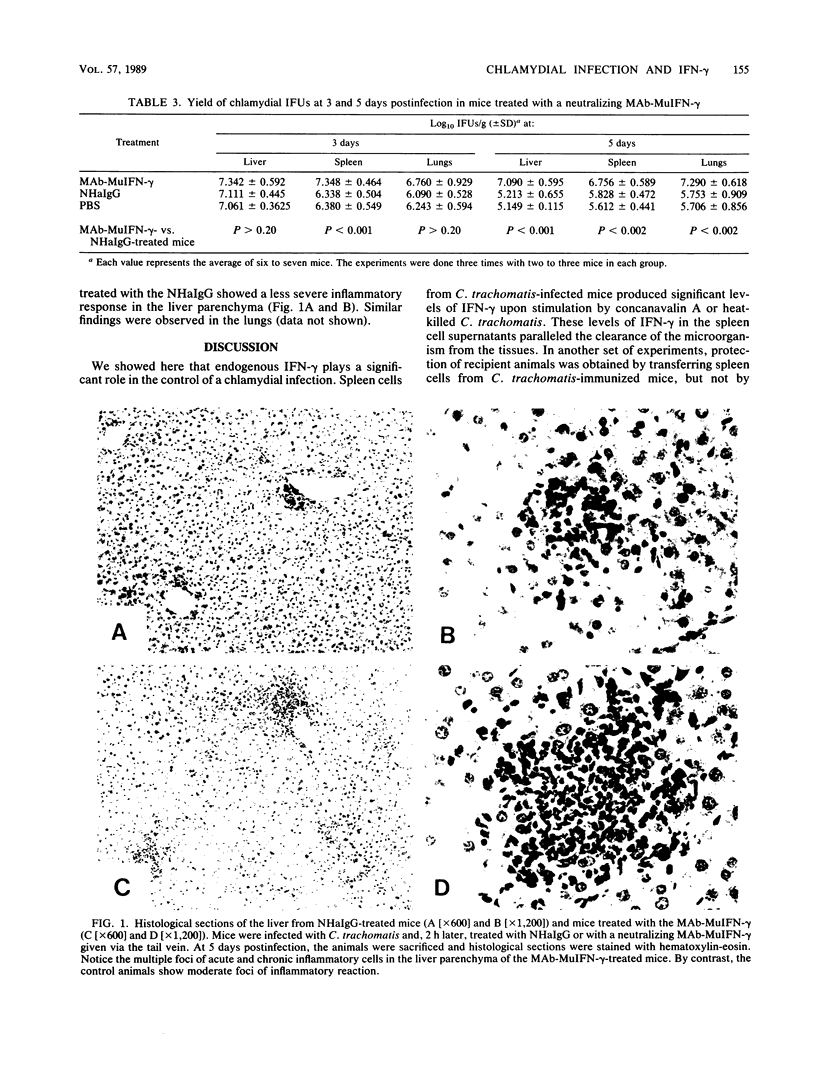
BALB/c mice (6 to 8 weeks old) infected with Chlamydia trachomatis serovar L1 were sacrificed, and the yield of Chlamydia inclusion-forming units from the liver and lungs was measured in HeLa 229 cells. The yield of inclusion-forming units reached a peak at 3 days postinfection and then progressively declined. The mice infected with C. trachomatis had no detectable levels of gamma interferon (IFN-gamma) in their sera. However, stimulation of their spleen cells with either concanavalin A or heat-killed C. trachomatis resulted in the release of high levels of IFN-gamma (600 to 900 IU/ml) at 5 to 8 days postinfection. The increased release of IFN-gamma from the spleen cells paralleled the clearance of chlamydia from the liver and lungs. Sera and spleen cells from animals immunized with live C. trachomatis were transferred to recipient mice that were subsequently challenged with C. trachomatis. Transfer of spleen cells resulted in a reduction of the infection in the recipient animal as measured by the yield of chlamydia from the spleen, but transfer of the sera did not confer protective immunity. In addition, mice infected with C. trachomatis serovar L1 were treated with a hamster neutralizing monoclonal antibody to recombinant murine IFN-gamma (MAb-MuIFN-gamma). In the animals receiving the MAb-MuIFN-gamma, the yield of chlamydia from the lungs, spleen, and liver was significantly higher than from the control groups of mice. Histopathological analysis of tissues from the chlamydia-infected mice showed that the animals treated with the MAb-MuIFN-gamma had a significantly more extensive inflammatory reaction in their lungs, liver, and spleen.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basham T. Y., Smith W. K., Merigan T. C. Interferon enhances antibody-dependent cellular cytotoxicity when suboptimal concentrations of antibody are used. Cell Immunol. 1984 Oct 15;88(2):393–400. doi: 10.1016/0008-8749(84)90172-2. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Remington J. S., Sharma S. D. Protective immunity in toxoplasmosis: correlation between antibody response, brain cyst formation, T-cell activation, and survival in normal and B-cell-deficient mice bearing the H-2k haplotype. Infect Immun. 1987 Apr;55(4):990–994. doi: 10.1128/iai.55.4.990-994.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Kuo C., Chen W. J. Systemic Chlamydia trachomatis infection in mice: a comparison of lymphogranuloma venereum and trachoma biovars. Infect Immun. 1985 Apr;48(1):78–82. doi: 10.1128/iai.48.1.78-82.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Inhibition of TRIC agents by virus-induced interferon. Proc Soc Exp Biol Med. 1966 Jun;122(2):417–421. doi: 10.3181/00379727-122-31150. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Spitalny G. L., Patel P. J. Enhanced production of murine interferon gamma by T cells generated in response to bacterial infection. J Exp Med. 1982 Jul 1;156(1):112–127. doi: 10.1084/jem.156.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkin H. M., Lu Y. K. Induction of interferon by the Bour strain of trachoma in HeLa 229 cells. Am J Ophthalmol. 1967 May;63(5 Suppl):1110–1115. doi: 10.1016/0002-9394(67)94091-3. [DOI] [PubMed] [Google Scholar]
- Kazar J., Gillmore J. D., Gordon F. B. Effect of Interferon and Interferon Inducers on Infections with a Nonviral Intracellular Microorganism, Chlamydia trachomatis. Infect Immun. 1971 Jun;3(6):825–832. doi: 10.1128/iai.3.6.825-832.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Jerrells T. R., Spitalny G. L., Walker D. H. Gamma interferon as a crucial host defense against Rickettsia conorii in vivo. Infect Immun. 1987 May;55(5):1252–1255. doi: 10.1128/iai.55.5.1252-1255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T., Seki H., Taga K., Taniguchi N. Interferon-gamma can augment expression ability of HLA-DR antigens on pokeweed mitogen-stimulated human T lymphocytes. Cell Immunol. 1984 Dec;89(2):300–309. doi: 10.1016/0008-8749(84)90332-0. [DOI] [PubMed] [Google Scholar]
- Oh J. O., Ostler H. B., Schachter J. Protective effect of a synthetic polynucleotide complex (poly I: C) on ocular lesions produced by trachoma agent in rabbits. Infect Immun. 1970 Jun;1(6):566–573. doi: 10.1128/iai.1.6.566-573.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata F., Wietzerbin J., Pons F. G., Falcoff E., Eisen H. Synergistic protection by specific antibodies and interferon against infection by Trypanosoma cruzi in vitro. Eur J Immunol. 1984 Oct;14(10):930–935. doi: 10.1002/eji.1830141013. [DOI] [PubMed] [Google Scholar]
- Richmond S. J., Caul E. O. Fluorescent antibody studies in chlamydial infections. J Clin Microbiol. 1975 Apr;1(4):345–352. doi: 10.1128/jcm.1.4.345-352.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueltenfuss E. A., Pollard M. Cytochemical Assay of Interferon Produced by Duck Hepatitis Virus. Science. 1963 Feb 15;139(3555):595–596. doi: 10.1126/science.139.3555.595. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Perez N., Arenzana-Seisdedos F., Devos R. Pure interferon gamma enhances class II HLA antigens on human monocyte cell lines. Eur J Immunol. 1984 Jan;14(1):106–108. doi: 10.1002/eji.1830140120. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Coalson J. J., Grubbs B. Cellular immunity to the mouse pneumonitis agent. J Infect Dis. 1984 Apr;149(4):630–639. doi: 10.1093/infdis/149.4.630. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Grubbs B., Sumaya C. V. The role of antibody in host defense against the agent of mouse pneumonitis. J Infect Dis. 1982 Feb;145(2):200–205. doi: 10.1093/infdis/145.2.200. [DOI] [PubMed] [Google Scholar]
- Zhong G. M., Peterson E. M., Czarniecki C. W., de la Maza L. M. Recombinant murine gamma interferon inhibits Chlamydia trachomatis serovar L1 in vivo. Infect Immun. 1988 Jan;56(1):283–286. doi: 10.1128/iai.56.1.283-286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Maza L. M., Goebel J. M., Czarniecki C. W., Peterson E. M. Ultrastructural analysis of the growth cycle of Chlamydia trachomatis in mouse cells treated with recombinant human alpha-interferons. Exp Mol Pathol. 1984 Oct;41(2):227–235. doi: 10.1016/0014-4800(84)90039-x. [DOI] [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M., Fennie C. W., Czarniecki C. W. The anti-chlamydial and anti-proliferative activities of recombinant murine interferon-gamma are not dependent on tryptophan concentrations. J Immunol. 1985 Dec;135(6):4198–4200. [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M., Goebel J. M., Fennie C. W., Czarniecki C. W. Interferon-induced inhibition of Chlamydia trachomatis: dissociation from antiviral and antiproliferative effects. Infect Immun. 1985 Mar;47(3):719–722. doi: 10.1128/iai.47.3.719-722.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



